Abstract
Background
Canine leishmaniosis caused by Leishmania infantum is an endemic disease in Spain. The dog is considered the main reservoir, and the detection of specific serum antibodies against L. infantum antigen is the most used technique for diagnosing this infection. The LEISCAN LEISHMANIA ELISA test is a commercialized enzyme‐linked immunosorbent assay for the detection and measurement of canine anti‐Leishmania serum antibodies.
Objectives
The aim of this study was to assess seroprevalence results of apparently healthy dogs in different areas of Spain using LEISCAN.
Methods
Collection of sera from 5451 apparently healthy dogs was performed between 2020 and 2021 in different areas of Spain. Dogs were of adult age (≥12 months), were not previously diagnosed with clinical leishmaniosis or vaccinated against Leishmania and did not present clinical signs compatible with L. infantum infection. LEISCAN was performed following the manufacturer's protocol.
Results
The overall seroprevalence was 5.5%. The highest seroprevalences were found in the Southeast of Spain: Comunidad Valenciana (14%) and Región de Murcia (14%), whereas the lowest seroprevalences were found in Northern Spain: Galicia (1%), Navarra (2%) and Castilla y León (2%) (p‐value <0.001).
Conclusions
In conclusion, the seroprevalence for L. infantum in apparently healthy dogs in Spain varied from almost no infection to being over 10%.
Keywords: canine, diagnosis, ELISA, leishmaniosis, seroprevalence
Graphical Abstract:
1. INTRODUCTION
Canine leishmaniosis caused by the protozoan Leishmania infantum is a zoonotic and endemic disease in Spain (Díaz‐Regañón et al., 2020; Gálvez et al., 2020; Montoya‐Alonso et al., 2020). Leishmania infantum is usually transmitted by the bite of a female phlebotomine sand fly following a digenetic cycle that alternates between two differentiated phases: (a) an extracellular and motile promastigote that colonizes the digestive tract of the vector sand fly and (b) an intracellular and non‐motile amastigote that colonizes the monocyte‐macrophage system of the vertebrate hosts (Dostálová & Volf, 2012). The dog is considered the main domestic and peridomestic reservoir for L. infantum infection in Spain (Dantas‐Torres, 2007; Solano‐Gallego et al., 2011), whereas other mammals, such as wild canids (Oleaga et al., 2018), rodents (Alcover et al., 2020) and lagomorphs (Molina et al., 2012), may be able to maintain a wild cycle.
Moreover, when a dog is infected, the development of clinical disease depends on the dog's immune response. Classically, two polarized outcomes have been described: (a) a protective T cell‐mediated immune response (the dog is infected but does not develop clinical disease) and (b) a non‐protective marked humoral immune response with a reduced or absent T cell mediated immunity (the dog develops clinical leishmaniosis) (Toepp & Petersen, 2020). Thus, a wide spectrum of clinical manifestations is documented in dogs with leishmaniosis (Solano‐Gallego et al., 2011). Furthermore, in endemic regions, such as Spain, there is a high prevalence of L. infantum infection in apparently healthy dogs (Baxarias, Homedes, et al., 2022; Müller et al., 2022), although the prevalence of clinical illness is frequently lower than 10% (Solano‐Gallego et al., 2009).
Detection of serum specific antibodies against Leishmania is the most frequently used technique for detecting infected dogs (Baxarias, Homedes, et al., 2022; Maurelli et al., 2020; Paltrinieri et al., 2016). Furthermore, as an anti‐Leishmania vaccine is available in Europe, serological screening is mandatory prior to vaccination in dogs (Solano‐Gallego et al., 2017). Several commercial serological techniques are available, such as immunochromatographic tests, enzyme‐linked immunosorbent assays (ELISA) and immunofluorescent antibody tests (IFI) (Baxarias, Homedes, et al., 2022; Maurelli et al., 2020; Miró et al., 2017; Paltrinieri et al., 2016; Solano‐Gallego et al., 2014). However, the interpretation of these techniques differs as ELISA and IFI allow the quantification of antibody levels, whereas immunochromatographic tests are only qualitative techniques and, thus, only give a positive or negative result (Maurelli et al., 2020; Solano‐Gallego et al., 2014). Regarding serological quantitative techniques, the difference lies in their interpretation as ELISA presents objective results depending on optical density (OD), whereas IFI presents subjective results depending on the operator's experience (Maurelli et al., 2020; Solano‐Gallego et al., 2014).
The LEISCAN LEISHMANIA ELISA test is an enzyme immunoassay for the detection and measurement of canine serum anti‐Leishmania antibodies (Rodríguez‐Cortés et al., 2013; Solano‐Gallego et al., 2014). Previous studies have evaluated LEISCAN in dogs and obtained good diagnostic sensitivity and specificity (Rodríguez‐Cortés et al., 2013; Solano‐Gallego et al., 2014).
The aim of this study was to assess seroprevalence results of apparently healthy dogs in different areas of Spain using LEISCAN.
2. MATERIALS AND METHODS
2.1. Dogs
Collection of sera from 5451 apparently healthy dogs was performed between June of 2020 and June of 2021 by veterinarians from 68 veterinary practices and 12 dog shelters in different areas of Spain (Table 1). The inclusion criteria of dogs enrolled were adult age (≥12 months), not have been previously diagnosed with clinical leishmaniosis nor vaccinated against Leishmania, and the absence of clinical signs based on clinical history and a full clinical examination that includes general appearance (physical body condition, mentation, posture), record of vital signs (temperature, pulse, heart and respiratory rates and capillary refill time) and to check all body from head to tail.
TABLE 1.
Seroprevalence and dubious rates of L. infantum infection in apparently healthy dogs classified by Spanish autonomous community.
