Abstract
Globally, the prevalence of prostate cancer is only the second to lung cancer. In Africa however, the commonest cancer among men is cancer of the prostate. The use of natural compounds from plants such as quercetin is being explored as a potential cure. Quercetin is a plant-based flavonoid that has anti-inflammatory, antioxidant and anticancer properties. Although quercetin has been extensively studied, its chemo preventive mode of action is not well-understood. The molecular targets and potential mechanisms underlying the action of quercetin against prostate cancer were identified and validated using network pharmacology and molecular docking methods. The biological targets of quercetin and targets associated with prostate cancer were obtained through database mining. Overlapping targets associated with quercetin and prostate cancer were identified and used to construct a compound–disease target (C-D) network and the targets were subjected to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and protein–protein interaction analysis (PPI). A disease target- pathway network was constructed and then merged with C-D network to form a compound–disease_target–pathway (C-D-P) network. Hub targets were obtained from the C-D-P and PPI networks. The binding affinities between quercetin and the retrieved hub targets were identified. Pathway enrichment analysis showed that prostate cancer associated quercetin targets were mainly linked with pathways such as the cancer signaling pathways (HIF-1 and ErbB) and hepatitis B. Basing on the PPI and C-D-P network analysis STAT3, TP53, MAPK1, MAPK3 and KRAS were identified as the main targets and were subjected to molecular docking. The results showed quercetin’s ability to stably bind to the key targets. In conclusion, this study showed the potential molecular targets and mode of action of quercetin in prostate cancer treatment. This can potentially inform the future use of quercetin in the treatment of prostate cancer.
Keywords: Prostate cancer, Molecular docking, Network pharmacology, Computer aided drug design, Quercetin
Introduction
Prostate cancer is the second most prevalent malignancy globally, following lung cancer (Hussain et al. 2021). In Africa, cancer of the prostate is the commonest cancer in men (Okuku et al. 2016). The relative burden of prostate cancer is higher in sub Saharan Africa (SSA), accounting for 20.3% of all cases of cancer in men (Parkin et al. 2014). In comparison to Zimbabwe and Uganda which have the highest incidence rates of 38.1 and 37.1 per 100,000 in SSA, black American men have much higher rates of 172.8 per 100,000 (Chu et al. 2011).
Prostate cancer can be cured by radical prostatectomy and radiation when it is confined or does not metastasize (Yang et al. 2015). However, most patients later suffer from local recurrence (Fleshner 2005), and several side effects from conventional therapy. Therefore, the search for new, safe and efficient anti-cancer agents is on-going and is a matter of urgency. Plant-based products provide a promising potential source of novel anticancer agents (Fridlender et al. 2015).
Quercetin is a plant derived flavonoid that is naturally abundant in a variety of plants such as apples, onions, tea, and red wine (Singh and Konwar 2012). Quercetin has antioxidant and free radical scavenging activity. Epidemiological studies have revealed that high consumption of flavanols such as quercetin is associated with reduced risk of cancer and coronary diseases (Mohammed et al. 2015). Quercetin is nontoxic and can effectively inhibit the growth and proliferation of different tumors (Yang et al. 2015).
Quercetin is an ideal therapeutic option to avoid cancer chemotherapy and can directly induce apoptosis in cancer cells without inhibiting normal cell growth (Hussain et al. 2021). Given the high incidence rate and limited treatment options for prostate cancer, the widespread use of quercetin in clinical settings can decrease the incidence and improve treatment outcomes, benefiting both patients and the healthcare system (Yang et al. 2015). Although there is a renewed interest in the pharmaceutical and nutraceutical benefits of quercetin, its mode of action at molecular level underlying its chemopreventive activities is not well understood (Michaud-Levesque et al. 2012; Murakami et al. 2008).
The drug discovery process is time consuming and costly. The current genomic era has been characterized by a large number of therapeutic targets amenable to investigation. This has in turn put pressure on pharmaceutical and biotechnology companies to prioritize drug discovery programs and conduct them in a highly efficient manner (Munuganti 2015). Among these drug discovery programs is Computer aided Drug discovery (CADD) which has the ability to shorten the drug discovery process and thus lower production costs. The CADD can be classified into structured-based drug design and ligand-based drug design (Anywar and Namukobe 2020; Ebhohimen et al. 2020).
