Abstract
Background:
One year of adjuvant durvalumab following concurrent chemoradiotherapy significantly improves progression-free survival (PFS) and overall survival (OS) for patients with stage III non-small cell lung cancer (NSCLC). However, the optimal length of adjuvant therapy has not been determined.
Methods:
We identified patients with stage III NSCLC treated with definitive chemoradiation and adjuvant durvalumab from November 2017 to April 2021 from the United States Veterans Affairs system. Predictors of early durvalumab discontinuation were evaluated with Cox proportional hazards regression. The effect of differing durations of durvalumab treatment (up to 6, 9, and 12 months) on PFS and OS were compared with a marginal structural model and time-dependent Cox modelling.
Results:
We included 1006 patients with stage III non-small cell lung cancer who received concurrent chemoradiotherapy and at least one dose of adjuvant durvalumab. The median duration of durvalumab treatment was 7 months (interquartile range 2.8–11.5) and 31% completed the intended durvalumab course. The most common reasons for early discontinuation were tumour progression (22%), immune-related adverse events (15%), and non-immune-related toxicity (6.0%), Marginal structural models suggested similar PFS for 9 months versus 12 months of durvalumab treatment and inferior PFS for 6 months versus 12 months.
Conclusions:
A substantial proportion of patients undergoing adjuvant durvalumab discontinue therapy early due to toxicity, and shorter durvalumab treatment durations may provide similar disease control to 12 months of therapy. Prospective randomised controlled studies are needed to characterise the optimal durvalumab treatment duration in locally advanced NSCLC patients.
Keywords: Non-small cell lung cancer, Immunotherapy, Durvalumab, De-escalation, Marginal structural modelling, Treatment duration
1. Introduction
With the publication of overall survival results from the PACIFIC trial in 2018 [1], 12 months of adjuvant durvalumab became the standard of care for patients with stage III non-small cell lung cancer (NSCLC) after definitive concurrent chemoradiotherapy (cCRT). Despite this exciting advance, treatment adherence and toxicity rates in real-world cohorts are commonly inferior to clinical trial populations [2]. While real-world outcomes of durvalumab maintenance have been presented in the PACIFIC-R study [3], the toxicity rates of durvalumab within the United States Veteran population, a patient population that was not included in the PACIFIC trial, have not been clearly defined. Further, though 12 months of adjuvant durvalumab remains the standard of care, it is unclear whether 12 months is the optimal therapy duration, and there have been no randomised comparisons of differing durations of adjuvant durvalumab to date. It is possible that durvalumab duration could be safely de-escalated to improve adherence, improve tolerance, decrease healthcare costs, and lower patient financial toxicity, without compromising cancer control [4].
In this study, we evaluate adjuvant durvalumab adherence, toxicity, and predictors for early discontinuation among a large, national, multicenter cohort drawn from the United States Veterans Affairs System. We further examine the influence of durvalumab treatment duration on oncologic outcomes to assess feasibility of a randomised controlled trial de-escalating durvalumab treatment duration.
2. Methods
2.1. Data source
We identified lung cancer patients using the Department of Veterans Affairs (VA) Informatics and Computing Infrastructure (VINCI). VINCI is an informatics platform that allows access to patient-level electronic health record information and administrative data for all veterans within the VA healthcare system. VINCI also incorporates tumour registry data uploaded from individual VA sites; these data are gathered by trained registrars according to standard protocols. This study was approved by the local institutional review board.
2.2. Cohort definition
We included patients with histologically confirmed stage III NSCLC (AJCC 7th–8th edition) treated with definitive cCRT and given at least one dose of adjuvant durvalumab between November 2017 and April 2021. The first and last durvalumab infusion dates were first identified with outpatient infusion records and confirmed by manual chart review. Staging and definitive treatment information were obtained by manual review of the medical record. These staging and treatment data were supplemented with data from the Veterans Affairs Cancer Registry System (VACRS) where available.
2.3. Durvalumab duration and early discontinuation
Durvalumab treatment duration was defined as the difference in days between the first and most recent infusion dates; this was defined as 14 days for patients with a single infusion. The number of durvalumab infusions and reason for early durvalumab discontinuation (classified as progression, immune-related adverse event [irAE], non-irAE toxicity, declining performance status, patient preference, lost-to-follow-up, death, or other/unknown) were obtained through manual review of physician notes. Every-two-week, weight-based durvalumab dosing was the standard of care through most of the study period; a small minority (2.5%) of patients started durvalumab therapy after November 2020, after the US Federal Drug Administration approved every-4-week dosing. In a chart review of 100 randomly selected patients from the cohort, 100% received every-two-week dosing. Patients were categorised as having durvalumab-related toxicity if the toxicity was possibly, probably, or definitely related to durvalumab in the judgement of the managing outpatient oncologist or inpatient physician. The presence of irAE was recorded if severe enough to warrant treatment discontinuation and was confirmed by manual review of oncology notes by a physician (K.S. and A.K.B).
