Abstract
This study evaluated the effects of antibiotics on the implantation rate (IR) as well as the optimal time interval from endometrial biopsy to subsequent embryo transfer (ET) to explore proper chronic endometritis (CE) management. We retrospectively analyzed the clinical data of patients who had failed 1 or 2 ET cycles and underwent hysteroscopy. CE was diagnosed when 3 or more cluster of differentiation138 - positive plasma cells were found per high-power field. We divided the patients into 3 groups: those with CE who received antibiotics (group 1), those with CE who did not receive antibiotics (group 2), and those without CE (group 3). We found that IR was significantly higher in Group 1 than in Group 2. Furthermore, while the IR in Groups 1 and 3 was significantly higher when the time interval was < 6 months than when the time interval was > 6 months, there were no significant differences in the IR when the time interval was < 2 months or ≥ 2 months but < 6 months. Postbiopsy oral antibiotic therapy significantly improved IR in patients with CE, whereas increasing the time interval from biopsy to ET reduced IR. This study may help to find a higher potential for success in the medical management of patients with CE.
Keywords: antibacterial agents, biopsy, embryo implantation, endometritis, hysteroscopy
1. Introduction
Good endometrial receptivity is essential for a successful embryo implantation.[1,2]Among the factors that can affect endometrial receptivity is chronic endometritis (CE) is a factor that can affect endometrial receptivity. However, the pathogenesis of CE is poorly understood. It may be associated with microbial infection as well as with noninfectious inflammatory conditions such as endometriosis, prolonged menstrual bleeding, the use of intrauterine devices, and autoimmune disorders.[3] CE can cause changes in the endometrial microenvironment that result in failed embryo transfer (ET),[4] and previous reports have shown that women with CE have lower implantation rate (IRs) than those without CE.[5]
CE is diagnosed by detecting plasma cell infiltration into the endometrial stroma via biopsy and staining for cluster of differentiation (CD)-138, a specific marker of plasma cells. At our reproductive health center, endometrial biopsy is recommended after 1 or 2 implantation failures during in vitro fertilization (IVF), and approximately 50% to 60% of these patients are diagnosed with CE by CD138 immunohistochemistry (IHC). However, there is no consensus regarding the number of plasma cells per high-power field (hpf), which is considered to represent CE, and investigators have used values ranging from 1 to 5 CD138 + cells per hpf or 5 or more plasma cells within at least 1 section of biopsy tissue.[6,7]
In addition to the lack of standardized diagnostic criteria for CE, it is unclear whether the damage caused to the endometrium by biopsy further decreases the endometrial receptivity for future embryo implantation. Historically, endometrial surgery was considered to represent an injury to the endometrium[8]; thus, some IVF centers advise delaying the next ET by 1 or 2 menstrual cycles after biopsy to allow endometrial recovery. However, there are no evidence-based guidelines for the optimal time intervals in this situation. Some studies have investigated the time interval (TI) after hysteroscopic polypectomy in patients with endometrial polyps, whereas others have studied TI after hysteroscopic exploration without endometrial biopsy.[9–11] However, there are very few reports of TI after hysteroscopic biopsy in patients diagnosed with CE.
Another challenge faced by women diagnosed with CE is that appropriate treatment remains unclear, with some practitioners advising on antibiotic treatment and others avoiding the use of antibiotics. Cicinelli et al[12] found that the pregnancy rate in CE patients (diagnosed using CD138 IHC) with implantation failure, who then received antibiotic treatment, significantly improved, demonstrating that oral antibiotic treatment is helpful in CE patients. Kotaro also found that oral antibiotic treatment for CE improves the live birth rate.[13] However, some researchers have reported that antibiotic treatment is ineffective in CE patients. Lamonica et al[14] diagnosed patients with CE when 1 or more CD138-positive plasma cells were found per hpf, and found that even when endometrial findings reverted to normal after antibiotic treatment, the IR for the subsequent ET cycle remained the same. Therefore, identification of patients who can benefit from antibiotics remains unclear.
Given the uncertainty regarding a number of factors related to CE and IVF, this study aimed to determine the optimal TI until the next cycle of ET as well as the effect of antibiotic treatment on IR in patients with CE.