Due to the promising advances in systems biology, network-based drug discovery is regarded as an efficient cost-effective route to drug development (Kim and Kim 2021). Such methods can be used to identify the potential mechanisms, key targets and synergy of herbal medicines and their ingredients. This can help in understanding and designing of herbal medicine formulae for clinical application (Li and Zhang 2013). Molecular docking is a structure-based drug design technique which predicts the preferred orientation of bound molecules in stable complex and is instrumental in providing insights into the ligand receptor interactions (Jemal 2019; Gu et al. 2021).
Materials and methods
Drug-likeness prediction, pharmacokinetics and toxicity of quercetin
To predict the drug-likeness and pharmacokinetics of quercetin, the SMILES format (Oc1cc (O)c2c(c1)oc(c(c2=O)O) c1ccc(c(c1)O)O) of quercetin was uploaded into SwissADME. Screening was then performed next using the default parameters. Swiss ADME is an open access web tool to evaluate drug-likeness, pharmacokinetics and medicinal chemistry friendliness of small molecules (https://www.swissadme.ch/). Similarly, the toxicity properties of quercetin were predicted by ProTox-II (http://tox.charite.de/protox_II/) webserver.
Pharmacokinetic evaluation and therapeutic targets associated with quercetin
The therapeutic targets of quercetin were retrieved from Genecards (https://www.genecards.org/), Drug bank (https://go.drugbank.com/) and NCBI gene databases (Home—Gene—NCBI (nih.gov). The NCBI gene database integrates information from a wide range of species worldwide. Drug bank is an open access online database that contains drugs and their targets. All searches were performed specific to Homo sapiens.
Retrieval of potential pathological target genes linked to prostate cancer
Prostate cancer associated targets were retrieved from NCBI and Genecards gene databases. Genecards is a searchable and integrative database that provides comprehensive user-friendly information on all annotated and predicted human genes (https://www.genecards.org/).
Construction of compound-disease-target (C-D) network
Overlapping genes between quercetin and prostate cancer were obtained using GeneVenn (http://genevenn.sourceforge.net/) and used to construct a network using Cytoscape software (v 3.9.1). Cytoscape (https://cytoscape.org/) is an open-source software platform for visualizing complex networks and integrating these with any types of data (Shannon et al. 2003).
KEGG pathway enrichment analysis
The Database for Annotation, Visualization and Integrated Discovery (DAVID) was used to retrieve pathways associated with the target proteins. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed through DAVID (https://david.ncifcrf.gov/). DAVID bioinformatics is an analytic tool used to extract biological meaning from large gene and protein lists.
Compounds–disease target–pathway (C-D-P) network construction
Cytoscape was used to construct and visualize compound–disease target and disease target–pathway networks. Pathways with p < 0.05 were used in this network. Two constructed networks were analyzed and merged to produce a compound–disease target–pathway (C-D-P) network.
Construction of protein–protein interaction (PPI) network
STRING is a database of known and predicted protein–protein interactions. The STRING database was used to obtain protein to protein interactions with a confidence score > 0.9 in order to have clear network (von Mering et al. 2005).
Molecular docking analysis
Analysis of the structural complexes of the target with ligands was done using Ligand-target docking analysis (Kim and Kim 2021). The three-dimensional (3D) structures of the targets MAPK1 (PDB ID: 6G54, 2.05 Å); STAT3 (PDB ID: 4ZIA, 2.70 Å); MAPK3 (PDB ID: 6GES, 2.07 Å); TP53: (PDB ID: 1TUP, 2.20 Å); KRAS (PBD ID: 4OBE, 1.24 Å) were downloaded from the RCSB protein data bank and were converted to Protein data bank files using Open Babel. The targets were read in Autodock Tools (ADT) and polar hydrogens were added, added waters were removed, added ligands removed and gasteiger charges added. The files were saved as .pdbqt extension.
