Abstract
BACKGROUND: Heart failure with preserved ejection fraction (HFpEF) imposes a high disease burden on patients, primarily because of multimorbidity and frequent hospitalizations. Recently, the American College of Cardiology Expert Consensus recommended treating all patients diagnosed with HFpEF with a sodium-glucose cotransporter 2 inhibitor, such as dapagliflozin or empagliflozin, to reduce the risk of cardiovascular death and hospitalization and improve health status. However, managing HFpEF can be expensive, highlighting the need to assess therapeutic alternatives that can minimize health care costs while optimizing patient outcomes.
OBJECTIVE: To compare the cost-effectiveness of dapagliflozin vs empagliflozin in managing patients with HFpEF from the US health care system perspective.
METHODS: We developed a Markov model to simulate a cohort of patients with HFpEF (defined as having a left ventricular ejection fraction ≥ 50%) treated with dapagliflozin or empagliflozin. Transition probabilities between 3 health states (HFpEF, hospitalization for heart failure, and death), costs, and quality of life weight input variables were obtained from the literature. In the base-case analysis, we estimated total expected costs, quality-adjusted life-years (QALYs) gained, and the incremental cost-effectiveness ratio (ICER) over a lifetime horizon. All future expected costs and QALYs were discounted at the annual rate of 3%. We conducted sensitivity analyses to demonstrate the robustness of the cost-effectiveness model findings.
RESULTS: Dapagliflozin had an incremental expected lifetime cost of $29,896 compared with empagliflozin, resulting in an ICER of $36,902/QALY. Value-based price threshold analysis suggested that for empagliflozin to be cost-effective, it would need a 29% discount on its annual price. In a probabilistic sensitivity analysis, dapagliflozin would be the most preferred cost-effective option at willingness-to-pay thresholds of $50,000/QALY about 72% of the time.
CONCLUSIONS: This cost-effectiveness analysis showed that, from the US health care system perspective, dapagliflozin was more cost-effective than empagliflozin, and its uptake may enhance long-term outcomes in patients with HFpEF.
Plain language summary
We created a model to simulate the lifetime progression of patients with heart failure and preserved ejection fraction with the goal of evaluating the cost-effectiveness of dapagliflozin compared with empagliflozin from the perspective of the US health care system. The study demonstrates that dapagliflozin was cost-effective over a lifetime compared with empagliflozin.
Implications for managed care pharmacy
By comparing the relative cost-effectiveness of 2 sodium-glucose cotransporter 2 inhibitors, this study helps managed care decision-makers in the judicious use of health care resources while choosing among alternative drugs.
Heart failure with preserved ejection fraction (HFpEF) is a growing concern in the United States accounting for more than 50% of all cases of HF.1,2 The incidence of HFpEF is rising, especially among older adults, and it is associated with high morbidity, mortality, and health care resource use.3-6 Although effective therapies have been developed for HF with reduced ejection fraction (HFrEF), therapies for HFpEF remain elusive.7,8 However, recent clinical trials have demonstrated the effectiveness of sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as empagliflozin and dapagliflozin in managing HFpEF.9-11
The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) trial and the Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) trial showed that these SGLT2 inhibitors significantly reduced cardiovascular death and HF hospitalization compared with the prior standard of care alone in patients with HFpEF.10-13 In light of these findings, the 2023 American College of Cardiology (ACC) expert consensus has recommended that all individuals with a diagnosis of HFpEF should be treated with an SGLT2 inhibitor, except in cases of contraindication.13 Although the cost of adding SGLT2 inhibitors like empagliflozin to the prior standard of care for HFpEF is higher, some studies have evaluated its cost-effectiveness compared with the prior standard of care alone.14,15 However, there are currently no studies comparing the relative lifetime cost-effectiveness of empagliflozin vs dapagliflozin from the US health care perspective.
This study aims to assess the relative cost-effectiveness of empagliflozin vs dapagliflozin in managing HFpEF from the US health care perspective. This study is crucial in determining the most cost-effective option to manage HFpEF, considering the economic burden associated with this condition. By providing evidence of cost-effectiveness, this study can guide clinicians and policymakers in making informed decisions about the management of HFpEF.
