Abstract
Bipolar disorder (BD) is a chronic and clinically complex disease, characterized by pathological disturbances in mood and energy. Cytokines can access the brain and their signaling pathways affect brain functions, such as neurotransmitter metabolism, neuroendocrine function, neural/synaptic plasticity, and mood neural circuitry. JAK 1 is the most common phosphorylation protein combined with the tyrosine kinase cytokine receptors; therefore, we investigated the association between the Janus family kinase 1 (JAK1) gene polymorphisms (rs2780895, rs4244165, and rs17127024) and susceptibility to BD. The case study population included 93 patients diagnosed with BD and 112 healthy controls, selected from the central coastal region of Tunisia. Polymerase chain reaction-restriction fragment length polymorphism was used to investigate these 3 JAK1 polymorphisms. We compared the sociodemographic and clinical parameters of 3 genotypes of this single nucleotide polymorphisms rs2780895, rs4244165, and rs17127024 of the JAK1 gene. The frequencies of the 3 genotypes were similar in the patient and control groups. One-way analysis of variance revealed a significant variation in rs4244165. After hospitalization, the average of the brief psychiatric rating scale score was significantly higher for the wild-type GG genotype than that for the double-mutation TT genotype (31.23% vs 22.85%, P = .043). The least significant difference post hoc test also showed a significant difference between the GG and TT genotypes at both hospital admission (P = .001) and after hospitalization (P = .012), with the GG genotype being associated with a higher brief psychiatric rating scale score. Haplotypic analysis revealed that the wild-type haplotype with the highest frequency (46.62%) was CTG. Our results showed no association between the 3 studied positions and bipolar disorder. However, the G-allele of rs4244165 in JAK1 is associated with the highest level of the brief psychiatric rating scale in patients with bipolar disorder. The JAK/signal transducer and activator of transcription pathway is an interesting therapeutic route that requires further investigations. Studying their regulatory regions can provide a clearer picture of all the interactions involved in the regulation of genetic expression in response to treatment.
Keywords: bipolar disorder, BPRS, JAK 1, RFLP-PCR, Tunisia
1. Introduction
Bipolarity is a chronic and clinically complex disease.[1] Its high misdiagnosis rate and long-term delayed diagnosis are due to doctors subjective judgment of the diagnosis procedure.[2] bipolar disorder (BD) is characterized by pathological disturbances in mood and energy.[3] The lifetime prevalence of the bipolar spectrum is estimated to be 2.4%,[4] making it one of the principal causes of disability among youth, leading to cognitive and functional deterioration and increased mortality, particularly death by suicide.[3] Approximately 59% of patients with BD experience their first symptoms during childhood or adolescence,[5] with a median age of 17.5 years in Kupfer cohort study.[6] This early onset is associated with shorter euthymic phases and a higher risk of suicide and violence.[7]
The immune system plays a significant role in the pathogenesis and aftermath of mental diseases.[8] A large body of plausible data suggests that innate inflammatory reactions may contribute to the induction of depression by interfering with stress-susceptible pathways that affect neuroendocrine and autonomic nervous systems.[9,10] This interaction between the inflammatory pathway and brain neurocircuits can induce typical behaviors in various neuropsychiatric disorders including depression. These behaviors include diminished motivation (anhedonia), avoidance or anxiety,[11] fatigue, psychomotor slowing, anorexia, cognitive dysfunction, and sleep impairment.[12] Cytokines can access the brain and interact with domains associated with depression. Their signaling pathways affect brain functions such as neurotransmitter metabolism, neuroendocrine function, neural/synaptic plasticity, and mood neural circuitry.[13,14]
Furthermore, genome-wide association studies have pointed to the implication of inflammation, abnormalities in signaling pathways, and mitochondrial dysfunction in BD.[15] This inflammation leads to substantial modifications in gene expression, and studies have revealed that alterations in mitochondrial function are associated with increased epidermal growth factor-JAK-signal transducer and activator of transcription (STAT)3 signaling.[16]
The main signaling pathway used by a broad range of cytokines and growth factors is the JAK/STAT pathway. Activated JAK drives cell proliferation, differentiation, migration, and apoptosis.[17] The central nervous system is involved in neurogenesis, synaptic plasticity, gliogenesis, and microglial activation, all of which are implicated in the pathophysiology of mood disorders. The JAK/STAT pathway could provide a novel treatment route for depressive disorders.[18]
The subgroup of the Janus family kinases (JAK) includes Janus family kinase 1 (JAK1), Janus family kinase 2 (JAK2), Janus family kinase 3, and Tyrosine Kinase (Tyk) 2. They can perform in homodimeric or heterodimeric complexes.[19,20]
1.1. Aim
JAK 1 is the most common phosphorylation protein combined with the tyrosine kinase cytokine receptors; therefore, the aim of our study was to investigate the association between 3 JAK1 polymorphisms (rs2780895, rs4244165, and rs17127024) and susceptibility to bipolar disorder.
