Abstract
The development of specific antibodies following primary Toxoplasma gondii infection during pregnancy was assessed by six different antibody assays: dye test, Platelia Toxo-IgG, Toxo-Screen DA IgG, Platelia Toxo-IgM, Toxo-ISAGA IgM, and Platelia Toxo-IgA. A total of 126 sera from 27 pregnant women, for whom the time of acquisition of infection could be estimated fairly accurately, were included. All tests showed great individual variation in the peak amounts of antibodies detected. The times elapsed after infection until the peak was reached also varied greatly from individual to individual: the ranges were 2 to 21 weeks for the dye test, 4 to 36 weeks for Platelia Toxo-IgG, 4 to 30 weeks for Toxo-Screen DA IgG, 2 to 18 weeks for Platelia Toxo-IgM, 1 to 6 weeks for Toxo-ISAGA IgM, and 2 to 21 weeks for Platelia Toxo-IgA. In the early phase of the infection the dye test and the specific-IgM tests were the most sensitive. Toxo-Screen DA IgG was more sensitive than Platelia Toxo-IgG in the acute phase, while Platelia Toxo-IgA was clearly the least sensitive assay. Of the sera collected 21 to 52 weeks after infection, all were positive by the dye test, all except one (which was negative by Platelia Toxo-IgG) were positive by the specific-IgG tests, approximately 80% were positive by the IgM tests, and 45% were positive by the IgA test. Due to the great individual variation it seems impossible to estimate when the infection occurred based on results obtained from a single serum, and it may even be difficult to assess when a titer increase in paired sera is detectable unless the first sample is only marginally positive. As a diagnostic criterion a dye test titer of ≥300 IU/ml has a low sensitivity for recent primary infection.
When a pregnant woman acquires a primary Toxoplasma gondii infection, the parasite may be transmitted to the fetus and cause serious damage (28). Antiparasitic treatment during pregnancy may prevent fetal transmission and sequelae (6, 17, 24, 28). The maternal infection is often asymptomatic or results in a clinical disease which is not recognized (18). Antibody screening programs aimed at the detection of T. gondii infection among pregnant women have therefore been introduced in several countries (1, 21, 32, 35). When seroconversion for specific immunoglobulin G (IgG) is detected in paired sera collected during pregnancy, the diagnosis is confirmed (23). But when the first serum sample, normally collected in early pregnancy, contains specific antibodies, the question of whether the infection occurred during pregnancy or prior to pregnancy arises. In the first case the fetus will be at risk of infection, whereas in the second case the fetus will most likely be protected (10). The ability to accurately determine when the infection occurred may therefore be crucial. Detection of specific IgM (5), detection of IgA (8), determination of T. gondii-specific IgG avidity (16, 19), measurement of acute-phase-specific IgG activity (7), and measurement of dye test titer (3, 28) have been introduced as supplementary methods to determine the time of infection. However, there is limited information on the development of specific antibodies among pregnant women in the first weeks after infection (22). In this study the emergence, variation in development, and duration of detection of different antibodies against T. gondii in pregnant women with primary infection are presented.
This study was part of the National Norwegian Study on Prevention of Congenital Toxoplasmosis approved by the Regional Committee for Ethics and Research (S-92039) and the Data Inspectorate (92/540-2).
MATERIALS AND METHODS
Sera.
A total of 126 sera with detectable T. gondii-specific antibodies collected from 27 pregnant women (2 to 8 samples per woman) with primary T. gondii infection were included in the study. The infections were detected by routine antenatal screening for specific IgG and IgM separately as described earlier (19). Only women with fourfold or greater increases in specific-IgG levels and for whom the times of acquisition of infection could be fairly accurately estimated were included in the study. Estimation was judged to be possible if one of two serological profiles was found: (i) the first serum sample was positive by either of the two specific-IgM tests used but was negative for specific IgG by enzyme immunoassay (EIA) (in this case the acquisition of infection was estimated to be 1 week prior to collection of this first IgM-positive sample [this profile was found in 15 women contributing 75 serum samples]), or (ii) the first sample was both positive for specific IgM and weakly positive for specific IgG (6 to 20 IU/ml) by EIA (in this case the infection was estimated to have occurred two weeks prior to collection of the first sample [this pattern was found in 12 women contributing 51 serum samples]). Prior to the sequence included in the study, 10 (37%) of the women had a serum sample collected that was negative in all the assays used. These women were thus true seroconverters.
