Abstract
Background
Fish in aquatic environments are end consumers of the food chain and are widely used for the evolution effects of environmental pollution and their interactions in aquatic ecosystem.
Objective
In the present study, common carp (Cyprinus carpio) fingerlings were selected to assess the potential risk and aquatic toxicity of meloxicam as a non‐steroidal anti‐inflammatory and a commonly used pharmaceutical drug.
Methods
In order to evaluate meloxicam toxicological effect on haematological, antioxidant status, enzymological and histological parameters, based on its LC50 24 h acute toxicity (10.05 mg L−1), fish fingerlings were exposed to four doses of meloxicam including; 0 (control), 0.1 (low), 1 (medium) and 2 mg L−1 (high) under static bioassay method for 28 days.
Results
The results showed that sublethal doses of meloxicam significantly decreased alanine aminotransferase, alkaline phosphatase, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) levels in comparison with the control group after 28 days (p < 0.05). However, red blood cell, haematocrit, haemoglobin and malondialdehyde values in fish exposed to meloxicam significantly increased alongside its concentration (p < 0.05) more than the control group after 28 days. SOD, CAT and GPX mRNA expression levels in gill, liver, kidney and brain organ of fish under meloxicam treatment were significantly down‐regulated compared to the control group (p < 0.05). Histopathological assessment showed the increased vacuolation in hepatocytes in liver of fish exposure to medium and high doses of meloxicam.
Conclusion
In conclusion, meloxicam induces oxidative stress in common carp which results a disruption of physiological and health status of this species based on our current findings.
Keywords: antioxidant status, Cyprinus carpio; haematology, meloxicam, relative mRNA expression
Graphical Abstract: In the present study, common carp (Cyprinus carpio) fingerlings were selected to assess the potential risk and aquatic toxicity of meloxicam as a non‐steroidal anti‐inflammatory and a commonly used pharmaceutical drug.
![]()
1. INTRODUCTION
In the last few decades with the increase of the world population, the variety and use of pharmaceutical and chemical substances have also been significantly increased for treatment and improvement of human health, agriculture, veterinary and aquaculture. Although many drugs have a short or medium half‐life, aquatic organisms are exposed to these pharmaceutical substances because they are continuously released into the aquatic environment (Li et al., 2016). In addition, the presence of active compounds of these pharmaceuticals and their metabolites in sources, including treated and untreated wastewater, ground and surface water, drinking water and estuaries, has been detected in concentrations ranging from μg L−1 to ng L−1 (Saravanan et al., 2012). Fish in aquatic ecosystems may be continuously exposed to pharmaceuticals contamination as non‐target organisms (Li et al., 2016).
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are widely used for treating postoperative pain and inflammation such as osteoarthritis and rheumatoid arthritis (Montesinos et al., 2015). Sometimes NSAIDs are more effective than opioids for relieving pain in cats and dogs.
Meloxicam, a derivative of enolic acid, belongs to NSAIDs that is widely used in the worldwide as an analgesic, antipyretic and anti‐inflammatory purpose in related to ankylosing spondylitis and acute rheumatoid arthritis (World Health Organization (WHO), 2010). In Iran and some countries, NSAIDs are widely prescribed by physicians and available in pharmacies without a prescription. Concerns and warnings about the risk of drug pollution in aquatic environments emerged in the late twentieth century, when the results of monitoring the conditions of aquatic ecosystems including rivers, streams, wastewater and surface water in Germany confirmed the presence of painkillers, anti‐inflammatory drugs, psychedelics and hormones in the tested samples (Sosnowska et al., 2009). Unfortunately, among the NSAIDs, there is no evidence of meloxicam concentration range in aquatic ecosystems, but its half‐life in nature is estimated to be 20 h approximately (Turck et al., 1996).
In principle, fish in aquatic environments are end consumers of the food chain and are widely used for evaluation the effects of environmental pollution and their interactions in aquatic ecosystem. The common carp (Cyprinus carpio) inhabit in the middle and down parts of the rivers and in shallow areas of water and sludge bottom and widely distributed in freshwater resources in the world (Hasan et al., 2007). On the other hand, due to large size availability of this species, makes it possible to have access to sufficient organic material for fundamental testing within organ structure, function and immune diagnosis. However, this is not possible in other model small fish species such as zebra (Danio rerio) and medaka (Oryzias latipes) (Henkel et al., 2012).
Currently, environmental contaminants of the pharmaceuticals are one of the most important factor causes of poisoning of fish and aquatic animals that may affect fish development stages even at low concentrations during long period. In order to monitoring the aquatic environment and investigate the effects of pollutants on the physiological and pathological status of the animals exposed, it is very necessary to analyse the fluctuations in blood biomarkers (Gharaei et al., 2010; Khandan Barani et al., 2019; Razeghi Mansour et al., 2012).
Different biomarkers can be studied at the enzymological, haematological, biochemical and molecular levels to investigate the interplay between a toxicant and biological system (Gharaei et al., 2011, 2010). Changes in biochemical factors of fish exposed to toxins can be beneficial to assess water quality, the determination of chemicals that require a more comprehensive risk assessment and also used as a potential bioindicators of exposure and effect (Suvetha et al., 2010; Gharaei et al., 2020).
The oscillations of these bioindicators are wide‐spreading used to determine toxicity stress, entirety of the immune system, antioxidant status and tissue damage (Kavitha et al., 2010; Jafarinejad et al., 2018). In addition, histopathological assay of target organs will help to identify the final effects of the toxin on the development of organs. The severity of these changes varies with environmental and biological conditions and provides useful information on the physiological status and pathology of the organism (Saravanan et al., 2012).
Medicinal products as harmful xenobiotic are constantly released in the surroundings, and limited information is known about their effects on non‐target organisms in onshore and aquatic environments (Ericson et al., 2010). In addition, there are few studies on pharmaceutical toxicity related to non‐target species during the chronic exposure (Han et al., 2010).
According to the literature review, no ecotoxicology studies of meloxicam and its chronic effects on aquatic organisms have been performed. Therefore, the aim of this study was to assess the chronic sublethal toxicity effect of meloxicam on the common carp using haematological, enzymological, molecular genetics biomarkers and histopathological examination.