2. Materials and methods
2.1. Ethical considerations
Our study was conducted in accordance with the tenets of the Declaration of Helsinki. The Health and Life Sciences Research Ethics Committee of the Higher Institute of Biotechnology of Monastir approved this study (CER-SVS/ISBM 006/2020).
2.2. Patients and controls
The subjects of our investigation were 93 individuals diagnosed with bipolar disorder based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria and 112 healthy controls without a family history of psychiatric disorders. The participants were recruited from the university hospital of Monastir in Tunisia and provided free and informed consent for the study. Recruitment was conducted between September 2014 and June 2017.
Of the 93 patients, 69 (74.19%) were male and 24 (25.8%) were female. In the control group, 77 (68%) were male and 36 (32%) were female, with a P value of .379. The mean age of the patients was 36.8 ± 10.8 years, which did not differ statistically from the mean age of controls (36.8 ± 10.8 years) with a P value of .106. The sociodemographic and clinical parameters were compared. The Brief Psychiatric Rating Scale (BPRS) was used at the start and end of hospitalization with the 3 genotypes of the single nucleotide polymorphisms (SNP) of the JAK1 gene.
2.3. Genomic deoxyribonucleic acid (DNA) extraction and investigation of the polymorphisms of JAK1
DNA was extracted from peripheral blood cells using the salting out method, and polymerase chain reaction-restriction fragment length polymorphism was used to investigate the 3 SNPs in JAK1. The PCR reaction volume was 25 μL and included 2 μL of DNA, 0.5 μL of each forward and reverse primer, 0.5 μL of deoxy Nucleotides triphosphate, 2.5 μL of buffer, 3.25 μL of MgCl2, 0.2 μL Taq DNA polymerase and water to complete the final volume.
The PCR reaction was performed using an Applied Thermal Cycler with a program consisting of a 10 minutes pre-denaturation step at 95°C, 35 cycles of denaturation at 95°C for 60 seconds, annealing at 55°C (for rs2780895 and rs4244165)/57°C (for rs17127024) for 1 minute and 15 seconds, extension at 72°C for 1 minute and 15 seconds, and a final extension step at 72°C for 10 minutes.
The amplified PCR products were visualized by 1.5% agarose gel electrophoresis, and the obtained PCR products were genotyped after incubation with a restriction enzyme. The primers, fragment sizes of the PCR products, and the restriction products are listed in Table 1. The 3 SNPs investigated in this study were located in the intronic region of the JAK1 gene, which is located on chromosome 1q31.3 and encodes the tyrosine kinase JAK1, which consists of 1154 amino acids.[21] We used the Strengthening the reporting of observational studies in epidemiology cross-sectional reporting guidelines.[22]
Table 1.
Primers and enzymatic digestion conditions for genotyping JAK1 SNPs.
| SNP | Position | Region | Primers | Restriction enzyme | Restriction product |
|---|---|---|---|---|---|
| rs2780895 | 64,843,717 | Intron | F: CACAGGTAGATTTGGAGGAG R: AAGACGCTGATTGAGGTGAG |
NdeI | CC: 676 CT: 676 + 314 + 362 TT: 314 + 362 |
| rs4244165 | 64,955,388 | Intron | F: GTGACTGCATAGTGGAGGTG R: CTTTAGAAGCCCCTATTGCC |
HaeIII | TT: 415 GT: 415 + 160 + 255 GG: 160 + 255 |
| rs17127024 | 64,837,448 | Intron | F: GTGAGCAGGTGGAGAGAATC R: TACTGGCACAAAGCAGGACC |
HaeIII | TT: 395 GT: 395 + 227 + 168 GG: 227 + 168 |
JAK1 = Janus family kinase 1, SNP = single nucleotide polymorphism.
2.4. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) software (version 26.0; SPSS Inc., Chicago, IL). Continuous variables are presented as mean values with standard deviation (±SD) and were compared using appropriate statistical tests, such as the independent-samples T test or 1-way analysis of variance. The power of the test was tested using R package (pwr).
3. Results
The study participants were predominantly of urban origin, with 86% coming from urban areas, and 52.4% of patients having a close relationship with psychiatric pathology. The majority of the patients had completed secondary education (44%) and were either unmarried (46.4%) or married (44%). A total of 58.3% of the patients had received some form of technical training. Most patients (70.2%) were either unemployed or unable to work, and nearly (90.5%) were unaffluent. The average age of onset of illness was 27.65 ± 9.3 years, with an average of 12.5 ± 10.6 years with the pathology. One-quarter of the patients (25.2%) had a concomitant physical pathology with psychiatric involvement.
