Abstract
We investigated the unrecognized patient-to-patient transmission of hepatitis C virus (HCV) in hemodialysis units by performing phylogenetic and serological analyses of hypervariable region 1 (HVR1) of HCV. Of the 62 patients in one center, 11 were positive for HCV RNA. A total of 24 HVR1 sequences, including the minor population of sequences of HCV isolates, from each patient were closely related and classified into five clusters by phylogenetic analysis. Of the 11 patients, 5 were infected with multiple clusters of HCV. Two patients were infected with HCV during an 18-month interval between examinations, and these HVR1 sequences fell into one of the five clusters. In another hemodialysis center, 5 of the 20 patients were HCV RNA positive, and two HVR1 sequences were found to be closely related and phylogenetically derived from the same cluster. The antibody responses of these patients to the HVR1 peptides representative of the genetic clusters revealed exactly the same clustering as that shown by phylogenetic analysis. These findings suggest that phylogenetic and serological analyses of HVR1 sensitively detect unrecognized and multiple transmission of HCV occurring within the same room in hemodialysis centers. Fingerprinting analyses using hypervariable regions of infectious agents are useful in identifying the precise route of transmission of infection.
Hepatitis C virus (HCV) is a major causative agent of posttransfusion non-A, non-B hepatitis. Screening and confirmatory assays for circulating antibodies to HCV became available (10, 27) after the molecular cloning of the HCV genome in 1989 (3). The second-generation enzyme immunoassay for detection of anti-HCV antibody has revealed a high prevalence of antibodies to HCV in hemodialysis (HD) patients (5). Most cases of HCV infection in HD patients are thought to be related to blood transfusions, but several reports from different parts of the world have also shown the presence of HCV infection in nontransfused HD patients as well (8, 22, 30), and the anti-HCV antibody-positive rates have been found to increase with the duration of dialysis (8, 22, 30). This suggests that nosocomial transmission of HCV occurs in HD units. Recently, patient-to-patient transmission of HCV in HD units has been demonstrated by molecular biology techniques, but the frequency of transmission was low (2, 20, 25). In these studies the sequences of HCV dominantly propagating in patients were determined and compared. Allander et al. (2) were the first to detect nucleotide sequence similarity in variable parts of the HCV genome in several HD patients. Sampietro et al. (20) and Stuyver et al. (25) found a rare HCV variant in several patients treated in the same HD unit by sequencing the relatively conserved region of the HCV genome, the 5′ untranslated region (5′-UTR), and the core region, respectively. However, no evidence of transmission had ever been demonstrated by sequence analysis of a minor population of HCV isolates.
The HCV genome exhibits different variability of nucleotide sequences in different regions. There is a hypervariable region, hypervariable region 1 (HVR1), in the putative second envelope glycoprotein (E2) of the HCV genome (7). HVR1 consists of 27 amino acid residues located in the N-terminal region of E2 and is the most variable region in the HCV genome. Accordingly, it appeared that HVR1 might be useful for discriminating HCVs the same as a polymorphic marker for genetic fingerprinting and for detecting nosocomial transmission of HCV. It was thought that not only rare but also common genotypes of HCV might be found to be transmitted. Furthermore, most patients with chronic hepatitis C possess antibody against the HVR1 of their own isolates (9, 29), and anti-HVR1 antibody was also thought to be useful for demonstrating nosocomial transmission. In this study, we examined HCV transmission in HD units by performing phylogenetic analysis of HCV HVR1 sequences and testing for antibodies to HVR1 peptides. We also analyzed multiple sequences of HVR1 from each individual and investigated nosocomial transmission of HCV, including a minor population of HCV isolates transmitted in HD patients.
MATERIALS AND METHODS
Patients.
We studied patients attending two dialysis centers. In one dialysis center, 62 patients were examined for HCV RNA twice, once in October 1994 and again in April 1996, by a two-step PCR amplifying the 5′-UTR. Nine patients were positive for HCV RNA the first time they were examined and two additional patients were found to be positive the second time. A total of 20 samples of patient serum were used for the sequence analysis of HCV HVR1 and the assay for anti-HVR1 antibody. Serial samples of serum from the patients were tested for alanine aminotransferase (ALT) and anti-HCV antibodies with a second-generation enzyme-linked immunosorbent assay (ELISA) (ELISA II; Ortho Diagnostic Systems, Tokyo, Japan). In the other center, 5 of the 20 patients were found to be positive for HCV, and their HCV HVR1 sequences were then examined. The HCV isolates from all patients were genotype 1b as determined by the SMI TEST HCV-Genotype (Sumitomo Metal Industries, Tokyo, Japan). All dialysis machines in each center were located in a single room. All patients were hemodialyzed against standard bicarbonate dialysate three times weekly, for 4 h each time. Dialysate was delivered from the central station, and the types of dialysis membranes used were regenerated cellulose membrane (AM-FP-15; Asahi Medical Co., Tokyo, Japan), polysulfone membrane (PS-1.3UW; Fresenius, Bad Homburg, Germany), and acrylonitrile-sodium methallyl membrane (Filtral-12; Hospal, Lyon, France). No dialyzers were reused. Informed consent was obtained from all patients examined in this study.
