Abstract
Emmonsia crescens, an agent of adiaspiromycosis, Blastomyces dermatitidis, the agent of blastomycosis, and Histoplasma capsulatum, the agent of histoplasmosis, are known to form meiotic (sexual) stages in the ascomycete genus Ajellomyces (Onygenaceae, Onygenales), but no sexual stage is known for E. parva, the type species of the genus Emmonsia. To evaluate relationships among members of the putative Ajellomyces clade, large-subunit ribosomal and internal transcribed spacer region DNA sequences were determined from PCR-amplified DNA fragments. Sequences were analyzed phylogenetically to evaluate the genetic variation within the genus Emmonsia and evolutionary relationships to other taxa. E. crescens and E. parva are distinct species. E. crescens isolates are placed into two groups that correlate with their continents of origin. Considerable variation occurred among isolates previously classified as E. parva. Most isolates are placed into two closely related groups, but the remaining isolates, including some from human sources, are phylogenetically distinct and represent undescribed species. Strains of B. dermatitidis are a sister species of E. parva. Paracoccidioides brasiliensis and Histoplasma capsulatum are ancestral to most Emmonsia isolates, and P. brasiliensis, which has no known teleomorph, falls within the Ajellomyces clade.
Adiaspiromycosis is primarily a respiratory disease of rodents and many other small mammals but affects humans on occasion (8, 15, 24, 41). The causative agents of the mycosis are Emmonsia parva and E. crescens. Spores in tissue, called adiaspores, resemble the parasitic spherules of Coccidioides immitis but differ in that they lack internal spores (41). The finding of large, round or oval, thick-walled adiaspores in histopathological sections of tissue is the main criterion for a diagnosis of adiaspiromycosis (8, 15, 24, 41). Confirmation by culture of the organism from human tissues has rarely been achieved (12, 24, 41). E. crescens forms larger adiaspores and has a broader host range and geographic distribution than E. parva. Most human infections are attributed to E. crescens, but a recent report documented disseminated disease caused by E. parva in a patient with AIDS (12). Because Emmonsia species rarely affect humans and do not cause fulminant disease, as do the other dimorphic fungi, these fungal pathogens have received little attention.
The pathogenesis, history of discovery, and controversial taxonomy of the etiologic agents of adiaspiromycosis have been reviewed recently in detail (40, 41). Emmons and Ashburn (14) observed C. immitis and a second fungus forming spherule-like structures measuring up to 20 μm in diameter in the lungs of rodents trapped in Arizona. Initially named Haplosporangium parvum (31), the fungus was later transferred to the new genus Emmonsia as E. parva (5). A second fungus producing larger adiaspores but having similar morphology was discovered in rodents trapped in Alberta, Canada (9), and later named E. crescens (13). Ensuing studies have shown that the etiologic agents differ in maximum growth temperature, size of parasitic spores, host range, and geographic distribution, but no consensus has been reached on whether these differences are indicative of species or varieties.
Aspects of the saprobic and parasitic stages have led some workers to consider Emmonsia species to be close relatives of the dimorphic fungi (4, 9, 14, 30, 40), but this relationship has not been widely accepted because of the unusual nature of the parasitic form and the type of infection caused. This has led, even in 1998, to the treatment of Emmonsia species apart from the other dimorphic fungi and together with unusual organisms having unknown affinities (41). Similarly, the endosporulating spherule of C. immitis has been a dominant character contributing to disagreement over its phylogenetic affinity. Recent molecular (2, 33) and morphologic (43) studies have provided evidence that C. immitis is closely related to a species of Malbranchea having a sexual stage in the genus Uncinocarpus (Onygenaceae, Onygenales), as suggested by similarities in its alternate arthroconidia (7, 42) and development of spirally coiled hyphae (43).
Sigler (30) observed ascomatal hyphae and hyphal coils in an E. parva-like isolate cultured from the lung of an Australian wombat (27) and predicted that the sexual stage of Emmonsia species would belong in Ajellomyces. Molecular phylogenetic studies supported this suggestion and showed that E. parva and Blastomyces dermatitidis are united on a single branch, with Histoplasma capsulatum a close relative (2, 25). The latter species have sexual states in the genus Ajellomyces known as Ajellomyces dermatitidis and A. capsulatus, respectively (23, 28, 29). In 1996, Sigler (40) demonstrated the development of an Ajellomyces sexual state among crossed strains of E. crescens; however, only a few strains demonstrated mating competence (with low fertility). No strains of E. parva demonstrated compatibility, and in this sense they appear to be distinct species, but no corroborating evidence has been found. Also unresolved is the degree of distinction between species of the genus Emmonsia and B. dermatitidis. With the finding of Ajellomyces sexual stages (teleomorphs) for the agents of blastomycosis and adiaspiromycosis and their location on the same clade (2, 25), there are grounds for combining the species of Emmonsia and Blastomyces within a single anamorphic genus. This proposal has been rejected (40) because of nomenclatural issues concerning the genus Blastomyces that could result in the loss of this important name in common use.
Peterson and Kurtzman (36) examined ribosomal DNA (rDNA) sequence variability between sibling species of yeasts, and among the six regions tested, they showed that only variable domain D2 of large-subunit (lsu) nuclear rDNA (5′ end) is sufficiently variable to distinguish between those sibling species. Berres et al. (1) also found this lsu region useful for analysis of closely related species of auriculariaceous basidiomycetes. Kretzer et al. (21) examined variation among Suillus species by using the internal transcribed spacer (ITS) regions of rDNA.
These two regions of the rDNA repeat unit are suitable for examining species level variability and have been used here to examine the genetic variability of Emmonsia isolates and to assess evolutionary relationships among several related taxa. DNA sequences from the ITS1, ITS2, and 5.8S rDNA regions and domains D1 and D2 of lsu rDNA were determined for strains of E. parva, E. crescens, B. dermatitidis, H. capsulatum, Paracoccidioides brasiliensis, and out group species from the families Onygenaceae and Arthrodermataceae (ca. 1,200 bp, 43 strains). These data were analyzed phylogenetically to determine whether the species of Emmonsia are distinct from one another and to provide evidence concerning the separation of the anamorphic states of Ajellomyces species into different genera.
MATERIALS AND METHODS
Fungal cultures and growth.