All samples were collected within 52 weeks after infection, and 80 (63%) samples were collected within 13 weeks after the estimated time of infection.
Serologic tests.
All sera were examined for toxoplasma-specific antibodies by the dye test (13, 29), for specific IgG by EIA (Platelia Toxo-IgG; Sanofi Diagnostics Pasteur, Marnes la Coquette, France) (26, 30) and direct agglutination assay (Toxo-Screen DA IgG; bioMérieux, Marcy l’Etoile, France) (12), for specific IgM by EIA (Platelia Toxo-IgM; Sanofi Diagnostics Pasteur) (5) and immunosorbent agglutination assay (Toxo-ISAGA IgM; bioMérieux) (11), and for specific IgA by EIA (Platelia Toxo-IgA; Sanofi Diagnostics Pasteur) (8). All the commercially available tests were performed according to the recommendations given by the manufacturers. The following values were regarded as positive: Platelia Toxo-IgG, titer of ≥6 IU/ml; Toxo-Screen DA IgG, titer of ≥40; Platelia Toxo-IgM and Platelia Toxo-IgA, ≥100% of the cutoff value; Toxo-ISAGA IgM, index of ≥9 (borderline, index of 6 to 8); and dye test, titer of ≥3 IU/ml.
For each woman, every new serum sample received was tested in parallel with the previous one. If repeated results were discordant all sera from that woman were reanalyzed in parallel after the collection of samples was completed, and these results were accepted. All the IgA analyses were run in parallel retrospectively.
RESULTS
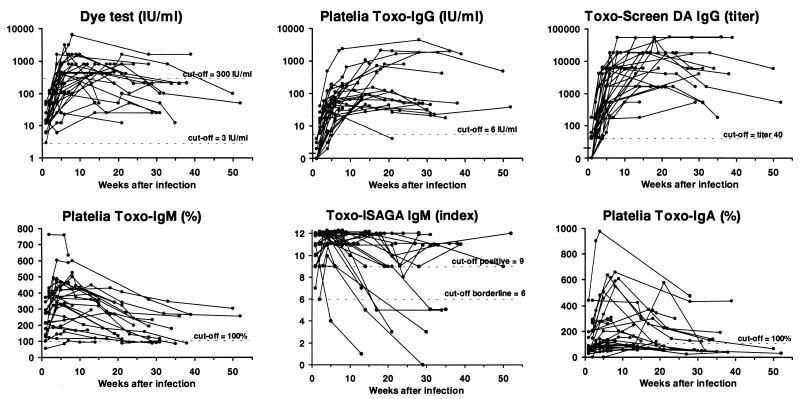
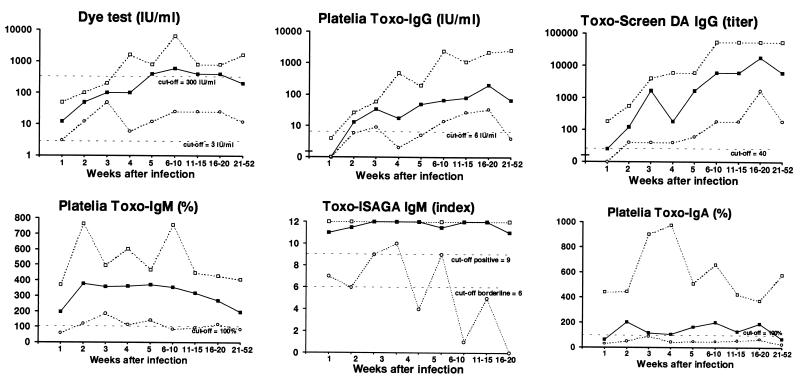
Diagrams showing the development of serum antibodies measured by the different assays for the 27 women are presented in Fig. 1. The median value and upper- and lower-range values for each assay according to the estimated number of weeks elapsed since infection are presented in Table 1 and Fig. 2. The proportions of sera exceeding defined cutoff values at different times after infection are presented in Table 2.
FIG. 1.
Development of T. gondii-specific antibodies for 27 pregnant women with primary infection as measured by six different assays. Each line represents one woman, and each dot represents the result for a serum specimen.