2.4. Outcomes and baseline covariates
The primary outcome measures were early treatment discontinuation, progression-free survival (PFS), and overall survival (OS). Early treatment discontinuation was defined as durvalumab discontinuation before the intended completion date for reasons other than tumour progression or death, and time to discontinuation was measured from the first durvalumab infusion to the last infusion. Patients with ongoing durvalumab therapy at the time of most recent follow-up were censored on the date of the most recent durvalumab infusion. Progression-free survival was defined as the date of radiographic progression or death, whichever occurred first. Date of radiographic progression was determined and confirmed by manual review of radiological reports by a licensed physician (M.D.G. and K.S.). Date of death was obtained from the VA Vital Status File (drawn from Medicare, Social Security Administration, and the internal VA death registry) and supplemented with the VA Master Patient Index for more recent deaths. OS and PFS were measured from the date of durvalumab initiation. Patients were censored at the date of last known follow-up, defined as the most recent encounter with a VA provider. Patients with ongoing follow-up past April 15, 2021 were administratively censored at that time.
Demographics including race, sex, and age were obtained through the Master Patient Index. Charlson Comorbidity Index (CCI) [5,6] was calculated from inpatient and outpatient ICD-10 diagnosis codes in the year before durvalumab start. Smoking status was obtained through Health Factors data [7,8]. Concurrent chemotherapy regimen was obtained through intravenous infusion records and supplemented with the VACRS where available.
2.5. Statistical analysis
Differences in baseline characteristics were assessed with the chi-square test for categorical variables and the t-test for continuous variables. Predictors of early treatment discontinuation were evaluated in multivariable Cox proportional hazards regression; in this analysis, patients were censored at the time of tumour progression, end of follow-up, or completion of planned therapy, whichever was first. This analysis adjusted for age (continuous, per 10 years), sex (male vs. female), race (African American, Caucasian, or other/unknown), smoking status (current, former, never, or unknown), CCI (0–2, 3–5, 6–8, or ≥ 9), AJCC summary stage (IIIA, IIIB, IIIC, or III not otherwise specified), concurrent chemotherapy regimen (carboplatin-paclitaxel vs other), and histology (adenocarcinoma, squamous cell carcinoma, or other).
To investigate the effect of durvalumab treatment duration on PFS and OS, we pursued a marginal structural modelling approach with the cloning, censoring, and weighting technique described by others [9–12]. This approach involves cloning each patient, assigning each clone to one arm of a hypothetical randomised clinical trial, artificially censoring each clone when they deviate from their assigned strategy, and assigning time-dependent weights to eliminate the selection bias introduced by artificial censoring. Our target trial compared up to 6, 9, and 12 months of durvalumab treatment. Patients were deemed non-compliant with each arm if they discontinued treatment early due to disallowed reasons or if they continued treatment beyond their assigned duration. Early discontinuation due to progression, irAE, durvalumab-related toxicity, or death were considered protocol-compliant; early discontinuation due to any other reason was disallowed. Patients with ongoing durvalumab therapy were censored on the date of the most recent durvalumab infusion. Time-dependent, stabilised inverse probability of censoring weights [9] were estimated with logistic regression using person-weeks of follow-up. The censoring model included all baseline covariates listed above plus treatment arm, follow-up time, and the interaction of time and treatment arm. Weighted Kaplan–Meier survival estimates including restricted mean survival time (RMST, truncated at 2 years of follow-up), 1- and 2-year PFS, and 1- and 2-year OS were generated for each arm. Weighted univariable Cox regression analysis was also performed to assess the effect of treatment arm. 95% confidence intervals (CI) for each metric and for the differences between duration groups in each metric were generated from 1000 bootstrap replicates.
To further analyse the effect of durvalumab treatment duration on PFS and OS, we performed sensitivity analyses using time-dependent Cox regressions. We first incorporated the cumulative months of durvalumab treatment completed as a time-dependent covariate in multivariable Cox regressions for PFS and OS. In addition to baseline covariates included in the primary analysis, time-dependent covariates in these models included the most common reasons for early discontinuation other than progression (irAE, declining performance status, and non-irAE toxicity). The relationship between cumulative months of durvalumab and PFS or OS was modelled as a penalised spline with three degrees of freedom. We used partial residual plots to assess departures from linearity in the relationship between cumulative durvalumab duration and the outcomes. Based on these analyses, we then discretized cumulative durvalumab duration into intervals of 0–2.9 months, 3–5.9 months, 6–8.9 months, 9–11.9 months, and 12+ months and assessed pairwise comparisons. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc, NC) and R v4.0.2 (R Core Team, Vienna).