Quercetin (PubChem CID: 5280343) was obtained from the PubChem database and converted to PDB file using the Open Babel. The file was then read in ADT and polar hydrogens were added, non polar hydrogens were merged, gasteiger charges were added, the root was detected automatically and it was saved with .pdbqt extension. Docking was performed in triplicates for 100 runs to get a better representation of the results using Autodock 4.2 (https://autodock.scripps.edu/) basing on the Lamarckian Genetic Algorithm and scoring functions. Visualization of the docking results was performed using Autodock Tools. Protein ligand complexes and their interactions were visualized using Pymol software (DeLano 2002).
Results
Drug-likeness, pharmacokinetics and toxicity of quercetin
Our results showed that the properties of quercetin are in conformity with Lipinski’s rule of five which shows that it has good druglikeness properties (Table 1). The results also showed that quercetin has moderate toxicity, can inhibit cytochrome p450 enzymes and cannot easily diffuse across cell membranes.
Table 1.
Drug-likeness, pharmacokinetics and toxicity prediction of quercetin
| Drug likeness Property | |
|---|---|
| Property | Value |
| Molecular weight | 302.24 |
| Polar surface area (PSA) | 131.36 A2 |
| Rotatable bonds | 1 |
| Hydrogen bond donors | 7 |
| Hydrogen bond acceptors | 5 |
| clogP | 1.54 |
| Molecular refractivity | 78.03 |
| Lipinski violations | 0 |
| Bioavailability score | 0.55 |
| Pharmacokinetic and toxicity properties | |
| LD50 | 159 mg/kg |
| Toxicity class | 3 |
| GI absorption | High |
| BBB permeant | No |
| Pgp substrate | No |
| CYP1A2 inhibitor | Yes |
| CYP2C19 inhibitor | No |
| CYP2C9 inhibitor | No |
| CYP2D6 inhibitor | Yes |
| CYP3A4 inhibitor | Yes |
| Log Kp (cm/s) | − 7.05 |
CYP, cytochrome-p450; GI, gatsrointenstinal; BBB, blood–brain barrier; Pgp, P-glycoprotein; LD50, Lethal dose at 50%; Kp, skin permeation coefficient
Biological targets of quercetin and compound–disease target network construction (C-D network)
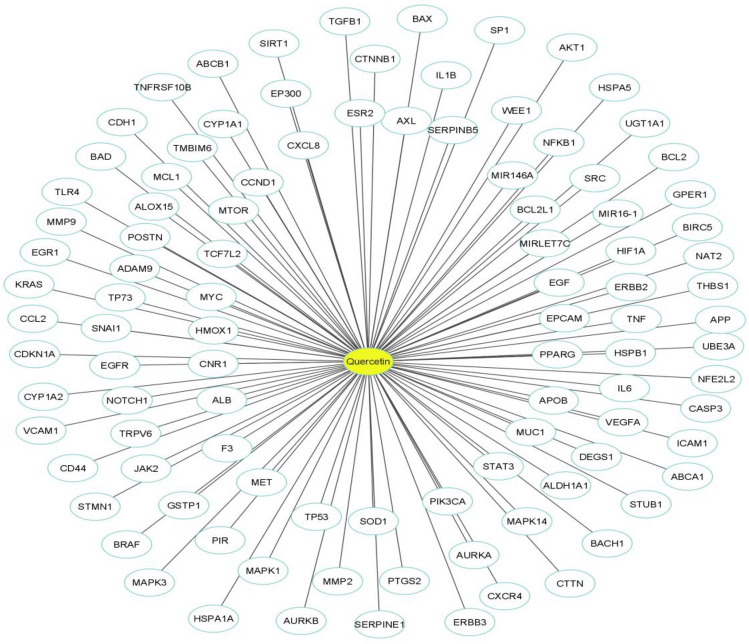
126 target genes (H. sapiens) associated with quercetin were retrieved from the DrugBank, GeneCards and NCBI databases. A total of 3,213 targets associated with prostate cancer were identified from the GeneCards and NCBI databases (Fig. 1). Between 126 quercetin associated targets and 3,213 prostate cancer associated targets, 101 overlapping target genes were obtained as prostate cancer associated quercetin targets.