TABLE 1.
Results of six different tests for detection of specific antibodies to T. gondii for 126 serum specimens from 27 pregnant womena
| No. of wksb (no. of serum specimens) | Median (range) for antibody level measured with:
|
|||||
|---|---|---|---|---|---|---|
| Dye test (IU/ml) | Platelia Toxo-IgG (IU/ml) | Toxo-Screen DA IgG (titer) | Platelia Toxo-IgM (%) | Toxo-ISAGA IgM (index) | Platelia Toxo-IgA (%) | |
| 1 (14) | 12 (3–50) | 0 (0–4) | 20 (0–180) | 195 (57–371) | 11 (7–12) | 62 (28–442) |
| 2 (13) | 50 (12–100) | 13 (6–26) | 120 (40–540) | 379 (121–764) | 11.5 (6–12) | 204c (49–443) |
| 3 (6) | 100 (50–200) | 33 (9–58) | 1,620 (40–4,000) | 358 (185–495) | 12 (9–12) | 117 (91–901) |
| 4 (10) | 100 (6–1,600) | 17 (2–488) | 180 (40–6,000) | 360 (115–602) | 12 (10–12) | 104 (42–976) |
| 5 (9) | 400 (12–800) | 48 (5–192) | 1,620 (60–6,000) | 371 (144–469) | 12 (4–12) | 164d (48–509) |
| 6–10 (22) | 400 (25–6,400) | 65 (14–2,331) | 6,000 (180–54,000) | 354 (85–758) | 11.5 (9–12) | 199 (48–660) |
| 11–15 (13) | 400 (25–800) | 78 (27–1,067) | 6,000 (180–54,000) | 318 (95–450) | 12 (1–12) | 125 (53–424) |
| 16–20 (10) | 400 (25–800) | 197 (33–2,168) | 18,000 (1,620–54,000) | 270 (116–430) | 12 (5–12) | 190 (66–373) |
| 21–52 (29) | 200 (12–1,600) | 66 (4–2,500) | 6,000 (180–54,000) | 194 (83–405) | 11 (0–12) | 67 (24–577) |
The assays are described in Materials and Methods.
Number of weeks elapsed after the estimated time of acquisition of infection.
Eleven specimens were examined.
Seven specimens were examined.
FIG. 2.
Development of T. gondii-specific antibodies for 27 pregnant women with primary infection as measured by six different assays and expressed as median values (■), upper-range values (□), and lower-range values (○).
TABLE 2.
Proportions of sera fulfilling different diagnostic criteria according to the time elapsed after estimated time of infection
| Diagnostic test and diagnostic criterion | % of samples fulfilling criterion at no. of wks after infection (no. of serum specimens examined)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (14) | 2 (13) | 3 (6) | 4 (10) | 5 (9) | 6–10 (22) | 11–15 (13) | 16–20 (10) | 21–52 (29) | |
| Dye test | |||||||||
| ≥3 IU/ml | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| ≥300 IU/ml | 0 | 0 | 0 | 40 | 56 | 68 | 62 | 70 | 31 |
| Platelia Toxo-IgG | |||||||||
| ≥6 IU/ml | 0 | 100 | 100 | 82 | 89 | 100 | 100 | 100 | 97 |
| Toxo-Screen DA IgG | |||||||||
| Titer, ≥40 | 50 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Platelia Toxo-IgM | |||||||||
| ≥100% | 93 | 100 | 100 | 100 | 100 | 91 | 92 | 100 | 76 |
| Toxo-ISAGA IgM | |||||||||
| Index, ≥6 | 100 | 100 | 100 | 100 | 89 | 100 | 85 | 90 | 79 |
| Index, ≥9 | 93 | 90 | 100 | 100 | 89 | 100 | 77 | 90 | 76 |
| Platelia Toxo-IgA | |||||||||
| ≥100% | 21 | 82a | 67 | 50 | 75b | 77 | 62 | 60 | 45 |
Eleven sera were examined.
Seven sera were examined.
Dye test.
