Abstract
From 7 to 24 March 1997, four patients developed Pseudomonas fluorescens bacteremia at the hospital; one on the oncology ward and the other three in the chemotherapy room. These patients all had underlying malignancies and had the Port-A-Cath (Smiths Industries Medical Systems, Deltec, Inc., St. Paul, Minn.) implants. Three patients had primary bacteremia, and one had Port-A-Cath-related infection. None of these patients had received a blood transfusion before the episodes of bacteremia. All patients recovered: two received antimicrobial agents with in vitro activity against the isolates, and the other two did not have any antibiotic treatment. A total of eight blood isolates were recovered from these patients during the febrile episodes that occurred several minutes after the infusion of chemotherapeutic agents via the Port-A-Cath. These isolates were initially identified as P. fluorescens or Pseudomonas putida (four), Burkholderia (Ralstonia) pickettii (three), and a non-glucose-fermenting gram-negative bacillus (one) by routine biochemical methods and the Vitek GNI card. These isolates were later identified as P. fluorescens on the basis of the characteristic cellular fatty acid chromatogram and the results of supplemental biochemical tests. The identification of identical antibiotypes by the E test and the random amplified polymorphic DNA patterns generated by arbitrarily primed PCR of the isolates showed that the outbreak was caused by a single clone of P. fluorescens. Surveillance cultures of the possibly contaminated infusion fluids and disinfectants, which were performed 7 days after recognition of the last infected patient, failed to isolate P. fluorescens. This report of a small outbreak caused by P. fluorescens suggests that timely, accurate identification of unusual nosocomial pathogens is crucial for early initiation of an epidemiological investigation and timely control of an outbreak.
Pseudomonas fluorescens and Pseudomonas putida are members of the fluorescent pseudomonad group (2). These organisms, unlike the well-known Pseudomonas aeruginosa, are generally considered to have a low level of virulence and to be of little clinical significance (1, 2, 13). Strains of P. fluorescens have been frequently identified as contaminants on the skin of humans and as agents causing pseudobacteremia and procedure-related infections in hospitalized patients (6, 7, 11–13). There is usually little need to differentiate among these organisms, except in blood isolates from patients who received blood transfusions because of the well-known association between P. fluorescens and contaminated blood components (2, 11, 12).
To the best of our knowledge, a nosocomial outbreak of bacteremia due to P. fluorescens unrelated to blood transfusions has never been reported in the literature. Prompt recognition of an outbreak caused by a rarely isolated pathogen is not feasible if the isolate is not accurately identified to the species level in time. The commercial semiautomated identification systems used in many clinical microbiology laboratories have successfully provided a supplemental means, in addition to conventional biochemical methods, to identify the majority of commonly encountered bacteria (2). However, the accuracy of these systems is often poor and is not known for some species of bacteria, particularly for non-glucose-fermenting gram-negative bacilli (1).
In the present study, we first document an outbreak of bacteremia caused by P. fluorescens in four oncology patients. Unfortunately, we were not able to identify the isolates early or trace the source of infections because the results of species identifications for the blood isolates from these patients were not concordant.
MATERIALS AND METHODS
Background of the outbreak.
National Taiwan University Hospital is a 2,000-bed teaching hospital in northern Taiwan at which 40 to 60 patients with malignancies receive scheduled intravenous chemotherapeutic agents in the chemotherapy room daily. From 17 to 24 March 1997, three patients, who had been treated in the chemotherapy room, developed fevers and chills and had one or more blood cultures positive for Burkholderia pickettii (Ralstonia pickettii) or for P. fluorescens or P. putida (Table 1). Seven isolates were recovered from blood specimens, including six from specimens from peripheral veins and one from the Port-A-Cath (Smiths Industries Medical Systems, Deltec, Inc., St. Paul, Minn.) implant, and one isolate was recovered from a Port-A-Cath tip (this isolate was not preserved for study) for these three patients during this period. A review of microbiological culture records revealed a blood isolate of a nonfermentative gram-negative bacillus which was recovered from a patient who had a febrile episode after the infusion of a chemotherapeutic agent during the period of admission (from 28 February to 27 March 1997) to the oncology ward. All eight isolates from the four patients were later identified as P. fluorescens.
