SUMMARY
OBJECTIVE:
The aim of this study was to analyze the second-trimester levels of vitronectin and plasminogen activator inhibitor-1 in gestational diabetes mellitus.
METHODS:
This study was conducted between September 2020 and December 2020 at the University of Health Sciences, Bursa Yuksek Ihtisas Research and Training Hospital, Department of Obstetrics and Gynecology. A total of 30 pregnant women with gestational diabetes mellitus and 60 healthy controls between 24 and 27/6 weeks of gestation were included. The inclusion criteria were as follows: being between 18 and 45 years old and 24–27/6 gestational weeks, having singleton pregnancy, diagnosed with gestational diabetes mellitus by using a two-step challenge test. The exclusion criteria of this study were as follows: chronic inflammatory or infectious disease, fasting blood glucose>126 mg/dL, intolerance to glucose tolerance testing, abnormal liver or kidney function tests, as well as pregnancy with pre-gestational diabetes history of adverse perinatal outcomes. Serum vitronectin and plasminogen activator inhibitor-1 levels were measured using the enzyme-linked immunosorbent assay method.
RESULTS:
Vitronectin and plasminogen activator inhibitor-1 levels were higher in the gestational diabetes mellitus group compared with controls [91.85 (23.08) vs. 80.10 (39.18) ng/mL, for vitronectin and 6.50 (1.05) vs. 4.35(1.0) ng/mL, for plasminogen activator inhibitor-1 (for both p<0.001)]. vitronectin >84.7 ng/mL was found to predict gestational diabetes mellitus with a sensitivity of 70% and specificity of 63.3%. Moreover, vitronectin had a significant positive correlation with fasting blood glucose (r=0.476, p<0.001), postprandial blood glucose (r=0.489, p<0.001), HbA1c (r=0.713, p<0.001), and plasminogen activator inhibitor-1 (r=0.586, p<0.001).
CONCLUSION:
This study revealed that second-trimester vitronectin and plasminogen activator inhibitor-1 are increased in gestational diabetes mellitus and vitronectin could be a candidate for the prediction of gestational diabetes mellitus.
KEYWORDS: Biomarkers, Gestational diabetes mellitus, Second trimester
INTRODUCTION
Gestational diabetes mellitus (GDM), the incidence of which varies from 2 to 10%, can be defined as glucose intolerance with onset or first recognition in pregnancy 1 . Beta-cell dysfunction in pancreatic tissue, insulin resistance, low-grade inflammation, and endothelial dysfunction are the main pathophysiological mechanisms in GDM 2,3 . As GDM is tightly associated with short- and long-term perinatal mortality and morbidity such as cardiovascular diseases, type 2 diabetes mellitus, birth complications, cesarean delivery, and endocrine disorders of neonate, new biomarkers elucidating the etiology of GDM have been suggested in the literature 4,5 .
Vitronectin (Vn), which is encoded by the Vn gene, is a 75-kDa cellular adhesion glycoprotein with its N-terminal somatomedin-B domain, central hemopexin-like domain, and C-terminal domain 6–8 . It has been found in many tissues including plasma, extracellular matrix, platelets, liver, blood vessels, embryonic lungs, renal basal membrane, muscles, and human skin 9 . Vn plays crucial roles in many processes such as regulation of coagulation cascade, oncogenic formation, fibrinolysis, inflammation, wound healing, fibrosis, and insulin signaling 10,11 . It interacts with integrins and urokinase plasminogen activators and leads to neutrophil adhesion and migration 12 . Somatomedin B domain of Vn stabilizes plasminogen activator inhibitor-1 (PAI-1) which plays a primary role in the inhibition of plasminogen activators and the in vivo conversion of plasminogen to plasmin 13 . In the literature, high PAI-1 levels have been demonstrated to predict the risk of type 2 diabetes, and deficiency in PAI-1 has a protective role against insulin resistance 14 . Recent studies have suggested that GDM triggers the expression and release of PAI-1, which is associated with GDM severity due to insulin resistance development and exaggerated proinflammatory and inflammatory cytokines. High PAI-1 levels in GDM may cause hypofibrinolysis and thrombotic complications 15–17 .