3. Results
3.1. Patient characteristics
We identified 1006 patients with stage III NSCLC who received cCRT followed by at least one dose of durvalumab. The median age was 69 years (interquartile range [IQR]: 64 to 72), and most patients were male (95%) and Caucasian (74%); 22% of the cohort was Black (Table 1). Most patients were current (43%) or former (40%) smokers, 48% had squamous cell histology, and 56% had stage IIIA disease. 2-year PFS was 42.7% (95% confidence interval [CI] 39.3–46.5) and 2-year OS was 61.9% (95% CI 58.5–65.5). Median follow-up was 19.9 months (IQR 10.7–27.1 months). The median PFS was 17.1 months and median OS was 34.3 months.
Table 1.
Characteristics of the sample.
| Characteristic | N = 1006a |
|---|---|
|
| |
| Age (in years) | 69 (64, 72) |
| Median follow-up time (in months) | 20 (12, 27) |
| Race | |
| White | 745 (74%) |
| Black | 221 (22%) |
| Other/unknown | 40 (4.0%) |
| Male | 959 (95%) |
| CCI | |
| 0–2 | 148 (15%) |
| 3–5 | 342 (34%) |
| 6–8 | 137 (14%) |
| 9+ | 379 (38%) |
| Smoking | |
| Current | 436 (43%) |
| Former | 402 (40%) |
| Never | 86 (8.5%) |
| Unknown | 82 (8.2%) |
| Summary stage | |
| IIIA | 559 (56%) |
| IIIB | 352 (35%) |
| IIIC | 66 (6.6%) |
| III NOS | 29 (2.9%) |
| Carboplatin/paclitaxel chemotherapy | 711 (71%) |
| Histology | |
| Adenocarcinoma | 490 (49%) |
| Other | 31 (3.1%) |
| Squamous cell carcinoma | 485 (48%) |
| Durvalumab treatment duration (in days) | 215 (84, 350) |
| Reason for durvalumab discontinuation | |
| Completed as planned | 314 (31%) |
| Tumour progression | 221 (22%) |
| irAE | 152 (15%) |
| Ongoing at last follow-up | 136 (14%) |
| Non-irAE toxicity | 60 (6.0%) |
| Other | 45 (4.5%) |
| Death | 24 (2.4%) |
| Declining performance status | 19 (1.9%) |
| Patient preference | 18 (1.8%) |
| Lost to follow-up | 17 (1.7%) |
| IrAE causing durvalumab discontinuation | |
| Pneumonitis | 106 (70%) |
| Other | 22 (14%) |
| Colitis | 12 (7.9%) |
| Arthritis | 6 (3.9%) |
| Myositis | 3 (2.0%) |
| Nephritis | 3 (2.0%) |
Abbreviations: CCI: Charlson comorbidity index; NOS: not otherwise specified; irAE: immune-related adverse events.
Median (IQR); n (%).
3.2. Treatment adherence and predictors of early durvalumab discontinuation
The median duration of durvalumab therapy was 215 days (IQR: 84 to 350) with 50 patients (4.9%) receiving a single infusion before discontinuation. 33% of the cohort discontinued durvalumab early due to reasons other than disease progression and 31% of the cohort completed therapy as planned. The most common reasons for early durvalumab discontinuation overall were disease progression (22%), irAE (15%), and non-irAE toxicity (6%) (Fig. 1). 136 patients (13.5%) had ongoing durvalumab therapy at the time of last follow-up. The most common irAE was pneumonitis (70% of irAE cases) followed by colitis (7.9%).
Fig. 1. Cumulative rates of early durvalumab discontinuation by underlying cause.

Abbreviations: PS: performance status; irAE: immune-related adverse events; f/u: follow-up.
In the multivariable Cox regression for early treatment discontinuation, older age was associated with increased hazard of early discontinuation (hazard ratio [HR] 1.24 per 10-year increase, 95% CI 1.04–1.47, p = 0.013; Table 2). Smoking status, histology, CCI, summary stage, sex, and chemotherapy regimen were not associated with early discontinuation. Predictors of increased risk of disease progression or death included adenocarcinoma histology, older age, and higher summary stage (Table 2).
Table 2.