The fungal isolates used in this study (Table 1) are on deposit in the University of Alberta Microfungus Collection, Edmonton, Alberta, Canada. Isolates were revived from either freeze-dried or frozen (vapor phase of liquid nitrogen) samples and grown at 25°C on petri plates containing pablum cereal agar (19). All cultures were handled within a class II biological safety cabinet. After 14 to 21 days of growth, blocks (1 by 1 cm) of mycelium and agar from cultures of Emmonsia species were excised from the culture plates and transferred to sterile snap-cap polypropylene tubes (12 by 75 mm; Fisher Scientific, Nepean, Ontario, Canada). The mycelial blocks were freeze-dried by using an Edwards Moduylo freeze-dryer (Edwards High Vacuum, Burlington, Ontario, Canada). B. dermatitidis, H. capsulatum, and P. brasiliensis isolates were treated differently because of their hazard level. Instead of being freeze-dried, the blocks of agar and mycelium were placed in tubes containing a 1% thimerosal [ethyl(2-mercaptobenzoato-5)mercury sodium salt] solution for 24 h to kill the fungi.
TABLE 1.
Origins and nucleotide sequence accession numbers of isolates used in this study
| Species and strain no. | Host and/or source | GenBank nucleotide sequence accession no. |
|---|---|---|
| Emmonsia parva (Emmons & Asburn) Ciferri & Montemartini | ||
| Genotypic group 1 | ||
| UAMHa 125 | Isolated in Arizona by C. W. Emmons (no. 2370) | AF038331 |
| UAMH 130 | Isolated in Arizona by C. W. Emmons, 1946 | AF038333 |
| UAMH 434 | Isolated from soil (?) (but see Carmichael [2]), Italy, by R. Ciferri | AF038332 |
| UAMH 134 | Isolated from Neotama micropus, Texas, by C. W. Emmons (no. 2372), 1949 | AF038326 |
| Genotypic group 2 | ||
| UAMH 139 | Isolated from weasel, Ravelli County, Mont., by W. L. Jellison (no.27274) | AF038328 |
| UAMH 4489 | Isolated from bird’s nest, Lost River Canyon, Alberta, by R. Currah, 1981 | AF038327 |
| UAMH 4770 | Isolated from coyote dung, Edmonton, Alberta, by R. Currah, 1983 | AF038325 |
| UAMH 6312 | Isolated from soil, Edmonton, Alberta, by J. Newton, 1988 | AF038330 |
| UAMH 7045 | Isolated from bronchial washings, Winnipeg, Manitoba, by R. Summerbell (no. FR 0091), 1991 | AF038329 |
| Emmonsia crescens Emmons & Jellison | ||
| Genotypic group 1 | ||
| UAMH 126 | Isolated from mouse lung, Lethbridge, Alberta, by E. S. Dowding (no. 10), 1946 (=CBSb 191.55; ATCCc 10784) | AF038319 |
| UAMH 127 | Isolated from mouse lung, Peace River, Alberta, by E. S. Dowding (no. 190), 1946 (=CBS 475.77; ATCC 10785) | AF038344 |
| UAMH 128 | Isolated from mouse lung, Red Deer, Alberta, by E. S. Dowding (no. F29), 1946 | AF038345 |
| UAMH 129 | Isolated from lung of Peromyscus maniculatus borealis, Athabasca, Alberta, by E. S. Dowding (no. 184), 1947 | AF038343 |
| UAMH 132 | Isolated from lung of Peromyscus maniculatus nebrascensis, Lethbridge, Alberta, by E. S. Dowding (no. 19?) | AF038351 |
| UAMH 136 | Isolated from skunk, Lake County, Mont., by W. L. Jellison (no. 27128) | AF038341 |
| UAMH 137 | Isolated from muskrat, Lake County, Mont., by W. L. Jellison (no. 27242) | AF038342 |
| UAMH 140 | Isolated from lung of Peromyscus maniculatus, Alberta, by E. S. Dowding (no. 32), 1950 | AF038350 |
| UAMH 1067 | Isolated from lung of wild field mouse, Edmonton, Alberta, by J. W. Carmichael, 1961 | AF038346 |
| UAMH 1140 | Isolated from lung of wild field mouse, Bittern Lake, Alberta, by J. W. Carmichael, 1961 | AF038347 |
| UAMH 4076 | Isolated from greenhouse soil, Edmonton, Alberta, by J. Weijer (no. V6), 1976 | AF038348 |
| UAMH 4077 | Isolated from moldy straw bales in a mushroom house by J. Weijer (no. 2), 1975 | AF038349 |
| Genotypic group 2 | ||
| UAMH 349 | Isolated from lung of mole (Talpa europaea), Exeter, United Kingdom, by P. K. Austwick (no. V.711), 1954 | AF038336 |
| UAMH 393 | Isolated from lung of Cleithrionomys sp., Korea, by W. L. Jellison (no. 2405), 1953 | AF038339 |
| UAMH 394 | Isolated from lung of Cleithrionomys sp., Korea, by W. L. Jellison (no. 2412), 1953 | AF038340 |
| UAMH 395 | Isolated from lung of Apodemus sp., Korea, by W. L. Jellison (no. 2409), 1953 | AF038338 |
| UAMH 3008 | Ex type strain, isolated from rodent (Arvicola terrestris) lung in Norway by Emmons and Jellison (=ATCC 13704; CBS 177.60) | AF038334 |
| UAMH 7268 | Isolated from lung of common brush-tail possum (Trichosurus vulpecula), New Zealand by A. Woodgyer, 1992 | AF038337 |
| UAMH 7365 | Isolated from lung of common brush-tail possum (Trichosurus vulpecula), New Zealand, by A. Woodgyer (no. MY 92.307), 1992 | AF038335 |
| Other Emmonsia sp. isolatesd | ||
| UAMH 141 | Isolated from barnyard soil, Boone County, Mo., by R. W. Menges (no. 3; Phillips strain soil 4), 1951 (28) | AF038321 |
| UAMH 2304 | Isolated from barnyard soil, Phillips barn, Kansas, by C. W. Emmons (no. 5117) (=CBS 273.77) | AF038320 |
| UAMH 7172 | Isolated from skin lesions of HIVe-positive patient, Saskatchewan, by H. Congly (no. 215M-92), 1992 | AF038322 |
| UAMH 7425 | Isolated from granulomatous lesions on lip, hands and soft palate of otherwise healthy patient, Israel, by I. Polachek (Kemna no. 407-93), 1993 | AF038323 |
| UAMH 7426 | Isolated from granulomatous lesions on lip, hands, and soft palate of otherwise healthy patient, Israel, by I. Polachek (Kemna no. 408-93), 1993 | AF038324 |
| Ajellomyces dermatitidis McDonough & Lewis UAMH 3538 | Ex type of Ajellomyces dermatitidis (+ mating type); isolated from human infection (=ATCC 18187; CBS 673.68) | AF038355 |
| UAMH 3604 | Isolated from E. S. McDonough (no. 49A), 1968 | AF038356 |
| UAMH 5438 | Isolated from dog lung, Alberta, 1986 | AF038358 |
| Ajellomyces capsulatus (Kwon-Chung) McGinnis & Katz UAMH 3536 | Centers for Disease Control and Prevention, Atlanta, Ga. (CDC B-1392) (− mating type) | AF038354 |
| UAMH 7141 | Clinical isolate, Alberta | |
| Paracoccidioides brasiliensis Almedia & Lacaz UAMH 8037 | Isolated from lung biopsy material, human, Alberta | AF038360 |
| Auxarthron californiense Orr & Kuehn UAMH 1889 | Isolated from pack rat dung, San Diego, Calif. Aphanoascus fulvescens (Cooke) Apinis | AF038352 |
| Aphanoascus fulvescens (Cooke) Apinis UAMH 5117 | Isolated from human toe cleft, New Zealand, 1985 Trichophyton mentagrophytes (Robin) Blanchard | AF038357 |
| Trichophyton mentagrophytes (Robin) Blanchard UAMH 6256 | Isolated from human, Edmonton, Alberta | AF038359 |
University of Alberta Microfungus Collection and Herbarium, Edmonton, Alberta, Canada.
