Abstract
Purpose
Cow’s milk protein allergy (CMPA) is a common condition in infants, but little is known about healthcare providers’ clinical experience treating infants with CMPA. To address this gap, we analyzed prospectively collected data from healthcare providers (HCPs) who treated infants under six months old with suspected CMPA using hypoallergenic formulas. The study focused on a commercial extensively hydrolyzed formula containing Lactobacillus rhamnosus GG (ATCC53103) (eHF-LGG) or a commercial amino acid formula (AAF).
Methods
In this secondary analysis of prospectively collected survey data, 52 HCPs treated 329 infants under six months old with suspected CMPA using hypoallergenic formulas. A series of two de-identified surveys per patient were collected by HCPs to assess short-term symptom relief in the patients and HCP’s satisfaction with the management strategies. The initial survey was completed at the initiation of treatment of CMPA, and the second survey was completed at a follow-up visit.
Results
The majority of HCPs (87%) in the study were general pediatricians, and most saw 2 to 10 CMPA patients weekly. Results showed that clinicians reported satisfaction with treatment in 95% of patients in the EHF cohort and 97% of patients in the AAF cohort and achieved expected clinical results in 93% and 97% of patients using eHF and AAF, respectively. Furthermore, few patients were switched from the hypoallergenic formula once initiated.
Conclusion
The study provides new insights into HCP perspectives on treating infants with CMPA and supports using hypoallergenic formulas to manage this condition. However, additional prospective controlled studies are needed to confirm these initial findings.
Keywords: Milk hypersensitivity, Infant formula, Infant, Lacticaseibacillus rhamnosus
INTRODUCTION
Cow’s milk protein allergy (CMPA) is a common food allergy in infants and young children, with an estimated 2 to 3% prevalence in developed countries [1,2,3,4,5,6,7]. CMPA induces different types of immune reactions that affect a wide range of organ systems, leading to cutaneous (50–60%), digestive (50–60%), and respiratory (20–30%) manifestations [7,8,9]. Symptoms include eczema, urticaria, angioedema, colic, vomiting, diarrhea, rectal bleeding, and stooling difficulties, among others [8,9]. CMPA occurs more frequently in non-exclusively breastfed infants than in breastfed infants. Symptoms typically develop before one month of age and within one week of introducing cow’s milk protein-based formula [7,10,11].
Proven treatment strategies for CMPA in breastfed infants include cow’s milk protein elimination from the maternal diet [8,11]. In non-exclusively breastfed infants with CMPA, the first-line treatment uses hypoallergenic formulas, which include extensively hydrolyzed formulas (eHF) for most cases. In contrast, amino acid formulas (AAF) are reserved for more severe cases [8,11].
Despite these recommended CMPA treatment guidelines, there is a paucity of literature examining healthcare providers’ (HCP) experience with these treatment options in the outpatient clinical setting. In this secondary analysis of prospectively collected survey data, we aimed to investigate the clinical experience of HCPs when treating infants with CMPA with either an eHF or an AAF to determine HCPs’ perceptions of effectiveness in two treatment strategies for CMPA in infants ≤6 months of age in a clinical setting. Specifically, we investigated the HCPs’ overall satisfaction with the two management strategies and examined whether the formula treatments achieved the expected outcomes at the next follow-up visit. This study provides insight into HCP perceptions of these two management strategies in a real-world clinical setting.
MATERIALS AND METHODS
Study design and ethics statement
This study is a Johns Hopkins All Children’s Hospital Institutional Review Board (IRB No. 00279920) approved prospective cohort analysis of de-identified survey data collected from HCPs. HCPs collected a series of two de-identified surveys for each patient between June 2021 and August 2021. The HCPs recorded data on their mobile device using the application ZS MomentsTM (ZS Associates), which is a mobile-based platform by ZS Associates that allows for rapid and secure data collection. The surveys evaluated short-term symptom relief in infants under six months of age with diagnosed or suspected CMPA who were initiated on a hypoallergenic formula, as well as HCP’s satisfaction with the management strategies. The first survey was completed at the first visit with the initiation of treatment of CMPA (Visit 1), and the second was completed at the next follow-up visit (Visit 2) following treatment initiation. Verbal informed consent was obtained from the patients during Visit 1.