Methods
OVERVIEW
We used a state-transition Markov cohort model to perform a cost-effectiveness analysis comparing dapagliflozin and empagliflozin in patients with HFpEF, following the guidance of the US Second Panel of Cost-Effectiveness in Health and Medicine.16-19 To ensure the transparent reporting of our findings, we followed the recommendations outlined in the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022).20 As this was a simulation modeling study that used aggregate secondary data sources from published studies as model inputs, institutional review board approval or consent was not required.
MODEL STRUCTURE AND ASSUMPTIONS
The Markov model comprised 3 distinct health states, HFpEF (defined as left ventricular ejection fraction [LVEF] ≥ 50%), hospitalization for HF (HHF), and death (Figure 1).18,19,21-23 Patients with HFpEF enter the model at the age of 65 and could transition to HHF or die. Those in the HHF state could be discharged from the hospital and return to the HFpEF state or die. We assumed patients could die because of cardiovascular and noncardiovascular causes. The model simulates how patients change or remain in a state over a lifetime horizon by recursively applying annual transition probabilities.
FIGURE 1.

Illustration of the Health State–Transition Markov Model
MODEL PARAMETER ESTIMATION
Transition Probabilities. To obtain relevant articles and estimate transition probabilities, we performed a methodic search of PubMed using keywords such as “heart failure,” “heart failure with preserved ejection fraction,” “HFpEF,” “sodium-glucose cotransporter-2 inhibitors,” “SGLT2 inhibitor,” “dapagliflozin,” “empagliflozin,” “randomized controlled trial,” “network-meta-analysis,” and “meta-analysis.” In addition, we identified and used the most recent and comprehensive network meta-analysis (NMA) of the comparative efficacy and safety of SGLT2 inhibitors and the DELIVER trial as sources for transition probabilities input.9,11 The relative risk estimates reported in the NMA were converted into probabilities using the formula described by Gidwani et al.24 Finally, we used the most recent publication of the US Life Table as the source of input for dying because of any cause for patients in HFpEF and HHF health states.25
Costs and Utilities. We used the RedBook (Truven Health Analytics) to obtain annual cost inputs for dapagliflozin (10mg daily) and empagliflozin (10mg daily).26 In addition, we obtained treatment-related costs and quality of life weights (utility values) for patients with HFpEF and HHF health states from the published literature.15,27 All model input parameters are reported in Supplementary Table 1 (available in online article).
MODEL OUTCOMES ANALYSES
Base-Case Analyses. The base-case analysis estimated the expected lifetime costs (expressed in 2022 US dollar value), expected lifetime quality-adjusted life-years (QALYs) gained, incremental cost-effectiveness ratios (ICERs), and expected net monetary benefits (NMBs) from the perspective of the US health care system.28 We applied an annual discount rate of 3% to both future costs and QALYs.16,29-31 ICERs were calculated as the incremental cost divided by the incremental QALY between the 2 treatment alternatives.32 To calculate the expected NMB of each drug,33,34 we multiplied the QALY by the willingness-to-pay (WTP) threshold and subtracted the total expected lifetime cost of treatment from the resulting estimate.
We considered WTP thresholds commonly used in the United States ($50,000, $100,000, and $150,000 per QALY) in determining the cost-effective treatment.35-38 We used the ACC/American Heart Association (AHA) guidance to characterize the value of a treatment relative to another as high value where the ICER is less than $50,000 per QALY and low value where the ICER is more than $150,000 per QALY.35 Using NMBs, we considered a treatment to be cost-effective compared with another if it had a higher NMB.
In addition to determining the most cost-effective treatment, we also performed a value-based price threshold analysis to determine at what price the treatment that is not cost-effective would be cost-effective.39,40 We conducted all analyses using the hēRo3 platform, a web-based, open-source modeling platform (hēRo LLC).