2. MATERIALS AND METHODS
2.1. Fish collection and maintenance
Common carp fingerlings with mean weight 10 ± 2.2 g were collected from Fish Reproduction Centre of Zahak, Sistan‐Iran. After transportation of fish to laboratory, they were acclimatized for 3 weeks in a stock tank (1500 L volume) before the start of trial. Pending the adaptation time, fish were fed with basal diet once daily. The proximate composition (% dry matter) of diet was protein 32, fat 13.4, moisture 4.8 and ash 14.5. The stock tank tap water was exchange by overflow of 500 L as a daily and the water had the following quality parameters; temperature (21.8 ± 1.5°C), pH (8.1 ± 0.2), hardness (255 ± 2 mg L−1) and dissolved oxygen (6.2 ± 0.6 mg L−1).
2.2. Determination of meloxicam LC50 24 h
Meloxicam sodium salt hydrate, purity 98% (Sigma‐Aldrich) was used for assessment its toxicity on fish. Stock solution for the exposure was made by dissolving meloxicam into dimethylsulphoxide (5 mg mL−1) due to its low solubility in water. Eighteen plexiglass aquaria (70 L volume) containing 10 fish per each aquarium were used to determine the LC50 24 h static acute toxicity of meloxicam. Different concentrations of the meloxicam 0, 0.5, 1, 5, 10, 20 and 50 mg L−1 were made up using the stock solution. After 24 h, the mortality of fish was recorded. All fish during the bioassay trial were not feeding. The LC50 24 h was determined by the Probit analysis method of Finney (1978). The LC50 value for 24 h was 10.05 ± 0.32 mg L−1 (Table 1). One‐fifth (2 mg L−1), one‐tenth (1 mg L−1) and one‐hundred (0.1 mg L−1) values of the LC50 24 h concentration were selected as the sublethal doses.
TABLE 1.
LC50 24 h of meloxicam on common carp.
| Fish group | Concentration (mg L−1) | Number of exposed | Number of death fish after 24 h |
|---|---|---|---|
| 1 | 0 | 10 | 0 |
| 2 | 0.5 | 10 | 0 |
| 3 | 1 | 10 | 1 |
| 4 | 5 | 10 | 2 |
| 5 | 10 | 10 | 3 |
| 6 | 20 | 10 | 7 |
| 7 | 50 | 10 | 10 |
| Point | Lethal concentration (mg L−1) | 95% confidence limit |
|---|---|---|
| LC1 | 0.626 | 0.421–0.831 |
| LC5 | 1.407 | 0.95–1.864 |
| LC10 | 2.169 | 1.912–2.426 |
| LC15 | 2.903 | 2.51–3.296 |
| LC50 | 10.05 | 9.73–10.37 |
| LC85 | 34.202 | 31.10–37.304 |
| LC95 | 70.55 | 64.42–76.68 |
2.3. Toxicity studies under sublethal concentration
2.3.1. Sublethal toxicity assay
At the end of acclimation period, 240 fish fingerlings were randomly distributed into 4 groups and 3 replicates per each group (20 fish per each replicate) in 12 plexiglass aquaria (70 L volume) and held under the same temperature and photoperiod. Each day, 100% of test solutions were renewed by water contained intended dose in order to retention the meloxicam dose at stable concentration. The test was performed base on the completely randomized design and directed for duration of 28 days and mortality was not recorded. At the end of trial, 16 fish were randomly decapitated from each group for further analysis. During the experimental period, water temperature, dissolved oxygen and pH were measured daily and maintained at 21.8 ± 1.5°C, 6.2 ± 0.6 mg L −1 and 8.1 ± 0.2, respectively.
2.3.2. Haematological analysis and enzymes assay
Blood collection was immediately done from caudal vein using 2 mL non‐heparinized syringes. A volume of 1 mL was transferred into the tubes containing 50 IU mL−1 sodium heparin (Alborz Darou Co.) for haematological assays. Values of red blood cell (RBC) and white blood cell (WBC) were measured (Jafarinejad et al., 2018). Haemoglobin (Hb) value was measured by cyanmethaemoglobin method. Hct was measured according to the method described by Jung et al., 2003. Indexes of erythrocyte, including MCV, MCH and MCHC, were measured following these standard formulas:
Moreover, 1 mL blood of six fish in each treatment pooled and then transferred into tubes without heparin for measurement serum enzymes, including alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX) and malondialdehyde (MDA) according to methods described by Saravanan et al. (2012) and Gharaei et al. (2020).
2.3.3. Measurement of gene expression
Total RNA extraction was carried out from the liver, gill, kidney and brain of fish at the experimental groups (n = 6 from each group) using the Takapou Zist kit following the manufacturer's instructions. RNA purity, cDNA synthesis and evaluation of mRNA expression level of CAT, SOD and GPX in different organs by fluorescent real‐time quantitative PCR were performed according to methods described by Gharaei et al. (2010). The special primers for CAT, SOD, GPX and β‐actin (as the housekeeping gene) were designed in accordance to the cDNA sequences of common carp in GenBank (Table 2) (Jiang et al., 2016). The conditions for PCR were initial denaturation at 95°C for 5 min and followed by 35 cycles of 95°C for 20 s, 60°C for 30 s and 72°C for 1 min and a final extension for 7 min at 72°C. Each reaction for PCR, the 20 μL contained 0.5 μL of each primer (15 mM), 2 μL of the diluted first strand cDNA product, 10 μL of green qPCR Master Mix (Yekta Tajhiz Azma Co.) and 7 μL of sterilized double‐distilled water. Three technical replicates for each sample were evaluated and the threshold cycle was characterized manually for each run. PCR efficiencies and gene expression data analysis were performed in accordance with methods explained by Gharaei et al. (2010).
TABLE 2.
Real‐time PCR primer sequences and thermocycling condition.
| Genes | Name primer | Primer sequence (5′–3′) | Accession no. | Tem. primer (°C) |
|---|---|---|---|---|
| SOD | F | TGGCGAAGAAGGCTGTTTGT | JF342355AJ | 60.4 |
| R | TTCACTGGACCCGTCT | |||
| CAT | F | CTGGAAGTGGAATCCGTTTG | JF411604 | 54.5 |
| R | CGACCTCAGCGAAATAGTTG | |||
| GPX | F | CCTTCCCATCCCACCAGTTT | FJ656211 | 60 |
| R | TGCGGAGTCACCGTTCACAT | |||
| β‐actin | F | CGTGATGGACTCTGGTGATG | M24113 | 60 |
| R | TCGGCTGTGGTGGTGAAG |
Abbreviations: CAT, catalase; GPX, glutathione peroxidase; SOD, superoxide dismutase.