2. Methods
2.1. Study participants
We analyzed the clinical data of 256 cycles from 218 women who had experienced 1 or 2 failed ET attempts and who underwent subsequent hysteroscopic biopsy followed by CD138 IHC analysis between January 2018 and May 2019 at the Affiliated Beijing Chaoyang Hospital of Capital Medical University. During the study period, 180 patients underwent only 1 ET cycle and 38 patients underwent 2 ET cycles. All patients underwent frozen embryo transfer after hysteroscopic biopsy, with TI ranging from 1 to 14 months. The inclusion criteria were as follows: IVF indications, including tubal or male factor infertility or unexplained infertility; Patients without any obvious intrauterine abnormalities on transvaginal ultrasound or hysterosalpingography, such as adhesions, submucosal uterine occupation, or uterine malformations; and Patients who had previously received 1 to 2 cleavage-stage embryos containing 5 to 10 cells with an endometrial thickness of 7 to 18 mm on the day of ET. The exclusion criteria were as follows: Previous uterine surgery; Ovarian endometrioma, corticosteroid treatment, known clinical autoimmune disease, or thrombophilia; and Genital tract infection.
To minimize variability in our study, we selected patients with CE who had no obvious intrauterine abnormalities and received cleavage-stage embryos during the ET cycle following biopsy. Serum hormone levels and endometrial thickness of the patients included in our study were strictly limited.
2.2. Biopsy procedure
All the patients underwent hysteroscopic biopsy during the early follicular phase. Prior to the operation, the patients were examined for the presence of infectious diseases and underwent routine blood and urine analyses, coagulation function testing, evaluation of vaginal discharge, and electrocardiography. The endocervical canal, uterine cavity, tubal orifices, and endometrium were inspected using a 6-mm (outer diameter) continuous-flow hysteroscope (Storz, Tuttlingen, Germany), after which an endometrial biopsy was performed using intrauterine curettage.
2.3. Processing of endometrial specimens for pathological analysis
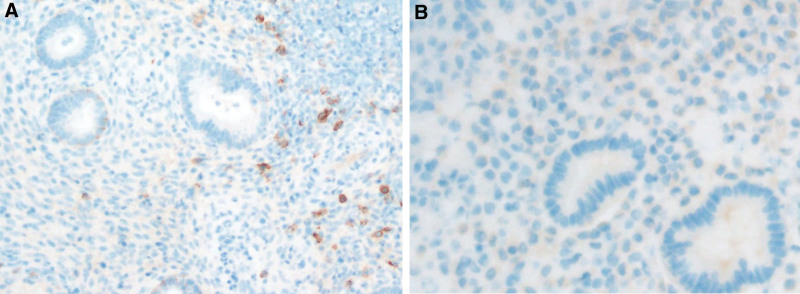
All specimens were sent to the same laboratory for analysis by a pathologist specializing in endometrial pathology. The biopsy samples were embedded in paraffin and serial slices were obtained and placed in xylene, anhydrous ethanol, 95% ethanol, and 70% ethanol successively for dewaxing and hydration. Immunohistochemical staining was performed by incubating with an undiluted rabbit monoclonal anti-CD138 antibody (Zhongshanjinqiao, Beijing, China) for 1 hour at room temperature, followed by gradient dehydration in 95% and anhydrous ethanol. We considered a sample to be CE-positive when 3 or more plasma cells (CD138 + cells) per hpf were observed in the endometrial stroma from at least 20 hpfs (using a Bx43 microscope, Olympus, Japan); if no cells or fewer than 3 plasma cells per hpf were observed, the specimen was designated CE-negative (Fig. 1).
Figure 1.
(A) CD138-positive IHC staining of endometrial biopsy tissue. The brown cells in the endometrial stroma are the plasma cells, (B) CD138-negative sample. CD = cluster of differentiation, IHC = immunohistochemistry.