Sensitivity Analyses. We conducted 2 types of sensitivity analyses to assess the robustness of our model results. Firstly, we performed deterministic sensitivity analysis (DSA) by varying 1 model parameter at a time, such as transition probabilities, costs, or utilities, while keeping others constant. This allowed us to determine the impact of each parameter on the incremental NMB of the treatments compared.41 We used a tornado diagram to visualize the results of the DSA showing how the incremental NMB is influenced as each parameter was varied within a plausible range of values.
Secondly, we conducted probabilistic sensitivity analysis to account for the uncertainty around all model parameters concurrently. To do this, we assigned probability distributions to all input parameters and conducted 1,000 Monte Carlo simulations. For each simulation, we calculated the expected costs, expected QALYs, and ICERs of the treatments to determine the probability that a treatment would be the most preferred cost-effective option.42,43 We used the λ distribution for cost and utility inputs, as this distribution is defined for positive values from 0 to infinity, and the β distribution for transition probabilities because this distribution is limited between 0 and 1.44
Results
BASE-CASE RESULTS
In the base-case analysis, it was found that dapagliflozin and empagliflozin were positioned on the efficiency frontier, indicating that either can be cost-effective treatment options for patients with HFpEF depending on the threshold for WTP (Figure 2). Over a lifetime time horizon, dapagliflozin had the higher expected cost ($191,202 vs $161,306) and expected QALY (4.953 vs 4.143) compared with empagliflozin. As a result, the incremental expected lifetime cost of dapagliflozin relative to empagliflozin was $29,896, resulting in an ICER of $36,902/QALY. Across all the different WTP thresholds ($50,000, $100,000, and $150,000/QALY), dapagliflozin had the highest expected NMB compared with empagliflozin, indicating that it was the most cost-effective treatment alternative in patients with HFpEF (Table 1).
FIGURE 2.

Cost-Effectiveness Plane
TABLE 1.
Cost-Effectiveness of Dapagliflozin and Empagliflozin for Treating Heart Failure With Preserved Ejection Fraction in the United States
| Treatment | QALY | Cost, USD | Comparator | ICER, USD | NMB at WTP of $50,000/QALY | NMB at WTP of $100,000/QALY | NMB at WTP of $150,000/QALY |
|---|---|---|---|---|---|---|---|
| Empagliflozin | 4.143 | 161,306 | — | — | 45,837 | 252,980 | 460,122 |
| Dapagliflozin | 4.953 | 191,202 | Empagliflozin | 36,902 | 56,449 | 304,100 | 551,750 |
Costs were rounded to the nearest whole number while QALYs were rounded to 3 decimal places. The ICER and NMBs were estimated as values prior to rounding the QALYs and costs.
ICER = incremental cost-effectiveness ratio; NMB = net monetary benefit; QALY = quality-adjusted life-year; WTP = willingness-to-pay; USD = US dollar.
The value-based price threshold analysis suggested that empagliflozin may need to be priced at $4,933 annually (corresponds to a 29% drop on its current price annually) at a WTP threshold of $50,000 per QALY to be the most cost-effective compared with dapagliflozin (Figure 3).
FIGURE 3.

Value-Based Price Threshold Analysis
Sensitivity Analyses. The DSA indicated that the incremental NMB of dapagliflozin vs empagliflozin was most sensitive to model parameters such as the prices of dapagliflozin and empagliflozin and the probability of transitioning to a death state from stable HFpEF while on dapagliflozin or empagliflozin (Supplementary Figure 1).
On the other hand, the probabilistic sensitivity analysis results showed that dapagliflozin had a higher probability of being cost-effective relative to empagliflozin at WTP thresholds between $50,000 and $150,000 per QALY (Figure 4). For example, at a WTP of $50,000/QALY, dapagliflozin had a 72.3% probability of being cost-effective, whereas empagliflozin had only a 27.7% probability of being cost-effective on the cost-effectiveness plane (Supplementary Figure 2).
FIGURE 4.