All sera were positive in the dye test. No serum collected less than 4 weeks after infection showed a titer of ≥300 IU/ml, a value often associated with recent infection (28), and for only 20 (74%) of the 27 women did the level reach this value in the dye test during the follow-up period. The median value reached the highest level at 5 weeks, after which it stayed constant for a further 15 weeks (Fig. 2). However, the peak value for individual women was detected from 2 to 21 weeks after infection. The titers at different times after infection and also the peak titers varied more than 100-fold among the women.
Platelia Toxo-IgG.
By definition the Platelia Toxo-IgG test was negative 1 week after infection (see Materials and Methods). Two (7%) women also had negative values for serum collected 4 to 5 weeks after infection (Fig. 1). One woman (4%) became negative after 21 weeks, while the others stayed clearly positive in the follow-up period. The peak value for each individual was reached from 4 to 36 weeks after infection. The titer increase was most pronounced in the first 5 weeks, after which the increase slowed down. For six women (22%) the peak value was detected more than 20 weeks after infection. The kinetics for all the immunoglobulins for these women are presented in Table 3.
TABLE 3.
Development of specific immunoglobulins following primary T. gondii infection among six pregnant women with an IgG peak value detected ≥20 weeks after infectiona
| Case no. | No. of wks after acquisition of infection | Test result
|
|||||
|---|---|---|---|---|---|---|---|
| Dye test (IU/ml)b | Platelia Toxo-IgG (IU/ml)c | Platelia Toxo-IgM (%)d | Toxo-Screen DA IgG (titer) | Toxo-ISAGA IgM (index)e | Platelia Toxo-IgA (%)d | ||
| 1 | 1 | 12 | 0 | 140 | 0 | 12 | 44 |
| 4 | 25 | 3 | 307 | 40 | 12 | 85 | |
| 6 | 100 | 22 | 322 | 540 | 12 | 104 | |
| 20 | 50 | 43 | 133 | 1,620 | 9 | 67 | |
| 21 | 100 | 26 | 86 | 1,620 | 9 | 60 | |
| 31 | 50 | 21 | 103 | 540 | 5 | 48 | |
| 35 | 12 | 18 | 83 | 180 | 5 | 43 | |
| 5 | −14f | 0 | 0 | 21 | 0 | 0 | 24 |
| 2 | 12 | 9 | 121 | 40 | 6 | 49 | |
| 4 | 6 | 17 | 115 | 180 | 10 | 68 | |
| 14 | 25 | 27 | 119 | 180 | 5 | 61 | |
| 29 | 25 | 48 | 87 | 540 | 0 | 59 | |
| 9 | 1 | 25 | 0 | 276 | 40 | 11 | 65 |
| 4 | 400 | 7 | 341 | 40 | 12 | 95 | |
| 5 | 200 | 7 | 371 | 540 | 12 | 80 | |
| 13 | 400 | 62 | 450 | 1,620 | 12 | 79 | |
| 21 | 800 | 119 | 342 | 18,000 | 12 | 577 | |
| 32 | 100 | 23 | 273 | 1,620 | 11 | 49 | |
| 52 | 50 | 38 | 256 | 540 | 12 | 33 | |
| 10 | 2 | 50 | 15 | 396 | 180 | 12 | 201 |
| 5 | 800 | 153 | 440 | 6,000 | 12 | 458 | |
| 8 | 1,600 | 317 | 525 | 18,000 | 12 | 589 | |
| 9 | 1,600 | 644 | 448 | 18,000 | 12 | 611 | |
| 22 | 400 | 1,651 | 279 | 54,000 | 12 | 224 | |
| 36 | 200 | 2,090 | 256 | 54,000 | 12 | 192 | |
| 11 | 2 | 100 | 20 | 410 | 180 | 11 | 280 |
| 4 | 800 | 480 | 602 | 6,000 | 12 | 482 | |
| 7 | 3,200 | 2,078 | 588 | 18,000 | 12 | 619 | |
| 8 | 6,400 | 2,331 | 600 | 54,000 | 12 | 660 | |
| 28 | 1,600 | 4,520 | 360 | 54,000 | 9 | 432 | |
| 39 | 1,600 | 1,680 | 265 | 54,000 | 11 | 439 | |
| 25 | 1 | 50 | 4 | 345 | 40 | 12 | 442 |
| 3 | 200 | 31 | 396 | 4,000 | 12 | 901 | |
| 4 | 1,600 | 119 | 486 | 6,000 | 12 | 976 | |
| 28 | 800 | 1,872 | 194 | 18,000 | 12 | 477 | |
Peak values are shown in bold.