TABLE 1.
Clinical characteristics of four patients with P. fluorescens bacteremia
| Patient no. | Age (yr)/ sexf | Underlying disease | Associated condition | Isolation data
|
Treatment (duration [days]) | |||
|---|---|---|---|---|---|---|---|---|
| Locationb | Isolate designa- tionc | Date (day/mo/yr) | Initial identification | |||||
| 1 | 50/F | Breast liposarcoma, liver and thyroid metastasis | Port-A-Cath inserted | OW | A | 7/3/97 | NFGNBd | Mefoxitin (5); ceftazidime plus amikacin (14) |
| 2 | 47/M | Non-Hodgkin’s lymphoma | Port-A-Cath inserted, leukopeniaa | CR | B1 | 17/3/97 | P. fluorescens or P. putida | Noe |
| CR | B2 | 17/3/97 | P. fluorescens or P. putida | |||||
| 3 | 26/M | Hodgkin’s disease | Port-A-Cath inserted, leukopenia | CR | C1 | 19/3/97 | B. pickettii | No |
| CR | C2 | 19/3/97 | P. fluorescens or P. putida | |||||
| 4 | 66/M | Gastric lymphoma | Port-A-Cath inserted, leukopenia | CR | D1 | 17/3/97 | B. pickettii | No |
| CR | D2 | 24/3/97 | P. fluorescens or P. putida | Ceftazidime (2); ciprofloxacin (7) | ||||
| CR | D3 | 24/3/97 | B. pickettii | Catheter removal | ||||
Defined as a leukocyte count <4.0 × 109/liter.
OW, oncology ward; CR, chemotherapy room.
Isolate D3 was recovered from a blood specimen collected from a Port-A-Cath, and others (A to D2) were recovered from blood specimens drawn from peripheral veins.
NFGNB, nonfermentative gram-negative bacilli.
No, no treatment.
M, male; F, female.
Epidemiological surveillance.
Epidemiological investigations started on 26 March 1997. Since the first three patients discussed above received infusion of various chemotherapeutic agents and intravenous injection of medications administered by nurses via their Port-A-Cath implants, three possible sources of infection were considered: (i) the solutions used to disinfect the skin of the Port-A-Cath; (ii) the fluids, including 5% glucose–water and normal saline, used to make up the infusate of chemotherapeutic agents; (iii) the medications simultaneously administered via the Port-A-Cath during the period of infusion, including granisetron, ondansetron, and methylprednisolone sodium succinate. All disinfectants used in the chemotherapy room as well as on the oncology ward and other possibly contaminated fluids that were used from 19 to 24 March in the chemotherapy room were also subjected to microbiological culture.
Bacterial isolates.
Isolates from these patients were initially identified on the basis of colonial morphology, oxidase reaction, and growth on triple-sugar iron agar, as well as by their biochemical profiles obtained with the Vitek GNI card (Table 1) (2). These isolates were subsequently identified as P. fluorescens by conventional methods on the basis of their growth at 4°C but not at 42°C, their production of pyoverdin, and their degradation of gelatin by the alkalinization of a gelatin agar slant (2, 10).
Cellular fatty acid analysis.
Isolates for cellular fatty acid analysis included those of P. fluorescens (8 blood isolates from the four patients, 3 from other clinical specimens, and 1 of P. fluorescens ATCC 13525) and those of P. putida (15 isolates from clinical specimens and 1 of P. putida ATCC 12633). These isolates were incubated in Trypticase soy agar (BBL Microbiology Systems, Cockeysville, Md.) for 24 h in ambient air. Procedures for bacterial cell lysis, saponification, methylation of fatty acids, and extraction of fatty acid methyl esters were performed as previously described (3). The software library used to identify the Pseudomonas species was TSA, version 3.9 (Microbial ID Inc., Newark, Del.). The similarity index (ranges from 0 to 1) was defined as the closeness of a match of the unknown bacterium to a library entry. A similarity index of >0.6 was defined as an excellent match.
