Abstract
Background
Despite the expansion of modernized poultry farming in Ethiopia, the presence of high prevalence of Eimeria species is the bottleneck in the sector causing high morbidity and mortality rate in poultry.
Objectives
The objectives of this study were to estimate the prevalence and identify Eimeria species and investigate the major risk factors.
Method
A cross‐sectional study was conducted from November 2019 to April 2020 in East Gojjam Zone, North West Ethiopia. A total of 384 chickens were used. Both floatation and McMaster coprological techniques were employed. Univariate and multinomial logistic regression was used to calculate the odds ratio for the associated risk factors. Analysis of variance was used to analyse differences in Eimeria oocyst counts among the groups.
Results
Overall prevalence of Eimeria species in poultry from the study area was 26.5%. Age (OR = 0.25, p = 0.001), management system (OR = 12.44, p = 0.001) and production system (OR = 0.37, p = 0.001) were found significantly (p < 0.05) associated with the risk of Eimeria species in poultry. The mean Eimeria oocyst count was significantly different by age and management system (F = 6.526, p = 0.002), (F = 5.369, p = 0.005), respectively. The mean Eimeria oocyst count was significantly greater in 6–12 weeks (p = 0.004) and <6 weeks of age (p = 0.025). A total of 6 Eimeria species were identified. Eimeria tenella (46.07%), Eimeria necatrix (24.5%) and Eimeria acervulina (8.82%) were the most common Eimeria species encountered.
Conclusion
The prevalence of Eimeria species was higher in poultry in North West Ethiopia. Therefore, tailor‐made intervention is required to mitigate risk factors and reduce the prevalence of Eimeria species in poultry from the study area.
Keywords: Eimeria species, Ethiopia, poultry, prevalence, risk factors
Despite the expansion of modernized poultry farming in different parts of Ethiopia, the presence of high prevalence of Eimeria species is the bottleneck in the sector causing high morbidity and mortality rate of poultry. The aim of this study was to estimate the prevalence and identify Eimeria species affecting poultry and investigate the major associated risk factors. A total of 384 chickens were used. Overall prevalence of Eimeria species in poultry in the study area was 26.5%. Eimeria tenella (46.07%), E. necatrix (24.5%) and E. acervulina (8.82%) were the most common Eimeria species encountered.

1. INTRODUCTION
Poultry production is one of the key livestock subsectors of Ethiopia. It plays an important roles in terms of generating employment opportunities, improving family nutrition and empowering women. It is a suitable business for low‐income households due to the small quantity of land needed and low investment costs required starting up and running the operation (Jordan, 2001). In Ethiopia, total population of chicken is estimated to be 56.38 million. Of the total population in Ethiopia, 94.31%, 3.21% and 2.49% are indigenous, hybrid and exotic breeds, respectively (Central Statistical Agency [CSA], 2017).
Despite the high number of chicken available in Ethiopia, poultry diseases are major constraints to productivity and production causing economic losses to poultry farm sector in Ethiopia. Several infectious agents like viruses, bacteria and protozoan parasites cause poultry morbidity and mortality (Alemu, 2012). Eimeria species are the most important protozoan parasites causing poultry coccidiosis characterized by enteritis and bloody diarrhoea (Adhikari et al., 2008).
Coccidiosis has been a major cause of poor performance and lost of productivity in poultry and other farm animals. The infectious process is rapid 4–7 days in a faecal–oral route and is characterized by parasites replication in host cells with extensive damage to the intestinal mucosa (Taylor et al., 2007). After ingestion of sporulated oocysts, sporozoites are released that enter asexual and sexual cycles of development resulting in the emergence of 1000 of new oocysts in the intestines (Taylor et al., 2007). Soon, they sporulate and become infective for chickens (Bowman, 2009). Affected birds become depressed, have ruffled feathers, the wings droop, have diarrhoea, and tend to huddle (Aarthi et al., 2010). Food and water consumption usually decreased and may become emaciated and dehydrated (Kinung et al., 2004). Laying hens will experience a reduction in the rate of egg production. Poultry coccidia are strictly host‐specific and parasitize specific parts of the intestine (Jordan et al., 2002). Eimeria acervulina, Eimeria brunetti, Eimeria maxima, Eimeria mitis, Eimeria necatrix, Eimeria praecox and Eimeria tenella are the most common Eimeria species which cause poultry coccidiosis (Taylor et al., 2007).