Centraalbureau voor Schimmelcultures, Baarn, The Netherlands.
American Type Culture Collection, Rockville, Md.
Strains identified previously as E. parva (40).
HIV, human immunodeficiency virus.
DNA isolation.
Freeze-dried blocks of agar and mycelium (100 mg) were placed in 1.5-ml microcentrifuge tubes and ground to a fine powder by using a 200-μl capacity pipettor tip. The fungal material was rehydrated with 500 μl of DNA extraction buffer (50 mM Tris, 10 mM EDTA, 1% sarcosyl, pH 8.0) with gentle agitation for 10 min. An equal volume of 1:1 chloroform-phenol was added to each tube, and an emulsion was formed by shaking. The emulsion was mixed for 20 min, and then the aqueous and organic phases were separated by centrifugation in a microcentrifuge for 5 min at full speed (14,000 × g). The aqueous phase was pipetted into a clean microcentrifuge tube, and 0.1 volume of 3 M sodium acetate (pH 6.0) and 1.3 volumes of ethanol were added. The tube was sealed, and the contents were mixed by inverting the tube several times. Precipitated nucleic acids were pelleted by centrifugation at full speed in a microcentrifuge for 1 min. Ethanol was decanted, and the pellet was dried by inverting the tube over absorbent paper for 5 min. Nucleic acids were dissolved in 100 μl of TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0), and 250 μl of a saturated NaI solution and 10 μl of glass milk (Gene Clean; Bio 101, Inc., La Jolla, Calif.) were added to the tube. The tube was inverted periodically, and DNA was adsorbed to the glass milk for 20 min. The glass milk was pelleted and rinsed in accordance with the manufacturer’s instructions, and the genomic DNA was eluted into 50 μl of 1/10-strength TE. DNA was stored at −20°C until used.
For the thimerosal-treated samples, excess thimerosal solution was decanted and agar blocks were placed in 1.5-ml microcentrifuge tubes. Glass beads (0.3 g; 0.5 mm in diameter) were added to each tube along with 0.5 ml of DNA extraction buffer. Cell walls were broken by vortexing the tube for 45 to 60 s at full speed on a vortex mixer. DNA extraction and purification procedures were the same as for the freeze-dried samples. To determine whether killing the fungi affected the quality of the DNA, the same protocol was applied to thimerosol-treated mycelium of 10 Aspergillus strains for which sequences have already been published. The DNA was amplified and sequenced, and the sequences were found to be the same.
Gene amplification and sequencing.
Genomic DNA (1 μl), 10 μl of 10× buffer (45), 1 μl of 50 μM primer ITS1 (45), 1 μl of 50 μM primer D2R (34), 0.5 μl (2.5 U) of Taq polymerase, 10 μl of a mixture containing each of the four deoxynucleoside triphosphates in a solution at a 1 mM concentration, and 76.5 μl of sterile deionized water (Milli-Q system) were placed in a reaction tube and overlaid with about 200 μl of mineral oil. The ca. 1,200-nucleotide fragment including ITS1, ITS2, 5.8S rDNA, and part of the 25S rDNA was amplified by 30 temperature cycles (96°C for 30 s, 53°C for 30 s, and 72°C for 150 s) followed by 10 min at 72°C. The amplified fragment was purified by using Gene Clean (Bio 101, Inc.) and eluted into 1/10-strength TE. The purified fragment was stored at −20°C until used in sequencing.
Sequencing was performed by using primers ITS1, ITS2, ITS3, ITS4, D1, D1R D2, and D2R and Applied Biosystems DyeDeoxy sequencing kits. The sequencing reaction mixture contained 1.5 pmol of primer, 200 to 400 ng of the purified DNA fragment, and sequencing reagents, in accordance with the manufacturer’s instructions. DNA sequences were read on an ABI 373 automated DNA sequencer. Alignment of overlapping and reverse complement strands to form a consensus sequence for each isolate was accomplished with an ASCII text editor. Alignment of sequences from different isolates was performed visually by using an ASCII text editor. Phylogenetic relationships of isolates were determined by using PAUP 3.1.1 (44) on a Macintosh IIcx computer. Some tests were performed by using programs from the PHYLIP package (17).
RESULTS
The suitability of combining the ITS1, ITS2, and 5.8S-plus-lsu rDNA regions into a combined data set was assessed by analyzing each region separately and comparing the trees. The search algorithm was heuristic (PAUP 3.1.1), and the reliability of each branch was estimated from bootstrap values (1,000 replicates). The resulting trees revealed no contradictory branching patterns. Because mutation rates differ in each region of the rDNA repeat unit, and because the sequence length of each region is different, trees are not expected to reveal exactly the same branching pattern. The data were combined for further analysis.