Healthcare provider selection
HCPs included in the study met the following inclusion criteria: 2–35 years of experience in a clinic/office-based setting and seeing at least two newly diagnosed CMPA patients per week, with at least 20% of their CMPA patients prescribed a hypoallergenic formula (eHF-LGG or AAF). HCP exclusion criteria included providers seeing fewer than two newly diagnosed CMPA patients per week, treating with hypoallergenic formula (eHF-LGG or AAF) in less than 20% of CMPA infants, or switching the patient’s treatment before the next follow-up visit (Visit 2).
Participant selection
Patients included in the study were diagnosed with or suspected to have CMPA by their HCP, were prescribed either an eHF-LGG (Nutramigen®, Mead Johnson Nutrition) or an AAF (PurAmino®, Mead Johnson Nutrition) as their treatment formula per HCP standard of care, were less than six months of age at the time of starting their treatment formula, and had survey data collected by their HCP at the time of treatment initiation for CMPA (Visit 1) and the subsequent follow-up visit (Visit 2). Infants who did not meet these criteria were excluded from the study.
Data collection
Data collected by the HCPs in both visits via ZS MomentTM were de-identified. Survey data collected at the first visit included: HCP demographics (specialty, average new CMPA patients weekly, and treatment formulas); location of visit (in person, telemedicine); patient characteristics [age, gender, height (percentile) and weight (percentile)]; and family history of allergies (mother, father, siblings, and types of allergies). Survey data collected at the follow-up visit (Visit 2) after initiation of dietary management of CMPA with either eHF LGG or AAF included: a review of the previously collected data, the date of the patient’s first visit, any changes to the patient’s treatment formula, and HCPs qualitative satisfaction with CMPA treatment strategy, including if the management strategy brought the expected results and if they would recommend the specific formula to another HCP.
RESULTS
Characteristics of HCPs
A total of 61 HCPs were surveyed across 24 states. Nine HCPs were excluded due to non-submission of Visit 2 information for any of their patients, leaving 52 (85.2%) HCPs included in the final analysis ( Fig. 1). Of those, twenty-nine were located on the east coast of the United States, with the most being from New York (n=12). Of the 52 HCPs, 45 (86.5%) were general pediatricians, and 6 were pediatric gastroenterologists ( Table 1). A combined total of 329 patients <6 months of age with symptoms of CMPA were included in the analysis; 222 patients were treated with eHF-LGG, and 107 patients were treated with AAF. General pediatricians collected the most patient charts, with 194 eHF-LGG charts and 88 AAF charts ( Table 2). HCPs saw a varying number of CMPA patients per week: twenty-two (42.3%) HCPs saw between 2–4, twenty (38.5%) saw between 5–10, nine (17.3%) saw between 11–20, and one HCP over 20 ( Table 1). The number of in-person patient visits was 211 (95.0%) and 93 (86.9%) for the eHF-LGG and AAF groups, respectively, while the remaining visits were telemedicine. Approximately two-thirds of the CMPA patients were seen in their follow-up visit between 3–5 weeks.
Fig. 1. Flow diagram of initial and final sample of HCPs.
HCP: healthcare providers.
Table 1. Healthcare provider demographics.
| Demographic | Value (n=52) | |
|---|---|---|
| Specialty | ||
| General pediatrics | 45 (86.5) | |
| Pediatric gastroenterologist | 3 (5.8) | |
| Pediatric allergy/immunology | 3 (5.8) | |
| Other specialties | 1 (1.9) | |
| Patient volume (#of CMPA patients/wk) | ||
| 2–4 | 22 (42.3) | |
| 5–10 | 20 (38.5) | |
| 11–20 | 9 (17.3) | |
| >20 | 1 (1.9) | |
Values are presented as number (%).