Positive, ≥3 IU/ml.
Positive, ≥6 IU/ml.
Positive, ≥100%.
Positive, index of ≥9; borderline, index of 5 to 8.
A negative sample acquired 14 weeks before infection is not included in the study.
Toxo-Screen DA IgG.
Seven of 14 serum samples (50%) collected 1 week after infection and all samples collected more than 1 week after infection were positive by Toxo-Screen DA IgG. The peak titer for each woman was reached between 4 and 30 weeks after infection. The titer increase was most pronounced in the first 5 to 10 weeks, after which the curves leveled off. After the fourth week of infection the titers in serum varied among individuals more than 100-fold.
Platelia Toxo-IgM.
All samples collected 1 or 2 weeks after infection were positive except one. This sample belonged to a woman who was found to be weakly positive (103%) by Platelia Toxo-IgM (Fig. 1) 7 weeks after infection but negative at 6, 10, and 14 weeks. (For this woman Toxo-ISAGA IgM was clearly positive for all samples while the other results were in the lower parts of the respective ranges. Tests for IgA were continuously negative. She gave birth to a severely affected infant.) All other women remained positive until the 21st week after infection, but the values varied greatly. The peak value was detected from 2 to 18 weeks after infection, but for 22 (81%) of the women the peak value was reached within 8 weeks (median interval to the peak value, 4 weeks).
Toxo-ISAGA IgM.
One serum sample collected 1 week after infection and one sample collected 2 weeks after infection gave borderline results (indices of 7 and 6, respectively). All other sera collected within the first 4 weeks after infection were positive. One woman (4%) was negative the 5th week after infection, and five (19%) more women became negative from the 14th to the 31st week (Fig. 1). For all women the index reached 11 or 12 within 6 weeks except those for two women whose highest detected indices were 9 and 10, detected the 2nd and 4th weeks after infection, respectively.
Platelia Toxo-IgA.
Only 3 of 14 (21%) serum samples collected 1 week after infection and 9 of 11 (82%) collected after 2 weeks after infection were positive for specific IgA. For four women (15%) specific IgA was not detected at all during the follow-up period, and for eight other women (30%) the peak value was only weakly positive (<200%). The peak value occurred at a median time after infection of 7 weeks (range, 2 to 21 weeks).
DISCUSSION
This study showed that the development of T. gondii-specific antibodies varies greatly among pregnant women with primary T. gondii infection. The individual variation was most pronounced with regard to the amounts of antibodies produced and the times of the antibody peaks. This variation was evident for all methods, but generally if a woman’s samples reacted weakly in one test, they reacted weakly in the other assays performed; likewise, if samples from a woman reacted strongly in one test, they reacted strongly in other assays.
An important factor in our study is the method for estimation of acquisition of infection. The incubation period in clinical cases has been reported to vary from 4 to 21 days (2, 34). The period from infection until specific IgM and IgG antibodies may be detected in subclinical cases is not known. The method of estimating the time of infection as 1 or 2 weeks prior to collection of the first serum sample with detectable specific antibodies probably underestimates the variation in the incubation period. This variation adds to the variations in emergence of antibodies and of the antibody peak times shown in the study.
The development of specific antibodies related to glandular and ocular toxoplasmosis has been presented earlier (4, 20, 25, 27, 36). These studies have also shown individual variation in antibody amount during the patients’ follow-up periods, but patients with weak reactions are not specifically mentioned. It may be possible that patients with a clinically evident disease have a stronger immune response than women with a T. gondii infection detected through a routine antibody screening program, who most often have a subclinical infection. In addition, the results of these previous studies were related to the time elapsed since the onset of clinical symptoms, which emerge some time after the acquisition of the infection. Therefore, these results may not be directly applicable to the diagnostic problems occurring in a screening program for pregnant women.
Many different factors may influence the immune response to T. gondii infection. The virulence of the parasite strain, the amount of parasites in the inoculum, and the infective form of the parasite (oocysts or cysts) play a role (18). On the other hand, the antigen used in the antibody test, the type of antibody examined for, and the analytic sensitivity of the test are among factors that may restrict the detection of the immune response.