In Ethiopia, E. necatrix, E. maxima and E. tenella are endemic in all parts of the country and affect many young growing birds (Hagos et al., 2004). In the past years coccidiosis used to be the most important cause of mortality in backyard and semi‐intensive farms. An incidence of the disease was as high as 80% usually occurring in the form of outbreaks (Safari et al., 2004). Although in Ethiopia quantitative losses due to coccidiosis are not well documented, reports from other countries indicate that the disease contributes to 8.4% and 11.86% loss in profit in large‐scale farms and small‐scale farms, respectively (Lew et al., 2003).
The specific identification of Eimeria species and strains is important for diagnosis and control, as well as for epidemiology and biological studies of the population (Morris & Gasser, 2006). The Eimeria species have been identified by morphology and/or morphometry of their sporocysts and oocysts as well as their modes of development, and assessing the site and extent of the pathological lesions in the intestine of chicken (Aarthi et al., 2010). However, these methods of identifications are costly, time‐consuming, require skilled personnel and can be unreliable under the circumstances of mixed‐field infections, particularly when the overlap in biological and morphological characteristics make the unambiguous identification and differentiation of Eimeria species impossible (Al‐Idreesi et al., 2013; Carvalho et al., 2011). Eimeria oocysts can be isolated according to the method of Davies et al. (1963) and can be identified by sporulation time using potassium dichromate solution for sporulation and preservation of the parasite (Ryley et al., 1976) where molecular methods like Polymerase chain reaction are not readily available. Although quite a lot of similar studies on poultry coccidiosis have been conducted to determine the prevalence and associated risk of poultry coccidiosis in different areas of Ethiopia, it is worth noting that Ethiopia is a large country with a large number and different types of poultry farms. Therefore, most of the studies are targeting only specific areas and not the whole country but the disease is still a major problem in both backyard and semi‐intensive chicken production system in Ethiopia. There was no recent study on epidemiology and species identification of Eimeria species in poultry in the selected districts of East Gojjam Zone. Therefore, this study was initiated to estimate the prevalence and identify Eimeria species and investigate the major risk factors.
2. MATERIALS AND METHODS
2.1. Study area
The present study was conducted in three purposely selected districts of Eastern Gojjam Zone, Amhara National Regional State, Ethiopia, which is located at 10°20′ N latitude and 37°43′ E longitudes, and an altitude range of 500–4154 m.a.s.l. The mean annual rainfall of the area ranges from 900 to 1800 mm and mean minimum and maximum temperature of 7.5 and 25°C, respectively. Mixed crop‐livestock production system is a common agricultural practice in the area; backyard and semi‐intensive production is practiced in each village and household. The livestock population of the area includes 1.84 million cattle, 1.14 million sheep, 0.4 million goats, 0.09 million horses, 0.36 million donkeys, 0.014 million mules and more than 1.24 million chicken (CSA, 2017).
2.2. Study design
A cross‐sectional study design was conducted from November 2019 to April 2020. Both local and exotic chicken breeds which are kept under the backyard and semi‐intensive husbandry system were included in the study.
2.3. Sample size determination and sampling procedures
The sample size used for this study was calculated according to Thrusfield (2005), assuming a 50% expected prevalence, with 95% confidence interval and 5% desired absolute precision. Accordingly, a total of 384 chickens were used for this study. The distribution of 384 samples to the districts and city was based on the number of peasant associations they have. Multistage sampling was used to select districts, peasant association, villages and household. Simple random sampling was employed to select peasant association from each districts, whereas village and household were selected purposely based on the potential availability of poultry, willingness of the owner and accessibility. Each backyard and semi‐intensive farm and chicken from each were selected by simple random sampling technique as shown in Figure 1.
FIGURE 1.
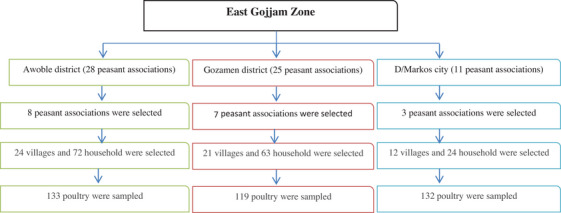
Chart showing stage of sample collection from the study areas.
2.4. Sample collection
About 3 g of faecal sample were collected from the upper surface of the litter immediately after the dropping of faeces using sterile disposable plastic gloves. During sampling, farm type, date of sampling, age, sex, breed, management system and production system were recorded for each chicken on a recording format. The sample was placed in a labelled clean universal bottle using 2.5% (w/v) potassium dichromate solution and transported in a cool box to Bahir Dar animal diseases diagnostic and investigation laboratory centre on the same day of collection, and preserved at refrigeration temperature until processing within 48 h of arrival.