CMPA: Cow’s milk protein allergy.
Table 2. Patient charts collected across specialties for each formula type.
| Specialty | eHF-LGG | AAF |
|---|---|---|
| General pediatrics | 194 | 88 |
| Pediatric gastroenterologist | 12 | 8 |
| Pediatric allergy/immunology | 11 | 6 |
| Other specialties | 5 | 5 |
| Total | 222 | 107 |
eHF-LGG: extensively hydrolyzed formula containing Lactobacillus rhamnosus GG, AAF: amino acid forula.
CMPA treatment course and HCP satisfaction
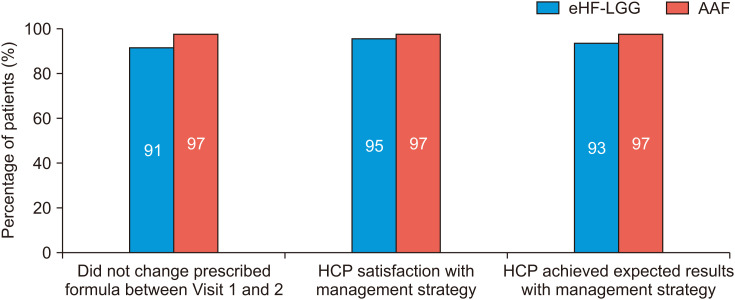
The majority of infants remained on the originally prescribed formula between visits 1 and 2 (eHF-LGG 202/222 [91.0%] and AAF 104/107 [97.2%]) ( Fig. 2). Of the 20 patients who switched from eHF-LGG, 13 changed to the study AAF, and seven changed to other formulas or treatments. All patients who changed from AAF switched to the study eHF-LGG. The main reasons for changing treatments were lack of improvement in CMPA symptoms, the infant not tolerating formula, and the severity of their condition. HCP’s satisfaction with the management strategy was 95% in patients treated with eHF-LGG and 97% in those treated with AAF ( Fig. 2). Among the 20 patients who were switched from the eHF-LGG, nine HCPs were still satisfied with eHF-LGG. HCPs reported that the management strategies achieved the expected results in 93% and 97% of patients treated with the eHF-LGG and AAF, respectively ( Fig. 2). All HCPs reported that they would recommend similar treatments to fellow HCPs.
Fig. 2. HCP experience and satisfaction with eHF-LGG and AAF.
eHF-LGG: extensively hydrolyzed formula containing Lactobacillus rhamnosus GG, AAF: amino acid forula, HCP: healthcare providers.
DISCUSSION
CMPA is one of the most prevalent food intolerances in infants and small children, and continues to be a challenge for HCPs in both diagnosis and treatment strategies [1,2,3,4,5,6,7]. The diagnostic criteria for CMPA in clinical practice include: typical CMPA symptoms, maternal cow’s milk elimination diet with symptom resolution, and an oral challenge test with the reemergence of symptoms after other abnormalities such as gastrointestinal infections are ruled out [9,12,13,14]. However, CMPA can be challenging to differentiate from gastroesophageal reflux disease and other functional disorders of infancy due to its many non-specific symptoms (abdominal pain, feeding difficulties, regurgitation, etc.) [9,12,13,14]. While the immunoglobulin E (IgE)-mediated subtype of CMPA presents with an immediate onset of symptoms and can be diagnosed using serology and skin prick tests, the non-IgE-mediated subtype of CMPA has a much gradual presentation and the diagnosis cannot be made using IgE-mediated assay [9,12,13,14]. HCPs also face challenges in deciding the proper treatment strategy to balance the efficacy and cost of the treatment. In non-breastfed infants, eHF is the first-line treatment formula due to its high efficacy and relatively lower cost [8,11,15]. Recent studies have shown that eHF containing the probiotic Lactobacillus rhamnosus GG (eHF-LGG) accelerates the development of tolerance to cow’s milk protein in infants [16,17]. AAF can be a first-line treatment in non-breastfed infants with severe CMPA. It is also an alternative treatment option in cases where eHF is discontinued due to complications, but AAF is more expensive [8,11,17,18].