2.4. Antibiotic treatment of patients diagnosed with CE
Out of 154 CE-positive cycles (as determined by IHC), 108 ET cycles were performed after patients received 0.2 g of levofloxacin (Shuanghe, Beijing, China) 3 times daily, and 0.2 g of metronidazole (Yabao, Shanxi, China) times daily, orally for 14 days. The remaining 46 ET cycles were performed without antibiotic treatment. Some studies have suggested that after antibiotic treatment, hysteroscopy should be performed again to determine whether CE has resolved before deciding whether to perform another ET cycle. However, this is not a consensus recommendation and repeat hysteroscopy was not performed in our study.
2.5. Protocols endometrial preparation and ET
Following our regular protocol for patients with normal ovulation, B-ultrasound monitoring (Philips, Boston) was initiated beginning on days 10 to 12 of the menstrual cycle, and ET was performed 3 days after ovulation. Patients without a regular ovulation cycle were injected with human menopausal gonadotropin (HMG; 75–150 IU/day; Lizhu, Zhuhai, China) from days 2 to 5 of the menstrual cycle. Recombinant human chorionic gonadotropin (hCG; 250 µg; Merck, Geneva, Switzerland) was administered to trigger ovulation when the follicle diameter was > 20 mm or the blood luteinizing hormone level was > 15 mIU/mL. Ovulation was determined using B-ultrasound, and ET was performed 3 days after ovulation. For hormone replacement cycles, the patients were treated with estradiol valerate orally (6–8 mg/day) from days 2 to 3 of the menstrual cycle. After 10 days, daily intramuscular injections of progesterone (60 mg; Tongyong, Shanghai, China) and didroxyprogesterone (20 mg, orally; Abbott, Netherlands) were administered for luteal support. ET was performed 3 days after administration of didroxyprogesterone. One or 2 thawed embryos were transferred under ultrasound guidance using a Cook catheter (Curved Embryo Transfer Catheter; Cook Medical, Bloomington). The number of embryos depended on the women’s age, embryo quality, and gynecological complications.
2.6. Follow-up examinations
Circulating hCG levels were measured 12 days after ET using peripheral venous blood testing, and patients with positive hCG levels (>60 mIU/mL) were considered pregnant. Transvaginal B-ultrasonography was performed 21 days after ET to determine fetal sac number and location. If fetal sacs were found, the implantation was considered successful.
2.7. Statistical analyses
All data were analyzed using the SPSS statistical software package (v. 18.0; IBM Corp., Armonk, NY). Independent paired-sample Student’s t tests were used if the data were normally distributed, as determined by the Kolmogorov–Smirnov test, and the Mann–Whitney nonparametric U test was used for data that were not normally distributed. The patients ages were compared using independent-sample t tests, and the duration of infertility, body mass index, and clinical outcomes were compared using the Mann–Whitney U test. The chi-squared test was used for categorical variables. P < .05 was considered significant.
3. Results
3.1. Study participant characteristics
The patients included in our study were aged between 23 and 43 years (median 33 years). The median duration of infertility was 3 years. In total, 74 (33.9%) patients had a history of pregnancy and 38 (17.4%) had experienced spontaneous miscarriage (Table 1).
Table 1.
Clinical characteristics of the participants at the time of each embryo transplantation cycle.
| G1 | G2 | G3 | Total | |
|---|---|---|---|---|
| Cycle (n) | 108 | 46 | 102 | 256 |
| Patients(n) | 90 | 38 | 90 | 218 |
| Previous pregnant history(n) | 28 | 15 | 31 | 74 |
| Spontaneous miscarriage (n) | 16 | 9 | 13 | 38 |
| Number of embryo transfers (n) | ||||
| 1 | 72 | 30 | 78 | 180 |
| 2 | 18 | 8 | 12 | 38 |
G1 = chronic endometritis with antibiotics, G2 = chronic endometritis without antibiotics, G3 = non-chronic endometritis.
In our study, we sought to analyze the effectiveness of antibiotic treatment in patients with infectious CE, excluding the role of noninfectious inflammatory conditions as much as possible. Thus, we tested the threshold value of 3 plasma cells per hpf for the diagnosis of CE. Based on a threshold value of 3 plasma cells per hpf, the incidence of CE in our study was 154/256 (60.2%) in the ET cycle and 128/218 (58.7%) in the population.