Amplification of HCV cDNA by reverse transcription-PCR.
RNA was extracted from 100 μl of serum with TRIzol (Life Technologies Inc., Gaithersburg, Md.) according to the manufacturer’s instructions. To synthesize cDNA, the RNA was reverse transcribed in 20 μl of reaction mixture containing 100 pmol of random primer, 20 U of ribonuclease inhibitor (Toyobo, Co., Osaka, Japan), and 100 U of Moloney murine leukemia virus reverse transcriptase (Life Technologies Inc.). Half of the synthesized cDNA was used for the PCR. The PCR was performed in 50 μl of solution containing 0.5 U of Taq DNA polymerase (Life Technologies Inc.), 50 μM concentrations of four deoxynucleoside triphosphates, and 0.3 μM concentrations of each primer by 35 cycles of 30 s at 94°C, 1 min at 60°C, and 1 min at 72°C, followed by 10 min of extension at 72°C. Five microliters of the product was used for the second-round PCR using nested primers under the same conditions as the first PCR. The primers used for the first PCR of HCV HVR1 were 5′-GCGGGCCTTGCCTACTATTC-3′ (sense; positions 1069 to 1088 [numbered from the ATG initiation codon]) and 5′-GGGCACCCGGACGAGTTGAA-3′ (antisense; positions 1339 to 1358), and those for the second were 5′-CATGGCGGGGAACTGGGCTAAGGT-3′ (sense; positions 1089 to 1112) and 5′-CAGGGCAGTCCTGTTGATGTGCCA-3′ (antisense; positions 1258 to 1281). The primer pairs for the first and second PCR of HCV 5′-UTR were 5′-CACTCCCCTGTGAGGAACTA-3′ (sense; positions −304 to −285) with 5′-GGTCTACGAGACCTCCCGGG-3′ (antisense; positions −20 to −1) and 5′-TTCACGCAGAAAGCGTCTCTAG-3′ (sense; positions −279 to −260) with 5′-CCCTATCAGGCAGTACCACA-3′ (antisense; positions −60 to −41), respectively.
Subcloning, sequencing, and phylogenetic analysis.
The PCR product was purified with phenol-chloroform, and then subcloned into T-tailed plasmid vector of pGEM-4Z by the T-A overhang cloning method (12). Ten recombinant clones were isolated from each sample and sequenced by the cycle sequencing method (16) using Taq DNA polymerase and 32P-labeled primer. The PCR product was also sequenced directly after the removal of excess primers by three cycles of centrifugation in a Microcon 100 centrifuge (Amicon, Inc., Beverly, Mass.).
The nucleotide sequences were translated by the DNASIS program (version 3.6; Hitachi Software Engineering Co., Ltd., Yokohama, Japan), and the amino acid sequences and the nucleotide sequences of HVR1, including unrelated HVR1 sequences from Japanese patients and blood donors, were aligned by using the CLUSTAL W program, version 1.5 (26). Phylogenetic trees were constructed by the neighbor-joining method (19), and 1,000 trials of bootstrap analysis were performed.
Peptide ELISA.
Six peptides of HVR1 sequences derived from five clusters were synthesized by the solid-phase method using a simultaneous multiple solid-phase peptide synthesizer (PSSM-8; Shimadzu Co., Kyoto, Japan). After the peptidyl resin was cleaved, crude peptides were purified by a preparatory reverse-phase high-performance liquid chromatography and the purified peptides were characterized by sequence analysis using a protein sequencer (PPSQ-10; Shimadzu Co.) in addition to analytical high-performance liquid chromatography. Using the synthesized peptides, indirect ELISA was performed as described previously (1). Briefly, microtiter wells (Nunc Immuno Plates, Maxisorp; Nunc A/S, Roskilde, Denmark) were coated with peptide (1 μg/ml), followed by blocking. Patient serum diluted 20 times was added to the wells, and reacting antibody was detected by incubation with peroxidase-conjugated goat F(ab)2 fragment to human immunoglobulin (Cappel Inc., Durham, N.C.) and colorization with tetramethylbenzidine (Sigma Co., St. Louis, Mo.) and H2O2. After stopping the reaction with H2SO4, absorbance at 450 nm (A450) was measured in a Multiskan Bichromatic System (Labsystems Inc., Helsinki, Finland). The data were estimated as an average of three independent experiments.