| Autonomous community (number of dogs) | Bioclimate (Gálvez et al., 2020) | Number of veterinary practices and dog shelters (total) | Mean of the metres of altitude (±SD) | Percentage of seroprevalence (95%CI) | Percentage of dubious results (95%CI) |
|---|---|---|---|---|---|
| Andalucía (1234) a | TM, MM, SM, OM and CM | 11 and 2 (13) | 468.8 (±287.4) | 4.5 (3.4–5.9) | 2 (1.3–2.9) |
| Aragón (516) | MM, SM, ST and OT | 7 and 1 (8) | 625.3 (±356) | 6.7 (4.7–9.2) | 1.6 (0.7–3) |
| Islas Baleares (189) | TM and MM | 3 and 0 (3) | 64.9 (±40.6) | 7.4 (4.1–12.1) | 3.7 (1.5–7.5) |
| Castilla‐La Mancha (18) b | MM, SM, OM and CM | 2 and 0 (2) | 552.2 (±10) | 41.2 (18.4–67.1) | 5.6 (0.1–27.3) |
| Castilla y León (216) | MM, SM, OM and CM | 3 and 0 (3) | 873.2 (±259.4) | 1.9 (0.5–4.8) | 2.3 (0.8–5.3) |
| Cataluña (851)a | TM, MM, SM, ST and OT | 12 and 1 (13) | 240.2 (±221.7) | 3 (1.9–4.3) | 0.8 (0.3–1.7) |
| Comunidad de Madrid (358) | MM, SM, OM and CM | 6 and 1 (7) | 599.4 (±45.9) | 5.7 (3.5–8.6) | 1.1 (0.3–2.8) |
| Comunidad Valenciana (325) | TM, MM, SM, OM and CM | 5 and 1 (6) | 65.6 (163.9) | 13.9 (10.3–18.2) | 2.5 (1.1–4.8) |
| Extremadura (472) | MM | 4 and 0 (4) | 298 (±89.8) | 3.8 (2.3–6) | 0.8 (0.2–2.2) |
| Galicia (235) | SM, MT and ST | 2 and 1 (3) | 38.8 (±16.3) | 0.9 (0.1–3.1) | 0.4 (0–2.4) |
| Navarra (414) | SM, MT and ST | 3 and 3 (6) | 362 (± 67.9) | 2.4 (1.2–4.4) | 0.2 (0–1.3) |
| País Vasco (35) b | SM, MT and ST | 1 and 0 (1) | 500 (±0) | 5.9 (0.7–19.7) | 2.9 (0.1–14.9) |
| Región de Murcia (468) | TM, MM, SM, OM and CM | 7 and 1 (8) | 254.7 (±176.9) | 13.7 (10.7–17.3) | 3.6 (2.1–5.8) |
| La Rioja (120) | SM, MT and ST | 2 and 1 (3) | 383.3 (±66.7) | 2.5 (0.5–7.1) | 0 (0–3) |
| Total (5451) | 68 and 12 (80) | 373.3 (±294) | 5.5 (4.9–6.1) | 1.6 (1.3–2) |
Abbreviations: CI; confidence interval; CM, crioro‐Mediterranean; MM, meso‐Mediterranean; MT, mesotemperate; OM, oro‐Mediterranean; OT, orotemperate; SD, standard deviation; SM, supra‐Mediterranean; ST, supratemperate; TM, thermo‐Mediterranean.
The Spanish autonomous communities from which high numbers of dogs were included were Andalucía and Cataluña.
bThe significance of results obtained in País Vasco and Castilla‐La Mancha remains to be further studied due to the limited number of dogs collected in these regions.
2.2. Detection of anti‐Leishmania antibodies using LEISCAN
LEISCAN (Ecuphar Veterinaria SLU, Spain) was performed to detect anti‐L. infantum antibodies in serum following the manufacturer's protocol. Briefly, samples were diluted using the dilution solution included in the kit and incubated for 10 min at room temperature in 96‐well plates. Then, washes were performed five times with the diluted washing solution, and afterwards, 100 μL of conjugate were added in each well. After incubating the plate for another 5 min at room temperature, washes were repeated, and 100 μL of substrate were added to each well. Finally, after an incubation of 10 min at room temperature in the dark, stop reaction solution was added to the plate, and the results were read at 450 nm in a spectrophotometer (MB‐580 HEALES; Shenzhen Huisong Technology Development Co., Ltd, Shenzhen, China).
LEISCAN results were calculated using the following formula: ratio sample = OD sample/OD low control positive. Samples were classified following the protocol as positive (when the ratio sample was ≥1.1), dubious (when the ratio sample was ≥0.9 and <1.1) and negative (when the ratio sample was <0.9). Furthermore, when samples were positive, samples were further classified between low positive (when the ratio sample was ≥1.1 and <2.0) and high positive (when the ratio sample was ≥2.0).
2.3. Statistical analysis
The statistical analysis was performed using the package Stats for the software R i386 3.6.1 for Windows, using t test to compare the altitudes of the centres between the LEISCAN results (positive or negative) and using chi‐square tests to compare seroprevalence and antibody levels between autonomous communities, the different areas of Spain (North, South, East and West) and the type of centre that collected the samples (veterinary practice or dog shelter). A p‐value of <0.05 was considered statistically significant. Maps were created using the Free and Open Source QGIS 3.10.4 for Windows. Information about altitudes of the centres were collected from Google Earth Web (https://earth.google.com/web/).
3. RESULTS
The overall seroprevalence and dubious results of the 5451 dogs and their geographical distribution are shown in Table 1. The highest seroprevalences were found in the Southeast of Spain: Comunidad Valenciana and Región de Murcia, whereas the lowest seroprevalences were found in Northern Spain: Galicia, Navarra and Castilla‐León (Table 1) (Figure 1) (chi‐square: χ 2 = 88.96, df = 1, p < 0.001). Furthermore, 170 (56.7%) of the seropositive dogs were considered low positive, whereas 130 (43.3%) were high positive. No differences in antibody levels were observed among dogs from different Spanish regions (p > 0.05).
FIGURE 1.
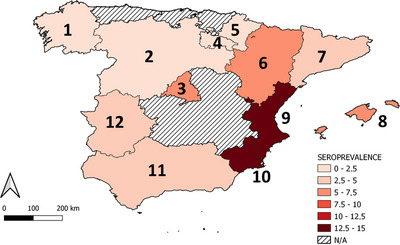
Geographical distribution of L. infantum seroprevalence in apparently healthy dogs in Spain: (1) Galicia, (2) Castilla y León, (3) Comunidad de Madrid, (4) La Rioja, (5) Navarra, (6) Aragón, (7) Cataluña, (8) Islas Baleares, (9) Comunidad Valenciana, (10) Región de Murcia, (11) Andalucía, (12) Extremadura. N/A, not applicable.
The majority of centres that collected samples were veterinary practices (68/80, 85%), whereas only a few were dog shelters (12/80; 15%). The geographical distribution of centres is shown in Table 1. The number of veterinary practices and dog shelters was similar in all regions studied (p > 0.05). Practitioners collected samples from 4733 apparently healthy dogs (86.8% of the total), whereas dog shelters collected 718 samples (13.2%). The majority of dog shelter samples were collected in Andalucía (147, 20.5%) and Navarra (137, 19.1%). Furthermore, there was a higher proportion of dogs sampled in dog shelters in Northern Spain (243, 28.1%) when compared to the Southeast of Spain (111, 13.7%) (chi‐square: χ 2 = 52.14, df = 1, p < 0.001). No differences in seroprevalence were detected between dogs from veterinary practices and dogs from shelters (p > 0.05).