As insulin resistance and inflammation are the main etiological mechanisms for GDM, we hypothesized that Vn and PAI-1 are increased in cases with GDM. There is no study evaluating the levels of these markers together for GDM in the second trimester. In this study, we first aimed to analyze second-trimester Vn and PAI-1 levels together in pregnant women.
METHODS
This observational case-control study was conducted between September 2020 and December 2020 at the University of Health Sciences, Bursa Yuksek Ihtisas Research and Training Hospital, Department of Obstetrics and Gynecology. This study was approved by the local ethics committee (2011-KAEK-25 2020/09-13), and written informed consent was obtained from all study participants.
Study population
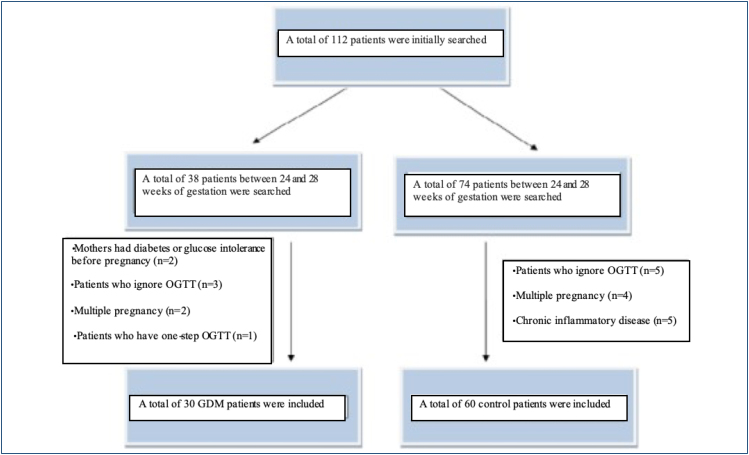
A power analysis was performed, and the analysis revealed that the minimum patient number was 30 for each group with 80% power to detect a 30% difference in cases with a value of 0.05. In this prospective case-control study, a total of 30 pregnant women with a diagnosis of GDM and 60 pregnant women without GDM were included in the study. GDM was diagnosed if the patient had a glucose level of >200 mg/dL at 50 g oral glucose challenge test or 95 mg/dL for fasting, 180 mg/dL at the first hour, 155 mg/dL at the second hour, and 140 mg/dL at the third hour in 100 g testing for patients who have 50 g challenge test value of 140–200 mg/dL. The control group was composed of pregnant women who had normal 50 g oral glucose testing. The inclusion criteria were as follows: being between 18 and 45 years old and 24–27/6 gestational week, having singleton pregnancy, diagnosed with GDM by using a two-step challenge test. The exclusion criteria of this study were as follows: chronic inflammatory or infectious disease, fasting blood glucose>126 mg/dL, intolerance to glucose tolerance testing, abnormal liver or kidney function tests, as well as pregnancy with pre-gestational diabetes history of adverse perinatal outcomes (Figure 1). The sociodemographic and obstetric features and laboratory characteristics were recorded.
Figure 1. Flowchart showing selection of gestational diabetes mellitus and non-gestational diabetes mellitus cohorts.
Definition of gestational diabetes mellitus
In our clinic, we routinely screen pregnant women for GDM between 24 and 28 gestational weeks by a two-step protocol that was suggested by the 2018 American College of Obstetricians and Gynaecologists Guidelines 18 . In a two-step protocol, 50 g oral glucose tolerance test was used in the first step followed by a 100 g oral glucose tolerance test if blood glucose levels were above 140 mg/dL at 1 h in 50 g testing. GDM was diagnosed if two abnormal glucose levels were detected according to the Carpenter and Coustan criteria in 100 g tolerance testing. The diagnostic glucose levels were 95 mg/dL for fasting,180 mg/dL at the first hour, 155 mg/dL at the second hour, and 140 mg/dL at the third hour in 100 g testing. Consequently, pregnant women who had normal 50 g oral glucose testing were assigned to the control group, whereas pregnant women diagnosed with GDM by a two-step protocol were assigned to the GDM group.