The dye test detects total specific antibodies, i.e., specific IgG, IgM, and IgA, directed towards the surface antigens. In the present study the dye test was consistently the first assay to yield a positive result, followed closely by a specific IgM test. By the Toxo-Screen DA IgG test, 50% of serum samples collected 1 week after the estimated time of infection were positive, and no negative result was detected later than 1 week after infection. The Toxo-Screen DA IgG test therefore seemed more sensitive in the acute phase than the Platelia Toxo-IgG test, which was negative by definition 1 week after infection and for some women remained negative even longer in the acute phase. The Platelia Toxo-IgA test was the least sensitive test. This is in agreement with the results of other studies (14, 15, 31, 33), although a higher sensitivity of specific-IgA tests than of IgM tests has been reported (8). However, in the detection of congenital infection the sensitivity for detection of specific IgA seems higher than that of IgM (8, 9, 14).
For all tests the increase in antibody amount in the acute phase of the disease was highest in the first 4 to 8 weeks after infection, but the peak value was reached earlier in the specific-IgM assays than in the IgG tests. With the IgG test several months may pass before the peak is reached for some women. This means that a titer increase may be detected in paired samples collected many weeks after the infection actually occurred (Table 3).
For most women specific IgM antibodies persisted for at least half a year after infection. However, the test may yield negative results earlier, a fact clearly demonstrated for the Toxo-ISAGA test.
Although all sera positive in the Platelia Toxo-IgA test were also positive in the Platelia Toxo-IgM assay, a positive result in the IgA test was not more helpful in estimating the time of acquisition of infection, since a positive result may persist for many months (Fig. 1).
Many important conclusions for the diagnosis of primary T. gondii infection during pregnancy can be drawn from these findings. (i) Specific IgM normally develops early, within 1 to 2 weeks after primary infection. (ii) Specific IgG will always develop, usually within 4 weeks after infection. (iii) The production and increase of the titers of specific antibodies normally culminate within 4 to 8 weeks, but in individual cases the increase in the level of specific IgM may continue for some more weeks and that of IgG may continue for several months. (iv) The amount of antibody measured in a single serum sample gives no clear indication of when the infection occurred. (v) The presence of IgM does not confirm a very recent infection. (vi) The amount of specific IgM may decrease to below the detection level less than 3 months after infection. (vii) Specific-IgA analysis is not an important contributor to a correct diagnosis, since a negative result does not exclude, nor does a positive result confirm, a recent primary T. gondii infection. (viii) As a diagnostic criterion a dye test titer of ≥300 IU/ml has a low sensitivity for recent primary infection. (ix) The Toxo-Screen DA IgG test seems more sensitive than the Platelia Toxo-IgG test.
REFERENCES
- 1.Aspöck H, Pollak A. Prevention of prenatal toxoplasmosis by serological screening of pregnant women in Austria. Scand J Infect Dis Suppl. 1992;84:32–37. [PubMed] [Google Scholar]
- 2.Benenson M W, Takafuji E T, Lemon S M, Greenup R L, Sulzer A J. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N Engl J Med. 1982;307:666–669. doi: 10.1056/NEJM198209093071107. [DOI] [PubMed] [Google Scholar]
- 3.Beverly J K A, Beattie C P. Glandular toxoplasmosis. A survey of 30 cases. Lancet. 1958;ii:379–384. doi: 10.1016/s0140-6736(58)90109-0. [DOI] [PubMed] [Google Scholar]
- 4.Brooks R G, McCabe R E, Remington J S. Role of serology in the diagnosis of toxoplasma lymphadenopathy. Rev Infect Dis. 1987;9:1055–1062. doi: 10.1093/clinids/9.5.1055. [DOI] [PubMed] [Google Scholar]