The mean altitude depending on the geographical distribution of the veterinary practices is also shown in Table 1. The highest altitudes were found in Castilla‐León (over 800 m) and Aragón (over 600 m), whereas the lowest altitudes were found in Galicia (under 40 m), Islas Baleares (under 70 m) and Comunidad Valenciana (a under 70 m) (Table 1). No differences in altitude were detected when comparing between seropositive and seronegative dogs (p > 0.05).
4. DISCUSSION
The seroprevalence of L. infantum infection in dogs in Spain has been previously investigated, and seroprevalences of around 10% between 2011 and 2020 have been described (Table 2) (Díaz‐Regañón et al., 2020; Gálvez et al., 2020; Miró et al., 2022; Montoya‐Alonso et al., 2020). The seroprevalence in the present study (5.5%) is lower than expected. However, the reason could be explained by the inclusion criteria as only apparently healthy dogs with no clinical signs were included in the study as it is well known that sick dogs presented higher seroprevalence when compared with apparently healthy dogs (Solano‐Gallego et al., 2006). Besides, some regions (País Vasco and Castilla‐La Mancha) sampled a limited number of dogs, and thus, the results obtained were not representative of the specific region. Furthermore, dubious results were not considered positive results and; therefore, the seroprevalence was lower in all Spanish regions. In addition, it is likely that the differences observed on seroprevalences might also be due to the variable diagnostic performance of previous studies performed (Maurelli et al., 2020; Rodríguez‐Cortés et al., 2013; Solano‐Gallego et al., 2014).
TABLE 2.
Seroprevalence rates of L. infantum infection in dogs in different countries with similar macroclimates.
| Country | Macroclimate (European Environment Agency, 2017) | Number of dogs | Type of dogs | Percentage of seroprevalence (serological test) (%) | References |
|---|---|---|---|---|---|
| Albania | Mediterranean and alpine | 602 | Dogs with and without clinical signs | 5.1 (IFI) | Hamel et al. (2016) |
| 308 | Dogs with and without clinical signs | 3.2 (ELISA) | Myrseli et al. (2016) | ||
| Algeria | Mediterranean and desertic | 4812 | Symptomatic and asymptomatic dogs | 21.2 (ELISA, IFI, DAT, rapid test) | Touhami et al. (2023) |
| 227 | Dogs with and without clinical signs | 35.7 (IFI, rapid test) | Medkour et al. (2020) | ||
| Bosnia and Herzegovina | Mediterranean, continental and alpine | 180 | No data | 16.7 (IFI) | Colella et al. (2019) |
| 172 | No data | 11 (ELISA) | Miró et al. (2022) | ||
| Croatia | Mediterranean and alpine | 200 | Asymptomatic dogs | 8 (IFI) | Zivicnjak et al. (2007) |
| 1761 | No data | 7 (ELISA) | Miró et al. (2022) | ||
| 435 | Apparently healthy dogs | 1.4 (rapid test) | Mrljak et al. (2017) | ||
| Cyprus | Mediterranean | 278 | Symptomatic and asymptomatic dogs | 1.1 (IFI) | Beyhan et al. (2016) |
| 281 | Symptomatic and asymptomatic dogs | 3.6 (IFI) | Çanakçı et al. (2016) | ||
| Egypt | Mediterranean and desertic | 450 | Asymptomatic dogs | 21.3 (ELISA) | Selim et al. (2021) |
| France | Mediterranean, Atlantic, continental and alpine | 80 | Symptomatic and asymptomatic dogs | 6.2 (IFI) | Davoust et al. (2013) |
| 5307 | No data | 8.7 (ELISA) | Miró et al. (2022) | ||
| Greece | Mediterranean | 2620 | Asymptomatic dogs | 20 (ELISA, IFI) | Athanasiou et al. (2012) |
| 9568 | No data | 18.5 (ELISA) | Miró et al. (2022) | ||
| Iran | Alpine, anatolian and steppic | 508 | Asymptomatic dogs | 23.4 (DAT) | Barati et al. (2015) |
| 384 | Symptomatic and asymptomatic dogs | 17.4 (DAT) | Moshfe et al. (2008) | ||
| Israel | Mediterranean and desertic | 60 | Dogs with and without clinical signs | 37 (ELISA) | Baneth et al. (2020) |
| Italy | Mediterranean and continental | 90,532 | No data | 11.9 (ELISA) | Miró et al. (2022) |
| 13,292 | Dogs with and without clinical signs | 6.7 (IFI) | Rombolà et al. (2021) | ||
| Kosovo | Mediterranean, continental and alpine | 125 | Dogs with and without clinical signs | 18.4 (ELISA) | Xhekaj et al. (2020) |
| 285 | Asymptomatic dogs | 4.2 (ELISA) | Xhekaj et al. (2023) | ||
| Malta | Mediterranean | 289 | No data | 15.9 (ELISA) | Miró et al. (2022) |
| Montenegro | Mediterranean and alpine | 1500 | Dogs with suspected leishmaniosis | 83 (IFI) | Andric et al. (2013) |
| Morocco | Mediterranean and desertic | 1514 | No data | 15.8 (rapid test) | El Berbri et al. (2020) |
| 3900 | Dogs with and without clinical signs | 14.9 (ELISA, IFI, rapid test | El‐Mouhdi et al. (2022) | ||
| Portugal | Mediterranean and Atlantic | 1860 | Dogs with and without clinical signs | 12.5 (DAT) | Almeida et al. (2022) |
| 1329 | No data | 13.8 (ELISA) | Miró et al. (2022) | ||
| Slovenia | Mediterranean, continental and alpine | 80 | Asymptomatic dogs | 3.7 (ELISA) | Dumitrache et al. (2016) |
| 465 | Asymptomatic dogs | 1.9 (ELISA) | Kotnik et al. (2021) | ||
| Spain | Mediterranean and Atlantic | 556 | Dogs with and without clinical signs suggestive of vector‐borne diseases | 9 (rapid test) | Díaz‐Regañón et al. (2020) |
| 1076 | Dogs with and without clinical signs | 8.1 (IFI) | Gálvez et al. (2010) | ||
| 1739 | Dogs with and without clinical signs | 10.1 (IFI) | Gálvez et al. (2020) | ||
| 439 | Dogs with and without clinical signs | 13 (IFI) | Martín‐Sánchez et al. (2009) | ||
| 1100 | Dogs with and without clinical signs | 15.7 (rapid test) | Miró et al. (2013) | ||
| 98,737 | No data | 10.7 (ELISA) | Miró et al. (2022) | ||
| 4643 | No data | 10.4 (rapid test) | Montoya‐Alonso et al. (2020) | ||
| 466 | Dogs with and without clinical signs | 30 (ELISA) | Solano‐Gallego et al. (2006) | ||
| Tunisia | Mediterranean and desertic | 317 | Dogs with and without clinical signs | 58.3 (IFI) | Bouattour et al. (2021) |
| Turkey | Mediterranean, black sea and anatolian | 490 | Dogs with and without clinical signs | 5.3 (IFI, DAT) | Ozbel et al. (2000) |
Abbreviations: DAT, direct agglutination test; ELISA, enzyme‐linked immunosorbent assay; IFI, indirect immunofluorescence assay.