Vitronectin and plasminogen activator inhibitor-1 measurement
Patients serum samples were obtained from the antecubital vein after 12 h of fasting and the sera were stored for Vn and PAI-1 measurement at −80°C after centrifuged at 3,500 rpm for 10 min to be analyzed after the patient was examined for GDM. Serum Vn and PAI-1 levels were measured using a commercially available kit, namely, Human Vn and PAI-1 kit, with the enzyme-linked immunosorbent assay method.
Statistical analysis
Statistical analyses were performed on the SPSS software. Shapiro-Wilk's test was used to determine whether the obtained data were normally distributed or not. Variables were defined as mean±standard deviation for normally distributed quantitative variables and median (IQR) for non-normally distributed quantitative variables. Student's t-test and Mann-Whitney U tests were used for two-group analysis. Categorical variables were compared with chi-square or Fisher's exact test. The correlation between Vn and clinical variables was evaluated by performing Spearman correlation analysis. The predictive value of Vn for GDM patients was determined by receiver operating curve analysis. A p-value<0.05 was considered statistically significant.
RESULTS
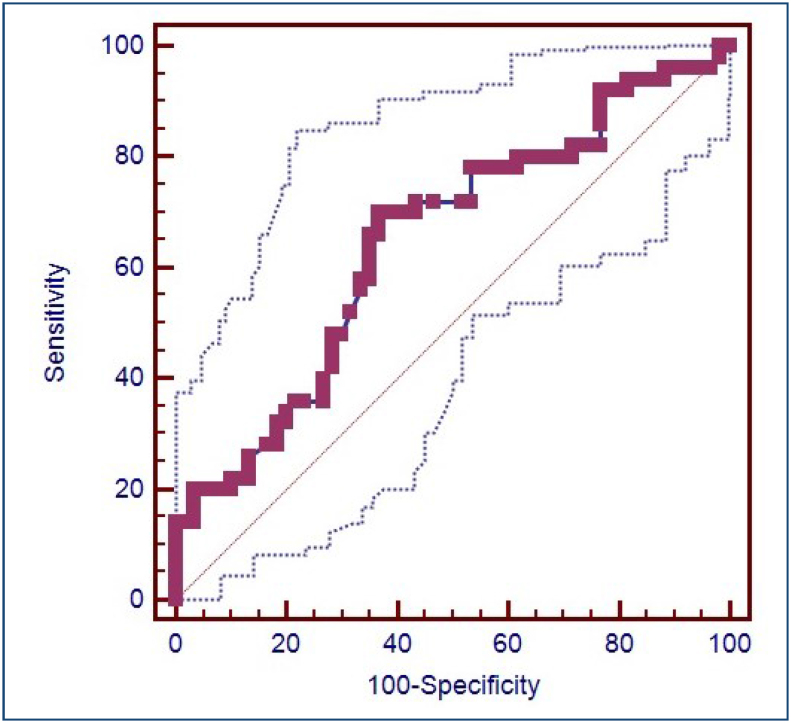
The sociodemographic features and the perinatal outcomes of the GDM (n=30) and control group (n=60) are demonstrated in Table 1. Gestational age at delivery was significantly lower in the GDM group compared with the control group [37 (1.47) vs. 38.18 (1.44) weeks, p<0.003]. The laboratory characteristics of the GDM and control groups are shown in Table 2. Fasting glucose postprandial glucose, HbA1c, C-reactive protein levels, and platelet levels were significantly higher in the GDM group (p<0.05). Furthermore, median Vn and PAI-1 levels were higher in the GDM group compared with the control group [91.85 (23.08) vs. 80.10 (39.18) ng/mL, for Vn and 6.50 (1.05) vs. 4.35 (1.0) ng/mL, for PAI-1 (for both of them p<0.001)]. The predictive value of Vn for GDM patients was determined by receiver operating curve analysis. Vn was found to predict GDM with a cutoff value >84.7 ng/mL with a sensitivity of 70% and specificity of 63.3%. The area under the curve was 0.647 and the 95% confidence interval was 0.550 and 0.736 with a p-value of 0.005. The receiver operating curve for the predictive value of Vn to diagnose GDM is presented in Figure 2.