- 5.Cesbron J Y, Capron A, Ovlaque G, Santoro F. Use of monoclonal antibody in a double sandwich ELISA for detection of IgM antibodies to Toxoplasma gondii major surface protein (P30) J Immunol Methods. 1985;83:151–158. doi: 10.1016/0022-1759(85)90068-7. [DOI] [PubMed] [Google Scholar]
- 6.Daffos F, Forestier F, Capella-Pavlovsky M, Thulliez P, Aufrant C, Valenti D, Cox W L. Prenatal management of 746 pregnancies at risk for congenital toxoplasmosis. N Engl J Med. 1988;318:271–275. doi: 10.1056/NEJM198802043180502. [DOI] [PubMed] [Google Scholar]
- 7.Dannemann B R, Vaughan W C, Thulliez P, Remington J S. Differential agglutination test for diagnosis of recently acquired infection with Toxoplasma gondii. J Clin Microbiol. 1990;28:1928–1933. doi: 10.1128/jcm.28.9.1928-1933.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decoster A, Darcy F, Caron A, Capron A. IgA antibodies against p30 as markers of congenital and acute toxoplasmosis. Lancet. 1988;ii:1104–1107. doi: 10.1016/s0140-6736(88)90523-5. [DOI] [PubMed] [Google Scholar]
- 9.Decoster A, Slizewicz B, Simon J, Bazin C, Darcy F, Vittu G, Boulanger C, Champeau Y, Demory J L, Duhamel M, Capron A. Platelia-Toxo IgA, a new kit for early diagnosis of congenital toxoplasmosis by detection of anti-P30 immunoglobulin A antibodies. J Clin Microbiol. 1991;29:2291–2295. doi: 10.1128/jcm.29.10.2291-2295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desmonts G, Daffos F, Forestier F, Capella-Pavlovsky M, Thulliez P, Chartier M. Prenatal diagnosis of congenital toxoplasmosis. Lancet. 1985;i:500–504. doi: 10.1016/s0140-6736(85)92096-3. [DOI] [PubMed] [Google Scholar]
- 11.Desmonts G, Naot Y, Remington J S. Immunoglobulin M-immunosorbent agglutination assay for diagnosis of infectious diseases: diagnosis of acute congenital and acquired Toxoplasma infections. J Clin Microbiol. 1981;14:486–491. doi: 10.1128/jcm.14.5.486-491.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmonts G, Remington J S. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J Clin Microbiol. 1980;11:562–568. doi: 10.1128/jcm.11.6.562-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman H A, Lamb G A. A micromodification of the toxoplasma dye test. J Parasitol. 1966;52:415. [Google Scholar]
- 14.Foundrinier F, Marx-Chemla C, Aubert D, Bonhomme A, Pinon J M. Value of specific immunoglobulin A detection by two immunocapture assays in the diagnosis of toxoplasmosis. Eur J Clin Microbiol Infect Dis. 1995;14:585–590. doi: 10.1007/BF01690729. [DOI] [PubMed] [Google Scholar]
- 15.Gross U, Bohne W, Schröder J, Roos T, Heesemann J. Comparison of a commercial enzyme immunoassay and an immunoblot technique for detection of immunoglobulin A antibodies to Toxoplasma gondii. Eur J Clin Microbiol Infect Dis. 1993;12:636–639. doi: 10.1007/BF01973647. [DOI] [PubMed] [Google Scholar]
- 16.Hedman K, Lappalainen M, Seppälä I, Mäkelä O. Recent primary Toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989;159:736–740. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- 17.Hohlfeld P, Daffos F, Thulliez P, Aufrant C, Couvreur J, MacAleese J, Descombey D, Forestier F. Fetal toxoplasmosis: outcome of pregnancy and infant follow-up after in utero treatment. J Pediatr. 1989;115:765–769. doi: 10.1016/s0022-3476(89)80660-2. [DOI] [PubMed] [Google Scholar]
- 18.Ho-Yen D O. Clinical features. In: Ho-Yen D O, Joss A W L, editors. Human toxoplasmosis. Oxford, United Kingdom: Oxford Medical Publications; 1992. pp. 56–78. [Google Scholar]