Previous serosurveys performed in dogs from other countries have documented different results (Table 2). In a study performed in Northwestern Iran (Barati et al., 2015), a higher seroprevalence in asymptomatic dogs (23%) was observed when compared to previous studies (17%) performed in the dog population in the same area (Moshfe et al., 2008). On the other hand, in a serosurvey performed in asymptomatic dogs in Kosovo, an overall seroprevalence of 4% was observed (Xhekaj et al., 2023), which was lower than the seroprevalence detected in both healthy and sick dogs in previous years (18%) (Xhekaj et al., 2020). In the present study, a similar result to Kosovo (Xhekaj et al., 2020, 2023) was observed as the overall seroprevalence (5.5%) was lower than the previous seroprevalences (around 10%) found in the Spanish dog population (Díaz‐Regañón et al., 2020; Gálvez et al., 2020; Miró et al., 2022; Montoya‐Alonso et al., 2020).
In terms of specific investigated regions, previous serological surveys in Spain have documented similar results, detecting lower seroprevalences in the North of Spain and higher in the Southeast (Díaz‐Regañón et al., 2020; Gálvez et al., 2020; Montoya‐Alonso et al., 2020) which is also the nearest region to the Mediterranean. However, the results found in Islas Baleares are lower than expected (7.4%) when compared to previous studies that found seroprevalences of around 20% (Baxarias, Viñals, et al., 2022; Gálvez et al., 2020; Montoya‐Alonso et al., 2020; Solano‐Gallego et al., 2001, 2006). This could be explained with the same reasons as the lower overall seroprevalence: only sampling apparently healthy dogs, not considering dubious results as positive and the test performed. Furthermore, the samples were collected all year around in the present study, whereas in previous studies, all the samples were collected in a specific time of the year (Baxarias, Viñals, et al., 2022; Solano‐Gallego et al., 2001) or, conversely, were collected for various years (Gálvez et al., 2020; Montoya‐Alonso et al., 2020). These results highlight the need to use preventive measures against L. infantum in any region of Spain (Baxarias, Homedes, et al., 2022; Miró et al., 2017) as well as to perform an annual health check‐up and serology for the detection of anti‐Leishmania antibodies in dogs living in L. infantum‐endemic countries (Miró et al., 2017; Solano‐Gallego et al., 2017).
Regarding the antibody levels of the seropositive dogs, surprisingly, there were similar percentages of low (57%) and high (43%) positive dogs. Apparently healthy dogs usually present low anti‐Leishmania antibody levels, whereas dogs with clinical leishmaniosis present high antibody levels (Solano‐Gallego et al., 2017, 2006). However, these percentages could be also affected by not considering dubious results as positive dogs and the test performed. Other tests with higher sensitivities (Solano‐Gallego et al., 2014) could detect a proportion of these dubious results as very low seropositive dogs increasing the number of low positive dogs of the study.
Interestingly, it has been described that owned dogs usually have a lower risk of infection than dogs living in dog shelters or kennels (Rombolà et al., 2021), which could be associated to environmental factors such as living outdoors, although it has also been described the opposite, being dogs living in kennels less likely to present L. infantum infection (Tamponi et al., 2021). In the present study, no differences in seroprevalence were detected between owned dogs from veterinary practices and dogs from shelters. However, a higher percentage of samples from dogs living in dog shelters was observed in Northern Spain, which was also the region with the lowest seroprevalences and could be affecting the overall seroprevalence of dog shelters. Another important factor could be that owned dogs are more frequently tested in the clinical setting (for clinical suspicion or annual health check‐up) than dogs living in dog shelters (usually only sampled at kennel admittance) (Rombolà et al., 2021). In endemic areas, it is appropriate to screen dogs for L. infantum antibodies at least every 6–12 months (Solano‐Gallego et al., 2009, 2017).
Regarding the vector importance over seroprevalences, the distribution and density of sand flies in a region affect directly the transmission of L. infantum and the rate of infected dogs, and thus, changes in the vector distribution can determine changes in the seroprevalence of a region (Ballart et al., 2014; Díaz‐Sáez et al., 2021). For example, in recent studies, phlebotomine sand flies have been described to be able to maintain L. infantum infection in regions above 1300 m (Díaz‐Sáez et al., 2021), which are higher altitudes than previously reported and, therefore, could be able to infect animal populations that were not previously affected by L. infantum (Alten et al., 2016; Hartemink et al., 2011). However, in the present study, all sampled areas were under 1000 m of altitude, and no differences were detected among different altitudes. Another factor that could also impact vector density and species is the bioclimate of the region and its climate changes (Ballart et al., 2014; Semenza & Suk, 2018). Spain presents several bioclimates such as supratemperate and mesotemperate in the North and meso‐Mediterranean and thermo‐Mediterranean in the Southeast (Ballart et al., 2014; Gálvez et al., 2020). For example, Ballart et al. (2014) reported that Phlebotomus perniciosus is mainly present in meso‐Mediterranean and supra‐Mediterranean bioclimates, whereas Phlebotomus ariasi preferred coline, subalpine and montane bioclimates. In the present study, higher seroprevalences were detected in the Southeast of Spain (meso‐Mediterranean bioclimate) and could be related to higher densities of sand flies in this region as previously reported (Ballart et al., 2014).
Dog populations of countries that have a Mediterranean macroclimate in some regions present a similar seropositivity to L. infantum with a range from 4% to 20% (Table 2). For example, Spain, Portugal, France and Italy have similar seroprevalence rates of around 10%. Interestingly, Greece, which is the country that only presents a Mediterranean macroclimate, reports higher seroprevalence rates of 20% (Table 2).
This study presents some limitations. First, there were some Spanish regions with a limited number of sampled dogs which could not be truly representative of that region. Second, there was access to limited information about the sampled dogs, and therefore, statistical analysis of dog characteristics such as age or breed was not possible to perform. Finally, LEISCAN protocol classified some samples as dubious and recommended to repeat the test in 6 months which was not possible in this study. Even when repeating the test with the same sample, the majority of samples presented a second dubious result which could not be further classified as positive and negative.