Table 1. Clinical characteristics and perinatal outcomes of the gestational diabetes mellitus and control groups.
| Variables | GDM group (n=30) | Control group (n=60) | p |
|---|---|---|---|
| Age (years) | 30.46 (5.37) | 28.45 (5.62) | 0.107a |
| Gravida (n) | 2 (1.25) | 2 (2) | 0.291b |
| Parity (n) | 1 (1.25) | 1 (2) | 0.993b |
| Body mass index (kg/m²) | 26.97 (1.87) | 26.39 (1.96) | 0.107a |
| Cesarean section (n, %) | 14 (46.7%) | 20 (33.3%) | 0.219c |
| Gestational age at delivery (weeks) | 37 (1.47) | 38.18 (1.44) | <0.003 a |
| Birth weight (g) | 3,200 (2,150–4,560) | 3,165 (2,060–4,350) | 0.840b |
| Polyhydramnios (n,%) | 4 (13.3%) | 5 (8.3%) | 0.474c |
| Macrosomia (n, %) | 4 (13.3%) | 5 (8.3%) | 0.474c |
| Apgar score first min | 7 (1) | 8 (2) | 0.430b |
| Apgar score fifth min | 9 (1) | 9 (1) | 0.682b |
| Apgar first min <7 | 4 (13.3%) | 6 (10%) | 0.726d |
| Apgar fifth min<7 | 2 (6.7%) | 2 (3.3%) | 0.598d |
| NICU admission (n, %) | 15 (30%) | 9 (15%) | 0.058c |
| Neonatal sepsis (n, %) | 3 (10%) | 5 (8.3%) | 1.000d |
| RDS (n, %) | 8 (16%) | 4 (6.7%) | 0.183c |
| Adverse perinatal outcome (n, %) | 10 (33.3%) | 12 (20%) | 0.165c |
NICU: neonatal intensive care unit; RDS: respiratory distress syndrome.
Independent-samples t-test
Mann-Whitney U test
Chi-square test
Fisher's exact test. Statistically significant value is indicated in bold.
Table 2. Laboratory characteristics of the gestational diabetes mellitus and control groups.
| Variables | GDM group (n=30) | Control group (n=60) | p |
|---|---|---|---|
| Fasting glucose (mg/dL) | 87 (15) | 75 (13) | <0.001 b |
| Postprandial glucose (mg/dL) | 165.5 (45.75) | 112 (26) | <0.001 b |
| HbA1c (%) | 6.1 (1.13) | 5.5 (0.97) | <0.001 b |
| C-reactive protein (mg/L) | 3.3 (1.43) | 2.4 (1.28) | <0.001 b |
| Urea (mg/dL) | 26.14 (7.39) | 25.73 (7.29) | 0.773a |
| Creatinine (mg/dL) | 0.71 (0.2) | 0.6 (0.2) | 0.077b |
| AST (IU/L) | 18 (14.25) | 17 (5.75) | 0.406b |
| ALT (IU/L) | 16 (8.25) | 13 (6) | 0.151b |
| Hemoglobin (g/dL) | 10.99 (1.46) | 10.82 (1.17) | 0.468a |
| Hematocrit (%) | 32.89 (4.47) | 32.43 (2.88) | 0.521a |
| Platelet count (103/L) | 238 (51.25) | 209 (51.75) | 0.024 b |
| Vn (ng/mL) | 91.85 (23.08) | 80.10 (39.18) | <0.001 b |
| PAI-1 (ng/mL) | 6.50 (1.05) | 4.35 (1.0) | <0.001 b |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; Vn: vitronectin; PAI-1: plasmınogen activator inhibitor-1.
Independent samples t-test
Mann-Whitney U test.
Statistically significant values are indicated in bold.