- 19.Jenum P A, Stray-Pedersen B, Gundersen A-G. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J Clin Microbiol. 1997;35:1972–1977. doi: 10.1128/jcm.35.8.1972-1977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karim K A, Ludlam G B. The relationship and significance of antibody titers as determined by various serological methods in glandular and ocular toxoplasmosis. J Clin Pathol. 1975;28:42–49. doi: 10.1136/jcp.28.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lappalainen M, Koskela P, Hedman K, Teramo K, Ämmälä P, Hiilesmaa V, Koskiniemi M. Incidence of primary Toxoplasma infections during pregnancy in Southern Finland: a prospective cohort study. Scand J Infect Dis. 1992;24:97–104. doi: 10.3109/00365549209048407. [DOI] [PubMed] [Google Scholar]
- 22.Lappalainen M, Koskela P, Koskiniemi M, Ämmälä P, Hiilesmaa V, Teramo K, Raivio K O, Remington J S, Hedman K. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J Infect Dis. 1993;167:691–697. doi: 10.1093/infdis/167.3.691. [DOI] [PubMed] [Google Scholar]
- 23.Lebech M, Joynson D H M, Seitz H M, Thulliez P, Gilbert R, Dutton G N, Øvlisen B, Petersen E. Classification system and case definitions of Toxoplasma gondii infection in immunocompetent pregnant women and their congenitally infected offspring. Eur J Clin Microbiol Infect Dis. 1996;15:799–805. doi: 10.1007/BF01701522. [DOI] [PubMed] [Google Scholar]
- 24.McAuley J, Boyer K M, Patel D, Mets M, Swisher C, Roizen N, Wolters C, Stein L, Stein M, Schey W, Remington J, Meier P, Johnson D, Heydeman P, Holfels E, Withers S, Mack D, Brown C, Patton D, McLeod R. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago collaborative treatment trial. Clin Infect Dis. 1994;18:38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 25.Montoya J G, Remington J S. Studies on the serodiagnosis of toxoplasmic lymphadenitis. Clin Infect Dis. 1995;20:781–789. doi: 10.1093/clinids/20.4.781. [DOI] [PubMed] [Google Scholar]
- 26.Naessens A, Heuninckx W, Foulon W, Lauwers S. Evaluation of seven commercially available enzyme immunoassays for immunoglobulin G and M antibody detection of Toxoplasma gondii. Immunol Infect Dis. 1993;3:258–262. [Google Scholar]
- 27.Naot Y, Guptill D R, Remington J S. Duration of IgM antibodies to Toxoplasma gondii after acquired toxoplasmosis. J Infect Dis. 1982;145:770. doi: 10.1093/infdis/145.2.770. [DOI] [PubMed] [Google Scholar]
- 28.Remington J S, McLeod R, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: The W. B. Saunders Company; 1995. pp. 140–267. [Google Scholar]
- 29.Sabin A B, Feldman H A. Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoon parasite (Toxoplasma) Science. 1948;108:660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- 30.Santoro F, Afchain D, Pierce R, Cesbron J Y, Ovlaque G, Capron A. Serodiagnosis of toxoplasma infection using a purified parasite protein (P30) Clin Exp Immunol. 1985;62:262–269. [PMC free article] [PubMed] [Google Scholar]
- 31.Sensini A, Pascoli S, Marchetti D, Castronari R, Marangi M, Sbaraglia G, Cimmino C, Favero A, Castelletto M, Mottola A. IgG avidity in the serodiagnosis of acute Toxoplasma gondii infection: a multicenter study. Clin Microbiol Infect. 1996;2:25–29. doi: 10.1111/j.1469-0691.1996.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 32.Stray-Pedersen B, Jenum P. Current status of toxoplasmosis in pregnancy in Norway. Scand J Infect Dis Suppl. 1992;84:80–83. [PubMed] [Google Scholar]
- 33.Sulahian S, Nugues C, Garin Y J F, Pelloux H, Longuet P, Slizewicz B, Derouin F. Serodiagnosis of toxoplasmosis in patients with acquired or reactivating toxoplasmosis and analysis of the specific IgA antibody response by ELISA, agglutination and immunoblotting. Immunol Infect Dis. 1993;3:63–69. [Google Scholar]
- 34.Teutsch S M, Juranek D D, Sulzer A, Bubey J P, Sikes R K. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979;300:695–699. doi: 10.1056/NEJM197903293001302. [DOI] [PubMed] [Google Scholar]
- 35.Thulliez P. Screening programme for congenital toxoplasmosis in France. Scand J Infect Dis Suppl. 1992;84:43–45. [PubMed] [Google Scholar]
- 36.Welch P C, Masur H, Jones T C, Remington J S. Serologic diagnosis of acute lymphadenopathic toxoplasmosis. J Infect Dis. 1980;142:256–264. doi: 10.1093/infdis/142.2.256. [DOI] [PubMed] [Google Scholar]