Of the patients, 88 (94.62%) had bipolar disorder type I , and 5 (5.37%) had bipolar disorder type II. Sleep issues and psychotic features were present in 86% and 39.78% of the cases, respectively. The average medication regimen consisted of 1.25 g mood stabilizers and 210 mg of the 2 types of neuroleptics. Sodium valproate was the first prescribed medication in 55.1% of the cases, and phenothiazine with typical or atypical neuroleptics was the second prescribed medication in 60.3% of the cases.
The patients had an average BPRS psychometric scale score of 42.96 ± 11.67 at the start of hospitalization, which improved to 25.72 ± 9.97 at the end of their stay. The mean SAPS score at the start of hospitalization was 18.19 ± 12.30, which was still present at the end of their stay, albeit with an improvement of 9.07 ± 7.76. The patients were hospitalized due to a manic episode in 83.87% of cases, with a corresponding psychometric mania assessment scale score of 20.54 ± 6.23 at the beginning of hospitalization, which significantly improved to 7.03 ± 4.20 by the end of their stay. In 12.2% of cases, patients were hospitalized due to depression, with a corresponding psychometric Hamilton Depression scale score of 21.64 ± 7.1 at the start of hospitalization, which significantly decreased to 8.62 ± 6.46 by the end of their stay.
The healthy control group showed Hardy-Weinberg equilibrium (rs2780895, P = .898, power = 0.07; rs4244165, P = .755, power = 0.093). However, we could not test the HW equilibrium for rs17127024 because of the luck of observations for TT genotype at this position. Additionally, HW equilibrium was validated for the patient group (P = .742 for rs2780895, power = 0.09; P = .707 for rs4244165, power = 0.10; and P = .167 for rs17127024, power = 0.37).
The JAK1 genotype and allele frequencies for 3 positions (rs2780895, rs4244165, and rs17127024) in both patient and control groups are shown in Table 2.
Table 2.
Distribution of genotype frequencies of rs2780895, rs4244165 and rs17127024 between controls and patients.
| Model | Genotype | Controls 112 (%) | Patients 93 (%) | OR (95% CL) | P value |
|---|---|---|---|---|---|
| rs2780895 | C/C | 55 (49.1%) | 49 (52.68%) | ||
| C/T | 49 (43.75 %) | 39 (41.93%) | 0.89 (0.5, 1.58) | .7 | |
| T/T | 8 (7.14%) | 5 (5.37 %) | 0.7 (0.19, 3.32) | .57 | |
| Alleles | C | 159 (70.98 %) | 137 (73.65 %) | 0.87 (0.56, 1.35) | .55 |
| T | 65 (29.01 %) | 49 (26.34 %) | |||
| rs4244165 | T/T | 40 (35.71 %) | 30 (32.25 %) | ||
| G/T | 57 (50.89 %) | 49 (52.68 %) | 1.145 (0.62, 2.17) | .66 | |
| G/G | 15 (13.39 %) | 14 (15.05 %) | 1.242 (0.51, 2.99) | .62 | |
| Alleles | T | 137 (61.16 %) | 109 (58.60 %) | 1.11 (0.74, 1.650) | .60 |
| G | 87 (38.83 %) | 77 (41.39 %) | |||
| rs17127024 | G/G | 102 (91.07%) | 85 (91.4%) | ||
| G/T | 10 (8.93%) | 7 (7.5 %) | 0.84 (0.29, 2.33) | .74 | |
| T/T | 0 (0%) | 1 (1.07%) | undefined (undefined) | .45 | |
| Alleles | G | 214 (95.53%) | 177 (95.16%) | ||
| T | 20 (8.92%) | 15 (8.06%) | 0.90 (0.44, 1.82) | .79 |
OR = odds ratio.
The frequencies of the wild-type homozygous genotype of rs2780895 (CC) and that of the mutated homozygote genotype (TT) were similar in the patient and control groups (49.1% vs 52.68% and 7.14% vs 5.37%, respectively). The allele frequency of rs2780895 (C) did not differ significantly between control subjects (70.98%) and those with BP (73.65%) (P > .05). We also conducted a binary regression analysis, but no significant result was found.
The frequency distributions of the wild-type homozygous genotype of rs4244165 (TT) and that of the mutated homozygote genotype (GG) were similar in the patient and control groups (35.71% vs 32.25% and 13.39% vs 14.05 %, respectively). Similarly, the allele frequency of rs4244165 (T) was 61.16% in the control subjects and 58.60% in the patients with BP (P > .05), indicating no significant difference. A binary regression analysis was also conducted, with no significant result found.