Antimicrobial susceptibility testing.
MICs for the eight blood isolates were determined by the E test (PDM Epsilometer; AB Biodisk, Solna, Sweden) on Mueller-Hinton agar (BBL Microbiology Systems). The results were read after 18 to 20 h of incubation in air. Antimicrobial agents (range of concentration for each antibiotic, 0.016 to 256 μg/ml) tested included piperacillin, cefoperazone, ceftazidime, aztreonam, imipenem, netilmicin, amikacin, minocycline, ofloxacin, and ciprofloxacin. MIC breakpoints for defining susceptibility were in accordance with the description by the National Committee for Clinical Laboratory Standards (8).
RAPD patterns.
The preparation of genomic DNA and the PCR conditions for determination of random amplified polymorphic DNA (RAPD) patterns generated by arbitrarily primed PCR were as described previously (4). Two oligonucleotide primers, M13 (5′-TTATGTAAAACGACGGCCAG-3′) and ERIC2 (5′-AAGTAAGTGACTGACTGGGGTGAGCG-3′), were used. Two of the three other clinical isolates of P. fluorescens (isolates E and F) were also included in this study as control strains. For interpreting RAPD patterns, both faint and intensive bands were included. Patterns differing by more than one band were considered to be different; otherwise, they were considered identical.
RESULTS
Clinical features.
Table 1 shows the clinical features of four patients with P. fluorescens bacteremia. Three patients presented with primary bacteremia, and one had a Port-A-Cath-related infection. Two patients (patients 3 and 4) had multiple sets of blood cultures, which initially yielded two different species of microorganisms. All patients recovered. Two patients (patients 1 and 4) received antimicrobial agents with in vitro activity against the isolates: patient 1 had defervescence 4 days after receiving the combination of ceftazidime and amikacin, and patient 4 had defervescence after the removal of the Port-A-Cath. The other two patients defervesced several hours later and did not have any antibiotic treatment. No recurrence of bacteremia due to P. fluorescens was found among these patients in the following 2 months. None of these patients had received a blood transfusion before the episodes of bacteremia.
Epidemiological investigation.
From 17 to 24 March, a total of 220 patients with underlying malignancies received chemotherapeutic agents intravenously in the chemotherapy room. Only three patients had evidence of infection related to the infusion of chemotherapeutic agents. Cultures of 5% glucose–water, normal saline, and disinfectants were all negative for P. fluorescens. Unfortunately, none of the possibly contaminated infusion fluids used from 17 to 18 March were available for bacterial cultures.
Identification of bacteria.
Because of incomplete identification or misidentification by the Vitek automated identification system, these isolates were identified as P. fluorescens or P. putida on the basis of a positive oxidase reaction, no production of yellowish green pigments on Mueller-Hinton agar (BBL Microbiology Systems), no growth at 42°C, and production of yellow-brown fluorescent pigment (pyoverdin) (2). P. fluorescens was differentiated from P. putida by the ability to grow at 4°C after 3 to 4 days of incubation and degradation of gelatin, which was observed after 5 to 6 days of incubation. Isolates C1, D1, and D3, initially identified as B. pickettii by the Vitek system, were then identified as P. fluorescens or P. putida by changing the negative reactions of the Vitek arginine dihydrolase to positive. All eight epidemiologically related isolates were subsequently identified as P. fluorescens.
Cellular fatty acid compositions.