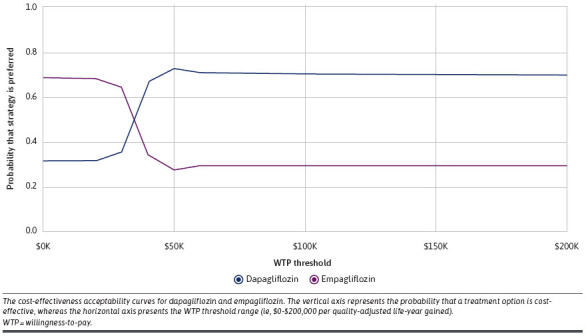
Cost-Effectiveness Acceptability Curve
Discussion
This study found that the expected lifetime cost of dapagliflozin in patients with HFpEF was higher than that of empagliflozin, and dapagliflozin use resulted in greater expected QALYs gained. The ICER comparing dapagliflozin with empagliflozin in treating patients with HFpEF at WTP thresholds is suggestive of a high-value medication per ACC/AHA guidance (< $50,000/QALY).35 Empagliflozin can be cost-effective relative to dapagliflozin if discounted at 29% of its current annual price.
To our knowledge, no study has compared the lifetime cost-effectiveness of dapagliflozin with empagliflozin from the US health care perspective before this study. A previous study compared the cost-effectiveness of empagliflozin with the standard of care from a US health system perspective and found that empagliflozin was not cost-effective even at a WTP of $150,000/QALY.15 This observation was attributed to the lack of empagliflozin’s effectiveness on noncardiovascular endpoints in HFpEF.15 Conversely, in another cost-effectiveness study conducted from the perspective of the Australian health care system, empagliflozin was found to be cost-effective compared with the standard of care alone in treating HFpEF.14 Although slightly lower, this study estimated the QALYs gained for empagliflozin comparable with those reported in the 2 previous cost-effectiveness studies.14,15
Although a recent cost-effectiveness analysis has suggested that adding an SGLT2 inhibitor to the prior standard of care in US adults with HFpEF was of intermediate or low economic value compared with standard of care alone,45,46 our study provides evidence of the relative value of the 2 SGLT2 inhibitors that have been evaluated in clinical trials. The results of the DELIVER trial made it possible for this study to assess the cost-effectiveness of dapagliflozin as another treatment option for patients with HFpEF.10,11 Our study added to the results of DELIVER trial and showed dapagliflozin to be more cost-effective than empagliflozin.10 Although treatment with dapagliflozin incurred an additional lifetime cost, this was offset by a greater gain in QALY than empagliflozin and an ICER suggestive of a medication with a higher economic value.35 The QALYs gained following treatment with dapagliflozin in this study are comparable to estimates from previous studies on HFrEF.47,48 This might be related to the fact that some patients across the spectrum of ejection fraction have a similar survival rate.49,50 Greater QALYs for dapagliflozin than empagliflozin in this study are supported with the consistent benefit of dapagliflozin for patients above and below an LVEF of 60% seen in the DELIVER trial.10 In contrast, the EMPEROR-Preserve trial showed that patients with LVEFs of 60% and higher treated with empagliflozin showed an attenuated response in a subgroup analysis.10,11
The 2022 AHA/ACC/Heart Failure Society of America guideline for managing HF recommended SGLT2 inhibitors in patients with HFpEF (class 2a [moderate] recommendation).51 This recommendation was supported by a 2023 ACC expert consensus suggesting that individuals with a diagnosis of HFpEF should receive an SGLT2 inhibitor unless there is a contraindication.13 Consequently, patients and clinicians need to make a choice between the 2 SGLT2 inhibitors with evidence from clinical trials to support their use, taking into account factors such as efficacy, safety/tolerability, and costs. Given the enormous economic burden associated with managing HFpEF, findings from this study can be used in decisions that consider which drug gives the greatest lifetime economic value. Moreover, because HFpEF is a chronic disease requiring lifelong management, studies like this enable patients and clinicians to make informed decisions that best optimize lifetime patient outcomes. Finally, as the out-of-pocket cost/insurance copays can influence a decision on treatment choices, findings from this study suggested that a discount on the current annual price of empagliflozin will improve its value relative to dapagliflozin and, ultimately, improve access to another effective medication recommended by the clinical guidelines.