The frequency distributions of the wild homozygote genotype of rs17127024 (GG) and that of the mutated homozygote genotype (TT) were similar in the patient and control groups (91.07% vs 91.4% and 0% vs 1.7 %, respectively). Similarly, the allele frequency of rs17127024 (G) was 95.53% in the control subjects and 95.16% in the patients with BP, which was not statistically significant (P > .05). A binary regression analysis revealed no significant results.
Statistically, the genotypic frequencies of the 3 SNPs were tested in accordance with the age of pathology onset, economic and social parameters, and psychometric and somatic scales, but no association was found.
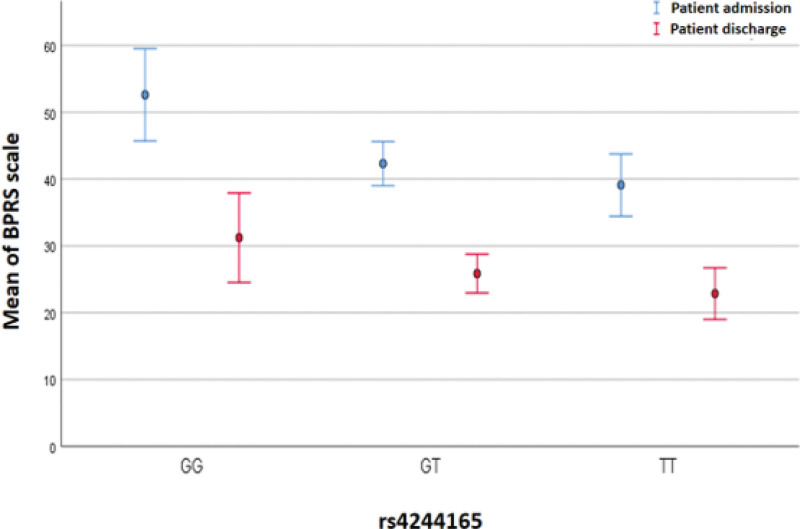
However, interesting findings were observed in this study, particularly regarding the association between rs4244165 of JAK1 and the BPRS psychometric scale. One-way analysis of variance revealed a significant variation in rs4244165. The average BPRS psychometric scale score at hospital admission differed according to genotype, with the highest score observed in individuals with the GG genotype (52.62%), followed by those with the GT genotype (42.32%), and the lowest score in individuals with the TT genotype (39.43%). After hospitalization, the average BPRS psychometric scale score was significantly higher for the wild-type GG genotype than for the double-mutation TT genotype (31.23% vs 22.85%, P = .043). The the least significant difference (LSD) post hoc test also showed a significant difference between the GG and TT genotypes at both hospital admission (P = .001) and after hospitalization (P = .012), and the GG genotype was associated with a higher BPRS score (Fig. 1). Furthermore, we conducted a multinomial regression analysis to eliminate the impact of confounding parameters (age and gender). We obtained a significant result for BPRS at hospital admission (P = .002), with (P = .011) for the GT genotype and (P = .002) for the TT genotype compared with the GG genotype.
Figure 1.
Juxtaposition of the mean of the BPRS psychiatric scale calculated on admission and discharge of patient in relation to the genotype of rs4244165 of JAK1 gene. BPRS = brief psychiatric rating scale, JAK1 = Janus family kinase 1.
Haplotypic analysis also revealed that the wild-type haplotype with the highest frequency (46.62%) was CTG, whereas the rare haplotypes CGT and CTT had a frequency of only 2% (Table 3).
Table 3.
Applotypic analysis for JAK 1 polymorphisms.
| JAK 1 polymorphisms | r2 | D’ | P value |
|---|---|---|---|
| rs2780895 vs rs4244165 | 0.237 | 0.313 | 0 |
| rs4244165 vs rs17127024 | 0.042 | 0.157 | .39 |
| rs17127024 vs rs2780895 | −0.136 | 0.99 | .0058 |
4. Discussion
We conducted an association study with Tunisian participants, and our statistical analysis showed that there was no link between the 3 studied positions and bipolar disorder. The absence of an association may be due to the small size of the study population or the variability in the distribution of SNPs among different ethnic groups. Likewise, the low level of power obtained for HW equilibrium was a result of the small size of the studied population. This is due to the difficulty in obtaining samples from patients suffering from psychiatric pathology. We collaborated with only 1 psychiatric service; however, we are considering expanding the study area by adding more psychiatric services as further studies on these polymorphisms may provide more insight into this matter.