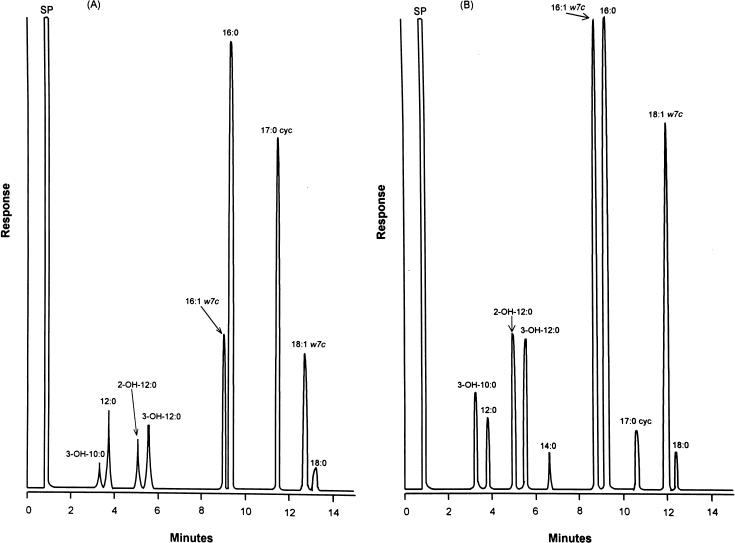
Cellular fatty acid chromatograms of P. fluorescens and P. putida isolates are shown in Fig. 1. All isolates had the same cellular fatty acid composition, including 3-OH-10:0 (3-hydroxydecanoic acid), 12:0 (dodecanoic acid), 2-OH-12:0 (2-hydroxydodecanoic acid), 3-OH-12:0 (3-hydroxydodecanoic acid), 16:1w7c (hexadecenoic acid), 16:0 (hexadecanoic acid), 17:0 cyc (cis-9; 10-methylenehexadecanoic acid), and 18:1w7c (octadecenoic acid). The ratio of 3-OH-10:0 to 12:0 was <1 (0.3 to 0.5) for isolates of P. fluorescens and was ≥1 (1.0 to 1.2) for isolates of P. putida. The ratio of 16:1w7c to 16:0 was <1 (0.4 to 0.5) for isolates of P. fluorescens but was approximately 1 (0.9 to 1.0) for P. putida isolates. The similarity indices for the identification of P. fluorescens were between 0.6 and 0.7, and those for the identification of P. putida were between 0.7 and 0.8.
FIG. 1.
Cellular fatty acid chromatograms of P. fluorescens (A) and P. putida (B). All eight epidemiologically related isolates, the three clinical isolates, and the control strain of P. fluorescens have chromatograms identical to the chromatograms shown for P. fluorescens. Chromatograms of 15 clinical isolates and the control strain of P. putida are identical.
Antimicrobial susceptibilities.
Table 2 shows the MICs for the P. fluorescens strain determined by the E test. The eight isolates had identical antibiotypes, and the MICs measured for them were within a twofold dilution for all agents tested. Among the tested agents, imipenem was the most active, followed by ceftazidime. Susceptibilities to other agents were poor.
TABLE 2.
Susceptibilities of the P. fluorescens strain to 10 antimicrobial agents by the E test
| Antibiotic | MIC (μg/ml) |
|---|---|
| Piperacillin | 96 |
| Cefoperazone | >256 |
| Ceftazidime | 8 |
| Aztreonam | 96 |
| Imipenem | 2 |
| Netilmicin | >32 |
| Amikacin | 24 |
| Ofloxacin | >32 |
| Ciprofloxacin | >32 |
| Minocycline | >256 |
RAPD patterns.
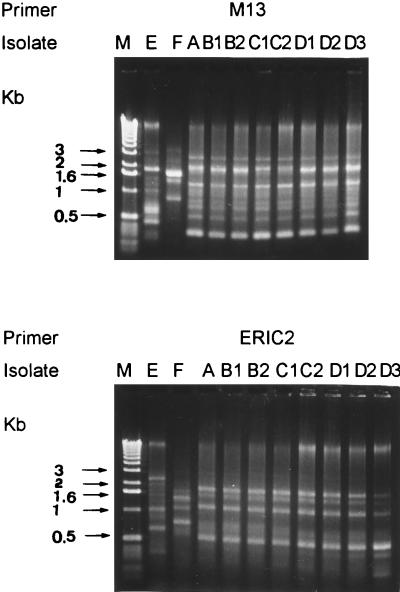
As shown in Fig. 2, the eight isolates of P. fluorescens produced identical RAPD patterns with the two primers, and these patterns were different from those produced by the two control strains with the two primers.
FIG. 2.