2.3.4. Histopathological assessment
At the end of the experiment for histopathological investigation, three fish were randomly selected from each group. All fish were euthanized using with 200 mg L−1 MS222 (Faggio et al., 2014) and dissected for tissue sampling with the size of 1 × 1 × 0.5 cm3 from liver, brain, kidney and gill organs. Tissue preparation with routine protocol (fixation in 10% neutral buffered formalin, embedding in paraffin, sectioning at 5 μm with microtome and staining with haematoxylin–eosin staining method) was performed (Sharifpour et al., 2004). Finally, all slides were submitted to light microscopy for examination of probably lesions.
2.4. Data analysis
All data were analysed for normality and homogeneity of variance by Shapiro–Wilk's and Leven's tests. The one‐way analysis of variance (ANOVA) was used for analysis the data related to haematological and enzymological indices and the mRNA levels followed by Tukey's HSD test for post hoc analyses. All statistical analyses were carried out using SPSS software v. 22.0 (SPSS, Inc.) and statistical significance was set at 5%. All data were showed as mean ± SE.
3. RESULTS
Long‐term meloxicam exposure had a dose dependent effect on some liver enzymes activity in common carp (Table 3). With increasing meloxicam concentration, ALT and ALP values decreased significantly (p < 0.05) in treatment groups as compared to the control after 28 days.
TABLE 3.
Serum values of liver enzymes activity in common carp exposed to four meloxicam levels: control (0 mg L−1), low (0.1 mg L−1), medium (1 mg L−1) and high (2 mg L−1) for a 28‐day period.
| Parameters | Control | Dose | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| AST | 122 ± 15.7 | 126.3 ± 13.3 | 117 ± 17.9 | 119 ± 11.8 |
| ALT | 6.7 ± 2.2a | 8.3 ± 1.5b | 10.5 ± 2.1c | 15 ± 2d |
| ALP | 35.7 ± 5.1a | 33.7 ± 4.3a | 63.5 ± 4.95b | 91 ± 6.7c |
| LDH | 732.3 ± 117.4 | 747.7 ± 112 | 736 ± 95.2 | 697 ± 108 |
Note: Values (mean ± SE; n = 6) are expressed in IU L−1. Statistically significant differences at the different doses are indicated by different letters (p < 0.05).
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase.
The results of haematologic parameters are shown in Table 4. After 28 days of exposure, the RBC, Hct and Hb level for all treatment groups were significantly higher (p < 0.05) than the control group. Exposure with the medium and high dose of meloxicam resulted in higher level of Hct compared to the control and low‐dose groups during the trial period (p < 0.05).
TABLE 4.
Haematologic parameters of common carp exposed to four meloxicam levels: control (0 mg L−1), low (0.1 mg L−1), medium (1 mg L−1) and high (2 mg L−1) for 28‐day period (mean ± SE; n = 6).
| Parameters | Control | Dose | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| WBC (×103 cells mm−3) | 93.93 ± 8.41 | 85.87 ± 4.62 | 89.6 ± 6.22 | 81.73 ± 2.64 |
| RBC (×106 cells mm−3) | 0.83 ± 0.23b | 1.43 ± 0.19ab | 1.3 ± 0.17ab | 1.64 ± 0.44a |
| Hb (g dL−1) | 6.03 ± 1.25b | 8.47 ± 0.32a | 10.05 ± 1.06a | 9.17 ± 0.42a |
| Hct (%) | 19.57 ± 4.18a | 20.1 ± 1.31a | 20.1 ± 2.83a | 21.9 ± 2.59a |
| MCV (fL) | 115.30 ± 4.8b | 140.55 ± 5.6a | 154.61 ± 8.1a | 133.53 ± 7.4a |
| MCH (pg) | 72.65 ± 3.6a | 59.23 ± 2.9b | 77.30 ± 4.7a | 55.91 ± 3.2b |
| MCHC (g dL−1) | 63.00 ± 4.1a | 42.13 ± 3.9b | 50.00 ± 2.7ab | 41.87 ± 3.1b |
Note: Statistically significant differences at the different doses are indicated by different letters (p < 0.05).
Abbreviations: Hb, haemoglobin; Hct, haematocrit; RBC, red blood cell.
The effects of different treatments on the CAT, GPX SOD enzyme activities as well as MDA level are shown in Table 5. Further analysis indicated that SOD and CAT enzyme activities of common carp exposed to the high dose of meloxicam were decreased significantly compared to the control group (p < 0.05). MDA level in common carp increased in groups received meloxicam dose dependently and achieved the highest level at the high dose of meloxicam. The level of GPX activity of common carp decreased significantly (p < 0.05) with increasing meloxicam concentration up to 2 mg L−1 on 28 days.
TABLE 5.
Antioxidant and non‐antioxidant capacity parameters in common carp exposed to four meloxicam levels: control (0 mg L−1), low (0.1 mg L−1), medium (1 mg L−1) and high (2 mg L−1) for 28‐day period (mean ± SE; n = 6).
| Parameters | Control | Dose | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Antioxidant | ||||
| CAT | 109.7 ± 5.5a | 100 ± 4.8ab | 97.7 ± 7.5ab | 94.3 ± 8.6b |
| GPX | 410.7 ± 21.9a | 354.5 ± 19.1b | 305.3 ± 9.5c | 206 ± 12.2d |
| SOD | 34.2 ± 2.4a | 26.7 ± 0.8b | 26.7 ± 3.7b | 22.8 ± 3.2b |
| Non‐antioxidant | ||||
| MDA | 20.7 ± 1.9b | 20.8 ± 1.3b | 22.3 ± 0.95ab | 24 ± 1.7a |
Note: Statistically significant differences at the different doses are indicated by different letters (p < 0.05).
Abbreviations: CAT, catalase; GPX, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
The relative mRNA expression of SOD, CAT and GPX gene in gill, liver, kidney and brain of common carp after 28 days is shown in Figures 1, 2, 3. ANOVA demonstrated that there was a significant effect of meloxicam doses in different tissues on the mRNA levels of SOD, CAT and GPX genes. SOD, CAT and GPX transcript levels decreased with increasing meloxicam doses in target tissues of common carp when compared with the control group. The lowest level of SOD, CAT and GPX mRNA was found in kidney, gill and gill of fish exposure to meloxicam after 28 days, respectively.
FIGURE 1.
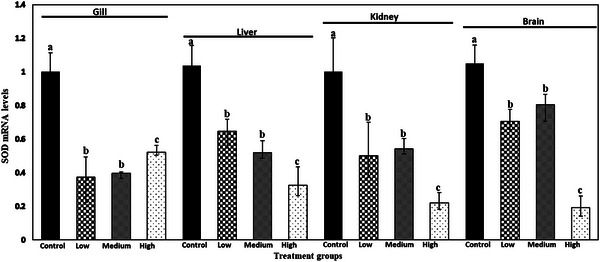
Superoxide dismutase (SOD) mRNA levels in gill, liver, kidney and brain of juvenile common carp exposed to four meloxicam levels: control (0 mg L−1), low (0.1 mg L−1), medium (1 mg L−1) and high (2 mg L−1) for 28 days. Results are the mean ± SD (n = 6). Means marked by different letters differ significantly (p < 0.05).