Alignment of the sequences was accomplished by visual inspection of the bases, with the addition of dashes to account for length differences. Sequences from most Emmonsia strains could be aligned easily, but two strains, UAMH 2304 and UAMH 141, previously determined as E. parva, and the outgroup species were quite different from the Emmonsia isolates and not all of their ITS1 and ITS2 regions could be satisfactorily aligned with the other strains. Unalignable regions were excised from the data set. The 5.8S-and-lsu rDNA could easily be aligned for all of the studied species.
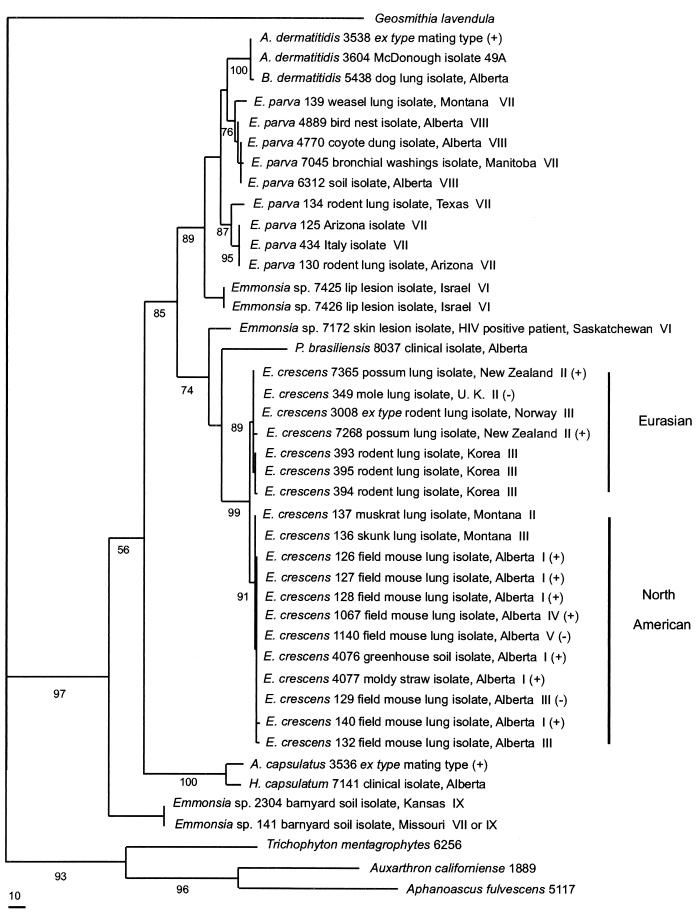
The data set with unalignable regions excised had 1,207 nucleotide positions, of which 703 were constant characters, 225 were variable but parsimony-uninformative characters, and 279 were parsimony-informative characters. Four equally parsimonious trees were found by heuristic searching (PAUP 3.1.1; random input order). The total length of each tree was 991 steps, the consistency index was 0.734, the homoplasy index was 0.266, the retention index was 0.788, and the rescaled consistency index was 0.578 for each tree. Tree topographies and branch lengths were the same for a data set that included all isolates, compared with a data set that excluded additional isolates having identical sequences. Bootstrap values were determined by using PAUP 3.1.1 (heuristic search, 1,000 replicates), and those values are placed on one of the four equally parsimonious trees (Fig. 1). The four trees differed in the lengths of the branch leading to P. brasiliensis and in the placement of B. dermatitidis strains as a sister group to one or both of the E. parva branches. The tree (Fig. 1) shows that all isolates of Emmonsia spp., A. capsulatus (H. capsulatum), P. brasiliensis, and A. dermatitidis (B. dermatitidis) are members of a single phylogenetic clade.
FIG. 1.
Phylogram representing one of the four equally parsimonious trees found in a heuristic search (PAUP 3.1.1) of the ITS1, ITS2, and 5.8S-plus-lsu rDNA sequence data. The aligned data set included 1,207 nucleotide positions for each of 43 strains. The sequence of Geosmithia lavendula was obtained from GenBank (AF033385) and was used to root the tree since this species has been phylogenetically placed among the perithecial ascomycetes (32). Unalignable segments of ITS1 and ITS2 were excised from the alignment before analysis. The number of steps between nodes is proportional to branch length, and a scale bar for 10 steps appears at the bottom. The number below each internode is the bootstrap value (1,000 replicates) for that node. Isolation data, colonial subgroups designated by roman numerals and based on growth rates and colonial features on potato dextrose agar after 28 days at 22°C (data from reference 40) and mating type (plus or minus) (40), when known, are listed to the right of the isolate numbers. B. dermatitidis isolates are a sister group to E. parva strains. E. parva strains form two phylogenetic groups closely related to B. dermatitidis. Three isolates from humans, previously identified as E. parva, are on distinct lineages and represent undescribed species. Isolates of E. crescens form a clade with two subgroupings. Strains in each subgroup were isolated from locales in Eurasia or North America. They may represent geographic variants or incipient species. Low bootstrap values on the basal branches leading to H. capsulatum and P. brasiliensis make the placement of these species in the tree equivocal. Two Emmonsia strains, UAMH 141 and UAMH 2304, previously identified as E. parva, branch basally in the tree and represent an undescribed species. HIV, human immunodeficiency virus.
The rDNA and ITS nucleotide sequences of E. crescens strains fall into one strongly supported clade (99% bootstrap value) with two closely related phylogenetic subgroups. The Eurasian group (Fig. 1, 89% bootstrap value) includes the ex type strain of E. crescens, UAMH 3008, and six other strains, UAMH 349, UAMH 393, UAMH 394, UAMH 395, UAMH 7268, and UAMH 7365. The DNA sequences of these strains were either identical to that of the ex type isolate or varied from it at no more than three nucleotide positions. These strains were isolated from animal lungs in Northern Europe, Asia, or New Zealand (Table 1). Hosts for E. crescens include small rodents that have panboreal distributions in Europe and Asia (38). The inclusion of strains from New Zealand in this clade probably reflects long-distance transport of infected animals mediated by human commerce, since all New Zealand mammals are species which have been introduced to that country, mostly from Northern Europe.