3.2. Antibiotic treatment increases the embryo IR in patients with CE
To investigate the utility of antibiotic treatment in patients with CE, we divided the 256 cycles into 3 groups based on the CD138 IHC results and whether or not the patients had received antibiotic therapy; we assessed ET outcomes in each group. Age, duration of infertility, and body mass index were similar between the groups (Table 2). Patients with and without CE, who received antibiotic treatment, exhibited comparable IRs. However, IR in patients with CE who received antibiotics was significantly higher than that in patients with CE who were left untreated (Table 2). These findings suggest that antibiotic therapy improves the embryo implantation efficacy in patients with CE.
Table 2.
Implantation rates among patients with or without chronic endometritis who received antibiotics or were left untreated.
| CE with antibiotics | CE without antibiotics | Non-CE | P value | P value | |
|---|---|---|---|---|---|
| (G1) | (G2) | (G3) | (G1&G3) | (G1&G2) | |
| Cycle (n) | 108 | 46 | 102 | ||
| Age, yr (mean ± SD)† | 32.2 ± 4.1 | 33.5 ± 4.3 | 33.1 ± 4.2 | .177 | .091 |
| Duration of infertility, yr (median, IQR)§ | 2, 3 | 3, 2 | 3, 3 | .167 | .359 |
| Body mass index, kg/m2 (median, IQR)§ | 23.13, 4.94 | 23.36, 5.38 | 23.05, 4.45 | .647 | .990 |
| Embryos transferred (n, %) | 185 (41.5) | 83 (18.6) | 178 (39.9) | ||
| Fetal sacs (n, %) | 81 (47.1) | 25 (14.5) | 66 (38.4) | ||
| Implantation rate (%)‡ | 43.8 (81/185) | 30.1 (25/83) | 37.1 (66/178) | .193 | .034* |
CE = chronic endometritis, SD = standard deviation, IQR = interquartile range.
P < .05.
Student’s t test.
Chi-squared test.
Mann–Whitney U test.
3.3. Increasing the time interval to embryo implantation after biopsy decreases the IR
To evaluate the effect of hysteroscopic biopsy on the subsequent IR, we analyzed the IR by TI in 210 ET cycles from the non-CE and CE with antibiotics groups. The CE group without antibiotics was excluded to reduce the bias of implantation failure caused by the lack of antibiotic treatment in these patients. A total of 173 ET cycles were performed within 6 months of hysteroscopic biopsy, and 37 were performed after 6 months. We found that the IR was significantly higher in the group that underwent ET < 6 months after biopsy (Table 3). Furthermore, among the 173 ET cycles, there was no significant difference between the IRs of patients who underwent ET < 2 months after biopsy and those who underwent ET ≥ 2 and < 6 months after biopsy (Table 4). Collectively, these findings suggested that hysteroscopic biopsy does not negatively affect endometrial receptivity during embryo implantation.
Table 3.
Implantation rates in patients with a TI < 6 months or ≥ 6 months.†
| <6 months | ≥6 months | t/X2/Z | P value* | |||||
|---|---|---|---|---|---|---|---|---|
| G1 | G3 | Total | G1 | G3 | Total | |||
| Cycle (n) | 90 | 83 | 173 | 18 | 19 | 37 | ||
| Fetal sac (n) | 72 | 58 | 130 | 9 | 8 | 17 | ||
| Embryos tran- sferred (n) | 157 | 145 | 302 | 28 | 33 | 61 | ||
| Implantation rate (%)† | 43.0 (130/302) | 27.9 (17/61) | 4.851 | 0.028* | ||||
TI = time interval.
P < .05.
Chi-squared test.
Table 4.
IR comparison in patients with a TI < 2 months and a TI ≥ 2 months and < 6 months.
| TI < 2 months | 2 months ≤ TI < 6 months | t/X2/Z | P value* | |||||
|---|---|---|---|---|---|---|---|---|
| G1 | G3 | Total | G1 | G3 | Total | |||
| Cycle (n) | 31 | 31 | 62 | 59 | 52 | 111 | ||
| Fetal sac (n) | 20 | 23 | 43 | 52 | 35 | 87 | ||
| Embryos transferred (n) | 56 | 56 | 112 | 101 | 89 | 190 | ||
| Implantation rate (%)† | 38.4 (43/112) | 45.8 (87/190) | 1.572 | 0.210 | ||||
IR = implantation rate, TI = time interval.