FIGURE 2.
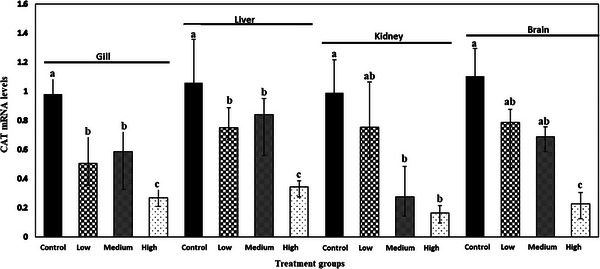
Catalase (CAT) mRNA levels in gill, liver, kidney and brain of juvenile common carp exposed to four meloxicam levels: control (0 mg L−1), low (0.1 mg L−1), medium (1 mg L−1) and high (2 mg L−1) at 28 days. Results are the mean ± SD (n = 6). Means marked by different letters differ significantly (p < 0.05).
FIGURE 3.
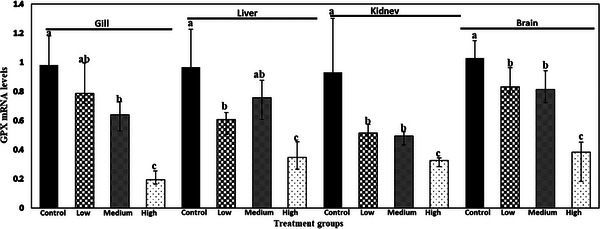
Glutathione peroxidase (GPX) mRNA levels in gill, liver, kidney and brain of juvenile common carp exposed to four meloxicam levels: control (0 mg L−1), low (0.1 mg L−1), medium (1 mg L−1) and high (2 mg L−1) at 28 days. Results are the mean ± SD (n = 6). Means marked by different letters differ significantly (p < 0.05).
After 28 days, assessment of hepatic tissue showed no prominent lesion in fish exposure to medium dose of meloxicam (0.2 mg kg−1) in comparison with the control group. Hepatocytes vacuolisation with pyknotic nuclei in centrilobular and periportal zones and disarrangement of hepatic cords were the significant lesions of the liver tissue in fish exposure to medium and high dose of meloxicam (1 and 2 mg kg−1) in comparison with the control group (Figure 4). Moreover, the brain, gill and kidney tissues were normal in all groups.
FIGURE 4.
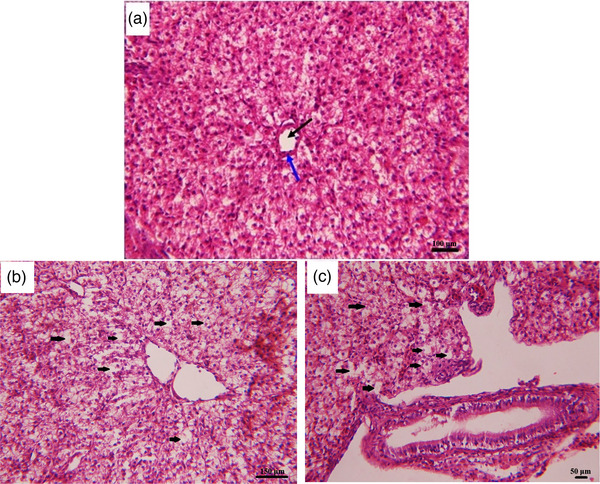
Histological structure of liver of common carp showing histopathological alterations due to 28 days meloxicam exposure at different concentrations. (A) Liver section of control fish shows normal construction of portal vein (black arrow) restricted by pancreatic tissue (blue arrow); (B) liver section of fish exposed to medium dose (1 mg L−1) of the meloxicam shows the increased vacuolation in hepatocytes (black arrows); (C) liver section of fish exposed to high dose (2 mg L−1) of the meloxicam, shows the increased vacuolisation in hepatocytes (black arrows); stain: H & E; 400× magnification.
4. DISCUSSION
Low doses of the drug lead to toxic effects on non‐target animals, so investigations on the acute toxicity of these compounds may provide prepare an active pattern and toxicity for drugs (Hoeger et al., 2008; Malarvizhi et al., 2012). In this study, the 24 h LC50 value of meloxicam to the common carp fingerlings was determined 10.05 mg L−1. Fish mortality during acute exposure may be because of abnormal physiological changes such as heart abnormalities and spinal deviation (Van Hecken et al., 2000; David & Pancharatna, 2009). Previous studies have clearly shown that long‐term exposure of meloxicam induces toxicity in different species and make the ecological risks (Nair et al., 2006; Tubbs et al., 2011; Burukoglu et al., 2014; Montesinos et al., 2015; Sriuttha et al., 2018). The toxicity of meloxicam on mortality, development, hatching rate, behaviour and breeding rate has not been stated in aquatic animals. However, chronic exposure meloxicam at environmental concentrations may have lesser effects on non‐target organisms (Furst et al., 2002).
The haematological and biochemical alterations are extensively exploited as markers for assessing toxic stress, health status and inner ambience of the organism (Lavanya et al., 2011; Li et al., 2016); moreover, these profiles may also be used for assessing the chronic toxicity of chemicals (Tellez‐Banuelos et al., 2009; Saravanan et al., 2011).
Parameters, such as Hb, RBC, WBC counts and Hct percentage, may be susceptible to certain contaminants and are often exploited to diagnose the physiological status of organisms (Van Vuren, 1986; Adhikari et al., 2004). In this study, the RBC value was enhanced in high dose group and Hb, Hct values were increased in different groups exposed to meloxicam for 28 days while WBCs that are involved in regulating the function of immune system was not changed. Similar studies showed that ibuprofen sublethal toxicity alterations in haematological parameters possibly change in RBC production in Indian major carp, Cirrhinus mrigala (Saravanan et al., 2012). Also, increasing Hb and Hct values might be due to replacing oxidized denatured Hb by the toxicant and to provide more oxygen for tissues (Nussey et al., 1995).
Another reason for increasing Hct level is RBCs swelling and respiratory capacity is impairment in fish due to gill damage (Nemcsok & Boross, 1982). Likewise, Li et al. (2016) indicated that chronic exposure of rainbow trout (Oncorhynchus mykiss) to carbamazepine drug causes increases in Hb, Hct, MCV and MCH contents.