Nucleotide sequence accession numbers.
The nucleotide sequences described in this work have been deposited in the DDBJ, GenBank, and EMBL databases under accession no. AB001390 to AB001416 and no. AB015321 to AB015326.
RESULTS
Phylogenetic analysis of HCV HVR1 sequences.
To examine the relationship among HCV isolates infecting HD patients in a dialysis center, HCV HVR1 sequences were amplified from the RNAs of patients’ sera and determined. The amplified cDNA was subcloned into plasmid DNA to determine the sequences of both major and minor HCV isolates in each patient.
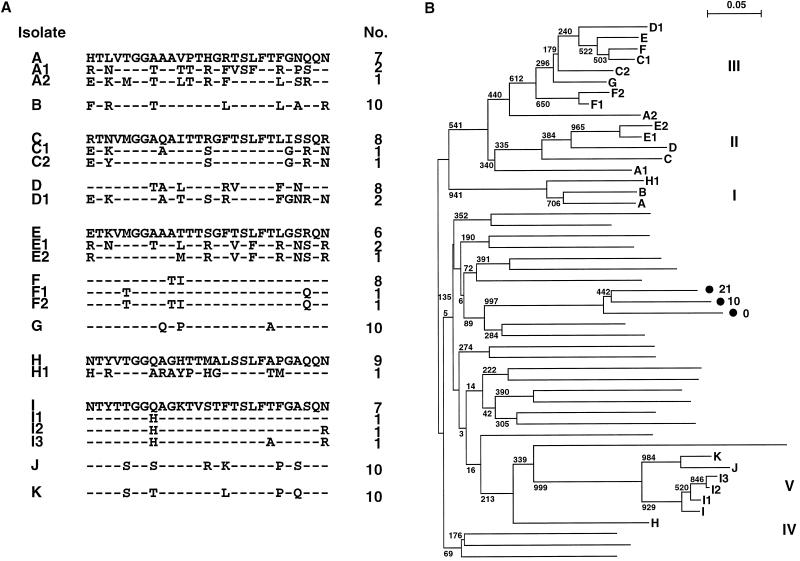
Nine patients were positive for HCV RNA among 62 patients examined at the center the first time. Unfortunately, 18 months after the first examination, two additional patients in the same unit were found to be positive. Figure 1A shows 24 sequences of 27 amino acids of HCV HVR1 isolated from these 11 patients. Each patient had a major clone of HCV individually accounting for more than 70% of the population. Patients F and I had minor clones related to their major clones, respectively, so-called quasispecies of HCV, whereas patients A, C, D, E, and H had clones unrelated to their own major species. A phylogenetic tree was constructed to determine the genetic relationship among these isolates (Fig. 1B). All 24 sequences, which were genotype 1b, were classified into five clusters. They were clearly separated from 25 unrelated sequences of HCV genotypes 1b, 2a, and 2b from Japanese patients. Major isolates A and B; C and D; E, F, and G; and I, J, and K were related and formed clusters I, II, III, and V, respectively. Since two isolates, J and K, from two additional patients were related to isolate I, HCV seemed to have been transmitted from patient I to patients J and K within 18 months. Minor isolates F1 and F2 and I1, I2, and I3, were quasispecies of their major isolates, F and I, respectively, and each grouped into the same cluster as its major isolate. On the other hand, minor isolates from the other patients were grouped into one of the three other clusters. As a control cluster, three related isolates which were major sequences from a single patient isolated at three different times, 0, 10, and 21 months (11), were included in this tree, and these actually formed an independent cluster. These results suggest that patient-to-patient transmission of HCV occurs in HD units, and the minor isolates from individuals in particular suggest that simultaneous transmission of mixed populations of HCV or multiple transmission may occur, since five patients, A, C, D, E, and H, were infected with one or two more isolates from different clusters. The phylogenetic tree of 19 HCV HVR1 sequences isolated from nine patients, A to I, during the first examination was also constructed. It revealed the same five genetic clusters of the sample sequences shown in Fig. 1B, with higher bootstrap values: 1,000, 887, 576, and 1,000 for clusters I, II, III, and V, respectively (data not shown). The phylogenetic tree of nucleotide sequences also confirmed these five clusters. In addition, phylogenetic analysis of all 43 sequences identified both times in the HD patients revealed that the classification into five clusters was maintained (data not shown). Amplifying the more conserved nonstructural protein 5a region for comparison with the results for HVR1, we found all nine sequences of the nonstructural protein 5a region from nine patients, A to I, to be within a single cluster (data not shown). Thus, HVR1 is sensitive for phylogenetic analysis of HCV transmission.