P < .05.
Chi-squared test.
4. Discussion
In this study, we found that antibiotic treatment improves IR in women with CE undergoing IVF, and that there is no need to delay ET after hysteroscopic biopsy. Patients who were diagnosed with CE based on a threshold value of 3 or more plasma cells per hpf and treated with antibiotics exhibited significantly higher IRs than those who were diagnosed with CE but were left untreated. Furthermore, IRs decreased if the next ET cycle took place >6 months after hysteroscopic biopsy in women with and without CE, compared with performing ET within 6 months of the procedure.
Our findings showed that antibiotic treatment improved IR in patients diagnosed with CE, which is similar to the findings of previous studies. Indeed, Cicinelli et al[12] found that the pregnancy rate in patients with CE who received oral antibiotics improved significantly, and Kotaro et al found that oral antibiotics improved the live birth rate.[13] Nevertheless, some studies, such as that performed by Xiong et al[15], have reported pregnancy outcomes even after antibiotic treatment in CE patients with a small number of CD138(≤4 per hpf).The conflicting results obtained in these studies may be related to different inclusion and diagnostic criteria, specifically different cutoff values for plasma cell counts. We found that antibiotic treatment significantly improved pregnancy outcomes in the subsequent ET cycle when CE was diagnosed based on the presence of 3 or more plasma cells per hpf.
However, in our study, patients diagnosed with CE did not undergo a second endometrial biopsy before the next ET; therefore, it was unclear whether the CE had been cured. Xiong et al[15] found that the cure rate was 74.4% after a second endometrial biopsy and 89.0% after a third biopsy in patients with CE with CD138+/HPF ≥ 5. However, this study did not consider TI and could not define whether CE was persistent or recurrent. It should be noted that if persistent CE patients receive antibiotic therapy again, their pregnancy outcomes may improve. However, because inflammation may recur over time, we believe that antibiotic therapy and TI should be considered and discussed together in patients with CE. If the TI is too long, the effect of antibiotic therapy can be compromised, or CE may recur. Therefore, it is important to determine the optimal TI value. We believe that the optimal time is crucial, and our study can provide a reference for whether and when it is appropriate to conduct a second endometrial biopsy.
Our findings suggest that delaying ET after hysteroscopic biopsy in patients with CE does not improve IRs and provide important evidence regarding the controversial question that, to date, has not been investigated. Some studies have investigated TI after hysteroscopic polypectomy,[10,16,17] intrauterine adhesions,[18] scratching for endometrial stimulation,[19] or hysteroscopic exploration without tissue biopsy.[9] Tu et al[10] retrospectively studied 102 IVF patients with endometrial polyps who underwent hysteroscopic polypectomy and found that a TI of > 4 months was associated with a decreased rate of successful pregnancy outcomes, thus recommending that these patients wait no longer than 4 months before their next ET cycle. Pereira et al[17] found that patients who underwent ET < 1, 1 to 3, and > 3 months after hysteroscopic polypectomy exhibited no differences in IR, suggesting that they could undergo ET within 1 month after hysteroscopic polypectomy biopsy. However, Eryilmaz et al[16] concluded that pregnancy outcomes were unrelated to TI, as no significant difference was found in pregnancy outcome rates between patients who underwent ET < 6 months and > 6 months after hysteroscopic polypectomy in their study. Deng et al[18] studied 312 patients with intrauterine adhesions who underwent hysteroscopic adhesiolysis before ET, and found that the optimal TI was 3 to 6 months. Zhu et al[11] demonstrated that performing ET and hysteroscopy during the same menstrual cycle did not impair pregnancy outcomes; however, the patients in this study were not classified according to intrauterine disease type. Rana et al divided patients into 3 groups according to TI (<50 days, 50 days to 6 months, and > 6 months after hysteroscopic biopsy) and found that the pregnancy rate was the highest when TI was < 50 days; however, the patients included in this study had normal hysteroscopic findings.[9] Kumbak et al[19] found that patients who underwent endometrial biopsy in the luteal phase on the day of GnRH agonist initiation for a long protocol and subsequent fresh ET (TI < 1 month) had significantly higher IR than the control group (1 month < TI < 6 months). Although the included patients did not experience ET failure, which was different from our study, the authors similarly proposed that endometrial biopsy did not negatively affect IR when TI was <1 month. The apparent discrepancies among these studies may be related to differences in intrauterine diseases and treatments, as well as differences in the embryo stage at ET; some studies used cleavage-stage embryos, whereas others used blastocysts. Moreover, some studies did not consider postoperative treatment when analyzing pregnancy outcomes. Based on our findings, we speculate that waiting too long before the next ET may lead to the recurrence of uterine inflammation, thus decreasing the chance of a successful pregnancy. Therefore, when patients are unable to undergo ET within 6 months of endometrial biopsy, repeated hysteroscopy should be recommended.