In the aquatic ecosystem, toxicants effect may appear at cellular or molecular level which can alter biochemical parameters of organism significantly (Kavitha et al., 2010). Enzymes like AST, ALT, ALP and LDH are good candidates for monitoring organism health (Suvetha et al., 2010; Lavanya et al., 2011). Among these enzymes, AST and ALT are widely used for assessing tissue damage and relevant stress indices in aquatic bioassays (Jung et al., 2003). In this study, serum AST and LDH levels showed no statistical differences among the groups. On the other hand, increasing doses of meloxicam significantly increased the ALT and ALP enzyme levels. Since NSAIDs cause releasing metabolites conjugated with glucuronic acid and hepatic immunological response (Bailey & Dickinson, 2003), alteration of ALT and ALP may show hepatotoxicity. Structural damage from exposure to toxicants in liver and kidney of fish causes leak these enzymes into blood stream and increase their plasma concentrations (Kavitha et al., 2012). The agglomeration and linkage of toxicants in cell membranes, cytoplasm and mitochondria may induce structural lesion and decomposition of cells which in results enhancing of AST and ALT enzymes into blood stream. On the other hand, under stress status, organisms may diminish the toxic effect via enhancing metabolism rate of protein and carbohydrate that may alter AST and ALT activities and their enhancement is a recovery process to spoiled metabolism (Reddy & Venugopal, 1991).
Antioxidant enzymes are the first defence system against oxidative stress in tissue injury by phagocytosis and radicalization (Jafarinejad et al., 2018). SOD, CAT and GPX enzymes play main role of neutralize of the reactive oxygen species (ROS) produced in damaged organs (Gharaei et al., 2020). SOD is the main enzyme responsible for compensating the toxic effects created by ROS presence. Moreover, SOD converses toxic superoxide anions into hydrogen peroxide (H2O2) as the first step of antioxidation defence (Gao et al., 2018). Afterwards, CAT and GPX enzymes convert H2O2 to H2O. CAT reduces H2O2 produced in metabolism of long‐chain fatty acids in peroxisomes and GPX acts as catalyzer in reduction of H2O2 and lipid peroxide (Winston & Digiulio, 1991; Mohammadi et al., 2020) and accordingly they remove ROS. In the present study, the activity of SOD, CAT and GPX decreased significantly in fish exposed to meloxicam compared with control group. This finding is consistent with that of Li et al. (2016) showed the decreasing of CAT enzyme activity in rainbow trout after exposure to carbamazepine because of the overwhelming production of H2O2 by SOD. Gao et al. (2018) demonstrated that the diclofenac, naproxen and ibuprofen decrease antioxidant enzyme activities (SOD, CAT and GPX) on common carp due to disruption of the antioxidant system and increasing lipid peroxidation and imbalance of GSH (reduced glutathione)/GSSG (oxidized glutathione) ratios.
MDA is a non‐enzymatic antioxidant and acts as a lipid peroxidation and health status of biological layers which rich unsaturated fatty acid (Khosravi‐Katuli et al., 2018). In this study, meloxicam in medium and high concentrations caused significant increase in the level of MDA compared to control group. This founding maybe relevant to agitation of oxidative stress by meloxicam, since MDA is the final product in lipid peroxidation process (Gharaei et al., 2020). The increasing MDA level in response to meloxicam exposure was also reported in previous studies (Villegas et al., 2002; Amin et al., 2017). It is demonstrated that increase in MDA level was closely accompanied by increased lipid peroxidation, DNA lesion, altered calcium and sulfhydryl homeostasis which results disturbances in antioxidant defence system (Ayala et al., 2014). In principle, antioxidant enzymes are inactivated by cross‐linking MDA to them, which results in increase of ROS accumulation and macromolecular damage aggravation (Khan & Rampal, 2014).
Relative mRNA expression of SOD, CAT and GPX gene indicated a significant fluctuation among all treatment groups, implying meloxicam involvement through antioxidant process and this effect was dose dependent. Previous studies proved that meloxicam induced liver and kidney toxicity by damage to protein synthesis, alteration in a number of enzymes in hepatocytes and renal functional capacity (Amin et al., 2017; Huerta et al., 2005). However, the effects of meloxicam on the expression of antioxidant enzyme mRNA levels in fish are not consistent with previous researches. In our research, decreasing SOD, CAT and GPX gene expression could be depend on expression of transcription factor Nrf2 and Keap1, which induce the expression of proantioxidant genes (Ristow & Schmeisser, 2011). This response may account for the decreased glutathione levels and antioxidant enzyme activities. However, decreasing the antioxidant gene expression by increasing meloxicam doses overwhelms disruption of antioxidant status perhaps because of the prolonged duration of drug treatment.
The results of the present study demonstrated that high doses of meloxicam lead to oxidative stress via a reduction of antioxidant enzymes production (SOD, CAT and GPX). Consequent of oxidative stress and free radical's redundant production, lipid peroxidation associated with DNA breakage occurs in affected cells. Hence, tissue damage and necrosis are histological markers (Muazzam et al., 2019). Hepatic lesions in the medium and high doses of meloxicam include hepatocytes vacuolisation (degeneration) with pyknotic nuclei in centrilobular zone, disarrangement of hepatic cords, caseous necrosis with haemorrhage and fibrin accumulation. These observations, although not specific, represent highly severe damage of liver tissue (Pal et al. 2012), which can lead to necrosis or apoptosis (Jarrar & Taib 2012).
In conclusion, the results from the present study indicate that exposure to meloxicam produces oxidative stress in common carp, as revealed by their elevated ALT, ALP, RBC, Hb, Hct, MCV and MDA levels and decreasing SOD, CAT and GPX activity and their gene expression. Moreover, the histological assay showed that the high dose of meloxicam cause to cellular damages and necrosis in liver of fish. Many studies have demonstrated the ameliorating outcome of NSAID on oxidative stress. However, in our study meloxicam with low, medium and high doses caused haematological, hepatic, renal and gene toxicities, which this response was dose dependent. The results indicate that a more identification on the end point of toxicology of this specific pharmaceutical drug and its toxicity effect on freshwater fish provide important knowledge on secure levels in the lentic ecosystems. So, more surveys are required, in order to provide more complementary knowledge about genotoxicity mechanisms of meloxicam including other metabolic and physiological pathways.