FIG. 1.
Comparison of deduced amino acid sequences of the HCV HVR1 isolated from 11 patients, A to K, 18 months after the first examination. Minor isolates from each patient are shown as patient letters with numbers. (A) Alignment of the amino acid sequences. Only amino acid residues differing from the reference sequences (A, C, E, H, and I) of each cluster are shown. The actual number of plasmid clones obtained from each patient is shown on the right. (B) Phylogenetic tree of HVR1 nucleotide sequences constructed by the neighbor-joining method using 24 isolates from 11 HD patients and 25 unrelated isolates. The Roman numerals on the right indicate the genetic clusters of 24 isolates from HD patients. Three related isolates (•) obtained from a single patient at different months, indicated by numbers, were included as a control cluster (11). Bootstrap analysis was performed for 1,000 trials, and calculated values are shown at each branch.
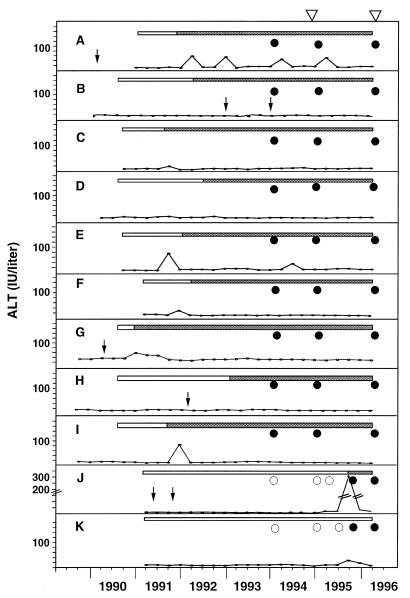
Figure 2 shows the epidemiological data for these 11 patients: serum ALT levels, seroconversion of anti-HCV antibody and HCV RNA, and history of blood transfusion. All patients seroconverted to HCV positivity after admission to the dialysis center. Since both patients J and K seroconverted for HCV RNA between July and October 1995, HCV transmission from patient I probably occurred at the same time. However, the other transmissions were not epidemiologically clear, since the time point of seroconversion for HCV RNA was not determined in each patient. In view of the pattern of anti-HCV antibody detection, HCV transmission probably occurred from patient A to B, from patient C to D, and from patient G to E and F, resulting in the generation of clusters I, II, and III, respectively. The second (and third) transmission of HCV from a different cluster, at least in patient A, was presumed to have occurred on the basis of results of the phylogenetic analysis of multiple isolates from each patient alone, with no substantiation by the epidemiological data. Thus, it is difficult to conclude that there was patient-to-patient transmission of HCV based on the epidemiological data alone. The phylogenetic tree is very informative in determining detailed transmission, including cases of multiple and mixed infection.
FIG. 2.
Serum ALT levels and HCV infection markers of 11 patients, A to K. Open bars, negative for anti-HCV antibody; shaded bars, positive for anti-HCV antibody; open circles, negative for HCV RNA; solid circles, positive for HCV RNA; arrows, blood transfusion; triangles, sequence examination.