Figure 2. Receiver operating curve for the predictive value of vitronectin to diagnose gestational diabetes mellitus.
Another finding of the study was the correlation of Vn with clinical parameters. Vn had a significant positive correlation with fasting blood glucose (r=0.476, p<0.001), postprandial blood glucose (r=0.489, p<0.001), HbA1c (r=0.713, p<0.001), CRP (r=0.245, p<0.001), and PAI-1 (r=0.586, p<0.001).
DISCUSSION
This study evaluated the role of Vn and PAI-1 in the second trimester of gestation in GDM. The main findings of the study demonstrated that both Vn and PAI-1 levels were increased in GDM. Vn was found to predict GDM with a cutoff value of >84.7 ng/mL with a sensitivity of 70% and specificity of 63.3%. Moreover, Vn levels were correlated with fasting blood glucose, postprandial glucose level, HbA1c, CRP, and PAI-1.
Gestational diabetes is a metabolic disorder that increases women's risk and likelihood of developing type 2 diabetes and cardiovascular disease following life. Inflammatory and metabolic changes that occur in normal pregnancy altered and exaggerated secondary to the excessive systemic inflammatory process in GDM initiated by diffuse endothelial dysfunction 19 . There are several factors regulating PAI-1 expression in GDM, such as hyperglycemia, hyperinsulinemia, proinflammatory cytokines, and elevated angiotensin II 19 . Vn is an adhesive extracellular glycoprotein with binding sites or PAI-1, the urokinase-type plasminogen activator receptor, and various integrins and is circulated as a high-molecular-weight complex 20 . In studies performed with fibroblast culture, it has been shown that PAI-1 inhibits the interaction of Vn and integrin and modulates the properties of Vn. Studies testified that Vn induces insulin secretion independently of glucose and a significant reciprocal decrease in insulin content in fetal beta cells 2 .
There are limited studies in the literature evaluating the role of Vn and PAI-1. Ekmekçi et al. reported high PAI-1, t-PA, and CRP levels and low Vn levels in patients with early- and late-onset preeclampsia (PE). They suggested that increased Vn complex formation led to the increment in PAI-1 level and PAI-1 activity, and decreased Vn levels contributed to the progression of inflammation and hypercoagulability in PE 21 . Contrary to this study, Blumenstein et al. found high Vn and high-molecular-weight quinolone levels in early pregnancy before the development of PE and small gestational age. They stated that Vn provides material endothelin repair by increasing platelet adhesion and aggregation following cell damage in areas with vascular endothelial damage developing in PE 22 .
Yaghoubi et al. reported high Vn levels in patients with coronary artery disease, which correlated significantly with the severity of the disease 23 . These results can be attributed to the regulatory role of Vn in the vascular hemostatic response and thrombus formation in vascular injury in atherosclerotic lesions. Evaluating the role of these markers in diabetes, Alessi et al. found higher basal Vn and PAI-1 levels in patients with metabolic syndrome (MetS) and type 2 diabetes mellitus compared with the control group in their 9-year follow-up. They concluded that Vn is a valuable predictive marker for MetS, independent of PAI-1 6 . In another recent study by Ravnsborg et al., it was confirmed that Vn significantly increased in GDM in their study in pregnant women with BMI>27 m2/kg in the early trimester of pregnancy 17 . In this study, we found higher Vn and PAI-1 levels in GDM, which supports the result of the previous study, and demonstrated a significant correlation with fasting blood glucose, postprandial glucose level, HbA1c, CRP, and PAI-1. Although the results are similar, according to our opinion, this study will contribute to the literature as it does not only consist of patients with high BMI but also includes second-trimester measurements. We suggest that this study could contribute to the literature by evaluating second-trimester Vn levels and assessing the patients regardless of BMI. Results regarding PAI-1 level in GDM are inconsistent. In a study, it was shown that t-PA level increased in GDM, but PAI-1 did not change 23 . Liu et al. verified that t-PA was lower and PAI-1 was significantly higher in GDM patients 24 .
In our study, similar to the literature, we found that the PAI-1 level was statistically significantly higher in the GDM group compared with healthy pregnant women.