Effect of baseline factors on early durvalumab discontinuation or progression-free survival.
| Characteristic | Risk of early discontinuation (not due to progression or death) |
Risk of progression or death |
||||
|---|---|---|---|---|---|---|
| HRa | 95% CIa | p-value | HRa | 95% CIa | p-value | |
|
| ||||||
| Age (per 10 years) Histology | 1.24 | 1.05, 1.47 | 0.013 | 1.15 | 1.00, 1.33 | 0.057 |
| Adenocarcinoma | - | - | - | - | ||
| Other | 1.06 | 0.54, 2.08 | 0.9 | 0.72 | 0.39, 1.30 | 0.3 |
| Squamous cell carcinoma | 1.03 | 0.82, 1.29 | 0.8 | 0.75 | 0.62, 0.90 | 0.002 |
| CCI | ||||||
| 0–2 | - | - | - | - | ||
| 3–5 | 1.33 | 0.93, 1.90 | 0.11 | 0.90 | 0.68, 1.19 | 0.5 |
| 6–8 | 1.42 | 0.94, 2.15 | 0.10 | 1.15 | 0.83, 1.59 | 0.4 |
| 9+ | 1.01 | 0.70, 1.45 | > 0.9 | 0.91 | 0.69, 1.20 | 0.5 |
| Summary stage | ||||||
| IIIA | - | - | - | - | ||
| IIIB | 1.25 | 0.99, 1.58 | 0.056 | 1.37 | 1.13, 1.65 | 0.001 |
| IIIC | 1.13 | 0.70, 1.80 | 0.6 | 1.59 | 1.12, 2.25 | 0.009 |
| III NOS | 1.26 | 0.64, 2.48 | 0.5 | 1.12 | 0.60, 2.08 | 0.7 |
| Male | 0.92 | 0.55, 1.55 | 0.8 | 1.75 | 1.02, 3.00 | 0.043 |
| Smoking | ||||||
| Current | - | - | - | - | ||
| Former | 1.15 | 0.90, 1.47 | 0.3 | 0.94 | 0.77, 1.15 | 0.5 |
| Never | 1.27 | 0.86, 1.88 | 0.2 | 1.08 | 0.78, 1.49 | 0.6 |
| Unknown | 0.84 | 0.53, 1.34 | 0.5 | 0.94 | 0.65, 1.35 | 0.7 |
| Race | ||||||
| Black | - | - | - | - | ||
| Other/unknown | 1.65 | 0.98, 2.80 | 0.061 | 1.24 | 0.77, 1.98 | 0.4 |
| White | 1.06 | 0.81, 1.39 | 0.7 | 1.05 | 0.84, 1.31 | 0.7 |
| Carboplatin/paclitaxel chemotherapy | 1.00 | 0.78, 1.28 | > 0.9 | 1.19 | 0.97, 1.46 | 0.10 |
Abbreviations: CCI: Charlson comorbidity index; NOS: not otherwise specified.
HR = Hazard Ratio, CI = Confidence Interval. Bolded results are statistically significant.
3.3. Effect of durvalumab treatment duration on survival outcomes
In the marginal structural model evaluating up to 6, 9, and 12 months of durvalumab treatment for PFS, compared to 12 months of therapy we observed similar PFS for 9 months and somewhat lower PFS for 6 months (Table 3). Compared to 12 months, 9 months of therapy showed similar 1-year PFS (9 months: 54.5%; 12 months: 61.8%) and 2-year PFS (9 months: 30.9%; 12 months: 35.7%), and the differences between the 9 and 12-month groups were non-significant (Table 3). 9 months of therapy also showed similar RMST (9 months: 14.6 months; 12 months: 15.4 months) and hazard of PFS (HR 1.06 for 9 vs. 12 months, 95% CI 0.86–1.25). 6 months of therapy showed larger declines in PFS and RMST compared to 12 months, though the HR for PFS was non-significant (HR 1.20 for 6 months vs. 12 months, 95% CI 0.97–1.43). In the model for OS, 9 months of therapy showed similar 2-year OS and hazard of death compared to 12 months (Table 3) while 6 months of therapy was associated with a significantly higher hazard of death compared to 12 months (HR 1.53, 95% CI 1.25–1.85).
Table 3.
Results of marginal structural models comparing up to 6, 9, and 12 months of durvalumab treatment.
| Metric | PFS |
OS |
|
|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | ||
|
| |||
| 1-year Kaplan–Meier estimate (in %) | 6 months | 44 (34.8–53.9) | 59.9 (53.5–65.9) |
| 9 months | 54.5 (48.6–61.3) | 72.9 (68.6–76.9) | |
| 12 months | 61.8 (58.2–65) | 78.9 (76.2–81.6) | |
| 6 vs. 12 months (difference) | −17.8 (−27.3 to −7.3) | −19 (−26 to −12.4) | |
| 9 vs. 12 months (difference) | −7.3 (−13.8 to 0.5) | −6.0 (−11.2 to −1.0) | |
|
| |||
| 2-year Kaplan–Meier estimate (%) | 6 months | 22.8 (14.5–36.4) | 36.4 (29–45.1) |
| 9 months | 30.9 (22.1–45) | 45.1 (38.1–52.7) | |
| 12 months | 35.7 (26–47) | 50.3 (44.2–56.9) | |
| 6 vs. 12 months (difference) | −12.9 (−26.6 to 4.4) | −13.9 (−23.5 to −3.5) | |
| 9 vs. 12 months (difference) | −4.8 (−18.7 to 12.7) | −5.3 (−14.8 to 4.5) | |
|
| |||
| Restricted mean survival time (in months) | 6 months | 12.8 (11.6–14.3) | 15.7 (14.7–16.6) |
| 9 months | 14.6 (13.8–15.7) | 17.6 (16.9–18.3) | |
| 12 months | 15.4 (14.6–16.2) | 18.3 (17.7–18.9) | |
| 6 vs. 12 months (difference) | −2.6 (−4.1 to −0.9) | −2.6 (−3.8 to −1.6) | |
| 9 vs. 12 months (difference) | −0.8 (−2.0 to 0.6) | −0.7 (−1.7 to 0.2) | |
|
| |||
| Cox hazard ratio | 6 vs. 12 months | 1.20 (0.97 to 1.43) | 1.53 (1.25–1.85) |
| 9 vs. 12 months | 1.06 (0.86 to 1.25) | 1.15 (0.96 to 1.40) | |
Abbreviations: PFS: progression-free survival; OS: overall survival; CI: confidence interval.