A strength of our study is that all endometrial samples were obtained and diagnosed histologically in the proliferative phase. This was important because a different prevalence of CE has been observed between the proliferative and secretory phases of the menstrual cycle.[20] Furthermore, there was a strict limit on the thickness of the endometrium on the day of ET, and it is believed that too thin or too thick an endometrium will affect IR.[21]
Our study has some limitations. Given that there is no unified worldwide standard for diagnosing CE, the diagnostic criteria that we selected may have yielded different outcomes than those of other studies that have used different criteria. None of the patients in this study underwent repeated hysteroscopy before the next ET to reduce the time to pregnancy and endometrial injury, and it was unclear whether CE had been cured before the next ET. In addition, the sample size was relatively small. Finally, the operative skills of the gynecologist and the biopsy method used may have influenced the pregnancy outcomes in our study population.
5. Conclusion
Defining CE as the presence of 3 or more typical plasma cells per hpf seemed to effectively identify patients in whom antibiotic therapy improved IR during the next ET cycle. The next ET cycle should not be delayed by 1 to 2 months after endometrial biopsy, and ET should ideally be performed within 6 months of hysteroscopic biopsy. Given that few studies have investigated the optimal TI for ET after biopsy in patients with CE, our results should be confirmed in large, multicenter, randomized controlled trials.
Acknowledgements
The authors would like to thank Dr Hui Su and Dr Danni Qu for their support and collaboration. The authors would like to thank Mr. Jiwen An for the language proofreading.
Author contributions
Conceptualization: Zhang Yinglan.
Data curation: Xue Li, Hui Su.
Formal analysis: Zhang Yinglan.
Investigation: Hui Su.
Methodology: Zhang Yinglan, Xue Li.
Resources: Xue Li, Hui Su.
Visualization: Xue Li.
Writing – original draft: Zhang Yinglan.
Writing – review & editing: Zhang Yinglan, Xue Li, Hui Su.
Abbreviations:
- CD
- cluster of differentiation
- CE
- chronic endometritis
- ET
- embryo transfer
- hpf
- high-power field
- IHC
- immunohistochemistry
- IR
- implantation rate
- IVF
- in vitro fertilization
- TI
- time interval
Data were retrospectively obtained from medical records or outpatient follow-ups, and informed consent was obtained from all the patients.
This study was reviewed and approved by the Ethics Committee of the Affiliated Beijing Chaoyang Hospital of the Capital Medical University (No.2021-2-1-52, date:01/03/2021).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Yinglan Z, Li X, Su H. Benefits of antibiotics and the optimal time interval between biopsy and the next embryo transfer in patients with chronic endometritis. Medicine 2023;102:37(e34650).