In conclusion, the seroprevalence for L. infantum as detected by the ELISA technique used in apparently healthy dogs in Spain varied from almost no infection in the Northern areas of Spain to being over 10% in the Southeast close to the Mediterranean basin. These results highlight the need to use preventive measures against L. infantum in any region of Spain and to perform an annual check‐up that includes a quantitative serological test.
AUTHOR CONTRIBUTIONS
Conceptualization; data curation; formal analysis; investigation; methodology; project administration; writing – original draft; writing – review and editing: Marta Baxarias. Funding acquisition; methodology; project administration; resources; supervision: Cristina Mateu. Investigation; resources; writing – review and editing: Guadalupe Miró. Conceptualization; funding acquisition; project administration; resources; supervision; validation; writing – review and editing: Laia Solano Gallego.
CONFLICT OF INTEREST STATEMENT
CM has financial competing interests as they receive a salary from Ecuphar Veterinaria SLU which markets and LEISCAN.
FUNDING INFORMATION
Ecuphar veterinaria SLU
ETHICS STATEMENT
Study authorization was obtained from the Spanish authority, Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) with the authorization number 008/EPA‐2383ESP.
CONSENT TO PARTICIPATE
Consent was obtained from the owner or the tutor of the dog(s) to collect the sample and perform LEISCAN.
TRANSPARENT PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1172.
ACKNOWLEDGEMENTS
This work was founded by Ecuphar Veterinaria SLU. The authors would like to thank the technicians of Ecuphar Veterinaria SLU that contacted all the shelters and the veterinary practices that participated in the study: Albaitari Clínica Veterinaria (País Vasco, Spain), Albayda Centro Veterinario (Andalucía, Spain), Alta Paterna Clínica Veterinaria (Comunidad Valenciana, Spain), Rioja Centro Veterinario (La Rioja, Spain), Animalex Clínica Veterinaria (Andalucía, Spain), Animalia Servet (Castilla y León, Spain), Animals! Serveis Veterinaris (Islas Baleares, Spain), Belén Carasa Clínica Veterinaria (Navarra, Spain), Bestioles Centre Veterinari (Cataluña, Spain), Bichos Clínica Veterinaria (Comunidad de Madrid, Spain), Bocairent Clínica Veterinaria (Comunidad Valenciana, Spain), Buztintxuri Clínica Veterinaria (Navarra, Spain), Caballero Clínica Veterinaria (Cataluña, Spain), Caminando con ellos Protectora (Región de Murcia, Spain), Centre d'Atenció d'Animals Domèstics (CAAD) del Maresme (Cataluña, Spain), Centro Comarcal de Protección Animal Ribera Baja Navarra (Navarra, Spain), Centro de Acogida de Animales (CAA) de Logroño (La Rioja, Spain), Centro de Protección Animal (CPA) de Zaragoza (Aragón, Spain), Centro de Protección Animal (CPA) del Gobierno de Navarra (Navarra, Spain), Ciempozuelos Clínica Veterinaria (Comunidad de Madrid, Spain), Clinivex Clínica Veterinaria (Extremadura, Spain), CVR Clínica Veterinaria (Aragón, Spain), Del Pla Clínica Veterinaria (Cataluña, Spain), Desvern Clínica Veterinaria (Cataluña, Spain), Dingo Clínica Veterinaria (Región de Murcia, Spain), El Acebo Clínica Veterinaria (Comunidad de Madrid, Spain), Elena Saenz Clínica Veterinaria (Aragón, Spain), Faunivet Clínica Veterinaria (Comunidad Valenciana, Spain), Francisco J Ramos Clínica Veterinaria (Extremadura, Spain), Fundación Benjamin Menhert (Andalucía, Spain), Galápago Clínica Veterinaria (Andalucía, Spain), Germanias Clínica Veterinaria (Comunidad Valenciana, Spain), Hospital Clínico Veterinario de la Facultad de Veterinaria de la Universidad Complutense de Madrid (UCM) (Comunidad de Madrid, Spain), Huellas Clínica Veterinaria (Galicia, Spain), Infantes Clínica Veterinaria (Castilla y León, Spain), Jaca Clínica Veterinaria (Aragón, Spain), Jara Clínica Veterinaria (Andalucía, Spain), La Casa Morada Clínica Veterinaria (Andalucía, Spain), La Corraliza Clínica Veterinaria (Navarra, Spain), La Reserva Protectora (Andalucía, Spain), Las Cigueñas Clínica Veterinaria (La Rioja, Spain), Lazareto (Navarra, Spain), Los Dolores Clínica Veterinaria (Región de Murcia, Spain), Malaka Clínica Veterinaria (Andalucía, Spain), Maresme Hospital Veterinari (Cataluña, Spain), Mataró Serveis Veterinaris (Cataluña, Spain), Miman's Hospital Veterinari (Cataluña, Spain), Mimascota Clínica Veterinaria (Castilla y León, Spain), Mirovet Clínica Veterinaria (Castilla‐La Mancha, Spain), Mistercan Clínica Veterinaria (Andalucía, Spain), Mivet Hospital Veterinari Manacor (Islas Baleares, Spain), Modepran Protectora (Comunidad Valenciana, Spain), Montaña del Sol Clínica Veterinaria (Andalucía, Spain), Monte Aloia Protectora (Galicia, Spain), Myvet Clínica Veterinaria (Aragón, Spain), Navalvet Clínica Veterinaria (Comunidad de Madrid, Spain), Navia Clínica Veterinaria (Galicia, Spain), Nutrizoo Clínica Veterinaria (Región de Murcia, Spain), Otto Can Coria Clínica Veterinaria (Extremadura, Spain), Otto Can Moraleja Clínica Veterinaria (Extremadura, Spain), Pacífico Clínica Veterinaria (Comunidad de Madrid, Spain), Pawspatas Clínica Veterinaria (Región de Murcia, Spain), Perrikus (Comunidad de Madrid, Spain), Pratdesaba Veterinaris Malla (Cataluña, Spain), Provença Clínica Veterinaria (Cataluña, Spain), Quipar Clínica Veterinaria (Región de Murcia, Spain), Rio Veral Clínica Veterinaria (Aragón, Spain), Safracan Clínica Veterinaria (Región de Murcia, Spain), San Bernardo Clínica Veterinaria (Comunidad Valenciana, Spain), San Francisco Hospital Veterinari (Andalucía, Spain), Santa Perpètua Clínica Veterinaria (Cataluña, Spain), Sevilla Clínica Veterinaria (Andalucía, Spain), Teruel Clínica Veterinaria (Aragón, Spain), Urgell Hospital Veterinari (Cataluña, Spain), Utrizoo Clínica Veterinaria (Aragón, Spain), Veteralia Movet (Cataluña, Spain), Victor Serena Clínica Veterinaria (Región de Murcia, Spain), Viladecans Clínica Veterinaria (Cataluña, Spain), Vilazoo Clínica Veterinaria (Islas Baleares, Spain) and Zeus Clínica Veterinaria (Región de Murcia, Spain).