Fig. 1.

Biological targets between quercetin and prostate cancer
Based on 101 targets, a compound (quercetin) – disease (prostate cancer) target (C-D) network (Fig. 2) was constructed using Cytoscape (Kim and Kim 2021).
Fig. 2.
Quercetin -prostate cancer target network
KEGG pathway analysis
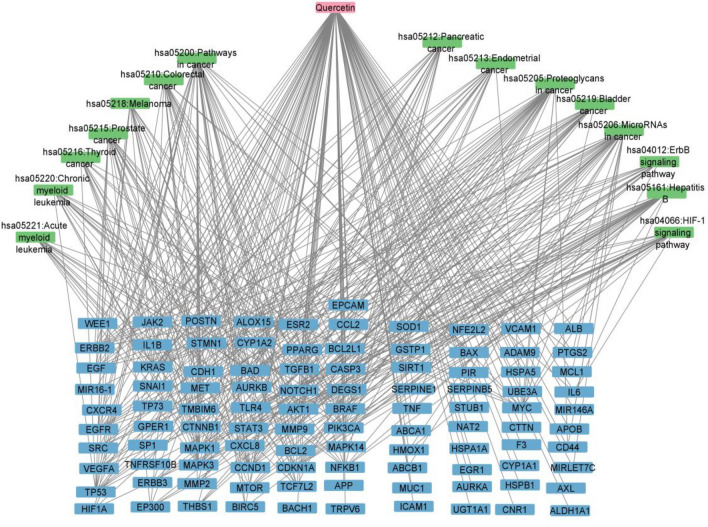
The 101 prostate cancer associated quercetin targets were further analyzed using DAVID for KEGG enrichment analysis. The top 15 enriched pathways and diseases based on the gene count (Table 2) were retrieved. Only enriched pathways and diseases with a p < 0.05 were chosen.
Table 2.
Top pathways basing on gene counts
| Pathways and diseases | Gene count |
|---|---|
| hsa05205: Proteoglycans in cancer | 30 |
| hsa05200: Pathways in cancer | 38 |
| hsa05219: Bladder cancer | 18 |
| hsa05161: Hepatitis B | 24 |
| hsa05206: MicroRNAs in cancer | 30 |
| hsa05215: Prostate cancer | 19 |
| hsa05210: Colorectal cancer | 17 |
| hsa05213: Endometrial cancer | 16 |
| hsa05212: Pancreatic cancer | 17 |
| hsa04066: HIF-1 signaling pathway | 19 |
| hsa05216: Thyroid cancer | 11 |
| hsa04012: ErbB signaling pathway | 15 |
| hsa05221: Acute myeloid leukemia | 13 |
| hsa05218: Melanoma | 14 |
| hsa05220: Chronic myeloid leukemia | 14 |
Compound–disease–target–pathway (C-D-P) network
The disease_target–pathway network (D–P) was constructed and merged with the C-D network giving the C-D-P network (Fig. 3). Nodes within the network represent different targets and the edges show intermolecular interactions between nodes. Cytoscape plug-in “Network Analyzer” was used to analyze the network. Among the target nodes, MAPK1, KRAS, and MAPK3 showed the highest degree of 15 which indicates the number of edges linked to a given node. Nodes with a high degree may represent the hub genes with important biological functions.
Fig. 3.