References
- [1].Buzzaccarini G, Vitagliano A, Andrisani A, et al. Chronic endometritis and altered embryo implantation: a unified pathophysiological theory from a literature systematic review. J Assist Reprod Genet. 2020;37:2897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Di Pietro C, Caruso S, Battaglia R, et al. MiR-27a-3p and miR-124-3p, upregulated in endometrium and serum from women affected by Chronic Endometritis, are new potential molecular markers of endometrial receptivity. Am J Reprod Immunol. 2018;80:e12858. [DOI] [PubMed] [Google Scholar]
- [3].Puente E, Alonso L, Laganà AS, et al. Chronic endometritis: old problem, novel insights and future challenges. Int J Fertil Steril. 2020;13:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu D, Kimura F, Zheng L, et al. Chronic endometritis modifies decidualization in human endometrial stromal cells. Reprod Biol Endocrinol. 2017;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Park HJ, Kim YS, Yoon TK, et al. Chronic endometritis and infertility. Clin Exp Reprod Med. 2016;43:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu Y, Chen X, Huang J, et al. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil Steril. 2018;109:832–9. [DOI] [PubMed] [Google Scholar]
- [7].Herlihy NS, Klimczak AM, Titus S, et al. The role of endometrial staining for CD138 as a marker of chronic endometritis in predicting live birth. J Assist Reprod Genet. 2022;39:473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ozgur K, Bulut H, Berkkanoglu M, et al. Six-month recovery needed after dilation and curettage (D and C) for reproductive outcomes in frozen embryo transfer. J Obstet Gynaecol. 2018;38:1150–7. [DOI] [PubMed] [Google Scholar]
- [9].Karayalçin R, Ozyer S, Ozcan S, et al. Office hysteroscopy improves pregnancy rates following IVF. Reprod Biomed Online. 2012;25:261–6. [DOI] [PubMed] [Google Scholar]
- [10].Tu YA, Yang PK, Chen SU, et al. Optimal time interval between hysteroscopic polypectomy and frozen-thawed blastocyst transfer: a retrospective study. PLoS One. 2020;15:e0240882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhu X, Ye H, Fu Y. The effect of frozen-thawed embryo transfer performed concurrently with hysteroscopy on the reproductive outcomes during assisted reproductive treatments. Sci Rep. 2017;7:11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cicinelli E, Matteo M, Tinelli R, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015;30:323–30. [DOI] [PubMed] [Google Scholar]
- [13].Kitaya K, Matsubayashi H, Takaya Y, et al. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am J Reprod Immunol. 2017;78. [DOI] [PubMed] [Google Scholar]
- [14].Lamonica R, Hartnett J, Engmann LR, et al. Immunohistochemistry confirms the presence of chronic endometritis in patients with recurrent implantation failure. Fertil Steril. 2006;86:S280. [Google Scholar]
- [15].Xiong Y, Chen Q, Chen C, et al. Impact of oral antibiotic treatment for chronic endometritis on pregnancy outcomes in the following frozen-thawed embryo transfer cycles of infertile women: a cohort study of 640 embryo transfer cycles. Fertil Steril. 2021;116:413–21. [DOI] [PubMed] [Google Scholar]
- [16].Eryilmaz OG, Gulerman C, Sarikaya E, et al. Appropriate interval between endometrial polyp resection and the proceeding IVF start. Arch Gynecol Obstet. 2012;285:1753–7. [DOI] [PubMed] [Google Scholar]
- [17].Pereira N, Amrane S, Estes JL, et al. Does the time interval between hysteroscopic polypectomy and start of in vitro fertilization affect outcomes? Fertil Steril. 2016;105:539–44.e1. [DOI] [PubMed] [Google Scholar]
- [18].Deng K, Song XH, Han XM, et al. Optimal waiting period for fresh embryo transfer after hysteroscopic adhesiolysis: a retrospective cohort study. Chin Med J (Engl). 2019;132:2333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kumbak B, Sahin L, Ozkan S, et al. Impact of luteal phase hysteroscopy and concurrent endometrial biopsy on subsequent IVF cycle outcome. Arch Gynecol Obstet. 2014;290:369–74. [DOI] [PubMed] [Google Scholar]
- [20].Adegboyega PA, Pei Y, McLarty J. Relationship between eosinophils and chronic endometritis. Hum Pathol. 2010;41:33–7. [DOI] [PubMed] [Google Scholar]
- [21].Craciunas L, Gallos I, Chu J, et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:202–23. [DOI] [PubMed] [Google Scholar]