LIMITATIONS
This study’s findings should be interpreted while considering the following limitations. First, effectiveness inputs, such as transition probabilities were obtained from the results of clinical trials. Second, we could not determine how patients’ adherence and tolerance affected outcomes, so we assumed adherence as expected in clinical trials. However, we do not expect our conclusion on the cost-effectiveness evidence to change unless there is a significant difference in adherence and tolerance between the 2 medications. Third, because of the high internal and limited external validity of most randomized clinical trials, the results of these trials may not accurately reflect what happens in the real world. Fourth, although an NMA demonstrated that SGLT2 inhibitors were effective in treating HF, regardless of ejection fraction or diabetes status, this cost-effectiveness analysis was limited to patients with HFpEF irrespective of having diabetes or not.11 Fifth, even though comorbidities are common in patients with HFpEF, we could not provide such evidence of cost-effectiveness by type and number of comorbid conditions because there are limited model input data from the literature. Sixth, although the clinical practice guideline recommended the SGLT2 inhibitor class, this study only compared the cost-effectiveness of 2 drugs in the class because they are the only treatment options for which clinical trials demonstrated their efficacy. There is no trial evidence to conduct a cost-effectiveness of canagliflozin and sotagliflozin (a dual SGLT1 and SGLT2 inhibitor). Lastly, because this study is conducted from the perspective of the US health care system, the results will not typically reflect what happens in other parts of the world, bearing in mind the variations in the cost of care and medical practice in different countries.
In addition to the limitations outlined, it is important to note that certain insurances or health care systems may negotiate specific pricing that favors one drug over the other. This may impact the relative value of the 2 drugs, for our value-based price threshold analysis was conducted based on the list prices of the drugs. In situations where such pricing negotiations have occurred, the value of the drugs may differ from what was suggested by our analysis. For example, if empagliflozin has been discounted by a rate equal to or greater than what our value-based price suggests, it could be considered of higher value. Therefore, additional research may be needed to account for such pricing agreements.
Conclusions
This study demonstrated that, from the perspective of the US health care system, dapagliflozin offers greater economic value than empagliflozin at commonly used cost-effectiveness thresholds. However, empagliflozin could still be deemed cost-effective if its current price is discounted. These findings provide valuable insights for decision-making regarding resource allocation, and further research should explore the impact of different pricing strategies on the cost-effectiveness of both medications, accounting for various contextual factors.
REFERENCES
- 1.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-19. doi:10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135(10):e146-603. doi:10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591-602. doi:10.1038/nrcardio.2017.65 [DOI] [PubMed] [Google Scholar]
- 4.Mureddu GF, Agabiti N, Rizzello V, et al. Prevalence of preclinical and clinical heart failure in the elderly. A population-based study in Central Italy. Eur J Heart Fail. 2012;14(7):718-29. doi:10.1093/eurjhf/hfs052 [DOI] [PubMed] [Google Scholar]
- 5.Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996-1004. doi:10.1001/jamainternmed.2015.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsao CW, Lyass A, Enserro D, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6(8):678-85. doi:10.1016/j.jchf.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-726. doi:10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 8.Shah SJ, Borlaug BA, Kitzman DW, et al. Research priorities for heart failure with preserved ejection fraction: National heart, lung, and blood institute working group summary. Circulation. 2020;141(12):1001-26. doi:10.1161/CIRCULATIONAHA.119.041886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou X, Shi Q, Vandvik PO, et al. Sodium-glucose cotransporter-2 inhibitors in patients with heart failure : A systematic review and meta-analysis. Ann Intern Med. 2022;175(6):851-61. doi:10.7326/M21-4284 [DOI] [PubMed] [Google Scholar]