RAPD patterns of the 10 isolates of P. fluorescens obtained with the two primers. Lane M, molecular size markers (1-kb ladder; Gibco BRL, Gaithersburg, Md.); lanes E and F, isolates E and F, respectively; lanes A to D3, isolates from patients 1 (A), 2 (B1 and B2), 3 (C1 and C2), and 4 (D1 to D3) (see Table 1 for the origins of the isolates). Molecular sizes in kilobase pairs are indicated to the left of the gel.
DISCUSSION
Four facets associated with this outbreak of bacteremia caused by P. fluorescens in four oncology patients are of particular importance. First, P. fluorescens should be considered in studies of the etiologies of infusion- or catheter-related infections, although the source of this organism in this outbreak is not known. Second, due to the difficulties in accurate and early identification of P. fluorescens by routine methods or commercially available, semiautomated identification instruments, timely recognition of outbreaks caused by these organisms is usually not feasible. Third, analysis of cellular fatty acid profiles was an excellent method to differentiate P. fluorescens and P. putida. Fourth, RAPD patterns generated by arbitrarily primed PCR were highly discriminatory for the epidemiological study of strain relatedness.
Several reports have appeared in the literature concerning cases of P. fluorescens causing pseudobacteremia or bacteremia associated with contaminated blood products (2, 6, 7, 11–13). This organism has also been isolated from the skin of donors (11, 12). However, no previous reports describing this organism were associated with Port-A-Cath-related sepsis. Previous experience indicated that the recovery of this rarely encountered organism from blood cultures from more than one patient over a short period of time might suggest that an outbreak of bacteremia due to contaminated infusion or injectable solutions had occurred (13). Our report was in accordance with this finding. As in many previous reports, no source of the organism responsible for the incident could be found (6, 7, 11–13).
Like some nonfermentative gram-negative bacilli, P. fluorescens is of low intrinsic pathogenicity (1). Bacteremia caused by P. fluorescens might appear to be a benign disease, even in patients with underlying malignancies involving leukopenia and patients infected with multiresistant isolates. However, two of our patients recovered only after the administration of appropriate antimicrobial agents and/or removal of the infected catheter. Scott et al. reported a fatal transfusion reaction due to contamination of platelet-depleted whole blood with P. fluorescens (12). Thus, we suggest prompt administration of effective antibiotics and investigating the possibility of catheter-related infection for patients with P. fluorescens bacteremia, particularly those with neoplastic diseases and with indwelling devices implanted.
Two key characteristics of P. fluorescens that differ from those of P. putida are the ability to degrade gelatin and to grow at 4°C (2, 10). However, negative gelatin results by a standard tube assay should be interpreted with caution, because accurate detection of gelatin degradation may require 14 days of incubation (10). More than 10% of P. fluorescens strains were reported to be negative for gelatin degradation when the results were read after 4 days of incubation (10). Furthermore, all our P. fluorescens isolates had evident growth at 4°C at least 4 days after beginning incubation. These findings indicate that use of these two tests for timely differentiation of these two species is not feasible in clinical microbiology laboratories.
A considerable volume of literature has reported on the use of whole-cell fatty acid analysis for the identification of microorganisms, including nonfermentative gram-negative bacilli (9, 14). However, no previous reports described the use of this technique for differentiation between P. fluorescens and P. putida. Though the overall cellular fatty acid compositions of these two species were similar, the differences in the relative amounts of 3-OH-10:0 and 12:0 and of 16:1w7c and 16:0 were significant. Nevertheless, these differences were not seen in the previous reports regarding the cellular fatty acid compositions of these two species (9, 14).
Strains of P. fluorescens are commonly susceptible to imipenem, meropenem, gentamicin, and tetracycline but are less susceptible to cefuroxime, cefmenoxime, cefotaxime, cefsulodin, and trimethoprim (5, 12, 15). The antimicrobial susceptibility patterns of our P. fluorescens isolates were partly in agreement with these findings. According to the limited susceptibility results for P. fluorescens, ceftazidime and carbapenems may be the drugs of choice for empiric treatment of severe infections caused by this organism (5, 15).