Bolded results reflect difference estimates for which the 95% CI crosses zero, suggesting no statistical difference between duration groups. 95% CIs were generated through 1000 bootstrap replicates.
In the sensitivity analysis using multivariable time-dependent Cox regression for PFS, cumulative durvalumab duration showed a non-linear association with progression, with the adjusted relative hazard of progression decreasing from 1 month to ~6 months of cumulative durvalumab therapy after which the relative hazard flattened (Fig. 2A). A more linear decreasing pattern was observed for OS (Fig. 2B). Given this non-linearity in PFS, cumulative duration was discretized into 3-month intervals and incorporated into the model; the results of the time-dependent Cox regressions are shown in Table 4. Overall, there was no significant difference between 12+ months of therapy and shorter durations except for 0–2.9 months (HR 1.91, 95% CI 1.08–3.39, p = 0.026). We observed larger differences in overall survival with shorter durations.
Fig. 2. Plateauing effect of cumulative durvalumab treatment on progression-free survival.
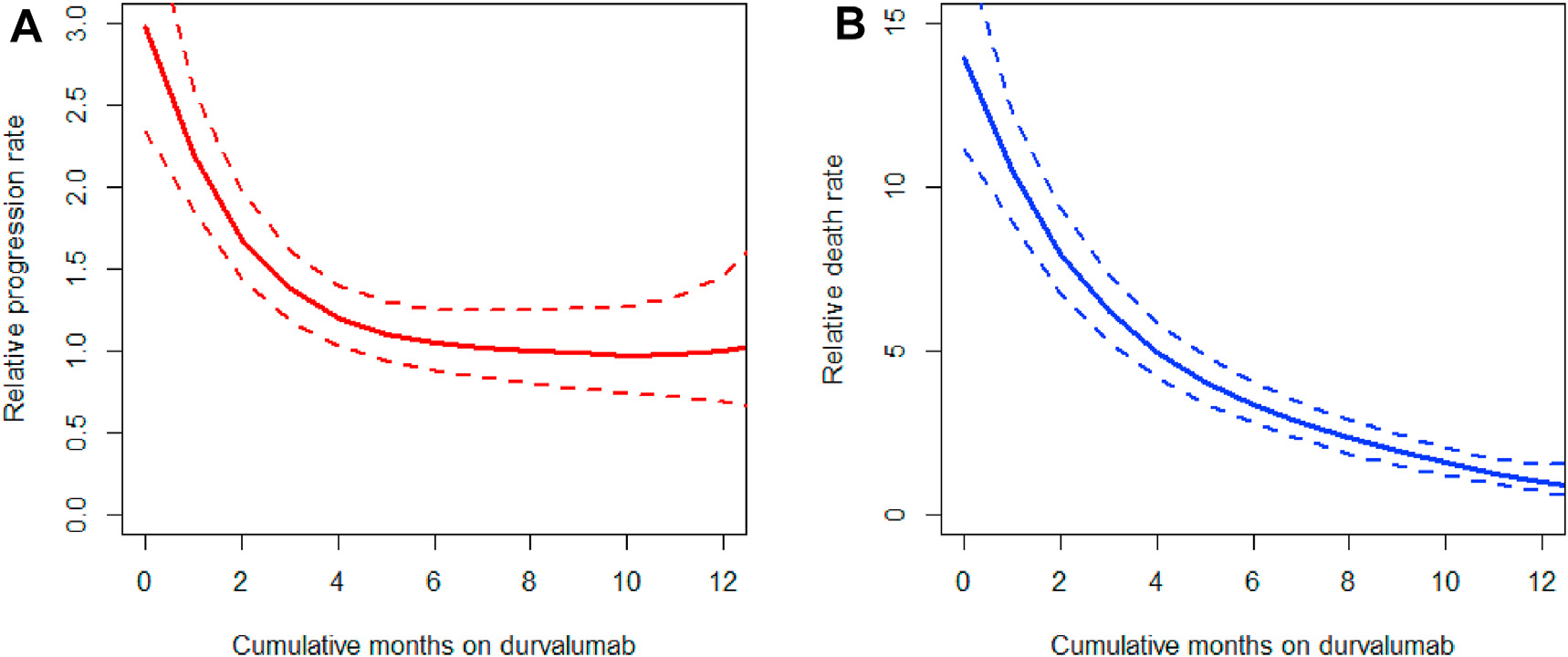
Partial residuals plots derived from multivariable time-dependent Cox regression for PFS (A) and OS (B), showing a non-linear relationship between cumulative months of durvalumab treatment and adjusted relative hazard for progression (A) and overall survival (B).