- 10.Dapagliflozin evaluation to improve the lives of patients with preserved ejection fraction heart failure. (DELIVER). ClinicalTrials.gov identifier NCT03619213. Updated April 27, 2022. Accessed December 20, 2022. https://clinicaltrials.gov/ct2/show/NCT03619213
- 11.Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089-98. doi:10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 12.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-61. doi:10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 13.Kittleson MM, Ruberg FL, Ambardekar AV, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: A report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2023;81(11):1076-126. doi:10.1016/j.jacc.2022.11.022 [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Liew D, Kaye DM, Zoungas S, Stub D. Cost-effectiveness of empagliflozin in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Qual Outcomes. 2022;15(10):e008638. doi:10.1161/CIRCOUTCOMES.121.008638 [DOI] [PubMed] [Google Scholar]
- 15.Zheng J, Parizo JT, Spertus JA, Heidenreich PA, Sandhu AT. Cost-effectiveness of empagliflozin in patients with heart failure with preserved ejection fraction. JAMA Intern Med. 2022;182(12):1278-88. doi:10.1001/jamainternmed.2022.5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-103. doi:10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 17.Neumann PJ, Sanders GD. Cost-effectiveness analysis 2.0. N Engl J Med. 2017;376(3):203-5. doi:10.1056/NEJMp1612619 [DOI] [PubMed] [Google Scholar]
- 18.Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: A report of the ISPOR-SMDM modeling good research practices task force--3. Value Health. 2012;15(6):812-20. doi:10.1016/j.jval.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 19.Sonnenberg FA, Beck JR. Markov models in medical decision making: A practical guide. Med Decis Making. 1993;13(4):322-38. doi:10.1177/0272989X9301300409 [DOI] [PubMed] [Google Scholar]
- 20.Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3-9. doi:10.1016/j.jval.2021.11.1351 [DOI] [PubMed] [Google Scholar]
- 21.Komorowski M, Raffa J. Markov models and cost effectiveness analysis: Applications in medical research. In: MIT Critical Data. Secondary Analysis of Electronic Health Records. Cham (CH): Springer Cham; 2016:351-67. [PubMed] [Google Scholar]
- 22.Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the universal definition of heart failure. J Card Fail. 2021;S1071-9164(21)00050-6. doi:10.1016/j.cardfail.2021.01.022 [Google Scholar]
- 23.Belkin MN, Cifu AS, Pinney S. Management of heart failure. JAMA. 2022;328(13):1346-7. doi:10.1001/jama.2022.16667 [DOI] [PubMed] [Google Scholar]
- 24.Gidwani R, Russell LB. Estimating transition probabilities from published evidence: A tutorial for decision modelers. Pharmacoeconomics. 2020;38(11):1153-64. doi:10.1007/s40273-020-00937-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arias E, Xu J. United States life tables, 2019. Natl Vital Stat Rep. 2022;70(19):1-59. [PubMed] [Google Scholar]
- 26.RED BOOK Online. Micromedex Healthcare Series [database online]. Truven Health Analytics, Greenwood Village, CO. Accessed November 30, 2022. https://www.ibm.com/watson-health/merative-divestiture [Google Scholar]
- 27.Urbich M, Globe G, Pantiri K, et al. A systematic review of medical costs associated with heart failure in the USA (2014-2020). Pharmacoeconomics. 2020;38(11):1219-36. doi:10.1007/s40273-020-00952-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann PJ, Cohen JT. QALYs in 2018-advantages and concerns. JAMA. 2018;319(24):2473-4. doi:10.1001/jama.2018.6072 [DOI] [PubMed] [Google Scholar]
- 29.Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluations. Pharmacoeconomics. 2018;36(7):745-58. doi:10.1007/s40273-018-0672-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz DA, Welch HG. Discounting in cost-effectiveness analysis of health care programmes. Pharmacoeconomics. 1993;3(4):276-85. doi:10.2165/00019053-199303040-00004 [DOI] [PubMed] [Google Scholar]
- 31.Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296(13):716-21. doi:10.1056/NEJM197703312961304 [DOI] [PubMed] [Google Scholar]