The North American E. crescens group (91% bootstrap value) includes UAMH 126, UAMH 127, UAMH 128, UAMH 129, UAMH 132, UAMH 136, UAMH 137, UAMH 140, UAMH 1067, UAMH 1140, UAMH 4076, and UAMH 4077. Seven of the strains display identical nucleotide sequences, and the other isolates vary from each other at four or fewer nucleotide positions. All strains were isolated from animal hosts or from inanimate substrates in western North America. The Eurasian and North American groups differ at 6 to 10 nucleotide positions, depending on the isolates being compared. Strains cited by Sigler (40) as sexually compatible and producing the Ajellomyces state have zero to eight nucleotide differences (Table 2) and are drawn from both phylogenetic and geographic groups of the species (Fig. 1).
TABLE 2.
E. crescens strains producing sequence ascospores in mating and numbers of nucleotide differences between paired strains
| Mating-negative UAMH strain crossed | No. of nucleotide differences from mating-positive strain:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 126 | 127 | 128 | 140 | 1067 | 4076 | 4077 | 7268 | 7365 | |
| 129 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 8 | 7 |
| 349 | 7 | 7 | 7 | 8 | 7 | 7 | 7 | 1 | 0 |
| 1140 | 0 | 0a | 0a | 1a | 0a | 0a | 0a | 8 | 7 |
Mating ability not tested.
Isolates previously identified as E. parva (Table 1) (40) are phylogenetically more diverse. Most isolates are placed into two strongly supported groups closely related to B. dermatitidis, while five other isolates are not part of either clade (Fig. 1). One set of related E. parva strains includes UAMH 139, UAMH 4489, UAMH 4770, UAMH 6312, and UAMH 7045, and these strains form a monophyletic group (76% bootstrap value). These strains were isolated from animate or inanimate substrates in Montana, Alberta, and Manitoba. A closely related second group (UAMH 134, UAMH 125, UAMH 434, and UAMH 130) was obtained from rodents in Arizona and Texas and from soil in Italy (Table 1) and appears to be monophyletic (87% bootstrap value). Each of the E. parva groups displays 0 to 13 ITS nucleotide substitutions and 0 or 1 lsu nucleotide substitution among its strains, with 0 to 24 ITS and 0 to 3 lsu differences between groups.
Three isolates from human sources are genetically distinct. UAMH 7425 and UAMH 7426, previously reported as atypical E. parva strains (Table 1) (40), were isolated from an otherwise healthy male in Israel with oral skin lesions. The strains have identical nucleotide sequences but differ from other E. parva isolates, including the other human isolate, UAMH 7172, at 30 to 59 ITS and 5 to 8 lsu nucleotide positions. These two strains were ancestral to E. parva and B. dermatitidis in all of the most parsimonious trees. Isolate UAMH 7172 was isolated as a presumptive contaminant from skin lesions on a human immunodeficiency virus-positive human (Saskatchewan) and differs from other E. parva isolates at 59 to 65 ITS and 8 to 9 lsu nucleotide positions. In Fig. 1, it branches closer to P. brasiliensis than to Emmonsia species isolates, but the low bootstrap value makes this placement equivocal. Its sequence differs from that of P. brasiliensis at 89 ITS positions and 11 lsu rDNA positions.
Isolates UAMH 141 and UAMH 2304, isolated from barnyard soils in Kansas and Missouri, are genetically vastly different from other E. parva isolates, and this distinction had strong support in bootstrap analysis. The isolates have identical sequences and differ from all other Emmonsia strains at 100 to 115 nucleotide positions in the ITS regions and 22 to 28 nucleotide positions in the lsu region (Fig. 1).
The B. dermatitidis isolates (strains UAMH 3538 and UAMH 3604 are identical, UAMH 5438 has a single nucleotide difference) form a strongly supported branch (100% bootstrap value) and are a sister group to the E. parva groups (Fig. 1). P. brasiliensis and H. capsulatum have a most recent ancestor in common with all of the Emmonsia isolates except UAMH 141 and UAMH 2304 (Fig. 1), but the lack of strong bootstrap support on the branches leading to these species means that their placement in the tree is equivocal. A significant genetic difference exists between the clinical H. capsulatum strain and the ex type mating strain of Ajellomyces capsulatus (11 ITS1 differences, 15 ITS2 differences, and 3 D2 differences). Such differences are suggestive of sibling species (35).
DISCUSSION
The genus Ajellomyces contains three heterothallic species, A. dermatitidis, A. capsulatus, and A. crescens, the anamorphs (asexual stages) of which are placed in different genera, Blastomyces, Histoplasma, and Emmonsia. Historically, the genera have been distinguished by differences in conidial features and the morphologies of structures produced in tissue (i.e., budding yeast cells or thick-walled, nonbudding adiaspores). Mating studies are of value in proving relatedness but are problematic within the group because of low fertility, poor reproducibility, and risks of working with the cultures. A molecular approach was used here to determine whether nonmating isolates of the genus Emmonsia are members of the Ajellomyces clade and to further resolve relationships between them and members of the genera Blastomyces and Histoplasma. E. crescens and E. parva were found to be distinct species, but some isolates formerly determined as E. parva were found to be genetically distinct. Although microscopic morphology varies little among Emmonsia isolates, colonial variation has long been recognized (3, 9, 13). The colonial subgroups observed by Sigler (40) (roman numerals in Fig. 1) are similar, but not identical, to the phylogenetic groupings of strains.
The two phylogenetic groups of E. crescens correlate with the continents from which the isolates were obtained. Strains from North America form one clade; strains isolated from hosts having a Eurasian panboreal distribution form a second clade. The inclusion of strains from New Zealand in this clade is enigmatic since, to our knowledge, E. crescens has not been found in soil or native animals. Two isolates were found in a species of marsupial (18) that was introduced from Australia, where E. parva, but not E. crescens, has been reported (6, 27). Johnstone et al. (18) mentioned that E. crescens may also have been the cause of adiaspiromycosis in a ferret. No mammal is indigenous to New Zealand, and many have been introduced from northern Europe, where E. crescens is well known. Interanimal transmission of adiaspiromycosis is unknown, but adiaspores can survive passage through the digestive tracts of mice, birds, and carnivores (11, 41).
Sigler’s mating data (40) help to place a taxonomic interpretation on the nucleotide substitution data. Isolates of E. crescens from a single geographic region displayed zero to two substitutions in the ITS regions and zero or one nucleotide substitution in the lsu regions. Comparisons of strains from the different geographic areas reveal five to eight ITS and one or two lsu region differences. The amount of DNA sequence divergence between the clades is similar to the amount of DNA difference observed in sexually isolated species of filamentous fungi and yeasts (26, 34–37). However, Sigler (40) found that isolates from different continents are sexually compatible, which indicates incomplete isolation. Additional mating experiments that assess viability in the F1 and F2 generations could resolve the degree of genetic isolation and provide good evidence of whether these geographically and phylogenetically defined clades represent sibling species or geographic variants (22).