Compound-disease-target-pathway network
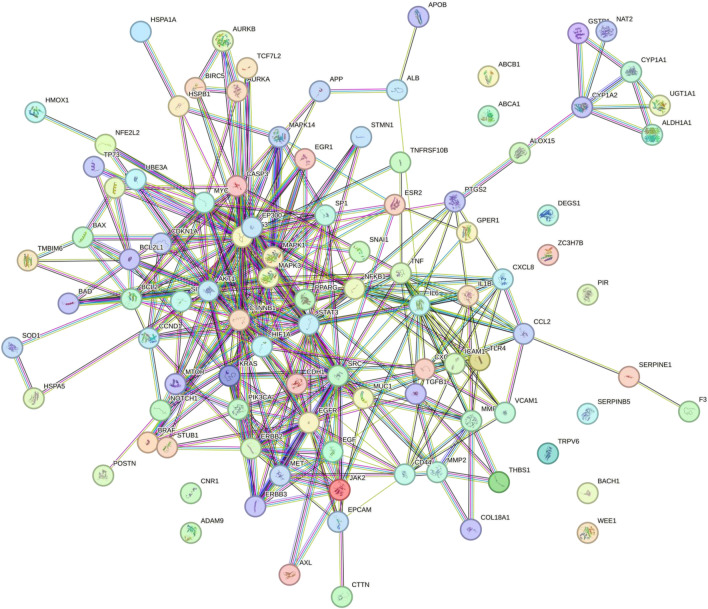
Protein–protein interaction of prostate cancer associated quercetin targets from the STRING database
The prostate cancer associated quercetin targets were inputted into STRING to retrieve a protein–protein interaction (PPI) network (Fig. 4). Cytoscape was used to analyze the network. The two main targets which showed the highest degree were identified as STAT3 (degree of 34) and TP53 (degree 33).
Fig. 4.
Protein to protein interactions of the prostate cancer associated quercetin targets generated using STRING
Molecular docking
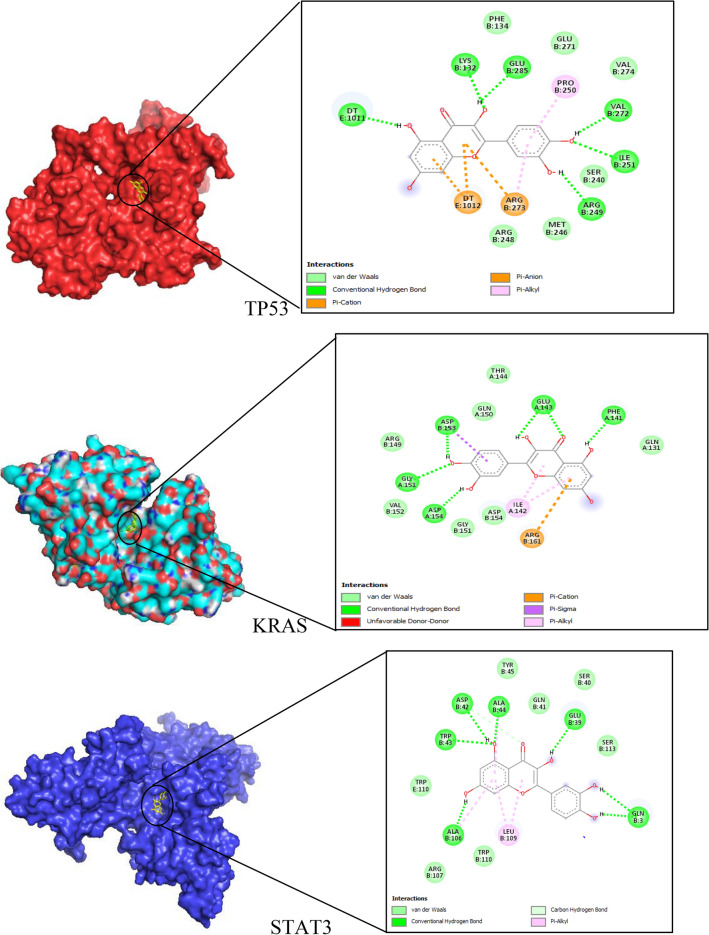
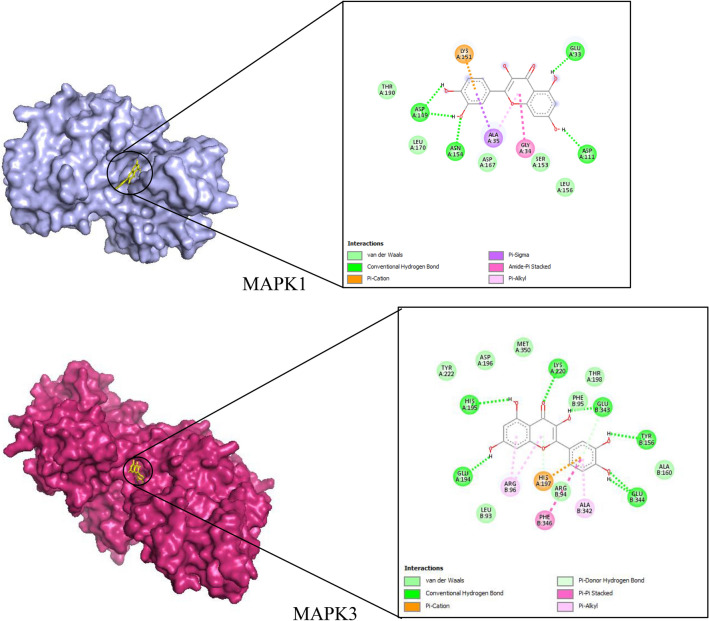
MAPK1, STAT3, MAPK3, KRAS, and TP53 were identified as five potential targets for quercetin against prostate cancer from the C-D-P and PPI networks’ analysis. A ligand–target docking method was used to analyze structural complexes of targets: MAPK1 (PDB ID: 6G54); STAT3 (PDB ID: 4ZIA); MAPK3 (PDB ID: 6GES); TP53: (PDB ID: 1TUP); KRAS (PBD ID: 4OBE) with ligand as quercetin and the results were visualized using Auto dock Tools and Pymol (Fig. 5). The binding affinities of quercetin-key target complexes and those of controls are shown in Table 3.
Fig. 5.
Quercetin-key target complexes and their associated interactions
Table 3.
Binding affinities and estimated inhibition constants of quercetin against key targets and standard known molecules against the targets
| Protein–ligand complex | Binding affinity (kcal/mol) | Estimated inhibition constant, Ki (µM) |
|---|---|---|
| Quercetin-MAPK3 | − 5.0767 | 5.49 |
| Quercetin-MAPK1 | − 5.0100 | 155.59 |
| Quercetin-STAT3 | − 5.6600 | 61.35 |
| Quercetin-KRAS | − 7.1367 | 4.10 |
| Quercetin-TP53 | − 6.2867 | 42.42 |
| PD98059-MAPK3(ctrl) | − 6.1400 | 31.63 |