Table 4.
Time-dependent Cox regression for effect of durvalumab duration on progression-free and overall survival.
| Characteristic | Progression-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|
| HRa | 95% CIa | p-value | HRa | 95% CIa | p-value | |
|
| ||||||
| Cumulative duration of durvalumab treatment | ||||||
| 12+ months | – | – | – | – | ||
| 0–2.9 months | 1.91 | 1.08, 3.39 | 0.026 | 11.8 | 5.56, 25.0 | < 0.001 |
| 3.0–5.9 months | 1.08 | 0.61, 1.91 | 0.8 | 5.38 | 2.49, 11.6 | < 0.001 |
| 6.0–8.9 months | 0.81 | 0.46, 1.43 | 0.5 | 2.60 | 1.18, 5.72 | 0.017 |
| 9.0–11.9 months | 0.79 | 0.48, 1.27 | 0.3 | 1.43 | 0.66, 3.07 | 0.4 |
| Age (per year) Histology | 1.01 | 1.00, 1.03 | 0.13 | 1.03 | 1.01, 1.04 | 0.005 |
| Adenocarcinoma | – | – | – | – | ||
| Other | 0.68 | 0.37, 1.23 | 0.2 | 0.58 | 0.27, 1.26 | 0.2 |
| Squamous cell carcinoma | 0.76 | 0.63, 0.91 | 0.003 | 0.76 | 0.61, 0.96 | 0.018 |
| CCI | ||||||
| 0–2 | – | – | – | – | ||
| 3–5 | 0.81 | 0.61, 1.08 | 0.2 | 0.80 | 0.57, 1.12 | 0.2 |
| 6–8 | 1.03 | 0.74, 1.43 | 0.9 | 0.93 | 0.63, 1.38 | 0.7 |
| 9+ | 0.87 | 0.66, 1.15 | 0.3 | 0.80 | 0.57, 1.12 | 0.2 |
| Summary stage | ||||||
| IIIA | – | – | – | – | ||
| IIIB | 1.31 | 1.08, 1.59 | 0.005 | 1.34 | 1.06, 1.69 | 0.013 |
| IIIC | 1.65 | 1.16, 2.33 | 0.005 | 1.31 | 0.85, 2.02 | 0.2 |
| III NOS | 1.03 | 0.55, 1.92 | > 0.9 | 1.03 | 0.49, 2.17 | > 0.9 |
| Male | 1.77 | 1.03, 3.04 | 0.040 | 1.76 | 0.93, 3.36 | 0.085 |
| Smoking | ||||||
| Current | – | – | – | – | ||
| Former | 0.91 | 0.74, 1.11 | 0.3 | 0.89 | 0.70, 1.15 | 0.4 |
| Never | 1.08 | 0.78, 1.50 | 0.6 | 1.03 | 0.70, 1.52 | 0.9 |
| Unknown | 0.99 | 0.69, 1.42 | > 0.9 | 0.85 | 0.55, 1.31 | 0.5 |
| Race | ||||||
| Black | – | – | – | – | ||
| Other/unknown | 1.03 | 0.64, 1.67 | 0.9 | 1.13 | 0.64, 2.00 | 0.7 |
| White | 1.04 | 0.83, 1.30 | 0.7 | 1.26 | 0.95, 1.68 | 0.10 |
| Carboplatin/paclitaxel chemotherapy | 1.25 | 1.01, 1.54 | 0.038 | 1.21 | 0.94, 1.55 | 0.13 |
| Durvalumab discontinuation due to irAE | 1.30 | 0.96, 1.77 | 0.086 | 0.86 | 0.63, 1.17 | 0.3 |
| Durvalumab discontinuation due to declining performance status | 2.25 | 1.21, 4.20 | 0.010 | 2.33 | 1.30, 4.18 | 0.005 |
| Durvalumab discontinuation due to non-irAE toxicity | 1.43 | 0.95, 2.14 | 0.083 | 1.03 | 0.69, 1.55 | 0.9 |
Abbreviations: CCI: Charlson comorbidity index; NOS: not otherwise specified.