Baxarias, M. , Mateu, C. , Miró, G. , & Solano‐Gallego, L. (2023). Serological survey of Leishmania infantum in apparently healthy dogs in different areas of Spain. Veterinary Medicine and Science, 9, 1980–1988. 10.1002/vms3.1172
DATA AVAILABILITY STATEMENT
The data is available from the corresponding author on reasonable request.
REFERENCES
- Alcover, M. M , Ribas, A. , Guillén, M. C , Berenguer, D. , Tomás‐Pérez, M. , Riera, C. , & Fisa, R. (2020). Wild mammals as potential silent reservoirs of Leishmania infantum in a Mediterranean area. Preventive Veterinary Medicine, 175, 104874. [DOI] [PubMed] [Google Scholar]
- Almeida, M. , Maia, C. , Cristóvão, J M. , Morgado, C. , Barbosa, I. , Ibars, R. F. , Campino, L. , Gonçalves, L. , & Cortes, S. (2022). Seroprevalence and risk factors associated with Leishmania infection in dogs from Portugal. Microorganisms, 10, 2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alten, B. , Maia, C. , Afonso, M. O. , Campino, L. , Jiménez, M. , González, E. , Molina, R. , Bañuls, A. L. , Prudhomme, J. , Vergnes, B. , Toty, C. , Cassan, C. , Rahola, N. , Thierry, M. , Sereno, D. , Bongiorno, G. , Bianchi, R. , Khoury, C. , Tsirigotakis, N. , … Dokianakis, E. (2016). Seasonal dynamics of Phlebotomine sand fly species proven vectors of Mediterranean leishmaniasis caused by Leishmania infantum . PLoS Neglected Tropical Diseases, 10, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andric, B. , Terzic, D. , Dupanovic, B. , & Andric, A. (2013). Public health aspects of visceral leishmaniasis in Montenegro. Open Journal of Clinical Diagnostics, 3, 195–201. [Google Scholar]
- Athanasiou, L. V. , Kontos, V. I. , Saridomichelakis, M. N. , Rallis, T. S. , & Diakou, A. (2012). A cross‐sectional sero‐epidemiological study of canine leishmaniasis in Greek mainland. Acta Tropica, 122, 291–295. [DOI] [PubMed] [Google Scholar]
- Ballart, C. , Guerrero, I. , Castells, X. , Barón, S. , Castillejo, S. , Alcover, M. M , Portús, M. , & Gállego, M. (2014). Importance of individual analysis of environmental and climatic factors affecting the density of Leishmania vectors living in the same geographical area: The example of Phlebotomus ariasi and P. perniciosus in Northeast Spain. Geospatial Health, 8, 389–403. [DOI] [PubMed] [Google Scholar]
- Baneth, G. , Nachum‐Biala, Y. , Zuberi, A. , Zipori‐Barki, N. , Orshan, L. , Kleinerman, G. , Shmueli‐Goldin, A. , Bellaiche, M. , Leszkowicz‐Mazuz, M. , Salant, H. , & Yasur‐Landau, D. (2020). Leishmania infection in cats and dogs housed together in an animal shelter reveals a higher parasite load in infected dogs despite a greater seroprevalence among cats. Parasites & Vectors, 13, 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barati, M. , Mohebali, M. , Alimohammadian, M. H. , Khamesipour, A. , Akhoundi, B. , & Zarei, Z. (2015). Canine visceral leishmaniasis: Seroprevalence survey of asymptomatic dogs in an endemic area of northwestern Iran. Journal of Parasitic Diseases, 39, 221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxarias, M. , Homedes, J. , Mateu, C. , Attipa, C. , & Solano‐Gallego, L. (2022). Use of preventive measures and serological screening tools for Leishmania infantum infection in dogs from Europe. Parasites & Vectors, 15, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxarias, M. , Viñals, J. , Fernández, A. Á. , Alcover, M. M. , & Solano‐Gallego, L. (2022). Detection of specific antibodies against Leishmania infantum in canine serum and oral transudate using an in‑house ELISA. Parasites & Vectors, 15, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyhan, Y. E. , Celebi, B. , Ergene, O. , & Mungan, M. (2016). Seroprevalance of leishmaniasis in dogs from Hatay and Burdur provinces of Turkey and Northern Cyprus. Turkiye Parazitolojii Dergisi, 40, 9–12. [DOI] [PubMed] [Google Scholar]
- Bouattour, A. , Amri, A. , Belkhiria, J. A. , Rhim, A. , Fezaa, O. , Gantier, J.‐C. , & M'ghirbi, Y. (2021). Canine leishmaniosis in Tunisia: Growing prevalence, larger zones of infection. PLoS Neglected Tropical Diseases, 15, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canakci, T. , Kurtdede, A. , Pasa, S. , Toz Ozensoy, S. , & Ozbel, Y. (2016). Seroprevalence of canine leishmaniasis in Northern Cyprus. Turkiye Parazitolojii Dergisi, 40, 117–120. [DOI] [PubMed] [Google Scholar]
- Colella, V. , Hodžić, A. , Iatta, R. , Baneth, G. , Alić, A. , & Otranto, D. (2019). Zoonotic leishmaniasis, Bosnia and Herzegovina. Emerging Infectious Diseases, 25, 385–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas‐Torres, F. (2007). The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis . Veterinary Parasitology, 149, 139–146. [DOI] [PubMed] [Google Scholar]
- Davoust, B. , Roqueplo, C. , Parzy, D. , Watier‐Grillot, S. , & Marié, J. L. (2013). A twenty‐year follow‐up of canine leishmaniosis in three military kennels in southeastern France. Parasites & Vectors, 6, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz‐Regañón, D. , Roura, X. , Suárez, M. , León, M. , & Sainz, A. (2020). Serological evaluation of selected vector‐borne pathogens in owned dogs from Northern Spain based on a multicenter study using a commercial test. Parasites & Vectors, 13, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz‐Sáez, V. , Corpas‐López, V. , Merino‐Espinosa, G. , Morillas‐Mancilla, M. J. , Abattouy, N. , & Martín‐Sánchez, J. (2021). Seasonal dynamics of phlebotomine sand flies and autochthonous transmission of Leishmania infantum in high‐altitude ecosystems in Southern Spain. Acta Tropica, 213, 1–7. [DOI] [PubMed] [Google Scholar]