This study has some limitations. It had a small sample size arising from the same center from a local region. Second, first-trimester measurements of these markers would be more beneficial for the early prediction of GDM. Finally, not knowing whether the cases of GDM were clinically controlled at the time when the Vn was evaluated was another challenging issue.
CONCLUSION
GDM is a progressive and long-term pregnancy complication with a risk of mortality and morbidity for both mother and fetus. The dynamics of the coagulation cascade and fibrinolysis mechanism at the pathophysiological level in GDM are still not fully clarified. By developing diagnostic biomarkers, elucidating the emerging pathogenic mechanisms for the development and consequences of GDM enables to improved early risk prediction. This study revealed that second-trimester Vn and PAI-1 are increased in GDM and vitronectin could be a candidate for the prediction of GDM.
Footnotes
ETHICAL APPROVAL
This study was approved by the local ethics committee (Number: 2011-KAEK-25; Date: 2020/09-13).
The study was carried out Department of Obstetrics and Gynecology University of Health Sciences, Bursa Yuksek Ihtisas Research and Training Hospital, Bursa, Turkey.
Funding: The expenses of the study were funded by the authors.
REFERENCES
- 1.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(1):13–28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 2.Abell SK, Courten B, Boyle JA, Teede HJ. Inflammatory and other biomarkers: role in pathophysiology and prediction of gestational diabetes mellitus. Int J Mol Sci. 2015;16(6):13442–73. doi: 10.3390/ijms160613442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrar D, Duley L, Dowswell T, Lawlor DA. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2017;8(8):CD007122. doi: 10.1002/14651858.CD007122.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harreiter J, Dovjak G, Kautzky-Willer A. Gestational diabetes mellitus and cardiovascular risk after pregnancy. Women's Health. 2014;10(1):91–108. doi: 10.2217/whe.13.69. [DOI] [PubMed] [Google Scholar]
- 5.Sudasinghe BH, Wijeyaratne CN, Ginige PS. Long and short-term outcomes of gestational diabetes mellitus (GDM) among South Asian women - a community-based study. Diabetes Res Clin Pract. 2018;145:93–101. doi: 10.1016/j.diabres.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Alessi MC, Nicaud V, Scroyen I, Lange C, Saut N, Fumeron F, et al. Association of vitronectin and plasminogen activator inhibitor-1 levels with the risk of metabolic syndrome and type 2 diabetes mellitus. Results from the D.E.S.I.R. prospective cohort. Thromb Haemost. 2011;106(3):416–422. doi: 10.1160/TH11-03-0179. [DOI] [PubMed] [Google Scholar]
- 7.Li T, Hao L, Li J, Du C, Wang Y. Insight into vitronectin structural evolution on material surface chemistries: the mediation for cell adhesion. Bioact Mater. 2020;5(4):1044–1052. doi: 10.1016/j.bioactmat.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puster LO, Stanley CB, Uversky VN, Curtis JE, Krueger S, Chu Y, et al. Characterization of an extensive interface on vitronectin for binding to plasminogen activator inhibitor-1: adoption of structure in an intrinsically disordered region. Biochemistry. 2019;58(51):5117–5134. doi: 10.1021/acs.biochem.9b00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park WU, Yeon GB, Yu MS, Goo HG, Hwang SH, Na D. A novel vitronectin peptide facilitates differentiation of oligodendrocytes from human pluripotent stem cells (Synthetic ecm for oligodendrocyte differentiation) Biology. 2021;10(12):1254–1254. doi: 10.3390/biology10121254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid C, Ignjatovic V, Pang B, Nie S, Williamson NA, Tingay DG, et al. Proteomics reveals region-specific hemostatic alterations in response to mechanical ventilation in a preterm lamb model of lung injury. Thromb Res. 2020;196:466–475. doi: 10.1016/j.thromres.