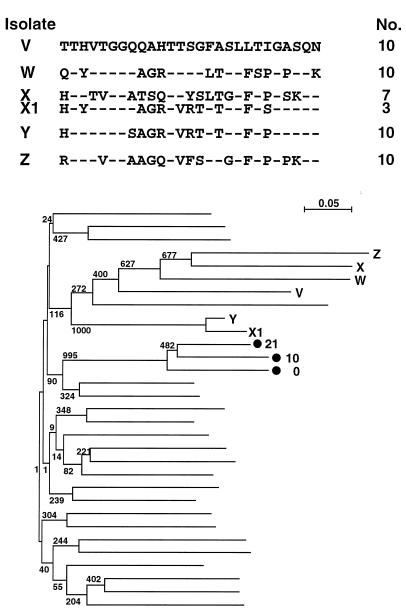
Figure 3 shows the HCV HVR1 sequences of five HD patients in another dialysis center. The phylogenetic tree indicates that minor isolate X1 from patient X was unrelated to major isolate X but was related to isolate Y. Four control clusters included in the phylogenetic tree revealed that isolates X1 and Y constructed a single genetic cluster. This transmission was not detected by sequence analysis of major isolates, e.g., direct sequencing of the amplified products. Sequence analyses following subcloning revealed unrecognized transmission of HCV in HD units.
FIG. 3.
Comparison of deduced amino acid sequences of the HCV HVR1 isolated from five patients, V to Z. Only residues differing from the reference sequence (V) are shown in the alignment. Isolate X1 was obtained from patient X as a minor clone. The actual number of plasmid clones obtained from each patient is shown to the right of the sequence. The phylogenetic tree of nucleotide sequences was constructed by the neighbor-joining method using 6 isolates from HD patients and 25 unrelated isolates, including a control cluster (•), as shown in Fig. 1B.
Serological analysis of anti-HVR1 antibody.
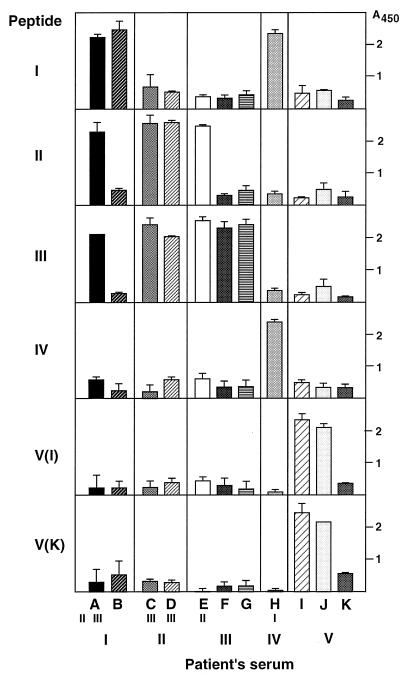
Most patients with chronic hepatitis C possess specific antibody against HVR1 of their own isolates, which is thought to be neutralizing antibody (9, 29). However, emergence of an escape mutant not selected by the host immune response seems to be involved in the development of chronicity of infection (9, 29). To assess whether anti-HVR1 antibody proves the footprinting of HCV transmission, the representative HVR1 peptides from five clusters were synthesized and used to detect anti-HVR1 antibody in patients’ sera. As shown in Fig. 4, the serum of all patients was specifically immunoreactive to HVR1 peptides related to their own major isolates individually. Furthermore, patients with mixed populations of HCV were also reactive to the HVR1 peptides derived from the cluster of minor isolates. For example, the antibody response of patient A was positive for not only peptide I but also peptides II and III. Patients C, D, E, and H were also specifically reactive to one more cluster peptide. Peptides V(I) and V(K) were derived from the same cluster, cluster V, but 5 amino acids were changed and phylogenetically distant, even within a cluster (Fig. 1). However, patients I and J were both immunoreactive to these two peptides. Patient K was still negative for the third generation of anti-HCV antibody and therefore had not produced antibody against his own HVR1 sequence.
FIG. 4.
Antibody responses of 11 patients, A to K, to six HVR1 peptides of different clusters, I to V. Patients’ sera were obtained at month 18. The amino acid sequences of the six peptides used are as follows: I, FTRVTGGAQAVPTHGLTSLFTFGAQQN; II, RTLVMGGAATLTTRGRVSLFTFINSQR; III, ETKVMGGAQAPTTSGFTSLFALGSRQN; IV, NTYVTGGQAGYTTMALSSLFAPGAQQN; V(I), NTYTTGGQAGKTVSTFTSLFTLGASQN; V(K), NTYTSGGTAGKTVSTLTSLFTPGQSQN. The small Roman numerals under A, C, D, E, and H indicate the cluster numbers of minor isolates from each patient, and the large Roman numerals at the bottom indicate those of predominant isolates from each patient. The A450 was estimated as the average ± standard deviation (error bar) of three independent experiments. Negative control values of the patients’ sera were 0.48 ± 0.13 (patient A), 0.46 ± 0.11 (patient B), 0.51 ± 0.13 (patient C), 0.53 ± 0.02 (patient D), 0.49 ± 0.09 (patient E), 0.47 ± 0.01 (patient F), 0.56 ± 0.07 (patient G), 0.50 ± 0.05 (patient H), 0.40 ± 0.16 (patient I), 0.48 ± 0.19 (patient J), and 0.52 ± 0.04 (patient K).