AUTHOR CONTRIBUTIONS
Zeynab Sheikhlangi conducted the experiments, data analysis and wrote the original draft. Ahmad Gharaei supervised, conceived and designed the research, data curation, writing, reviewing and editing. Javad Mirdar Harijani supervised, designed the research, edited the draft. Seyedeh Ayda Davari, Parisa Hassanein and Abdolali Rahdari verified the analytical methods, analysed the data, discussed the results and contributed to the final manuscript. The authors have prepared the article at their ‘personal capacity’ and this research is a collaborative work of the authors in the form of Ms. Sheikh Langi's master's thesis without the use of government financial aid.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. All procedures were carried out in accordance with the Animal Care and Use Committee guidelines at the Faculty of Sciences of the University of Zabol.
CONSENT TO PARTICIPATE
The authors agree to collaborate and publish this article.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1207.
ACKNOWLEDGEMENTS
We thank Dr. Fatma El‐Demardash for manuscript review, Dr. Karami for their time and energy and all staff of Hamoon International Wetland Research Institute for financial support and cooperation. The research project was funded by University of Zabol (Grant cod: IR‐UOZ‐GR‐7925).
Sheikhlangi, Z. , Gharaei, A. , Mirdar Harijani, J. , Davari, S. A. , Hassanein, P. , & Rahdari, A. (2023). Toxicological effects of meloxicam on physiological and antioxidant status of common carp (Cyprinus carpio). Veterinary Medicine and Science, 9, 2085–2094. 10.1002/vms3.1207
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from [third party]. Restrictions apply to the availability of these data, which were used under license for this study. Data are available [from the authors / at URL] with the permission of [third party].
REFERENCES
- Adhikari, S. , Sarkar, B. , Chatterjee, A. , Mahapatra, C. T. , & Ayyappan, S. (2004). Effects of cypermethrin and carbofuran on certain haematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton). Ecotoxicology and Environmental Safety, 58, 220–226. 10.1016/j.ecoenv.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Amin, H. M. , El‐Feki, M. A. , Abdalla, A. A. , & Youssef, M. A. (2017). Hematological and biochemical effects of meloxicam in male albino rats. Current Science International, 6, 23–33. [Google Scholar]
- Ayala, A. , Muñoz, M. F. , & Argüelles, S. (2014). Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4‐hydroxy‐2‐nonenal. Oxidative Medicine and Cellular Longevity, 2014, 360–438. 10.1155/2014/360438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burukoglu, D. , Baycu, C. , Taplamacioglu, F. , Sahin, E. , & Bektur, E. (2014). Effects of nonsteroidal anti‐inflammatory meloxicam on stomach, kidney, and liver of rats. Toxicology and Industrial Health, 32, 980–986. 10.1177/0748233714538484 [DOI] [PubMed] [Google Scholar]
- David, A. , & Pancharatna, K. (2009). Developmental anomalies induced by a non‐selective COX inhibitor (ibuprofen) in zebrafish (Danio rerio). Environmental Toxicology and Pharmacology, 27, 390–395. 10.1016/j.etap.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Ericson, H. , Thorsen, G. , & Kumblad, L. (2010). Physiological effects of diclofenac, ibuprofen and propranolol on Baltic Sea blue mussels. Aquatic Toxicology, 99, 223–231. 10.1016/j.aquatox.2010.04.017 [DOI] [PubMed] [Google Scholar]
- Faggio, C. , Fedele, G. , Arfuso, F. , Panzera, M. , & Fazio, F. (2014). Haematological and biochemical response of Mugil cephalus after acclimation to captivity. Cahiers de Biologie Marine, 55, 31–36. [Google Scholar]
- Finney, D. J. (1978). Statistical methods in biological assay (3rd ed., pp. 508). Griffin Press. 10.1038/172925a0 [DOI] [Google Scholar]
- Furst, D. E. , Kolba, K. S. , Fleischmann, R. , Silverfield, J. , Greenwald, M. , Roth, S. , Hall, D. B. , Roszko, P. J. , & Meloxicam Rheumatoid Arthritis Investigators . (2002). Dose response and safety study of meloxicam up to 22.5 mg daily in rheumatoid arthritis: A 12 week multicenter, double blind, dose response study versus placebo and diclofenac. Journal of Rheumatology, 29, 436–446. [PubMed] [Google Scholar]
- Gao, X. , Geng, J. , Du, Y. , Li, S. , Wu, G. , Fu, Y. , & Ren, H. (2018). Comparative study of the toxicity between three non‐steroidal anti‐inflammatory drugs and their UV/Na2S2O8 degradation products on Cyprinus carpio . Scientific Reports, 8, 13512. 10.1038/s41598-018-29524-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaei, A. , Ghaffari, M. , Keyvanshokooh, S. , & Akrami, R. (2011). Changes in metabolic enzymes, cortisol and glucose concentrations of beluga (Huso huso) exposed to dietary methylmercury. Fish Physiology and Biochemistry, 37, 485–493. 10.1007/s10695-010-9450-3 [DOI] [PubMed] [Google Scholar]
- Gharaei, A. , Khajeh, M. , khosravanizadeh, A. , Mirdar Harijani, J. , & Fadaei, R. (2020). Fluctuation of biochemical, immunological and antioxidant biomarkers in blood of beluga (Huso huso) under effect of dietary ZnO and chitosan‐ZnO NPs. Fish Physiology and Biochemistry, 46(2), 547–561. 10.1007/s10695-019-00726-2 [DOI] [PubMed] [Google Scholar]
- Gharaei, A. , Mahboudi, F. , Esmaili‐Sari, A. , Edalat, R. , Adeli, A. , & Keyvanshokooh, S. (2010). Molecular cloning of cDNA of mammalian and chicken II gonadotropin‐releasing hormones (mGnRHs and cGnRH‐II) in the beluga (Huso huso) and the disruptive effect of methylmercury on gene expression. Fish Physiology and Biochemistry, 36, 803–817. 10.1007/s10695-009-9356-0 [DOI] [PubMed] [Google Scholar]
- Han, S. , Choi, K. , Kim, J. , Ji, K. , Kim, S. , Ahn, B. , Yun, J. , Choi, K. , Khim, J. S. , Zhang, X. , & Giesy, J. P. (2010). Endocrine disruption and consequences of chronic exposure to ibuprofen in Japanese medaka (Oryzias latipes) and freshwater cladocerans Daphnia magna and Moina macrocopa . Aquatic Toxicology, 98, 256–264. 10.1016/j.aquatox.2010.02.013 [DOI] [PubMed] [Google Scholar]