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Goyal U, Ta M. A novel role of vitronectin in promoting survival of mesenchymal stem cells under serum deprivation stress. Stem Cell Res Ther. 2020;11(1):1–14. doi: 10.1186/s13287-020-01682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciregia F, Deroyer C, Cobraiville G, Plener Z, Malaise O, Gillet P, et al. Modulation of αVβ6 integrin in osteoarthritis-related synovitis and the interaction with VTN(381-397 a.a.) competing for TGF-β1 activation. Exp Mol Med. 2021;53(2):210–222. doi: 10.1038/s12276-021-00558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batiha GE, Al-Kuraishy HM, Al-Maiahy TJ, Al-Buhadily AK, Saad HM, Al-Gareeb AI, et al. Plasminogen activator inhibitor 1 and gestational diabetes: the causal relationship. Diabetol Metab Syndr. 2022;14(1):127–127. doi: 10.1186/s13098-022-00900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai J, Li Z, Zhou Y, Yang X. The role of plasminogen activator inhibitor-1 in gynecological and obstetrical diseases: an update review. J. Reprod. Immunol. 2022;150:103490–103490. doi: 10.1016/j.jri.2022.103490. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Li X, Lu C, Zhan X. The relationship between vitronectin and hepatic insulin resistance in type 2 diabetes mellitus. Endocr J. 2018;65(7):747–753. doi: 10.1507/endocrj.EJ17-0504. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Lin Z, Zhan X, Gao J, Sun L, Cao Y, Qiu H. RNA-seq analysis of the transcriptome of the liver of cynomolgus monkeys with type 2 diabetes. Gene. 2018;651:118–125. doi: 10.1016/j.gene.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Ravnsborg T, Andersen LL, Trabjerg ND, Rasmussen LM, Jensen DM, Overgaard M. First-trimester multimarker prediction of gestational diabetes mellitus using targeted mass spectrometry. Diabetologia. 2016;59(5):970–979. doi: 10.1007//s00125-016-3869-8. [DOI] [PubMed] [Google Scholar]
- 18.ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):49–64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 19.Valero P, Cornejo M, Fuentes G, Wehinger S, Toledo F, Beek EM, et al. Platelets and endothelial dysfunction in gestational diabetes mellitus. Acta Physiol (Oxf) 2023;237(4):13940–13940. doi: 10.1111/apha.13940. [DOI] [PubMed] [Google Scholar]
- 20.Teliga-Czajkowska J, Sienko J, Zareba-Szczudlik J, Malinowska-Polubiec A, Romejko-Wolniewicz E, Czajkowski K. Influence of glycemic control on coagulation and lipid metabolism in pregnancies complicated by pregestational and gestational diabetes mellitus. Adv Exp Med Biol. 2019;1176:81–88. doi: 10.1007/5584_2019_382. [DOI] [PubMed] [Google Scholar]
- 21.Balcı Ekmekçi Ö, Ekmekçi H, Güngör Z, Tüten A, Toprak MS, Korkmaz M, et al. Evaluation of Lp-PLA2 mass, vitronectin and PAI-1 activity levels in patients with preeclampsia. Arch Gynecol Obstet. 2015;292(1):53–58. doi: 10.1007/s00404-014-3601-1. [DOI] [PubMed] [Google Scholar]
- 22.Blumenstein M, Prakash R, Cooper GJ, North RA, SCOPE Consortium Aberrant processing of plasma vitronectin and high-molecular-weight kininogen precedes the onset of preeclampsia. Reprod Sci. 2009;16(12):114452–114452. doi: 10.1177/1933719109342756. [DOI] [PubMed] [Google Scholar]
- 23.Yaghoubi A, Ghojazadeh M, Abolhasani S, Alikhah H, Khaki-Khatibi F. Correlation of serum levels of vitronectin, malondialdehyde and Hs- CRP with disease severity in coronary artery disease. J Cardiovasc Thorac Res. 2015;7(3):113–117. doi: 10.15171/jcvtr.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu BY, Jian YL, Zhong M, Yu YH, Wang Q, Zhang J. Value of coagulation function and fibrinolytic system assessment in patients with gestational diabetes mellitus. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(1):35–37. [PubMed] [Google Scholar]