These results confirm the fidelity of phylogenetic clustering of these HCV isolates and suggest that the anti-HVR1 antibody assay is simple and useful for detecting HCV transmission in a unit only if the patients are immunocompetent.
DISCUSSION
In this study we demonstrated that multiple instances of unrecognized transmission of HCV occurred in HD units by constructing the phylogenetic tree of multiple HVR1 sequences isolated from each patient. In addition to several reports of molecular evidence of nosocomial transmission of HCV in HD units (2, 4, 14, 15, 18, 20, 25, 31), we found that patient-to-patient transmission of HCV occurred more frequently than expected in HD units, as shown by our amplifying the HVR1 sequences as individual markers of HCV isolates and comparing multiple clones isolated from each sample. However, there is some concern, as indicated by Munro et al. (15), that the high and inconstant mutation rate of HVR1 sequences causes spurious clustering or unclustering in the phylogenetic analysis. To dispel such concerns in this study, the phylogenetic analysis was performed in the presence of numerous unrelated sequences together with bootstrap analysis (Fig. 1B) and was performed by using sequences from different time points and constructing a mixed tree to confirm the genetic clustering. Recently, phylogenetic analysis using the HVR1 sequences clearly proved that a cardiac surgeon with chronic hepatitis C transmitted HCV to patients during open-heart surgery (6). Thus, HVR1 sequences may be a useful probe for fingerprinting of HCV isolates, provided that patients have been infected with HCV for less than 5 years, as in this study, because of differences in the selective forces and the mutation rate of HVR1.
The second point in this study is that the sequence analysis of multiple clones from each patient revealed complicated HCV infection; one possibility is that mixed populations of HCV (clusters II and III) were simultaneously transmitted in patients A, C, D, and E. Another is that transmission occurred more than once in these patients, especially in patient A. These infections are not detected by the epidemiological data or direct sequence analysis of the amplified products. Multiple sequence analysis in each patient suggests that patient-to-patient transmission of HCV occurs more frequently than expected in HD units. As shown in Fig. 4, we also found that the antibody response to HVR1 peptides monitored the historical transmission of HCV, including unrecognized and multiple infection. For analysis of multiple plasmid clones, however, we have to carefully consider artificial variants which were produced by high error rates of reverse transcriptase and Taq DNA polymerase during reverse transcription-PCR of the virus genome, as indicated by Smith et al. (24).
The transmission mechanism in HD units has not yet been identified. A possible cause of transmission is thought to be a lack of careful infection control by HD personnel. Okuda et al. (17) found that transmission of HCV was associated with the location of the HD console and suggested strict aseptic precautions by the staff to prevent nosocomial spread of HCV infection. The location of the consoles occupied by the 11 patients in this study indicates that transmission occurred more frequently between patients treated during the same shift than between those occupying the same dialysis machine. The consoles of patients J and K were located side by side and near that of patient I in the same shift; thus, transmission probably occurred as a result of the practices of a staff member common to these three patients. It is important to educate HD personnel to be aware of the risk of unexpected HCV transmission from HCV-positive patients. In order to precisely identify HCV-positive patients, regular testing for HCV RNA should be performed, because there are some populations of HD patients who are negative for anti-HCV antibody and do not show ALT elevation but who carry HCV RNA (23).
Patient-to-patient transmission of human immunodeficiency virus (28) and hepatitis GB virus C (13, 21) has also recently been suspected in HD units. Thus, there is a high possibility that not only HCV but also other infectious viruses in blood are transmitted in HD units. The phylogenetic analysis of the variable region of these infectious agent clones may be useful for sensitive detection of the agents and identification of the transmission route.
ACKNOWLEDGMENTS
We thank M. Yanai, Department of Clinical Pathology, Nihon University School of Medicine, and M. Mizokami, Nagoya City University Medical School, for helpful discussion, and C. Yamamoto for technical assistance.
This work was supported by Health Science Research Grants (Non-A, Non-B Hepatitis Research Grants), the Kurozumi Medical Foundation, and an Interdisciplinary General Joint Research Grant for Nihon University (DA 97-003).