2.5. Coproscopical examination
Qualitative faecal examination was conducted using flotation technique for the detection of the oocysts of Eimeria using concentrated sucrose solution (Sheather's sucrose solution) with specific gravity of 1.27 as described by Hendrix (1998). The slides were examined using a compound microscope (10× objectives). For identification of Eimeria species, positive samples were taken to the National Animal Health Diagnostic and Investigation Center, Addis Ababa, Ethiopia. Identification of Eimeria oocysts was based on the morphological features of the oocysts (size of oocyst and sporocysts, shape, colour and texture of oocyst wall, presence or absence of micropyle and polar cap) with the aid of taxonomic keys (Kennedy & Kralka, 1987; Sommer, 1998; Soulsby, 2005). Oocysts size was measured under ocular eye piece that is calibrated with a micrometre under a 40× objective of a microscope. Quantitative faecal examination was performed on positive samples using McMaster technique to determine the number of oocysts of Eimeria per gram of faeces (OPG) (Hendrix, 1998; Kaufman, 1996; Carvalho et al., 2011). The severity of the Eimeria infection was evaluated as mild, moderate and severe with OPG level of <1800, 1800–6000 and >6000 OPG, respectively (Carvalho et al., 2011).
2.6. Data management and analysis
Statistical Package for the Social Sciences (SPSS) statistical software version 20 was used to run logistic regression. Initially, the association of eight individual risk factors with an outcome variable was screened by univariate logistic regression. Those variables significantly associated with the outcome variable at p < 0.05 significance level in the univariate analysis were recruited for multiple logistic regression to see their independent effect. In the multinomial logistic regression analysis, a model was fitted for each outcome variable by stepwise backward elimination of insignificant variables (p > 0.05). ANOVA was used to analyse differences in mean oocyst count. It is not possible to perform any factor analysis for each Eimeria species, since the estimation of the relative percentage of each species, since only a limited number of oocyst were identified for each sample. Therefore, both presence and OPG values of the single species suffer from a high uncertainty that is going to affect the analysis if referred to single species. A statistically significant association between all variables was set at p < 0.05.
3. RESULTS
3.1. Association of Eimeria species prevalence with risk factors
A total of 384 pooled faecal samples were examined for Eimeria species from the 3 selected areas of East Gojjam Zone, Northwest Ethiopia. The overall prevalence of Eimeria species found in this study was 26.6% (102/384). In the present study, the highest prevalence of Eimeria species in poultry (10.2%) was recorded in Gozamen district. Besides, higher prevalence values were found in exotic breed, in younger animals, in females, and in farms characterised by a poor management and a semi‐intensive system (Table 1).
TABLE 1.
Prevalence of Eimeria species in poultry in the subgroups of animals identified by the associated risk factors.
| Variables categories | N | No of positive | Prevalence (%) | |
|---|---|---|---|---|
| Breed | Local | 136 | 24 | 6.3 |
| Exotic | 248 | 78 | 20.3 | |
| Age | <8 weeks | 148 | 57 | 14.8 |
| 8–20 weeks | 151 | 30 | 7.8 | |
| >20 weeks | 85 | 15 | 3.9 | |
| Management | Good | 89 | 10 | 2.6 |
| Medium | 182 | 32 | 8.3 | |
| Poor | 113 | 60 | 15.6 | |
| Production system | Semi‐intensive | 185 | 70 | 18.2 |
| Backyard | 199 | 32 | 8.3 | |
| Sex | Male | 138 | 41 | 10.7 |
| Female | 246 | 61 | 15.9 | |
| Districts | Gozamen | 119 | 39 | 10.2 |
| Awobel | 132 | 28 | 7.3 | |
| D/Markos city | 133 | 35 | 9.1 | |
Note: N, number of animal sampled.
A total of six potential risk factors were tested by using multinomial logistic regression analysis. Of these, three potential risk factors were significantly (p < 0.05) associated with Eimeria species in poultry. These included animals’ age, quality of the management and production system (Table 2). Odds ratio of Eimeria positivity in poor management system was 12.44 times higher than good management system (Table 2).
TABLE 2.