- 32.Sanders GD, Maciejewski ML, Basu A. Overview of cost-effectiveness analysis. JAMA. 2019;321(14):1400-1. doi:10.1001/jama.2019.1265 [DOI] [PubMed] [Google Scholar]
- 33.Eckermann S, Willan AR. Presenting evidence and summary measures to best inform societal decisions when comparing multiple strategies. Pharmacoeconomics. 2011;29(7):563-77. doi:10.2165/11587100-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 34.Paulden M. Correction to: Calculating and interpreting ICERs and net benefit. Pharmacoeconomics. 2020;38(10):1147. doi:10.1007/s40273-020-00950-2 [DOI] [PubMed] [Google Scholar]
- 35.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: A report of the American College of Cardiology/American Heart Association Task Force on performance measures and task force on practice guidelines. Circulation. 2014;129(22):2329-45. doi:10.1161/CIR.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 36.Vanness DJ, Lomas J, Ahn H. A. health opportunity cost threshold for cost-effectiveness analysis in the United States. Ann Intern Med. 2021;174(1):25-32. doi:10.7326/M20-1392 [DOI] [PubMed] [Google Scholar]
- 37.Willke RJ, Neumann PJ, Garrison LP, Jr., Ramsey SD. Review of recent US value frameworks-a health economics approach: An ISPOR Special Task Force Report [6]. Value Health. 2018;21(2):155-60. doi:10.1016/j.jval.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 38.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-7. doi:10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 39.Claxton K, Briggs A, Buxton MJ, et al. Value based pricing for NHS drugs: An opportunity not to be missed? BMJ. 2008;336(7638):251-4. doi:10.1136/bmj.39434.500185.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danzon P, Towse A, Mestre-Ferrandiz J. Value-based differential pricing: Efficient prices for drugs in a global context. Health Econ. 2015;24(3):294-301. doi:10.1002/hec.3021 [DOI] [PubMed] [Google Scholar]
- 41.Rich MW, Nease RF. Cost-effectiveness analysis in clinical practice: The case of heart failure. Arch Intern Med. 1999;159(15):1690-700. doi:10.1001/archinte.159.15.1690 [DOI] [PubMed] [Google Scholar]
- 42.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479-500. doi:10.2165/00019053-200017050-00006 [DOI] [PubMed] [Google Scholar]
- 43.Hunink MG, Bult JR, de Vries J, Weinstein MC. Uncertainty in decision models analyzing cost-effectiveness: the joint distribution of incremental costs and effectiveness evaluated with a nonparametric bootstrap method. Med Decis Making. 1998;18(3):337-46. doi:10.1177/0272989X9801800312 [DOI] [PubMed] [Google Scholar]
- 44.Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force--6. Value Health. 2012;15(6):835-42. doi:10.1016/j.jval.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 45.Cohen LP, Isaza N, Hernandez I, et al. Cost-effectiveness of sodium-glucose cotransporter-2 inhibitors for the treatment of heart failure with preserved ejection fraction. JAMA Cardiol. 2023;8(5):419-28. doi:10.1001/jamacardio.2023.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandhu AT, Cohen DJ. Cost-effectiveness of sodium-glucose cotransporter-2 inhibitors for patients with heart failure and preserved ejection fraction—Living on the edge. JAMA Cardiol. 2023;8(5):415-6. doi:10.1001/jamacardio.2023.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isaza N, Calvachi P, Raber I, et al. Cost-effectiveness of dapagliflozin for the treatment of heart failure with reduced ejection fraction. JAMA Netw Open. 2021;4(7):e2114501. doi:10.1001/jamanetworkopen.2021.14501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parizo JT, Goldhaber-Fiebert JD, Salomon JA, et al. Cost-effectiveness of dapagliflozin for treatment of patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6(8):926-35. doi:10.1001/jamacardio.2021.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476-86. doi:10.1016/j.jacc.2017.08.074 [DOI] [PubMed] [Google Scholar]
- 50.Abebe TB, Gebreyohannes EA, Tefera YG, Abegaz TM. Patients with HFpEF and HFrEF have different clinical characteristics but similar prognosis: A retrospective cohort study. BMC Cardiovasc Disord. 2016;16(1):232. doi:10.1186/s12872-016-0418-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):e876-94. doi:10.1161/CIR.0000000000001062 [DOI] [PubMed] [Google Scholar]