REFERENCES
- 1.Ahmed M, Shikata T, Esumi M. Murine humoral immune response against recombinant structural proteins of hepatitis C virus distinct from those of patients. Microbiol Immunol. 1996;40:169–176. doi: 10.1111/j.1348-0421.1996.tb03321.x. [DOI] [PubMed] [Google Scholar]
- 2.Allander T, Medin S, Jacobson S H, Grillner L, Persson M A A. Hepatitis C transmission in a hemodialysis unit: molecular evidence for spread of virus among patients not sharing equipment. J Med Virol. 1994;43:415–419. doi: 10.1002/jmv.1890430417. [DOI] [PubMed] [Google Scholar]
- 3.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 4.de Lamballerie X, Olmer M, Bouchouareb D, Zandotti C, de Micco P. Nosocomial transmission of hepatitis C virus in haemodialysis patients. J Med Virol. 1996;49:296–302. doi: 10.1002/(SICI)1096-9071(199608)49:4<296::AID-JMV7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.de Medina M, Ortiz C, Krenc C, Leete J, Vallari D, Hill M, LaRue S, Jimenez M, Anderson W, Schiff E, Perez G. Improved detection of antibodies to hepatitis C virus in dialysis patients using a second-generation enzyme immunoassay. Am J Kidney Dis. 1992;20:589–591. doi: 10.1016/s0272-6386(12)70224-x. [DOI] [PubMed] [Google Scholar]
- 6.Esteban J I, Gomez J, Martell M, Cabot B, Quer J, Camps J, Gonzalez A, Otero T, Moya A, Esteban R, Guardia J. Transmission of hepatitis C virus by a cardiac surgeon. N Engl J Med. 1996;334:555–560. doi: 10.1056/NEJM199602293340902. [DOI] [PubMed] [Google Scholar]
- 7.Hijikata M, Kato N, Ootsuyama Y, Nakagawa M, Ohkoshi S, Shimotohno K. Hypervariable regions in the putative glycoprotein of hepatitis C virus. Biochem Biophys Res Commun. 1991;175:220–228. doi: 10.1016/s0006-291x(05)81223-9. [DOI] [PubMed] [Google Scholar]
- 8.Irie Y, Hayashi H, Yokozeki K, Kashima T, Okuda K. Hepatitis C infection unrelated to blood transfusion in hemodialysis patients. J Hepatol. 1994;20:557–559. doi: 10.1016/s0168-8278(05)80506-9. [DOI] [PubMed] [Google Scholar]
- 9.Kato N, Sekiya H, Ootsuyama Y, Nakazawa T, Hijikata M, Ohkoshi S, Shimotohno K. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993;67:3923–3930. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo G, Choo Q L, Alter H J, Gitnick G L, Redeker A G, Purcell R H, Miyamura T, Dienstag J L, Alter M J, Stevens C E, Tegtmeier G E, Bonino F, Colombo W S, Lee W S, Kuo C, Berger K, Shuster J R, Overby L R, Bradley D W, Houghton M. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 11.Kurosaki M, Enomoto N, Marumo F, Sato C. Evolution and selection of hepatitis C virus variants in patients with chronic hepatitis C. Virology. 1994;205:161–169. doi: 10.1006/viro.1994.1631. [DOI] [PubMed] [Google Scholar]
- 12.Marchuk D, Drumm M, Saulino A, Collins F S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991;19:1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuko K, Mitsui T, Iwano K, Yamazaki C, Okuda K, Meguro T, Murayama N, Inoue T, Tsuda F, Okamoto H, Miyakawa Y, Mayumi M. Infection with hepatitis GB virus C in patients on maintenance hemodialysis. N Engl J Med. 1996;334:1485–1490. doi: 10.1056/NEJM199606063342301. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin K J, Cameron S O, Good T, McCruden E, Ferguson J C, Davidson F, Simmonds P, Mactier R A, McMillan M A. Nosocomial transmission of hepatitis C virus within a British dialysis centre. Nephrol Dial Transplant. 1997;12:304–309. doi: 10.1093/ndt/12.2.304. [DOI] [PubMed] [Google Scholar]
- 15.Munro J, Briggs J D, McCruden E A B. Detection of a cluster of hepatitis C infections in a renal transplant unit by analysis of sequence variation of the NS5a gene. J Infect Dis. 1996;174:177–180. doi: 10.1093/infdis/174.1.177. [DOI] [PubMed] [Google Scholar]