- Hasan, M. R. , Hecht, T. , De Silva, S. S. , & Tacon, A. G. J. (Eds.). (2007). Study and analysis of feeds and fertilizers for sustainable aquaculture development (pp. 510) [FAO Fisheries Technical Paper No. 497]. Food and Agriculture Organization of the United Nations. [Google Scholar]
- Henkel, C. V. , Dirks, R. P. , Jansen, H. J. , Forlenza, M. , Wiegertjes, G. F. , Howe, K. , van den Thillart, G. E. E. J. M. , & Spaink, H. P. (2012). Comparison of the exomes of common carp (Cyprinus carpio) and zebrafish (Danio rerio). Zebrafish, 9, 59–67. 10.1089/zeb.2012.0773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger, B. , Dietrich, D. R. , Schmid, D. , Hartmann, A. , & Hitzfeld, B. (2008). Distribution of intraperitoneally injected diclofenac in brown trout (Salmo trutta fario). Ecotoxicology and Environmental Safety, 71, 412–418. 10.1016/j.ecoenv.2007.10.020 [DOI] [PubMed] [Google Scholar]
- Huerta, C. , Castellsague, J. , Varas‐Lorenzo, C. , & García Rodríguez, L. A. (2005). Nonsteroidal anti‐inflammatory drugs and risk of ARF in the general population. American Journal of Kidney Diseases, 45, 531–539. 10.1053/j.ajkd.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Jafarinejad, R. , Gharaei, A. , & Mirdar Harijani, J. (2018). Dietary ginger improves growth performance, blood parameters, antioxidant capacity and gene expression in Cyprinus carpio . Iranian Journal of Fisheries Sciences, 19(3), 1237–1252. 10.22092/ijfs.2018.119876 [DOI] [Google Scholar]
- Jarrar, B. M. , & Taib, N. T. (2012). Histological and histochemical alterations in the liver induced by lead chronic toxicity. Saudi Journal of Biological Sciences, 19(2), 203–210. 10.1016/j.sjbs.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, S. H. , Sim, D. S. , Park, M. S. , Jo, Q. T. , & Kim, Y. (2003). Effects of formalin on hematological and blood chemistry in olive flounder, Paralichthys olivaceus (Temminck et Schlegel). Aquaculture Research, 34, 1269–1275. 10.1046/j.1365-2109.2003.00936.x [DOI] [Google Scholar]
- Kavitha, C. , Malarvizhi, A. , SenthilKumaran, S. , & Ramesh, M. (2010). Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla . Food and Chemical Toxicology, 48, 2848–2854. 10.1016/j.fct.2010.07.017 [DOI] [PubMed] [Google Scholar]
- Kavitha, C. , Ramesh, M. , Senthil Kumaran, S. , & Audhi Lakshmi, A. (2012). Toxicity of Moringa oleifera seed extract on some hematological and biochemical profiles in a freshwater fish, Cyprinus carpio . Experimental and Toxicologic Pathology, 64, 681–687. 10.1016/j.etp.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Khan, A. M. , & Rampal, S. (2014). Effects of repeated oral administration of pazufloxacin mesylate and meloxicam on the antioxidant status in rabbits. Journal of the American Association for Laboratory Animal Science, 53, 399–403. 10.1177/0960327116637111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandan Barani, H. , Gharaei, A. , Sanchooli, N. , & Miri, M. (2019). Blood biochemistry fluctuations as influenced by feed provision in juvenile Snow trout (Schizothorax zarudnyi). Iranian Journal of Fisheries Sciences, 18, 735–744. 10.22092/ijfs.2019.118286 [DOI] [Google Scholar]
- Khosravi‐Katuli, K. , Lofrano, G. , Nezhad, H. P. , Giorgio, A. , Guida, M. , Aliberti, F. , Siciliano, A. , Carotenuto, M. , Galdiero, E. , Rahimi, E. , & Libralato, G. (2018). Effects of ZnO nanoparticles in the Caspian roach (Rutilus rutilus caspicus). Science of The Total Environment, 626, 30–41. 10.1016/j.scitotenv.2018.01.085 [DOI] [PubMed] [Google Scholar]
- Lavanya, S. , Ramesh, M. , Kavitha, C. , & Malarvizhi, A. (2011). Hematological, biochemical and ionoregulatory responses of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere, 82, 977–985. 10.1016/j.chemosphere.2010.10.071 [DOI] [PubMed] [Google Scholar]
- Li, Q. , Wang, P. , Chen, L. , Gao, H. , & Wu, L. (2016). Acute toxicity and histopathological effects of naproxen in zebra fish (Danio rerio) early life stages. Environmental Science and Pollution Research, 23, 18832–18841. 10.1007/s11356-016-7092-4 [DOI] [PubMed] [Google Scholar]
- Malarvizhi, A. , Kavitha, C. , Saravanan, M. , & Ramesh, M. (2012). Carbamazepine (CBZ) induced enzymatic stress in gill, liver and muscle of a common carp, Cyprinus carpio . Journal of King Saud University – Science, 24, 179–186. 10.1016/j.jksus.2011.01.001 [DOI] [Google Scholar]
- Mohammadi, M. , Imania, A. , Farhangi, M. , Gharaei, A. , & Hafeziehd, M. (2020). Replacement of fishmeal with processed canola meal in diets for juvenile Nile tilapia (Oreochromis niloticus): Growth performance, mucosal innate immunity, hepatic oxidative status, liver and intestine histology. Aquaculture, 518, 734–824. 10.1016/j.aquaculture.2019.734824 [DOI] [Google Scholar]
- Montesinos, A. , Ardiaca, M. , Juan‐Sallés, C. , & Tesouro, M. A. (2015). Effects of meloxicam on hematologic and plasma biochemical analyte values and results of histologic examination of kidney biopsy specimens of African grey parrots (Psittacus erithacus). Journal of Avian Medicine and Surgery, 29, 1–8. 10.1647/2013-056 [DOI] [PubMed] [Google Scholar]
- Muazzam, B. , Munawar, K. , Ahmad Khan, I. , Jahan, S. , Iqbal, M. , Rafique Asi, M., Farooqi, A. , Nazli, A. , Hussain, I. , & Iqbal Zafar, M. (2019). Stress response and toxicity studies on zebrafish exposed to endosulfan and imidacloprid present in water. Journal of Water Supply: Research and Technology‐Aqua, 68(8), 718–730. 10.2166/aqua.2019.077 [DOI] [Google Scholar]
- Nair, P. , Singh Kanwar, S. , & Nath Sanyal, S. (2006). Effects of non‐steroidal anti‐inflammatory drugs on the antioxidant defense system and the membrane functions in the rat intestine. Nutricion Hospitalaria, 21, 638–649. [PubMed] [Google Scholar]