Nine of the 14 isolates originally determined as E. parva were placed into two closely related groups and demonstrated phylogenetic diversity similar to that in E. crescens. One group of five closely related strains, including three authentic isolates examined by Emmons, was isolated in the northern short-grass prairie regions of North America, while another group of four related strains contains isolates from the desert southwest of the United States or from Italy (Fig. 1). The molecular data correlate, to some extent, with colonial subgroups observed by Sigler (40), who found that group VIII isolates UAMH 4489, UAMH 4770, and UAMH 6312 grew faster than group VII isolates UAMH 125, UAMH 130, UAMH 134, and UAMH 434. Carmichael (3) also recorded phenotypic variation and placed UAMH 139 in a position intermediate between subgroupings. These E. parva groups are closely related to B. dermatitidis. Guého et al. (16) reported similar findings of close relationship between authentic E. parva and B. dermatitidis isolates. There is no known sexual state in E. parva, but the phylogenetic diversity from one group to the other and to B. dermatitidis strains suggests diverging groups that may be varieties or sibling species.
Although literature reports suggest that E. crescens is the main cause of human adiaspiromycosis, primarily based on the larger size of the adiaspores observed in tissue (8, 11, 15, 24, 41), no isolate of E. crescens from a human source was available for this study, nor could we obtain for comparison the E. parva isolate described as causing disseminated infection in a patient with AIDS (12). Three isolates from human skin lesions, originally identified as E. parva, are shown here to be phylogenetically distinct and not closely related to any environmental or animal isolates of E. parva. UAMH 7172 was ancestral to E. crescens isolates and appears to represent an undescribed species. However, it requires comparison with an Emmonsia-like isolate that was recently isolated from an Italian patient also with AIDS and described as forming yeast cells in vivo (10). Guého et al. (16) showed their isolate to be phylogenetically related to E. crescens, similar to the placement of UAMH 7172. Two other slowly growing atypical isolates (40), UAMH 7425 and UAMH 7426, represent an undescribed species, according to the phylogenetic analysis. Both isolates came from oral skin lesions on a single male patient in Israel (Table 1) whose clinical and histopathological findings were suggestive of blastomycosis (20), but these isolates demonstrated morphology more consistent with that of an Emmonsia species, grew restrictedly at 37°C, failed to convert to a yeast phase in vitro, and demonstrated a negative exoantigen test with B. dermatitidis antiserum. The only other human isolate, UAMH 7045 (from bronchial washings in Manitoba), was included in a group of North American environmental isolates of E. parva (Fig. 1). It is notable that all of the human isolates examined in this study fall outside of the E. crescens clade, with pathogenicity known only for the Israeli isolates.
In the parsimony tree (Fig. 1), B. dermatitidis has a most recent ancestor in common with E. parva but is distinct from that species. Similar findings of phylogenetic relatedness between single isolates of B. dermatitidis and E. parva have been reported in prior studies (2, 25), but a direct comparison cannot be made because some of the isolates examined have been reidentified. Bowman and Taylor (2) examined UAMH 1067, and Leclerc et al. (25) used CBS 191.55 (=UAMH 126), both listed as E. parva, but both of these isolates have now been determined to be E. crescens (Table 1). Our study (Fig. 1) placed B. dermatitidis strains and E. parva isolates in the same clade, with high bootstrap support (89%), and E. crescens on a distinct branch of the tree. The study of Guého et al. (16) showed results similar to ours, but again two of their E. parva isolates (CBS 191.55 = UAMH 126 and CBS 475.77 = UAMH 127) have been reidentified as E. crescens (Table 1).
The distant phylogenetic position of isolates UAMH 141 and UAMH 2304, both from barnyard soils in the central United States, is surprising since they are basal to all other members of the clade. The isolates have identical sequences but differ from all other Emmonsia strains at 100 to 115 nucleotide positions in the ITS regions and 22 to 28 nucleotide positions in the lsu regions. These data prompted a reexamination of morphological data not reported in Sigler’s previous study (40). UAMH 141 was slower growing on potato dextrose agar (40-mm diameter after 28 days at 22°C) than members of colonial subgroup VII, including UAMH 125, UAMH 130, UAMH 134, and UAMH 434 (average diameter, 53 mm), differed in colonial features, and produced larger adiaspores than other strains of E. parva. Carmichael (3) suggested that the species identification of this strain was in doubt. The colonial features of UAMH 2304 appeared to be intermediate between those of group VII and strain UAMH 141. Additionally, it produced slightly larger conidia and differed from all others in expressing acidity on bromcresol purple-milk solids-glucose agar (19). There is a possibility that these strains have a common origin. UAMH 141 is referred to as “Philips strain” in the study by Menges and Habermann (31). Although isolated in Missouri, the strain was used for skin testing of animals in Kansas. UAMH 2304 was received as “soil, Phillips barn, Kansas.”
The other two systemic human pathogens, H. capsulatum and P. brasiliensis, cannot be placed in the tree with any statistical certainty, except to say that they belong in the same clade with the Emmonsia species and B. dermatitidis. Because P. brasiliensis is in a clade that contains Ajellomyces sexual states, it is likely that if a teleomorph is found for P. brasiliensis, it will be an Ajellomyces species. The data of Guého et al. (16) also show that Blastomyces, Histoplasma, Paracoccidioides, and Emmonsia are part of a strongly supported clade but that the relationships of these four groups are unknown. Our findings are also similar to theirs in placing members of the Ajellomyces clade distant from out group species belonging to the families Onygenaceae (Auxarthron californiense and Aphanoascus fulvescens) and Arthrodermataceae (Trichophyton mentagrophytes). The muriculate ascospores of all Ajellomyces species appear to set them apart and are known elsewhere in the order Onygenales only in the genus Polytolypa (40).