Potential risk factors significantly associated with the prevalence of Eimeria species in poultry using multinomial logistic regression analysis in the study area.
| Variables | Categories | OR | 95% CI | p‐Value |
|---|---|---|---|---|
| Age | <8 weeks a | |||
| 8—20 weeks | 0.25 | 0.13–0.48 | ||
| >20 weeks | 0.16 | 0.07–0.37 | 0.001 | |
| Management | Good a | |||
| Medium | 3.52 | 1.47–8.44 | 0.001 | |
| Poor | 12.44 | 5.12–30.19 | ||
| Production system | Backyard a | |||
| Semi‐intensive | 0.37 | 0.22–0.66 | 0.001 |
Abbreviation: OR, odd ratio.
Reference.
3.2. Association of Eimeria oocyst output with risk factors
Mean counts for each subgroup of animals and the significance of the differences are shown in Table 3. The ANOVA indicated that there was the existence of significant differences in the mean Eimeria oocysts counts among the age categories of poultry (F = 6.526, p = 0.002), with significantly greater counts in younger animals (age of <8 weeks), both compared to the ones with 8–20 weeks (p = 0.025) and to the oldest ones with >20 weeks (p = 0.004), as indicated by the Bonferroni pair‐wise comparison. Similarly, different management systems were significantly different in the mean count of Eimeria oocysts (F = 5.369, p = 0.005), with the poor management system showing higher outputs, both compared to the medium (p = 0.001) and to good (p = 0.0001) ones. However, chicken under good and medium management system did not show a significant difference (p > 0.05). Instead, there were no significant difference in the mean Eimeria oocysts count in poultry between sexes (F = 0.062, p = 0.8040), breeds (F = 3.108, p = 0.079) and production systems (F = 3.173, p = 0.076).
TABLE 3.
Potential risk factors associated with the Eimeria oocyst output (OPG) using ANOVA test.
| Variables | N | Mean | SE | F | p‐Value | Bonferroni result | p‐Value |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <8 weeks | 148 | 476.968 | 69.549 | <8 and 8–20 weeks | 0.025 | ||
| 8–20 weeks | 151 | 218.358 | 73.314 | 6.526 | 0.002 | <8 and >20 weeks | 0.004 |
| >20 weeks | 85 | 31.415 | 106.441 | >20 and 8–20 weeks | 0.950 | ||
| Breed | |||||||
| Local | 136 | 154.932 | 80.641 | 3.108 | 0.079 | ||
| Exotic | 248 | 329.562 | 57.894 | ||||
| Management | |||||||
| Good | 89 | 86.196 | 96.859 | 5.369 | 0.005 | Good and medium | 1.000 |
| Medium | 182 | 162.85 | 65.516 | Good and poor | 0.001 | ||
| Poor | 113 | 477.6 | 89.891 | Poor and medium | 0.001 | ||
| Sex | |||||||
| Male | 138 | 257.700 | 79.830 | 0.062 | 0.804 | ||
| Female | 246 | 233.055 | 62.195 | ||||
| Production system | |||||||
| Semi‐intensive | 185 | 322.941 | 66.482 | 3.173 | 0.076 | ||
| Backyard | 199 | 161.545 | 68.264 | ||||
3.3. Eimeria species identification
During sampling, 384 pooled faecal samples were collected according to the established protocol and 102 samples were tested positive for Eimeria species. For each sample the coproculture and identification of species was performed. A total of six Eimeria species were identified as displayed Figure 2a–f. E. tenella and E. necatrix were the more prevalent species and accounted for 46.1% and 24.5%, respectively. Other species found were E. acervulina (8.8%), E. mitis (5.9%), E. maxima (4.9%) and E. brunetti (2.9%). Mixed infections were recorded due to E. tenella together with E. maxima (2.94%), or E. necatrix (3.92%). The distributions of Eimeria species as a single and mixed infection are shown Figure 3.
FIGURE 2.
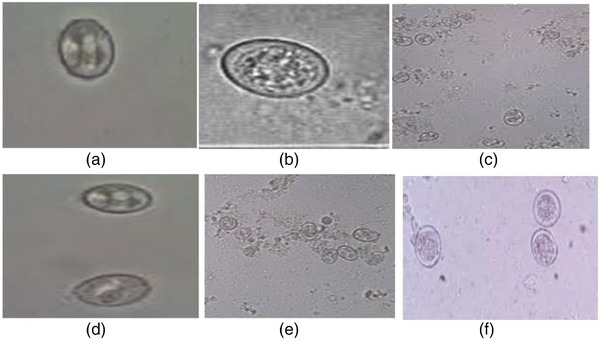
Identified Eimeria species; (a) Eimeria tenella, (b) Eimeria mitis, (c) Eimeria necatrix, (d) Eimeria acervulina, (e) Eimeria maxima and (f) Eimeria brunetti.