The genetic origin of an SNP and its position within a gene can determine its potential impact on SNP.[23] Not only do exonic regions play an important role in the production of functional proteins, but the cis-regulatory steps that occur before protein production are also critical. Up to 90% of transcript segments in human genes are intronic regions in pre- Ribonucleic acid messengers.[24] Mammalian gene expression is essential for eliminating introns from primary transcripts through splicing.[25] Intronic regions serve as attachment sites for cis-acting Ribonucleic acid elements that regulate alternative splicing in diverse human tissues.[26] Alternative splicing performs regulatory functions that can be abolished by nucleotidic changes, thus inducing various diseases.[27]
The rs2780895 SNP of JAK1 has only been studied in 3 studies worldwide, with 2 focusing on Asian ethnicity. A Chinese study found no association with hepatitis B,[21] whereas an Iraqi investigation revealed an association between a heterozygous genotype and hepatitis C.[23] In Taiwanese children, the C-related allele is highly associated with asthma and can serve as a biomarker.[28]
Regarding the JAK1 SNP rs4244165, the comparison of genotypic and allelic frequencies did not show a significant difference between patients and controls. The results obtained allowed us to calculate a P value of .426 (> 0.05) and an odds ratio of 1.068, suggesting the absence of an association between the rs4244165 polymorphism of JAK1 and bipolar disorder in the Tunisian population studied. However, we observed a significantly higher average level of the BPRS psychometric scale for the wild-type genotype compared to the double-mutated genotype, with the LSD post hoc test at the beginning of hospitalization (P = .001) and after hospitalization (P = .012). The rs4244165 locus is located 1545 nucleotides away from the splice site and may be a regulatory or treatment response region. The BPRS is an assessment tool that is widely used by clinicians to evaluate psychiatric symptoms and outcomes. It consists of 18 items,[29–31] with the aim of providing a method to assess the functioning and behavior of patients and estimate their degree of symptomatology.[32]
Compared to international studies that have investigated this SNP, a Korean team found no association between rs4244165 and rs17127024 and Behçet’s disease, a systemic vasculitis that affects young adults.[33,34] An Iraqi study also found no association between this SNP and HCV infection.[23] Additionally, a single study conducted in Sfax, North Africa, did not find a correlation between this SNP and the development of erythematous disseminated lupus.[34]
In contrast, a Chinese investigation into the involvement of rs4244165 and rs17127024 in viral hepatitis B infection and the response to interferon alpha therapy showed an association between these SNPs and the consequences of infection, but not with the response to Interferon alpha therapy as a first-line treatment for hepatitis B.[21] To date, no investigation of these 3 SNPs has been published in the European, American, or Australian populations.
Immune dysfunction, particularly chronic low-grade inflammation, plays an important role in the development and progression of mood disorders.[18] The emergence of mood symptoms in response to chronic immune-inflammatory activation indicates a relationship between immune-inflammatory signaling and the pathophysiology of mood disorders. These mechanisms represent the dysfunction of 2 central lines: the metabolism of serotonin and the hypothalamic-pituitary-adrenal axis, which can lead to progressive atrophy of the white and gray matter.[35]
In bipolar disorder, structural and connectivity abnormalities are associated with higher levels of serum inflammatory markers.[36] Cytokines can interact with other systems in the human body, including the nervous system.[37] A study of patients with bipolar disorder in the severe manic phase revealed an increase in the levels of some pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interferon gamma, and Interleukin-6 (IL-6), compared with controls.[38] Inflammation can affect mood and cognition through various mechanisms, with pro-inflammatory cytokines playing a direct role in affecting monoamine levels.[39] As a result, the coordination between inflammation and mood can lead to sickness behavior, induced by secreted pro-inflammatory cytokines like Interleukin-1, IL-6, TNF-α,[40] and interferons (IFNs).[41] Symptoms of sickness behavior include a lack of motivation for habitual actions, an abnormal spectrum of behavior, and a lack of self-care activities, which are associated with the infection response of the host.[42]