- 16.Murray V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 1989;17:8889. doi: 10.1093/nar/17.21.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okuda K, Hayashi H, Kobayashi S, Irie Y. Mode of hepatitis C infection not associated with blood transfusion among chronic hemodialysis patients. J Hepatol. 1995;23:28–31. doi: 10.1016/0168-8278(95)80307-6. [DOI] [PubMed] [Google Scholar]
- 18.Olmer M, Bouchouareb D, Zandotti C, de Micco P, de Lamballerie X. Transmission of the hepatitis C virus in a hemodialysis unit: evidence for nosocomial infection. Clin Nephrol. 1997;47:263–270. [PubMed] [Google Scholar]
- 19.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 20.Sampietro M, Badalamenti S, Salvadori S, Corbetta N, Graziani G, Como G, Fiorelli G, Ponticelli C. High prevalence of a rare hepatitis C virus in patients treated in the same hemodialysis unit: evidence for nosocomial transmission of HCV. Kidney Int. 1995;47:911–917. doi: 10.1038/ki.1995.136. [DOI] [PubMed] [Google Scholar]
- 21.Sampietro M, Badalamenti S, Graziani G, Como G, Buccianti G, Corbetta N, Ticozzi A, Archenti A, Lunghi G, Penso D, Pizzuti A, Fiorelli G, Ponticelli C. Hepatitis G virus infection in hemodialysis patients. Kidney Int. 1997;51:348–352. doi: 10.1038/ki.1997.43. [DOI] [PubMed] [Google Scholar]
- 22.Schlipkoter U, Roggendorf M, Ernst G, Rasshofer R, Deinhardt F, Weise A, Gladziwa U, Luz N. Hepatitis C virus antibodies in haemodialysis patients. Lancet. 1990;335:1409. [Google Scholar]
- 23.Silini E, Bono F, Cerino A, Slocia E, Piazza V, Mondelli M U. Virological features of hepatitis C virus infection in hemodialysis patients. J Clin Microbiol. 1993;31:2913–2917. doi: 10.1128/jcm.31.11.2913-2917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith D B, McAllister J, Casino C, Simmonds P. Virus ‘quasispecies’: making a mountain out of a molehill? J Gen Virol. 1997;78:1511–1519. doi: 10.1099/0022-1317-78-7-1511. [DOI] [PubMed] [Google Scholar]
- 25.Stuyver L, Claeys H, Wyseur A, Van Arnhem W, De Beenhouwer H, Uytendaele S, Beckers J, Matthijs D, Leroux-Roels G, Maertens G, De Paepe M. Hepatitis C virus in a hemodialysis unit: molecular evidence for nosocomial transmission. Kidney Int. 1996;49:889–895. doi: 10.1038/ki.1996.122. [DOI] [PubMed] [Google Scholar]
- 26.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Poel C L, Cuypers H T, Reesink H W, Weiner A J, Quan S, Di Nello R, Van Boven J J, Winkel I, Mulder-Folkerts D, Exel-Oehlers P J, Schaasberg W, Leentvaar-Kuypers A, Polito A, Houghton M, Lelie P. Confirmation of hepatitis C virus infection by new four-antigen recombinant immunoblot assay. Lancet. 1991;337:317–319. doi: 10.1016/0140-6736(91)90942-i. [DOI] [PubMed] [Google Scholar]
- 28.Velandia M, Fridkin S K, Cardenas V, Boshell J, Ramirez G, Bland L, Iglesias A, Jarvis W. Transmission of HIV in dialysis centre. Lancet. 1995;345:1417–1422. doi: 10.1016/s0140-6736(95)92603-8. [DOI] [PubMed] [Google Scholar]
- 29.Weiner A J, Geysen H M, Christopherson C, Hall J E, Mason T J, Saracco G, Bonino F, Crawford K, Marion C D, Crawford K A, Brunetto M R, Barr P J, Miyamura T, McHutchinson J, Houghton M. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci USA. 1992;89:3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi K, Nishimura Y, Fukuoka N, Machida J, Ueda S, Kusumoto Y, Futami G, Ishii T, Takatsuki K. Hepatitis C virus antibodies in haemodialysis patients. Lancet. 1990;335:1409–1410. [Google Scholar]
- 31.Zeuzem S, Scheuermann E H, Waschk D, Lee J-H, Blaser C, Franke A, Roth W K. Phylogenetic analysis of hepatitis C virus isolates from hemodialysis patients. Kidney Int. 1996;49:896–902. doi: 10.1038/ki.1996.123. [DOI] [PubMed] [Google Scholar]