FIGURE 3.
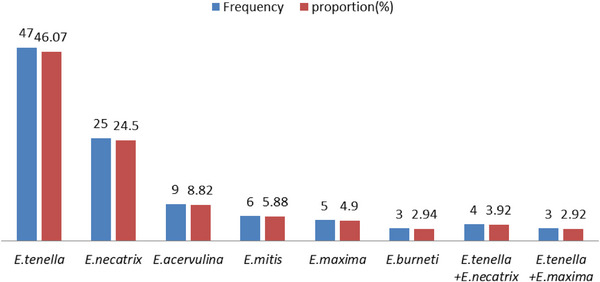
Distribution of Eimeria species as a single and mixed infection.
4. DISCUSSION
In the present study, the overall prevalence of Eimeria species in poultry was 26.6% which is comparable to what reported in a study done in Arsi zone (22.6%) by Getachew et al. (2008) and in another study performed in central Ethiopia (25.8%) by Hagos et al. (2004). Generally, these prevalence values are halfway in the Eimeria species prevalence rates encountered in poultry in Ethiopia, with values ranging from about 10% to 80%. Higher prevalence values were reported by Dinka and Yacob (2012) in Debre Zeit (71.1%), by Bereket and Abdu (2015) in Kombolcha (48.5%) and by Alemargot (1987) in Addis Ababa (80.0%). At the same time, lower values were found by Fikre et al. (2005) in central Ethiopia (11.0%) and by Firmaye et al. (2015) in the same area (19.5%). The variation of these findings may be due to the differences in agro‐ecology and climatic conditions in the study areas, although most of these studies were conducted in the highland part of Ethiopia. Most probably, many confounding factors influenced the outcome of the different studies, such as different management system, seasonal variation, study design and different target animal.
Concerning risk factors, there was a significant (p < 0.05) association of age of chicken with the risk of Eimeria species infection in the present investigation. This finding is in agreement with most of the international literature, which consider the young chickens at major risk for Eimeria species infection (Taylor et al., 2007), and with other reports in Ethiopia. In particular, both Firmaye et al. (2015) and Hagos et al. (2004) reported higher prevalence of Eimeria species infection in younger than adults; and Muluken and Liuel (2017) reported a significant higher mean count of Eimeria oocyst in older animals. However, few other studies, both in Ethiopia (Temesgen et al., 2018) and in other countries worldwide (Julie, 1999; Muhammad, 2019), reported higher prevalence of Eimeria species infection in adult than in growers, either similar values. Also in this case, the presence of confounding factors, or the adoption of different age‐classes, can justify. The higher prevalence of Eimeria species at the age of <6 weeks is probably associated to fact that the immune system is still not fully developed at this age. Immunity to Eimeria species is acquired gradually and is not complete until 7 weeks of age (Allen & Fetterer, 2002). However, it might be associated also with the presence of another immunosuppressive disease, such as Gumboro diseases (Hachimi et al., 2008).
The more important risk factor for Eimeria species infection found in this study was the management system that resulted highly influencing the presence of the infection (animals kept with a poor management are 12.44 times at risk compared to the ones with good management) and the oocyst output (p < 0.01), resulting in a higher environmental contamination. This finding is in line with other reports in Ethiopia (Anteneh et al., 2019; Belaynew et al., 2016; Tekalign & Teshome, 2018) that found poor management systems at high risk of acquiring Eimeria species infection than good and medium management systems. This might be due to high exposure to environmental oocysts due to many managerial factors, such as lack of clean litter, poor ventilation, absence of isolation pen or defective feeders and waterers that increase the risk of oocyst ingestion. Besides, in poultry farm with poor management, the lack of vaccination for other immunosuppressive diseases like Marek's disease may reduce resistance to Eimeria species by interference with coccidial immunity. Consequently, coccidiosis may not respond to either preventive or curative chemotherapy in a normal way because the normal self‐limiting immunity to Eimeria species fails to become established.