The type I IFNs result from upstream innate immune regulatory events, primed by the cytosolic DNA sensors Cyclic guanosine monophosphate and cyclic adenosine monophosphate synthase and the stimulator of interferon genes, via the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells and interferon regulatory factor transcription pathway.[43–45] Type 1 IFNs and their targeted receptors downstream activate the JAK/STAT pathways that drive the transcription of IFN-stimulated genes.[46] The JAK/STAT pathways play critical roles in orchestrating the immune system.[47] The immune reaction via pro-inflammatory cytokines leads to sickness behavior; therefore, the persistence of the immune reaction could lead to the exacerbation of the disease and the onset of depressive symptoms.[48]
Defective processing of information at the synapse and critical circuits levels is due to plasticity anomalies in synaptic and neuronal cascades. Therefore, increased data supports the conclusion that bipolarity emerges from these anomalies.[49] Cluster of differentiation4+ CD25+T regulatory cells, monocyte-derived macrophages, microglia, and astrocytes play essential roles in managing neuroplasticity, such as long-term potentiation, neural stem cell survival, synaptic branching, neurotrophin regulation, and neurogenesis, specifically by neuroimmune factors, including Interleukin-1, IL-6, and TNF-α. These neuroimmunology mechanisms play an active role in the neuroplastic changes associated with depression.[50]
One of the architecturally simplest paradigms presenting direct communication from transmembrane receptors to the nucleus is the JAK/STAT pathway; it is not surprising that it becomes a therapeutic target.[51] Janus kinase inhibitors (JAKi) are very effective small oral medications.[52] The JAKi exhibit enhanced selectivity within the JAK family by targeting specific amino acid residues.[53] Presently the JAKi are potentially therapeutic agents under development and are testing at 25.[18] Ruxolitinib was the first Food and Drug Administration approved JAKi, a potent inhibitor of JAK1 and JAK2.[51] The second 1 was tofacitinib, having a competitive behavior with adenosine triphosphate [53]. The ability of tofacitinib to inhibit JAK1 and JAK2 may allow utilization and exploration across a range of immune-mediated diseases and autoimmunity.[51] In addition, a few herbal compounds, such as berbamine, inhibit JAK/STAT signaling, bringing about beneficial effects in neuroinflammatory diseases.[54]
The JAK/STAT pathway mediates the mechanisms of antidepressant actions, and inhibition of specific JAK/STAT pathways may be a promising novel treatment for depressive disorders.[18] Amitriptyline is a Janus family kinase 3 phosphorylation-reducing tricyclic antidepressant.[55] Tofacitinib exhibits some side effects from its inhibitory activity on the immune pathway involved in detecting and eliminating tumor cells and fighting infections. In this context, JAKi can increase cancer development risk by inhibiting IFNs and natural killer cells.[18] Moreover, Winthrop and colleagues evaluated the risk of opportunistic infections during treatment with tofacitinib in 5671 subjects. They found that Tuberculosis is the most frequent among the 60 opportunistic infections detected.[56] However, it depends on the incidence of tuberculosis in the studied regions. Another OI, herpes zoster caused serious infections in all 7061 patients receiving tofacitinib for the treatment of rheumatoid arthritis.[57] On the other hand, JAKi has some advantages. In rheumatoid arthritis, impaired motility and work capacity are due to one of their most critical symptoms, pain. Tóth and colleagues assumed the efficacy and safety of JAKi especially on pain.[58] JAKi also has a relatively shorter half-life compared to other biological products (3.3 hours vs 12.4 days) and can be discontinued more quickly in emerging infections.[18]
5. Conclusion
In conclusion, our results showed no link between the 3 studied positions of JAK1 and bipolar disorder in the Tunisian population. However, we observed a significantly higher average level of the BPRS psychometric scale for the wild-type genotype of rs4244165 (GG) than for the double-mutated genotype, with an LSD post hoc test at the beginning of hospitalization (P = .001) and after hospitalization (P = .012). The JAK/STAT pathway is an interesting therapeutic route that requires further investigation. Studying their regulatory region can provide a clearer picture of all the interactions involved in the regulation of genetic expression in response to treatment.
Acknowledgments
We thank the Department of Psychiatry and Vulnerability of the Psychoses Laboratory, Fattouma Bourguiba University Hospital of Monastir. We are also grateful for the Tunisian Association of Psycho-Neuro-Endocrine-Immunology. In addition, I wish to express my gratitude to my statistics professor Nabil ABID and my English teacher Christine Campanella.
Author contributions
Conceptualization: Ferid Zaafrane, Lotfi Gaha.
Data curation: Akila Ahlem Elouaer Benkortbi-Elouaer.
Formal analysis: Bochra Ben Mohamed, Lotfi Gaha.
Project administration: Akila Ahlem Elouaer Benkortbi-Elouaer.
Supervision: Besma Bel Hadj Jrad Tensaout.
Writing – original draft: Akila Ahlem Elouaer Benkortbi-Elouaer.
Writing – review & editing: Akila Ahlem Elouaer Benkortbi-Elouaer.