- Nemcsok, J. , & Boross, L. (1982). Comparative studies on the sensitivity of different fish species to metal pollution. Acta Biologica Hungarica, 33, 23–27. [PubMed] [Google Scholar]
- Nussey, G. , van Vuren, J. H. , & Du Preez, H. H. (1995). Effect of copper on blood coagulation of Oreochromis mossambicus (Cichlidae). Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 111, 359–367. 10.1016/0742-8413(95)00062-3 [DOI] [PubMed] [Google Scholar]
- Pal, S. , Kokushi, E. , Koyama, J. , Uno, S. , & Ghosh, A. R. (2012). Histopathological alterations in gill, liver and kidney of common carp exposed to chlorpyrifos. Journal of Environmental Science and Health, Part B, 47(3), 180–195. 10.1080/03601234.2012.632285 [DOI] [PubMed] [Google Scholar]
- Razeghi Mansour, M. , Akrami, R. , Ghobadi, S. H. , Amani Denji, K. , Ezatrahimi, N. , & Gharaei, A. (2012). Effect of dietary mannan oligosaccharide (MOS) on growth performance, survival, body composition, and some hematological parameters in giant sturgeon juvenile (Huso huso Linnaeus, 1754). Fish Physiology and Biochemistry, 38, 829–835. 10.1007/s10695-011-9570-4 [DOI] [PubMed] [Google Scholar]
- Reddy, S. N. , & Venugopal, N. B. R. K. (1991). In vivo effects of cadmium chloride on certain aspects of protein metabolism in tissues of a freshwater field crab, Barytelphusa guerini . Bulletin of Environment Contamination and Toxicology, 46, 583–590. 10.1007/BF01688203 [DOI] [PubMed] [Google Scholar]
- Ristow, M. , & Schmeisser, S. (2011). Extending life span by increasing oxidative stress. Free Radical Biology and Medicine, 51, 327–336. [DOI] [PubMed] [Google Scholar]
- Saravanan, M. , Karthika, S. , Malarvizhi, A. , & Ramesh, M. (2011). Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: Hematological, biochemical, ionoregulatory and enzymological responses. Journal of Hazardous Materials, 195, 188–194. 10.1016/j.jhazmat.2011.08.029 [DOI] [PubMed] [Google Scholar]
- Saravanan, M. , Usha Devi, K. , & Malarvizhi, A. , Ramesh, M. (2012). Effects of Ibuprofen on hematological, biochemical and enzymological parameters of blood in an Indian major carp, Cirrhinus mrigala . Environmental Toxicology and Pharmacology, 34, 14–22. 10.1016/j.etap.2012.02.005 [DOI] [PubMed] [Google Scholar]
- Sharifpour, I. , Soltani, M. , & Javadi, M. (2004). Determination of LC50 and histopathological changes due to endosulfan in beluga (Huso huso) . Iranian Scientific Fisheries Journal, 12(4), 69–84. [Google Scholar]
- Sosnowska, K. , Styszko‐Grochowiak, K. , & Gołaś, J. (2009). Leki w środowisku – źródła, przemiany, zagrożenia. IV Krakowska Konferencja Młodych Uczonych (pp. 395–404). AGH University of Science and Technology; (in Polish). [Google Scholar]
- Sriuttha, P. , Sirichanchuen, B. , & Permsuwan, U. (2018). Hepatotoxicity of nonsteroidal anti‐inflammatory drugs: A systematic review of randomized controlled trials. International Journal of Hepatology, 2018, 5253623. 10.1155/2018/5253623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvetha, L. , Ramesh, M. , & Saravanan, M. (2010). Influence of cypermethrin toxicity on ionic regulation and gill Na+/K+‐ATPase activity of a freshwater teleost fish Cyprinus carpio . Environmental Toxicology and Pharmacology, 29, 44–49. 10.1016/j.etap.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Tellez‐Banuelos, M. C. , Santerre, A. , Casas‐Solis, J. , Bravo‐Cuellar, A. , & Zaitseva, G. (2009). Oxidative stress in macrophages from spleen of Nile tilapia (Oreochromis niloticus) exposed to sublethal concentration of endosulfan. Fish & Shellfish Immunology, 27, 105–111. 10.1016/j.fsi.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Tubbs, J. T. , Kissling, G. E. , Travlos, G. S. , Goulding, D. R. , Clark, J. A. , King‐Herbert, A. P. , & Blankenship‐Paris, T. L. (2011). Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. Journal of the American Association for Laboratory Animal, 50, 185–191. [PMC free article] [PubMed] [Google Scholar]
- Turck, D. , Roth, W. , & Busch, U. (1996). A review of the clinical pharmacokinetics of meloxicam. British Journal of Rheumatology, 35, 13–16. 10.1093/rheumatology/35.suppl_1.13 [DOI] [PubMed] [Google Scholar]
- Van Hecken, A. , Schwartz, J. I. , Depré, M. , De Lepeleire, I. , Dallob, A. , Tanaka, W. , Wynants, K. , Buntinx, A. , Arnout, J. , Wong, P. H. , Ebel, D. L. , Gertz, B. J. , & De Schepper, P. J. (2000). Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX‐2 versus COX‐1 in healthy volunteers. Journal of Clinical Pharmacology, 40, 1109–1120. 10.1590/S0021-75572006000700011 [DOI] [PubMed] [Google Scholar]
- Van Vuren, J. H. (1986). The effect of toxicants on the haematology of Labeo umbratus (Teleostei: Cyprinidae). Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 83, 155–159. 10.1016/0742-8413(86)90029-0 [DOI] [PubMed] [Google Scholar]
- Villegas, I. , Martín, M. J. , La Casa, C. , Motilva, V. , & De La Lastra, C. A. (2002). Effects of oxicam inhibitors of cyclooxygenase on oxidative stress generation in rat gastric mucosa. A comparative study. Free Radical Research, 36, 769–777. 10.1080/10715760290032575 [DOI] [PubMed] [Google Scholar]
- Winston, G. W. , & Digiulio, R. T. (1991). Prooxidant and antioxidant mechanisms in aquatic organisms. Aquatic Toxicology, 19, 137–161. 10.1016/0166-445X(91)90033-6 [DOI] [Google Scholar]
- World Health Organization (WHO) (2010). WHO model list of essential medicines . World Health Organization (WHO) [16th list (updated), March 2010]. http://www.who.int/medicines/publications/essentialmedicines/en/index.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from [third party]. Restrictions apply to the availability of these data, which were used under license for this study. Data are available [from the authors / at URL] with the permission of [third party].