- Dostálová, A. , & Volf, P. (2012). Leishmania development in sand flies: Parasite‐vector interactions overview. Parasites & Vectors, 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrache, M. O. , Nachum‐Biala, Y. , Gilad, M. , Mircean, V. , Cazan, C. D. , Mihalca, A. D. , & Baneth, G. (2016). The quest for canine leishmaniasis in Romania: The presence of an autochthonous focus with subclinical infections in an area where disease occurred. Parasites & Vectors, 9, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Mouhdi, K. , Boussaa, S. , Chahlaoui, A. , & Fekhaoui, M. (2022). Prevalence and risk factors of canine leishmaniasis in Morocco: A systematic review and meta‐analysis. Journal of Parasitic Diseases, 46, 967–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Berbri, I. , Mahir, W. , Shaw, A. , Ducrotoy, M. J. , Lhor, Y. , Dehhaoui, M. , Petavy, A. F. , Dakkak, A. , Bouslikhane, M. , Boué, F. , & Fassi Fihri, O. (2020). Evaluation of integrated control of three dog transmitted zoonoses: Rabies, visceral leishmaniasis and cystic echinococcosis, in Morocco. Acta Tropica, 212, 105689. [DOI] [PubMed] [Google Scholar]
- European Environment Agency . (2017). https://www.eea.europa.eu/data‐and‐maps/figures/biogeographical‐regions‐in‐europe‐2
- Gálvez, R. , Montoya, A. , Cruz, I. , Fernández, C. , Martín, O. , Checa, R. , Chicharro, C. , Migueláñez, S. , Marino, V. , & Miró, G. (2020). Latest trends in Leishmania infantum infection in dogs in Spain, Part I: Mapped seroprevalence and sand fly distributions. Parasites & Vectors, 13, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez, R. , Miró, G. , Descalzo, M. A. , Nieto, J. , Dado, D. , Martín, O. , Cubero, E. , & Molina, R. (2010). Emerging trends in the seroprevalence of canine leishmaniasis in the Madrid region (central Spain). Veterinary Parasitology, 169, 327–334. [DOI] [PubMed] [Google Scholar]
- Hamel, D. , Shukullari, E. , Rapti, D. , Silaghi, C. , Pfister, K. , & Rehbein, S. (2016). Parasites and vector‐borne pathogens in client‐owned dogs in Albania. Blood pathogens and seroprevalences of parasitic and other infectious agents. Parasitology Research, 115, 489–499. [DOI] [PubMed] [Google Scholar]
- Hartemink, N. , Vanwambeke, S. O. , Heesterbeek, H. , Rogers, D. , Morley, D. , Pesson, B. , Davies, C. , Mahamdallie, S. , & Ready, P. (2011). Integrated mapping of establishment risk for emerging vector‐borne Infections: A case study of canine leishmaniasis in Southwest France. PLoS One, 6, e20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotnik, T. , Moreno, J. , Šoba, B. , Krt, B. , Skvarč, M. , Vergles Rataj, A. , Gorišek Bajc, M. , & Ravnik Verbič, U. (2021). Canine leishmaniasis prevalence in the Slovenian dog population. Journal of Veterinary Research (Poland), 65, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín‐Sánchez, J. , Morales‐Yuste, M. , Acedo‐Sánchez, C. , Barón, S. , Díaz, V. , & Morillas‐Márquez, F. (2009). Canine leishmaniasis in southeastern Spain. Emerging Infectious Diseases, 15, 795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli, M. , Bosco, A. , Foglia Manzillo, V. , Vitale, F. , Giaquinto, D. , Ciuca, L. , Molinaro, G. , Cringoli, G. , Oliva, G. , Rinaldi, L. , & Gizzarelli, M. (2020). Clinical, molecular and serological diagnosis of canine leishmaniosis: An integrated approach. Veterinary Sciences, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medkour, H. , Laidoudi, Y. , Lafri, I. , Davoust, B. , Mekroud, A. , Bitam, I. , & Mediannikov, O. (2020). Canine vector‐borne protozoa: Molecular and serological investigation for Leishmania spp., Trypanosoma spp., Babesia spp., and Hepatozoon spp. in dogs from Northern Algeria. Veterinary Parasitology: Regional Studies and Reports, 19, 100353. [DOI] [PubMed] [Google Scholar]
- Miró, G. , Montoya, A. , Roura, X. , Gálvez, R. , & Sainz, A. (2013). Seropositivity rates for agents of canine vector‐borne diseases in Spain: A multicentre study. Parasites & Vectors, 6, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miró, G. , Petersen, C. , Cardoso, L. , Bourdeau, P. , Baneth, G. , Solano‐Gallego, L. , Pennisi, M. G. , Ferrer, L. , & Oliva, G. (2017). Novel areas for prevention and control of canine leishmaniosis. Trends in Parasitology, 33, 718–730. [DOI] [PubMed] [Google Scholar]
- Miró, G. , Wright, I. , Michael, H. , Burton, W. , Hegarty, E. , Rodón, J. , Buch, J. , Pantchev, N. , & von Samson‐Himmelstjerna, G. (2022). Seropositivity of main vector‐borne pathogens in dogs across Europe. Parasites & Vectors, 15, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina, R. , Jiménez, M. I. , Cruz, I. , Iriso, A. , Martín‐Martín, I. , Sevillano, O. , Melero, S. , & Bernal, J. (2012). The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Veterinary Parasitology, 190, 268–271. [DOI] [PubMed] [Google Scholar]
- Montoya‐Alonso, J. A. , Morchón, R. , Costa‐Rodríguez, N. , Matos, J. I. , Falcón‐Cordón, Y. , & Carretón, E. (2020). Current distribution of selected vector‐borne diseases in dogs in Spain. Frontiers in Veterinary Science, 7, 564429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfe, A. , Mohebali, M. , Edrissian, G. H. , Zarei, Z. , Akhoundi, B. , Kazemi, B. , Jamshidi, S. , & Mahmoodi, M. (2008). Seroepidemiological study on canine visceral leishmaniasis in Meshkin‐Shahr district, Ardabil province, northwest of Iran during 2006–2007. Iranian Journal of Parasitology, 3, 1–10. [Google Scholar]
- Mrljak, V. , Kuleš, J. , Mihaljević, Ž. , Torti, M. , Gotić, J. , Crnogaj, M. , Živičnjak, T. , Mayer, I. , Šmit, I. , Bhide, M. , & Barić Rafaj, R. (2017). Prevalence and geographic distribution of vector‐borne pathogens in apparently healthy dogs in Croatia. Vector‐Borne and Zoonotic Diseases, 17, 398–408. [DOI] [PubMed] [Google Scholar]