HR = Hazard Ratio, CI = Confidence Interval, Bolded results are statistically significant.
4. Discussion
In this study of over 1000 veterans with stage III NSCLC treated with cCRT and adjuvant durvalumab, only 31% completed a full year of adjuvant therapy as planned. 33% discontinued durvalumab early due to reasons other than progression, and older age was associated with early discontinuation. We and others have shown that real-world patients receive a shorter median duration of durvalumab therapy as compared to patients on the PACIFIC trial and experience higher rates of toxicity, particularly pneumonitis [13]. Due to this substantial toxicity burden and low completion rate, we investigated the effect of decreasing durvalumab treatment duration with a marginal structural modelling and time-dependent Cox modelling. These analyses suggested that shorter durations of adjuvant durvalumab therapy may provide similar disease control to the standard 12 months; this may offer the possibility of decreased toxicity, lower healthcare costs, and reduced treatment burden on patients. While preliminary and limited by their retrospective nature, our data suggest that prospective investigation of de-escalating durvalumab duration may be warranted.
While recent high-profile studies have shown a substantial clinical benefit with adjuvant immunotherapy in multiple cancer types such as renal cell carcinoma [14], muscle-invasive bladder cancer [15], melanoma [16], and non-small cell lung cancer [1], these studies typically prescribe 1 year of adjuvant immunotherapy, imposing logistical, financial, and social burdens on patients while causing substantial costs to healthcare systems [17]. Adjuvant therapy duration has been successfully de-escalated for many patients with stage III colon cancer, where a series of large, randomised studies suggested near-equivalence of 3 versus 6 months of adjuvant chemotherapy for OS [18] and substantial reductions in toxicity, cost, and patient inconvenience with shorter regimens [19,20]. No randomised studies to date have investigated the optimal duration of adjuvant immunotherapy, and it is possible that shorter durations would provide similar oncologic benefit while decreasing patient burden and cost [4].
Using a marginal structural modelling approach which involves the emulation of a hypothetical randomised clinical trial, we found that shorter treatment durations may preserve the oncologic benefit of 12 months of durvalumab therapy with regards to PFS. This was supported by sensitivity analyses using a time-dependent Cox modelling approach. Our findings are generally supported by a recent report of 113 patients treated with adjuvant durvalumab, in which patients who discontinued durvalumab after a median of approximately 4 months had similar outcomes to patients who never discontinued treatment for toxicity [21]. While we did observe larger differences in OS with shorter durations compared to PFS, we believe that unmeasured confounders may be responsible for this discrepancy, as it does not seem plausible that true OS differences would emerge without concomitant PFS differences. Compared to OS, PFS is also less likely to be affected by unmeasured baseline confounders (such as unmeasured comorbidity, performance status, or frailty) and time-dependent confounders (such as tumour progression, which is associated both with shorter durvalumab duration and death, and aggressiveness of post-progression therapy). Overall, our data are suggestive that shorter durations of durvalumab may be provide similar disease control to the current standard of care of 12 months.
Strengths of our analysis include large patient numbers, the national and multicenter setting within an integrated healthcare system, and the availability of patient charts to manually confirm treatment dates, capture disease progression, and ascertain reasons for durvalumab discontinuation. The primary limitation of our study is its retrospective nature, which limits the certainty of our conclusions regarding treatment duration and oncologic outcomes. The difficulties of using observational data to analyse the causal effect of treatment duration on time-to-event outcomes are well-known and have been described extensively by others [11,22,23]. A major concern is time-dependent confounding, in which time-varying patient factors influence both the decision to discontinue treatment and affect oncologic outcomes; the situation is even more complex when prior treatment influences the confounder. We attempted to mitigate this concern using both marginal structural models, which were developed to accommodate complex time-dependent confounding [24], and with time-dependent Cox modelling, which explicitly controls for the reasons for early durvalumab discontinuation. Though hazard ratios from time-dependent Cox models of treatment duration require careful interpretation, as ‘only patients who survive for a long time can be treated for a long time,’ [11] the results of this analysis are broadly consistent with the marginal structural model. The majority of patients in our sample were treated with concurrent carboplatin and paclitaxel, which may not be representative of typical concurrent chemotherapy regimens at other centers. Other limitations include the low representation of women in the VHA database (though we note the substantial representation of African American men in our study [22%] compared to PACIFIC [1.9%]) and the well-described demographic and comorbidity differences between veteran and civilian populations [25], which may limit generalisability.