This report of a small outbreak of bacteremia caused by P. fluorescens suggests that accurate identification of infrequently isolated nosocomial pathogens is crucial for early recognition of the outbreak, prompt initiation of epidemiological surveillance, and timely control of the outbreak.
REFERENCES
- 1.Blazevic D J, Koepcke M H, Matsen J M. Incidence and identification of Pseudomonas fluorescens and Pseudomonas putida in the clinical laboratory. Appl Microbiol. 1973;25:107–110. doi: 10.1128/am.25.1.107-110.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilligan P H. Pseudomonas and Burkholderia. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 509–519. [Google Scholar]
- 3.Hsueh P-R, Teng L-J, Ho S-W, Hsieh W-C, Luh K-T. Clinical and microbiological characteristics of Flavobacterium indologenes infections associated with indwelling devices. J Clin Microbiol. 1996;34:1908–1913. doi: 10.1128/jcm.34.8.1908-1913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsueh P R, Teng L J, Lee P I, Yang P C, Huang L M, Chang S C, Lee C Y, Luh K T. Outbreak of scarlet fever at a hospital day care centre: analysis of strain relatedness with phenotypic and genotypic characteristics. J Hosp Infect. 1997;36:191–200. doi: 10.1016/s0195-6701(97)90194-8. [DOI] [PubMed] [Google Scholar]
- 5.Jones R N, Aldridge K E, Allen S D, Barry A L, Fuchs P C, Gerlach E H, Pfaller M A. Multicenter in vitro evaluation of SM-7338, a new carbapenem. Antimicrob Agents Chemother. 1989;33:562–565. doi: 10.1128/aac.33.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarus H M, Magalhaes-Silverman M, Fox R M, Creger R J, Jacobs M. Contamination during in vitro processing of bone marrow for transplantation: clinical significance. Bone Marrow Transplant. 1991;7:241–246. [PubMed] [Google Scholar]
- 7.Murray A E, Bartzokas C A, Shepherd A J N, Roberts F M. Blood transfusion associated Pseudomonas fluorescens septicaemia: is this an increasing problem? J Hosp Infect. 1987;9:243–248. doi: 10.1016/0195-6701(87)90120-4. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Performance standard for antimicrobial susceptibility testing: eighth informational supplement. M100-S8. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 9.Osterhout G J, Shull V H, Dick J D. Identification of clinical isolates of gram-negative nonfermentative bacteria by an automated cellular fatty acid identification system. J Clin Microbiol. 1991;29:1822–1830. doi: 10.1128/jcm.29.9.1822-1830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickett M J, Greenwood J R, Harvey S M. Tests for detecting degradation of gelatin: comparison of five methods. J Clin Microbiol. 1991;29:2322–2325. doi: 10.1128/jcm.29.10.2322-2325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puckett A, Davison G, Entwistle C C, Barbara J A J. Posttransfusion septicemia 1980–1989: importance of donor arm cleansing. J Clin Pathol. 1992;45:155–157. doi: 10.1136/jcp.45.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott J, Boulton F E, Govan J R W, Miles R S, McClelland D B L, Prowse C V. A fatal transfusion reaction associated with contamination with Pseudomonas fluorescens. Vox Sang. 1988;54:201–204. doi: 10.1111/j.1423-0410.1988.tb03905.x. [DOI] [PubMed] [Google Scholar]
- 13.Simor A E, Ricci J, Lau A, Bannatyne R M, Ford-Jones L. Pseudobacteremia due to Pseudomonas fluorescens. Pediatr Infect Dis. 1985;4:508–512. doi: 10.1097/00006454-198509000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Veys A, Callewaert W E, Waelkens E, Abbeele K V D. Application of gas-liquid chromatography to the routine identification of nonfermentative gram-negative bacteria in clinical specimens. J Clin Microbiol. 1989;27:1538–1542. doi: 10.1128/jcm.27.7.1538-1542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe N-A, Katsu K, Moriyama M, Kitoh K. In vitro evaluation of E1040, a new cephalosporin with potent antipseudomonal activity. Antimicrob Agents Chemother. 1988;32:693–701. doi: 10.1128/aac.32.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]