Because the three species with Ajellomyces teleomorphic states occur in the same clade, as shown in this study (Fig. 1) and other studies (16), there are grounds for placing all of the anamorphic states in the same genus. Arguments supporting this approach have been made previously (4), but the genera have been retained because (i) phenotypic differences can be recognized, (ii) the names are in widespread use, and (iii) emphasis has been placed historically on the differences in their in vivo forms. In other genera, where different anamorphic states occur among species from the same teleomorphic genus (e.g., Talaromyces with anamorphs in Geosmithia and Penicillium [39]), identification of strains is aided by recognition of the phenotypic differences displayed by the anamorphs. The same rationale could be applied to the retention of separate anamorphic names for these members of the Ajellomyces clade. However, with the results of this study, which shows that isolates of E. parva are closer to B. dermatitidis than to E. crescens, and with the discovery that human-associated Emmonsia-like isolates have a propensity to form yeast cells in tissue (this study, 10, 20), there appears to be little basis for maintaining Blastomyces and Emmonsia as separate genera. Blastomyces is the older and best-known name, and its retention is important for the maintenance of a stable nomenclature. Normally, it would be a simple matter to transfer Emmonsia species, as has recently been suggested (16). However, an impediment to this proposal comes from the fact that the genus Blastomyces Gilchrist and Stokes is invalid under the International Code of Botanic Nomenclature and requires conservation first. A proposal for conservation is being initiated.
ACKNOWLEDGMENTS
We thank I. Polachek, I. F. Salkin, and A. Woodgyer for sending interesting isolates. S.P. gratefully acknowledges the skilled technical assistance of Paul A. Bonneau.
L.S. gratefully acknowledges operating grants from the Natural Sciences and Engineering Research Council of Canada and the University of Alberta Small Faculties Fund Support for the Advancement of Scholarship.
REFERENCES
- 1.Berres M E, Szabo L J, McLaughlin D J. Phylogenetic relationships in auriculariaceous basidiomycetes based on 25S ribosomal DNA sequences. Mycologia. 1995;87:821–840. [Google Scholar]
- 2.Bowman B H, Taylor J W. Molecular phylogeny of pathogenic and nonpathogenic Onygenales. In: Reynolds D R, Taylor J W, editors. The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics—1993. Wallingford, United Kingdom: CAB International; 1993. pp. 169–178. [Google Scholar]
- 3.Carmichael J W. The pulmonary fungus Haplosporangium parvum. II. Strain and generic relationships. Mycologia. 1951;43:605–624. [Google Scholar]
- 4.Carmichael J W. Chrysosporium and some other aleuriosporic hyphomycetes. Can J Bot. 1962;40:1137–1173. [Google Scholar]
- 5.Ciferri R, Montemartini A. Taxonomy of Haplosporangium parvum. Mycopathol Mycol Appl. 1959;10:303–316. doi: 10.1007/BF02051638. [DOI] [PubMed] [Google Scholar]
- 6.Connole M D. Review of animal mycoses in Australia. Mycopathologia. 1990;111:133–164. doi: 10.1007/BF02282798. [DOI] [PubMed] [Google Scholar]
- 7.Currah R S. Taxonomy of the Onygenales: Arthrodermataceae, Gymnoascaceae, Myxotrichaceae, Onygenaceae. Mycotaxon. 1985;24:1–216. [Google Scholar]
- 8.de Almeida Barbosa A, Moreira Lemos A C, Severo L C. Acute pulmonary adiaspiromycosis. Report of three cases and a review of 16 other cases collected from the literature. Rev Iberoam Micol. 1997;14:177–180. [PubMed] [Google Scholar]
- 9.Dowding E S. The pulmonary fungus Haplosporangium parvum, and its relationship with some human pathogens. Can J Res Sect E Med Sci. 1947;25:195–206. doi: 10.1139/cjr47e-020. [DOI] [PubMed] [Google Scholar]
- 10.Drouhet E, Guého E, Gori S, Huerre M, Borgers M, Dupont B. Mycological, ultrastructural and experimental aspects of a new dimorphic fungus, isolated from a cutaneous disseminated mycosis in AIDS. Salsomaggiore, Italy: International Society for Human and Animal Mycology; 1997. p. 372. [Google Scholar]
- 11.Dvorak J, Otcenasek M, Rosicky B. Adiaspiromycosis caused by Emmonsia crescens Emmons and Jellison 1960. Acta Univ Palacki Olomuc Fac Med. 1973;70:1–120. [Google Scholar]
- 12.Echaverria E, Cano E L, Restrepo A. Disseminated adiaspiromycosis in a patient with AIDS. J Med Vet Mycol. 1993;31:91–97. doi: 10.1080/02681219380000101. [DOI] [PubMed] [Google Scholar]
- 13.Emmons C S, Jellison W L. Emmonsia crescens sp. n. and adiaspiromycosis (haplomycosis in mammals) Ann N Y Acad Sci. 1960;89:91–101. doi: 10.1111/j.1749-6632.1960.tb20133.x. [DOI] [PubMed] [Google Scholar]
- 14.Emmons C W, Ashburn L L. The isolation of Haplosporangium parvum n. sp. and Coccidioides immitis from wild rodents. Public Health Rep. 1942;57:1715–1727. [Google Scholar]
- 15.England D M, Hochholzer L. Adiaspiromycosis: an unusual fungal infection of the lung. Am J Surg Pathol. 1993;17:876–886. [PubMed] [Google Scholar]
- 16.Guého E, Leclerc M C, de Hoog G S, Dupont B. Molecular taxonomy and epidemiology of Blastomyces and Histoplasma species. Mycoses. 1997;40:69–81. doi: 10.1111/j.1439-0507.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 17.Felsenstein J. PHYLIP (phylogeny inference package) version 3.5c. Seattle: Department of Genetics, University of Washington; 1993. . (Distributed by the author.) [Google Scholar]
- 18.Johnstone A C, Hussein H M, Woodgyer A. Adiaspiromycosis in suspected cases of pulmonary tuberculosis in the common brush-tail possum (Trichosurus vulpecula) N Z Vet J. 1993;41:175–180. doi: 10.1080/00480169.1993.35765. [DOI] [PubMed] [Google Scholar]