Abbreviations:
- BD
- bipolar disorder
- BPRS
- brief psychiatric rating scale
- DNA
- genomic deoxyribonucleic acid
- IFN
- Interferon
- IL-6
- Interleukin-6
- JAK
- Janus family kinase
- JAK1
- Janus family kinase 1
- JAK2
- Janus family kinase 2
- JAKi
- Janus kinase inhibitors
- LSD
- the least significant difference
- OI
- opportunistic infection
- SNP
- single nucleotide polymorphism
- STAT
- Signal transducer and activator of transcription
- TNF-α
- tumor necrosis factor alpha
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
How to cite this article: Benkortbi Elouaer AAE, Ben Mohamed B, Zaafrane F, Gaha L, Bel Hadj Jrad Tensaout B. Case control study: G-allele of rs4244165 in JAK1 gene correlated with high-level brief psychiatric rating scale in bipolar patients. Medicine 2023;102:37(e34652).
Contributor Information
Bochra Ben Mohamed, Email: benmed520@yahoo.fr.
Ferid Zaafrane, Email: fdzaafrane@yahoo.fr.
Lotfi Gaha, Email: gaha.lotfi@yahoo.fr.
Besma Bel Hadj Jrad Tensaout, Email: bbhj2002@yahoo.fr.
References
- [1].Mutz J. Brain stimulation treatment for bipolar disorder. Bipolar Disord. 2023;25:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hu X, Yu C, Dong T, et al. Biomarkers and detection methods of bipolar disorder. Biosens Bioelectron. 2023;220:114842. [DOI] [PubMed] [Google Scholar]
- [3].Grande I, Berk M, Birmaher B, et al. Bipolar disorder. Lancet. 2016;387:1561–72. [DOI] [PubMed] [Google Scholar]
- [4].Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lish JD, Dimemeenan S, Whybrow PC, et al. The National Depressive and Manic-Depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31:281–94. [DOI] [PubMed] [Google Scholar]
- [6].Kupfer DJ, Frank E, Grochocinski VJ, et al. Demographic and clinical characteristics of individuals in a bipolar disorder case registry. J Clin Psychiatry. 2002;63:120–5. [DOI] [PubMed] [Google Scholar]
- [7].Perlis RH, Miyahara S, Marangell LB, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry. 2004;55:875–81. [DOI] [PubMed] [Google Scholar]
- [8].Miller AH, Manji HK. On redefining the role of the immune system in psychiatric disease. Biol Psychiatry. 2006;60:796–8. [DOI] [PubMed] [Google Scholar]
- [9].Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Karaoulanis SE, Angelopoulos NV. The role of immune system in depression. Psychiatriki. 2010;21:17–30. [PubMed] [Google Scholar]
- [11].Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature. 2016;16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacology implications. Pharmacol Ther. 2011;130:226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Salim S, Chugh G, Asghar M. Inflammation in anxiety. Adv Protein Chem Struct Biol. 2012;88:1–25. [DOI] [PubMed] [Google Scholar]
- [15].Wartchow KM, Cordeiro RC, Scaini G. Advances in the pathophysiology of bipolar disorder. Curr Opin Psychiatry. 2023;36:20–7. [DOI] [PubMed] [Google Scholar]
- [16].Downton P, Sanna F, Maidstone R, et al. Chronic inflammatory arthritis drives systemic changes in circadian energy metabolism. Proc Natl Acad Sci USA. 2022;119:e2112781119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rawlings JS, Rosler KM, Douglas AH. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–3. [DOI] [PubMed] [Google Scholar]
- [18].Shariq AS, Brietzke E, Rosenblat JD, et al. Therapeutic potential of JAK/STAT pathway modulation in mood disorders. Rev Neurosci. 2018;30:1–7. [DOI] [PubMed] [Google Scholar]
- [19].Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spinelli, FR, Colbert RA, Gadina M. Jak1: number one in the family; number one in inflammation? Rheumatology. 2021;60(supplement_2):ii3–ii10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen K, Min H, Wu X, et al. JAK1 gene polymorphisms are associated with the outcomes of hepatitis B virus infection, but not with α interferon therapy response in a Han Chinese population. Genet Test Mol Biomarkers. 2012;16:1206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) Statement: guidelines for reporting observational studies; 18:800–4 [DOI] [PubMed] [Google Scholar]
- [23].Abood Chaloob F. Genetic variants in JAK1 gene and susceptibility to hepatitis c viral infection in Iraq. J Biotech Res Center. 2016;10:37–4. [Google Scholar]
- [24].Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the] decoding machinery. Nat Rev Genet. 2007;8:749–61. [DOI] [PubMed] [Google Scholar]