Finally, there was difference of prevalence of Eimeria species in poultry also between sex, with a slightly high prevalence of coccidiosis were recorded in male than female chicken. This finding is in line with previous reports in Ethiopia, which reported higher prevalence in male than female (Anteneh et al., 2019; Kefyalew & Hailegebrael, 2018), although other studies disagree with our results (Eshetu & Nigussu, 2019) reporting a higher prevalence of Eimeria species in female than in male. The absence of a statistically significant difference between males and females might be due to an equal chance of exposure for the parasite infection. This indicates there is no significant biological resistance variation with the sex (Pinard‐Van Der Laan et al., 1998).
In the present study, positive samples were further investigated for Eimeria species identification based on morphological features of different Eimeria oocysts. Worldwide, more than 13 Eimeria species are reported in chicken. However, in the present study, six Eimeria species were isolated, with E. tenella (47%) followed by E. necatrix (25%) and E. acervulina (9%) as the most prevalent species. Except E. mitis, this result is in line with Solomon (2006), who reported the other five Eimeria species. We may consider that the six species of Eimeria, namely E. acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. tenella, found in this study are the most common species of Eimeria that infect poultry in Ethiopia.
5. CONCLUSION
The present study revealed that there was a prevalence of 26.6% of Eimeria species in poultry from the study area, which is in the middle of the prevalence values reported to date in different regions of Ethiopia. Age, management and production system of poultry were significantly associated with the prevalence of Eimeria species and partly with oocyst output. The average level of OPG and the widespread presence of two highly pathogenic species E. tenella and E. necatrix is particularly alarming. Based on the findings of this study, awareness creation for both the backyard and semi‐intensive poultry farm owners about all‐in and all‐out strategy and prophylactic treatment should be implemented.
AUTHOR CONTRIBUTIONS
Conceptualization; data curation; investigation; methodology: Hailehizeb Cheru. Formal analysis; investigation; methodology; supervision; writing – original draft; writing – review and editing: Habtamu Tamrat. Supervision; writing – review and editing: Mussie Hailemelekot. Validation; writing – original draft; writing – review and editing: Rudi Cassini.
CONFLICT OF INTEREST STATEMENT
The other authors declare no conflicts of interest.
FUNDING INFORMATION
No funding was obtained for this study.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required, as this study required no animal experiments.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1002/vms3.1243.
ACKNOWLEDGEMENTS
The authors’ would like to thank Bahir Dar animal diseases diagnostic and investigation laboratory centre and National Animal Health Diagnostic and Investigation Center, Addis Ababa, Ethiopia for allowing their laboratory to conduct coproscopical examination.
Cheru, H. , Tamrat, H. , Hailemelekot, M. , Cassini, R. , & Belayneh, N. (2023). Epidemiology and identification of Eimeria species affecting poultry in East Gojjam Zone, North West Ethiopia. Veterinary Medicine and Science, 9, 2160–2167. 10.1002/vms3.1243
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aarthi, S. , Dhinakar Raj, G. , Raman, M. , Gomathinay Agam, S. , & Kumanan, K. (2010). Molecular prevalence and preponderance of Eimeria spp. among chickens in Tamil Nadu, India. Parasitology Research, 107(4), 1013–1017. 10.1007/s00436-010-1971-2 [DOI] [PubMed] [Google Scholar]
- Adhikari, A. , Gupta, R. , & Pant, G. R. (2008). Prevalence and identification of coccidian parasite (Eimeria spp) in layer chicken of Ratnanagar Municipality, Chitwan district, Nepal. Journal of the Natural History Museum, 23, 45–50. 10.3126/jnhm.v23i0.1838 [DOI] [Google Scholar]
- Alamargot, J. (1987). Avian pathology of industrial poultry farms in Ethiopia. First National Livestock Improvement Conference, Institute of Agricultural Research, Addis Ababa.