- 19.Kane J, Summerbell R C, Sigler L, Krajden S, Land G. Laboratory handbook of dermatophytes. A clinical guide and laboratory manual of dermatophytes and other filamentous fungi from skin, hair and nails. Belmont, Calif: Star Publishing Co.; 1997. [Google Scholar]
- 20.Kemna M E, Weinberger M, Sigler L, Zeltser R, Polachek I, Salkin I F. Abstracts of the 94th General Meeting of the American Society for Microbiology 1994. Washington, D.C: American Society for Microbiology; 1994. A primary oral blastomycosis-like infection in Israel, abstr. F-75; p. 601. [Google Scholar]
- 21.Kretzer A, Li Y, Szaro T, Bruns T D. Internal transcribed spacer sequences from 38 recognized species of Suillus sensu lato: phylogenetic and taxonomic implications. Mycologia. 1996;88:776–785. [Google Scholar]
- 22.Kurtzman C P, Smiley M J, Johnson C J. Emendation of the genus Issatchenkia Kudriavzev and comparison of species by deoxyribonucleic acid reassociation, mating reaction, and ascospore ultrastructure. Int J Syst Bacteriol. 1980;30:503–513. [Google Scholar]
- 23.Kwon-Chung K J. Emmonsiella capsulata: perfect state of Histoplasma capsulatum. Science. 1972;177:368–369. doi: 10.1126/science.177.4046.368. [DOI] [PubMed] [Google Scholar]
- 24.Kwon-Chung K J, Bennett J W. Medical mycology. Malvern, Pa: Lea & Febiger; 1992. [Google Scholar]
- 25.Leclerc M C, Philippe H, Guèho E. Phylogeny of dermatophytes and dimorphic fungi based on large subunit ribosomal RNA sequence comparison. J Med Vet Mycol. 1994;32:331–341. doi: 10.1080/02681219480000451. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Kurtzman C P. Phylogenetic relationships among species of Williopsis and Saturnospora gen. nov. as determined from partial rRNA sequences. Antonie van Leeuwenhoek. 1991;60:21–30. doi: 10.1007/BF00580437. [DOI] [PubMed] [Google Scholar]
- 27.Mason R W, Gauhwin M. Adiaspiromycosis in South Australian hairy-nosed wombats. J Wildl Dis. 1982;18:3–8. doi: 10.7589/0090-3558-18.1.3. [DOI] [PubMed] [Google Scholar]
- 28.McDonough E S, Lewis A L. Blastomyces dermatitidis: production of the sexual stage. Science. 1967;156:528–529. doi: 10.1126/science.156.3774.528. [DOI] [PubMed] [Google Scholar]
- 29.McGinnis M R, Katz B. Ajellomyces and its synonym Emmonsiella. Mycotaxon. 1979;8:157–164. [Google Scholar]
- 30.McGinnis, M. R., L. Sigler, B. H. Bowman, M. Masuda, and C. J. K. Wang. 1992. Impact of conidiogenesis, teleomorph connections, pleomorphism and molecular genetics on evolving hyphomycete systematics. J. Med. Vet. Mycol. 29(Suppl):261–270. [DOI] [PubMed]
- 31.Menges R W, Habermann R T. Isolation of Haplosporangium parvum from soil and results of experimental inoculations. Am J Hyg. 1954;60:106–116. doi: 10.1093/oxfordjournals.aje.a119708. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa H, Yoshimura A, Sugiyama J. Polyphyletic origins of species of the anamorphic genus Geosmithia and the relationship of the cleistothecial genera: evidence from 18S, 5S and 28S rDNA sequence analysis. Mycologia. 1997;89:756–771. [Google Scholar]
- 33.Pan S, Sigler L, Cole G T. Evidence for a phylogenetic connection between Coccidioides immitis and Uncinocarpus reesii (Onygenaceae) Microbiology. 1994;140:1481–1494. doi: 10.1099/00221287-140-6-1481. [DOI] [PubMed] [Google Scholar]
- 34.Peterson S W. Molecular genetic assessment of relatedness of Penicillium subgenus Penicillium. In: Reynolds D R, Taylor J W, editors. The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics—1993. Wallingford, United Kingdom: CAB International; 1993. pp. 121–128. [Google Scholar]
- 35.Peterson S W. Species concepts, molecular systematics and the rapid identification of toxigenic hyphomycetes. In: Eklund M, Richard J L, Mise K, editors. Molecular approaches to food safety—1995. Fort Collins, Colo: Alaken, Inc.; 1995. pp. 3–18. [Google Scholar]
- 36.Peterson S W, Kurtzman C P. Ribosomal RNA sequence divergence among sibling species of yeasts. Syst Appl Microbiol. 1991;14:124–129. [Google Scholar]
- 37.Peterson S W, Logrieco A. Ribosomal RNA sequence variation among interfertile strains of some Gibberella species. Mycologia. 1991;83:397–402. [Google Scholar]
- 38.Piechocki R. Mouse-like rodents. In: Grzimek H C B, editor. Grzimek’s animal life encyclopedia. Vol. 11. New York, N.Y: Van Nostrand Reinhold Company; 1975. pp. 296–406. [Google Scholar]
- 39.Pitt J I. Geosmithia gen. nov. for Penicillium lavendula and related species. Can J Bot. 1979;57:2021–2030. [Google Scholar]
- 40.Sigler L. Ajellomyces crescens sp. nov., taxonomy of Emmonsia spp., and relatedness with Blastomyces dermatitidis (teleomorph Ajellomyces dermatitidis) J Med Vet Mycol. 1996;34:303–314. [PubMed] [Google Scholar]
- 41.Sigler L. Agents of adiaspiromycosis. In: Ajello L, Hay R, editors. Topley & Wilson’s microbiology and microbial infections. 9th ed. Vol. 4. London, United Kingdom: Arnold; 1998. pp. 571–583. [Google Scholar]
- 42.Sigler L, Carmichael J W. Taxonomy of Malbranchea and some other hyphomycetes with arthroconidia. Mycotaxon. 1976;4:349–488. [Google Scholar]
- 43.Sigler, L., A. L. Flis, and J. W. Carmichael. The genus Uncinocarpus (Onygenaceae) and its synonym Brunneospora: new concepts, combinations and connections to anamorphs in Chrysosporium, and further evidence of relationship with Coccidioides immitis. Can. J. Bot., in press.
- 44.Swofford D L. PAUP: phylogenetic analysis using parsimony, version 3.1.1. Urbana: Illinois Natural History Survey; 1993. [Google Scholar]
- 45.White T J, Bruns T D, Lee S B, Taylor J W. Amplification and direct sequencing of fungal ribosomal DNA for phylogenetics. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols: a guide to the methods and applications. New York: Academic Press, Inc.; 1990. pp. 315–322. [Google Scholar]