- [25].Padgett RA. New connections between splicing and human disease. Trends Genet. 2012;28:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang X, Wang K, Radovich M, et al. Genome-wide prediction of cis-acting RNA elements regulating tissue-specific pre-mRNA alternative splicing. BMC Genomics. 2009;10(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Heyd F. Alternative splicing - principles, functional consequences and therapeutic implications. Dtsch Med Wochenschr. 2014;139:339–42. [DOI] [PubMed] [Google Scholar]
- [28].Hsieh YY, Chang CC, Hsu CM, et al. JAK-1 rs2780895 C-related genotype and allele but not JAK-1 rs10789166, rs4916008, rs2780885, rs17127114, and rs3806277 are associated with higher susceptibility to asthma. Genet Test Mol Biomarkers. 2011;15:841–7. [DOI] [PubMed] [Google Scholar]
- [29].Bell M, Milstein R, Beam-Goulet J, et al. The positive and negative syndrome scale and the brief psychiatric rating scale: reliability, comparability, and predictive validity. J Nerv Ment Dis. 1992;180:723–8. [DOI] [PubMed] [Google Scholar]
- [30].Faustman WO, Overall JE. Brief Psychiatric Rating Scale. In: Maruish ME. (eds). The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers. 1999:791–830 [Google Scholar]
- [31].Ishii Y, Tomotake M, Chiba S, et al. Relationship between quality of life and clinical factors in inpatients with schizophrenia. J Med Investigat. 2022;69:80–5. [DOI] [PubMed] [Google Scholar]
- [32].Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- [33].Kang EH, Choi, JY, Lee YJ, et al. Single nucleotide polymorphisms in IL-10-mediated signaling pathways in Korean patients with Behçet’s disease. Clin Exp Rheumatol. 2014;32(4 Suppl 84):S27–32. [PubMed] [Google Scholar]
- [34].Fourati H, Bouzid D, Abida O, et al. Genetic factors contributing to systemic lupus ery. 2012.
- [35].McNamara RK, Lotrich FE. Elevated immune-inflammatory signaling in mood disorders: a new therapeutic target? Expert Rev Neurother. 2012;12:1143–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tu PC, Li CT, Lin WC, et al. Structural and functional correlates of serum soluble IL-6 receptor level in patients with bipolar disorder. J Affect Disord. 2017;219:172–7. [DOI] [PubMed] [Google Scholar]
- [37].Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies. Neurosci Biobehav Rev. 2005;29:891–909. [DOI] [PubMed] [Google Scholar]
- [38].Radtke FA, Chapman G, Hall J, et al. Modulating neuroinflammation to treat neuropsychiatric disorders. Biomed Res Int. 2017;5071786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39:125–37. [DOI] [PubMed] [Google Scholar]
- [40].Hosoi T, Okuma Y, Nomura Y. The mechanisms of immune-to-brain communication in inflammation as a drug target. Curr Drug Targets Inflamm Allergy. 2002;1:257–62. [DOI] [PubMed] [Google Scholar]
- [41].Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–34. [DOI] [PubMed] [Google Scholar]
- [42].Kent S, Bluthé RM, Kelley KW, et al. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–8. [DOI] [PubMed] [Google Scholar]
- [43].Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signaling. Nature. 2008;455:674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–9. [DOI] [PubMed] [Google Scholar]
- [45].Tao J, Zhou X, Jiang Z. cGAS-cGAMP-STING: the three musketeers of cytosolic DNA sensing and signaling. IUBMB Life. 2016;68:858–70. [DOI] [PubMed] [Google Scholar]
- [46].Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schloesser RJ, Huang J, Klein PS, et al. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:110–33. [DOI] [PubMed] [Google Scholar]
- [50].Eyre H, Baune BT. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. 2012;37:1397–416. [DOI] [PubMed] [Google Scholar]
- [51].O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Singh JA. The Emerging safety profile of jak inhibitors in rheumatic diseases. BioDrugs. 2023. [online publication]. [DOI] [PubMed] [Google Scholar]
- [53].Clark JD, Flanagan ME, Telliez J-B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57:5023–38. [DOI] [PubMed] [Google Scholar]
- [54].Yan Z, Gibson SA, Buckley JA, et al. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin Immunol. 2018;189:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gulbins A, Grassmé H, Hoehn R, et al. Role of janus-kinases in major depressive disorder. Neurosignals. 2016;24:71–80. [DOI] [PubMed] [Google Scholar]
- [56].Winthrop KL, Park S-H, Gul A, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020;6:e001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tóth L, Juhász MF, Szabó L, et al. Janus kinase inhibitors improve disease activity and patient-reported outcomes in rheumatoid arthritis: a systematic review and meta-analysis of 24,135 Patients. Int J Mol Sci . 2022;23:1246. [DOI] [PMC free article] [PubMed] [Google Scholar]