This secondary analysis of prospectively collected survey data suggests that HCPs are satisfied with the short-term management strategies of CMPA using eHF-LGG and AAF. Survey data collected via the mobile app ZS MomentTM allowed HCPs to enter data conveniently in real-time. This study collected and analyzed patient chartings of 329 infants with CMPA. Individual symptom improvement in both the EHF and AAF cohorts have previously been reported [19,20]. Clinicians in this cohort reported satisfaction in 95% and 97% of patients using eHF-LGG and AAF formulas for symptom management, respectively. Furthermore, HCPs achieved the expected results in treating patients with CMPA using eHF-LGG (93%) and AAF (97%) management strategies, and all HCPs reported that they would recommend these treatments to their colleagues. At Visit 2, there were 20 patients (9.0%) that switched from eHF-LGG (13 to the study AAF, 7 to other treatment formulas), and there were three patients (2.8%) that switched from AAF (all to the study eHF-LGG). The top reasons reported for changing treatments included no improvement in CMPA symptoms, infants not tolerating the formula, and the severity of the condition. Almost half (9/20) of the patients that switched from eHF-LGG were still satisfied with eHF-LGG as a treatment option, suggesting that HCPs were still assured even if there were unexpected outcomes. The increased number of patients switched from eHF-LGG compared to AAF could be related to eHF-LGG being the first-line treatment option and AAF being reserved for more severe cases of CMPA in infants [8,11].
Current treatments for CMPA include starting the mother on a strict cow’s milk protein-free diet in breastfed infants and starting the infant on eHF as a first-line formula choice with AAF reserved for severe CMPA in non-breast-fed infants [8,9]. Hypoallergenic formulas such as these have been recognized as effective long-term treatments of CMPA [9,10,21]. Although there is data demonstrating the efficacy of these formulas, there is a gap in the literature regarding the clinician’s experience and satisfaction with the current treatment options. This study provided further insight into the HCP’s experience and satisfaction with eHF-LGG and AAF as treatments for CMPA in infants over two patient visits.
Limitations
There are several limitations to this study. First, this study lacked a control group, which limits the ability to determine if symptoms improved spontaneously or were due to treatment formula intervention. Secondly, this cohort included a small sample size of HCPs and, due to the short-term focus of the study endpoint, gathered provider responses from only two visits. Increasing the number of providers and the study duration may broaden the HCP’s experience. This study also only examined one type of eHF and one type of AAF.
In conclusion, this secondary analysis of prospective survey data addresses the knowledge gap in healthcare providers’ perspectives and clinical experience in managing infants with CMPA using hypoallergenic formulas. At the next follow-up visit, clinicians in this cohort obtained the expected results and were satisfied with managing CMPA using eHF-LGG or AAF. Very few patients were switched from the hypoallergenic formula initially prescribed. The study provides new insights into HCP perspectives on treating infants with CMPA and supports using hypoallergenic formulas to manage this condition. However, additional prospective controlled studies are needed to confirm these initial findings.