In summary, we show substantial rates of early treatment discontinuation of adjuvant durvalumab among veterans with stage III NSCLC who completed a course of cCRT and suggest that shorter durations of durvalumab treatment may provide similar benefit to the standard 12 months. These data are suggestive that patients may derive most of the benefits of adjuvant durvalumab within the first 6 months, but they cannot inform if some patients should receive a longer course beyond 12 months. Prognostic biomarkers are needed to predict who can be safely treated with shorter courses of adjuvant durvalumab, as this would reduce healthcare costs and decrease financial, logistical, and social burdens on patients. Prospective randomised studies are needed to further evaluate opportunities to de-escalate the duration of adjuvant durvalumab.
Funding statement
Lung Precision Oncology Program, VA Ann Arbor Healthcare System. NCI, Lungevity, Melanoma Research Alliance, Rogel Cancer Center.
Footnotes
Conflict of interest statement
The authors declare the following financial interests/personal relationships that may be considered as potential competing interests:
A.K.B. serves as a consultant for Boston Consulting Group. G.W.S. serves an uncompensated position on the Board of Directors for the Optimal Cancer Alliance. D.M. participated in a steering committee for AstraZeneca in June 2020. M.J.K. has received research funding from Novartis, AstraZeneca, Bristol-Myers Squibb, Regeneron, and Genentech. K.S., L.Z., D.E., M.D.G. and N.R. do not have any conflicts of interest.
References
- [1].Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379(24):2342–50. [DOI] [PubMed] [Google Scholar]
- [2].Cramer-van der Welle CM, Verschueren MV, Tonn M, et al. Real-world outcomes versus clinical trial results of immunotherapy in stage IV non-small cell lung cancer (NSCLC) in the Netherlands. Sci Rep 2021;11(1):6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Girard N, Smit HJM, Sibille A, et al. PACIFIC-R real-world study: treatment duration and interim analysis of progression-free survival in unresectable stage III NSCLC patients treated with durvalumab after chemoradiotherapy. Ann Oncol 2021;32(suppl_5):S939–48. [Google Scholar]
- [4].Stav I, Gyawali B, Goldstein DA. Duration of adjuvant immunotherapy-biologic, clinical and economic considerations. Med Oncol 2018;35(12):160. [DOI] [PubMed] [Google Scholar]
- [5].Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- [6].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- [7].Golden SE, Hooker ER, Shull S, et al. Validity of Veterans Health Administration structured data to determine accurate smoking status. Health Informatics J 2020;26(3):1507–15. [DOI] [PubMed] [Google Scholar]
- [8].Melzer AC, Pinsker EA, Clothier B, et al. Validating the use of veterans affairs tobacco health factors for assessing change in smoking status: accuracy, availability, and approach. BMC Med Res Methodol 2018;18(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11(5):550–60. [DOI] [PubMed] [Google Scholar]
- [10].Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11(5):561–70. [DOI] [PubMed] [Google Scholar]
- [11].Hernán MA. How to estimate the effect of treatment duration on survival outcomes using observational data. BMJ 2018;360:k182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huitfeldt A, Kalager M, Robins JM, Hoff G, Hernán MA. Methods to estimate the comparative effectiveness of clinical strategies that administer the same intervention at different times. Curr Epidemiol Rep 2015;2(3):149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sankar K, Bryant AK, Strohbehn GW, et al. Real world outcomes versus clinical trial results of durvalumab maintenance in veterans with stage III non-small cell lung cancer [Internet] Cancers 2022;14(3). 10.3390/cancers14030614. Available from:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 2021;385(8):683–94. [DOI] [PubMed] [Google Scholar]
- [15].Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med 2021;384(22):2102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378(19):1789–801. [DOI] [PubMed] [Google Scholar]
- [17].Tran G, Zafar SY. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Ann Transl Med 2018;6(9):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].André T, Meyerhardt J, Iveson T, et al. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol 2020;21(12):1620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Iveson T, Boyd KA, Kerr RS, et al. 3-month versus 6-month adjuvant chemotherapy for patients with high-risk stage II and III colorectal cancer: 3-year follow-up of the SCOT non-inferiority RCT. Health Technol Assess 2019;23(64):1–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Iveson TJ, Kerr RS, Saunders MP, et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol 2018;19(4):562–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shaverdian N, Offin M, Shepherd AF, et al. Association between the early discontinuation of durvalumab and poor survival in patients with stage III NSCLC. JTO Clin Res Rep 2021;2(7):100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Redmond C, Fisher B, Wieand HS. The methodologic dilemma in retrospectively correlating the amount of chemotherapy received in adjuvant therapy protocols with disease-free survival. Cancer Treat Rep 1983;67(6):519–26. [PubMed] [Google Scholar]
- [23].Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- [24].Shinozaki T, Suzuki E. Understanding marginal structural models for time-varying exposures: pitfalls and tips. J Epidemiol 2020;30(9):377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schult TM, Schmunk SK, Marzolf JR, Mohr DC. The health status of veteran employees compared to civilian employees in Veterans Health Administration. Mil Med 2019;184(7–8):e218–24. [DOI] [PubMed] [Google Scholar]