- Müller, A. , Montoya, A. , Escacena, C. , de la Cruz, M. , Junco, A. , Iriso, A. , Marino, E. , Fúster, F. , & Miró, G. (2022). Leishmania infantum infection serosurveillance in stray dogs inhabiting the Madrid community: 2007–2018. Parasites & Vectors, 15, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrseli, T. , Mersini, K. , Nachum‐Biala, Y. , Bino, S. , & Baneth, G. (2016). A survey of canine leishmaniosis in Albania. In The 3rd Conference on Neglected Vectors and Vector‐Borne Diseases (EurNegVec) with Management Committee and Working Group Meetings of the COST Action TD1303 (p. 8). Zaragoza, Spain. [Google Scholar]
- Oleaga, A. , Zanet, S. , Espí, A. , Pegoraro De Macedo, M. R. , Gortázar, C. , & Ferroglio, E. (2018). Leishmania in wolves in Northern Spain: A spreading zoonosis evidenced by wildlife sanitary surveillance. Veterinary Parasitology, 255, 26–31. [DOI] [PubMed] [Google Scholar]
- Ozbel, Y. , Oskam, L. , Ozensoy, S. , Turgay, N. , Alkan, M. Z , Jaffe, C. L , & Ozcel, M. A (2000). A survey on canine leishmaniasis in western Turkey by parasite, DNA and antibody detection assays. Acta Tropica, 74, 1–6. [DOI] [PubMed] [Google Scholar]
- Paltrinieri, S. , Gradoni, L. , Roura, X. , Zatelli, A. , & Zini, E. (2016). Laboratory tests for diagnosing and monitoring canine leishmaniasis. Veterinary Clinical Pathology, 45, 552–578. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Cortés, A. , Ojeda, A. , Todolí, F. , & Alberola, J. (2013). Performance of commercially available serological diagnostic tests to detect Leishmania infantum infection on experimentally infected dogs. Veterinary Parasitology, 191, 363–366. [DOI] [PubMed] [Google Scholar]
- Rombolà, P. , Barlozzari, G. , Carvelli, A. , Scarpulla, M. , Iacoponi, F. , & Macrì, G. (2021). Seroprevalence and risk factors associated with exposure to Leishmania infantum in dogs, in an endemic Mediterranean region. PLoS One, 16, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim, A. , Shoulah, S. , Abdelhady, A. , Alouffi, A. , Alraey, Y. , & Al‐Salem, W. (2021). Seroprevalence and risk factors associated with canine leishmaniasis in Egypt. Veterinary Sciences, 8, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza, J. C. , & Suk, J. E. (2018). Vector‐borne diseases and climate change: A European perspective. FEMS Microbiology Letters, 365, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano‐Gallego, L. , Morell, P. , Arboix, M. , Alberola, J. , & Ferrer, L. (2001). Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. Journal of Clinical Microbiology, 39, 560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano‐Gallego, L. , Koutinas, A. , Miró, G. , Cardoso, L. , Pennisi, M. G. , Ferrer, L. , Bourdeau, P. , Oliva, G. , & Baneth, G. (2009). Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Veterinary Parasitology, 165, 1–18. [DOI] [PubMed] [Google Scholar]
- Solano‐Gallego, L. , Cardoso, L. , Pennisi, M. G. , Petersen, C. , Bourdeau, P. , Oliva, G. , Miró, G. , Ferrer, L. , & Baneth, G. (2017). Diagnostic challenges in the era of canine Leishmania infantum vaccines. Trends in Parasitology, 33, 706–717. [DOI] [PubMed] [Google Scholar]
- Solano‐Gallego, L. , Llull, J. , Osso, M. , Hegarty, B. , & Breitschwerdt, E. (2006). A serological study of exposure to arthropod‐borne pathogens in dogs from northeastern Spain. Veterinary Research, 37, 231–244. [DOI] [PubMed] [Google Scholar]
- Solano‐Gallego, L. , Miró, G. , Koutinas, A. , Cardoso, L. , Pennisi, M. G. , Ferrer, L. , Baneth, G. , Bourdeau, P. , Oliva, G. , & The LeishVet Group . (2011). LeishVet guidelines for the practical management of canine leishmaniosis. Parasites & Vectors, 4, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano‐Gallego, L. , Villanueva‐Saz, S. , Carbonell, M. , Trotta, M. , Furlanello, T. , & Natale, A. (2014). Serological diagnosis of canine leishmaniosis: Comparison of three commercial ELISA tests (Leiscan, ID Screen and Leishmania 96), a rapid test (Speed Leish K) and an in‐house IFAT. Parasites & Vectors, 7, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamponi, C. , Scarpa, F. , Carta, S. , Knoll, S. , Sanna, D. , Gai, C. , Pipia, A. P. , Dessì, G. , Casu, M. , Varcasia, A. , & Scala, A. (2021). Seroprevalence and risk factors associated with Leishmania infantum in dogs in Sardinia (Italy), an endemic island for leishmaniasis. Parasitology Research, 120, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toepp, A J. , & Petersen, C A. (2020). The balancing act: Immunology of leishmaniosis. Research in Veterinary Science, 130, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelifi Touhami, N. A. , Ouchene, N. , Ouchetati, I. , & Naghib, I. (2023). Animal leishmaniasis in Algeria: A systematic review and meta‐analysis. Comparative Immunology, Microbiology and Infectious Diseases, 93, 101930. [DOI] [PubMed] [Google Scholar]
- Xhekaj, B. , Alishani, M. , Rexhepi, A. , Jakupi, X. , & Sherifi, K. (2020). Serological and clinical survey of canine leishmaniasis in southwestern region of Kosovo. Veterinaria Italiana, 24, 56. [DOI] [PubMed] [Google Scholar]
- Xhekaj, B. , Stefanovska, J. , Sherifi, K. , Rexhepi, A. , Bizhga, B. , Rashikj, L. , Nikolovski, M. , Kniha, E. , & Cvetkovikj, A. (2023). Seroprevalence of canine leishmaniosis in asymptomatic dogs in Kosovo. Parasitology Research, 122, 607–614. [DOI] [PubMed] [Google Scholar]
- Zivicnjak, T. , Martinkovic, F. , Beck, R. , & Marinculic, A. (2007). Canine leishmaniosis spread in Croatia: Feasibilities of PCR‐based and serological monitoring activities. In International Meeting on Emerging Diseases and Surveillance .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available from the corresponding author on reasonable request.