- Alemu, Y. (2012). Poultry production in Ethiopia. World's Poultry Science Journal, 51(2), 197–201. 10.1079/WPS19950014 [DOI] [Google Scholar]
- Al‐Idreesi, S. , Kweider, M. , & Katranji, M. M. (2013). Efficacy of Eimeria tenella (oocyst and sporozoite) proteins as a vaccine in broilers against cocidiosis. International Journal of Poultry Science, 12(3), 157–163. [Google Scholar]
- Allen, P. C. , & Fetterer, R. H. (2002). Recent advances in biology and immunology of Eimeria species and in diagnosis and control of infection with coccidian parasites of poultry. Clinical Microbiology Reviews, 15, 58–65. 10.1128/CMR.15.1.58-65.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anteneh, W. , Ermias, M. , & Yehualashet, B. (2019). Prevalence of poultry coccidiosis and associated risk factors in intensive farming system of Gondar town, Ethiopia. Veterinary Medicine International, 2019, 5748690. 10.1155/2019/5748690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaynew, A. , Wudu, T. , Mengestie, A. , Ayalew, N. , Kassa, D. , Mebrie, Z. , & Genene, G. (2016). Study on the prevalence, species identification and risk factors associated with poultry coccidiosis in Condar town, north Ethiopia. Natural Sciences, 14(9), 35–40. [Google Scholar]
- Bowman, D. (2009). Georgis’ parasitology for veterinarians (9th ed.). Saunders Elsevier. [Google Scholar]
- Brekete, M. , & Abdu, A. (2015). Epidemiological study on poultry coccidiosis: Prevalence, species identification and post mortem lesions in grower chicken in Kombolcha, North‐Eastern Ethiopia. Journal of Veterinary Medicine and Animal Health, 7(1), 1–8. [Google Scholar]
- Carvalho, F. S. , Wenceslau, A. A. , Teixeira, M. , Matos Carneiro, J. A. , Melo, A. D. , & Albuquerque, G. R. (2011). Diagnosis of Eimeria species using traditional and molecular methods in field studies. Veterinary Parasitology, 176, 95–100. [DOI] [PubMed] [Google Scholar]
- Central Statistical Agency (CSA) . (2017). Central statistical authority, statistical report on livestock and livestock characteristics . CSA. [Google Scholar]
- Davies, S. F. M. , Joyner, L. P. , & Kendall, S. B. (1963). Coccidiosis. Edinburgh & London. https://books.google.com.et/books?id=WWrnMgEACAAJ [Google Scholar]
- Dinka, A. , & Yacob, H. (2012). Coccidiosis in Fayoumi chickens at Debre Zeit Agricultural Research Center Poultry Farm, Ethiopia. European Journal of Applied Sciences, 4(5), 191–195. [Google Scholar]
- Eshetu, K. , & Nigussu, F. (2019). Study on prevalence and associated risk factors of poultry coccidiosis in and around alage at vet college, Southwestern Ethiopia. Dairy and Veterinary Sciences Journal, 11(1), 555805. 10.19080/JDVS.2019.11.555805 [DOI] [Google Scholar]
- Fikre, L. , Worku, N. , & Abebe, W. (2005). Study on coccidiosis in Kombolcha poultry farm, Ethiopia. Tropical Animal Health and Production, 37(3), 245–250. [DOI] [PubMed] [Google Scholar]
- Firmaye, G. , Asmamaw, T. , & Mezene, W. (2015). Study on prevalence of coccidiosis in Nekemte town East Wollega, Ethiopia. African Journal of Agricultural Research, 50, 328–333. [Google Scholar]
- Getachew, G. , Getachew, T. , & Dorchies, P. (2008). Study on poultry coccidiosis in Tiyo district, Arsi Zone, Ethiopia. International Journal of Poultry Science, 7, 251–256. [Google Scholar]
- Hachimi, M. , Belghyti, D. , El Kharrim, K. , & El Guamri, Y. (2008). Coccidioses du poulet dans la région du Gharb (Maroc). Preventive Veterinary Medicine, 147(1), 49–60. [Google Scholar]
- Hagos, A. , Tadess, S. , Medhin, G. , & Tibbo, M. (2004). Study on coccidiosis of scavenging indigenous chicken in the central Ethiopia. Tropical Animal Health and Production, 36(7), 693–701. [DOI] [PubMed] [Google Scholar]
- Hendrix, C. (1998). Diagnostic veterinary Kashmir Valley. Global Veterinarian, 7, 27–30. [Google Scholar]
- Jordan, F. , Pattison, M. , Alexander, D. , & Faragher, T. (2002). Parasitic diseases. In: Poultry disease (5th ed., pp. 405–420). W.B. Saunders. [Google Scholar]
- Jordan, F. T. W. (2001). Poultry diseases (3rd ed.). The Cambridge University Press. [Google Scholar]