Footnotes
Funding: A grant (RB2073) from Reckitt supported article processing and presentation fees for this research. Additionally, Reckitt provided funding for data collection through ZS Associates.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Fiocchi A, Dahdah L, Albarini M, Martelli A. Cow’s milk allergy in children and adults. Chem Immunol Allergy. 2015;101:114–123. doi: 10.1159/000375415. [DOI] [PubMed] [Google Scholar]
- 2.Hill DJ, Firer MA, Shelton MJ, Hosking CS. Manifestations of milk allergy in infancy: clinical and immunologic findings. J Pediatr. 1986;109:270–276. doi: 10.1016/s0022-3476(86)80384-5. [DOI] [PubMed] [Google Scholar]
- 3.Høst A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy. 1990;45:587–596. doi: 10.1111/j.1398-9995.1990.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 4.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Warren CM, Jhaveri S, Warrier MR, Smith B, Gupta RS. The epidemiology of milk allergy in US children. Ann Allergy Asthma Immunol. 2013;110:370–374. doi: 10.1016/j.anai.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Høst A. Frequency of cow’s milk allergy in childhood. Ann Allergy Asthma Immunol. 2002;89(6 Suppl 1):33–37. doi: 10.1016/s1081-1206(10)62120-5. [DOI] [PubMed] [Google Scholar]
- 8.Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55:221–229. doi: 10.1097/MPG.0b013e31825c9482. [DOI] [PubMed] [Google Scholar]
- 9.Vandenplas Y, Marchand J, Meyns L. Symptoms, diagnosis, and treatment of cow’s milk allergy. Curr Pediatr Rev. 2015;11:293–297. doi: 10.2174/1573396311666150731113059. [DOI] [PubMed] [Google Scholar]
- 10.Guest JF, Fuller GW. Effectiveness of using an extensively hydrolyzed casein formula supplemented with Lactobacillus rhamnosus GG compared with an extensively hydrolysed whey formula in managing cow’s milk protein allergic infants. J Comp Eff Res. 2019;8:1317–1326. doi: 10.2217/cer-2019-0088. [DOI] [PubMed] [Google Scholar]
- 11.Vandenplas Y. Prevention and management of cow’s milk allergy in non-exclusively breastfed infants. Nutrients. 2017;9:731. doi: 10.3390/nu9070731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiocchi A, Brozek J, Schünemann H, Bahna SL, Von Berg A, Beyer K, et al. World allergy organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. Pediatr Allergy Immunol. 2010;21(Suppl 21):1–125. doi: 10.1111/j.1399-3038.2010.01068.x. [DOI] [PubMed] [Google Scholar]
- 13.Caffarelli C, Baldi F, Bendandi B, Calzone L, Marani M, Pasquinelli P EWGPAG. Cow’s milk protein allergy in children: a practical guide. Ital J Pediatr. 2010;36:5. doi: 10.1186/1824-7288-36-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenplas Y, Brough HA, Fiocchi A, Miqdady M, Munasir Z, Salvatore S, et al. Current guidelines and future strategies for the management of cow’s milk allergy. J Asthma Allergy. 2021;14:1243–1256. doi: 10.2147/JAA.S276992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer R, Groetch M, Venter C. When should infants with cow’s milk protein allergy use an amino acid formula? a practical guide. J Allergy Clin Immunol Pract. 2018;6:383–399. doi: 10.1016/j.jaip.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Berni Canani R, Nocerino R, Terrin G, Coruzzo A, Cosenza L, Leone L, et al. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: a randomized trial. J Allergy Clin Immunol. 2012;129:580–582. 582 e1–585. doi: 10.1016/j.jaci.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Berni Canani R, Nocerino R, Terrin G, Frediani T, Lucarelli S, Cosenza L, et al. Formula selection for management of children with cow’s milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013;163:771–7.e1. doi: 10.1016/j.jpeds.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Lozinsky AC, Meyer R, Anagnostou K, Dziubak R, Reeve K, Godwin H, et al. Cow’s milk protein allergy from diagnosis to management: a very different journey for general practitioners and parents. Children (Basel) 2015;2:317–329. doi: 10.3390/children2030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilsey MJ, Florio J, Beacker J, Lamos L, Baran JV, Oliveros L, et al. Extensively hydrolyzed formula improves allergic symptoms in the short term in infants with suspected cow’s milk protein allergy. Nutrients. 2023;15:1677. doi: 10.3390/nu15071677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilsey MJ, Baran JV, Lamos L, Beacker J, Florio J, Oliveros L, et al. Short-term symptom improvement in infants with suspected cow’s milk protein allergy using amino acid formula: a prospective cohort analysis. Front Nutr. 2023;10:1208334. doi: 10.3389/fnut.2023.1208334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludman S, Shah N, Fox AT. Managing cows’ milk allergy in children. BMJ. 2013;347:f5424. doi: 10.1136/bmj.f5424. [DOI] [PubMed] [Google Scholar]