- Julie, D. H. (1999). Coccidiosis in poultry livestock. In Poultry Health Programs, Clemson, Columbia (Vol. 17, pp. 191–199). [Google Scholar]
- Kaufmann, J. (1996). Parasitic infections of domestic animals, a diagnostic manual. Birkhäuser Basel. [Google Scholar]
- Kefyalew, L. , & Hailegebrael, B. (2018). Coprological epidemiology of chicken coccidiosis in and around Gondar town, North West Ethiopia. Journal of Animal Husbandry and Dairy Science, 1(3), 23–31. [Google Scholar]
- Kennedy, M. J. , & Kralka, R. A. (1987). A survey of Eimeria species in cattle in central Alberta. Canadian Veterinary Journal, 28(3), 124–125. [PMC free article] [PubMed] [Google Scholar]
- Kinung, S. M. , Getachew, T. M. H. , Hafez, W. , Moges, K. , Moses, G. , & Mathias, G. (2004). Assessment of economic impact caused by poultry coccidiosis in small and large poultry farms in Debre Zeit, Ethiopia. International Journal of Poultry Science, 3, 715–718. [Google Scholar]
- Lew, E. , Anderson, G. R. , Minchin, C. M. , Jeston, P. J. , & Jorgensen, W. K. (2003). Inter‐ and intra‐strain variation and PCR detection of the internal transcribed spacer 1 (ITS‐1) sequences of Australian isolates of Eimeria species from chickens. Veterinary Parasitology, 112(1–2), 33–50. 10.1016/S0304-4017(02)00393-X [DOI] [PubMed] [Google Scholar]
- Morris, G. M. , & Gasser, R. B. (2006). Biotechnological advances in the diagnosis of avian coccidiosis and theanalysis of genetic variation in Eimeria . Biotechnology Advances, 24, 590–603. [DOI] [PubMed] [Google Scholar]
- Muhammad, S. (2019). Prevalence of coccidiosis in commercial broiler chicken in district Abbottabad Khyber Pakhtunkhwa, Pakistan. EC Veterinary Science, 4(7), 482–487. [Google Scholar]
- Muluken, G. , & Liuel, Y. (2017). The prevalence of poultry coccidiosis in intensive farm and individual small holder poultry farm in Hawassa town district. International Journal of Advanced Research in Biological Sciences, 4(4), 57–66. 10.22192/ijarbs.2017.04.04.009 [DOI] [Google Scholar]
- Pinard‐Van Der Laan, M. H. , Monvoisin, J. L. , Pery, P. , Hamet, N. , & Thomas, M. (1998). Comparison of outbred lines of chickens for resistance to experimental infection with coccidiosis (Eimeria tenella). Poultry Science, 77, 185–191. [DOI] [PubMed] [Google Scholar]
- Ryley, J. F. , Meade, R. , Judith, H. , & Thelma, E. R. (1976). Methods in coccidiosis research: Separation of oocysts from faeces. Parasitology, 40, 35–48. [DOI] [PubMed] [Google Scholar]
- Safari, M. H. , Kinung, T. , Getachew, W. , Hafez, K. , & Mathios, G. (2004). Assessment of economic impact caused by poultry coccidiosis in small and large scale poultry farm in Debre Zeit, Ethiopia. International Journal of Poultry Science, 3, 715–725. [Google Scholar]
- Solomon, S. (2006). Study on prevalence of poultry coccidiosis in and around Ambo, West Shewa Zone, Oromia regional state, Ethiopia. International Journal of Poultry Science, 7, 251–256. [Google Scholar]
- Sommer, C. (1998). Quantitative characterization, classification and reconstruction of oocyst shapes of Eimeria species from cattle. Parasitology, 116, 21–28. [DOI] [PubMed] [Google Scholar]
- Soulsby, E. J. L. (2005). Helminths, arthropods and protozoa of domesticated animals (7th ed.). Bailliere Tindall. [Google Scholar]
- Taylor, M. A. , Coop, R. L. , & Wall, R. L. (2007). Veterinary parasitology (3rd ed.), Blackwell Publishing. [Google Scholar]
- Tekalign, T. , & Teshome, L. (2018). A study on copro‐epidemiology of poultry coccidiosis in and around Jimma town, Oromia regional state, Ethiopia. Journal of Veterinary Medicine and Research, 5(11), 1167. [Google Scholar]
- Temesgen, W. M. , Fentahun, W. M. , & Anuar, N. (2018). Prevalence of coccidiosis in back yard chicken in and around DebereTabere town, South Gondar Zone, Amhara regional state, Ethiopia. Cohesive Journal of Microbiology & Infectious Disease, 1(5). 10.31031/CJMI.2018.01.000525 [DOI] [Google Scholar]
- Thrusfield, M. (2005). Veterinary epidemiology (2nd ed.). Blackwell Science. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