| PD98059-MAPK1 (ctrl) | − 5.4000 | 109.40 |
| Sotorasib-KRAS (ctrl) | − 6.9500 | 8.10 |
| STA-21-STAT3 (ctrl) | − 6.0500 | 36.58 |
| APR-246 -TP53 (ctrl) | − 3.5800 | 2.39 mM |
Discussion
In this study, a systems biology approach integrating target identification, druglikeness evaluation, network construction, KEGG pathway enrichment analysis, protein–protein interaction analysis and molecular docking was set up to analyze the possible molecular mode of quercetin in treating prostate cancer.
Druglikeness, phamacokinetics and toxicity prediction
According to the results of Lipinski’s rule of five, quercetin had good druglikeness properties which imply that it can act as a potential treatment for prostate cancer. Lipinski’s rule of 5 predicts that poor absorption or permeation is more likely when there are more than five hydrogen-bond donors, 10 hydrogen-bond acceptors, a molecular mass greater than 500, the calculated logP (clogP) is greater than 5, the polar surface area (PSA) less than or equal to 140 A2 and number of rotatable bonds is less than 10 (Ivanović et al. 2020). Furthermore, quercetin had a Log Kp of − 7.05 cm/s implying that it cannot readily diffuse through cell membranes. In addition, our current results show that quercetin can inhibit CYPA12, CYP2D6 and CYP3A4 implying that it can have an effect on co-administered drugs (Daina et al. 2017). The results showed that quercetin has a LD50 of 159 mg/kg implying that it has moderate toxicity and should thus be taken following proper guidelines. Quercetin has been found to have moderate toxicity in mice (Dibal et al. 2020).
KEGG pathway enrichment analysis
The prostate cancer associated quercetin targets were mainly linked with pathways such as cancer (bladder, colorectal, endometrial, pancreatic, thyroid, acute myeloid leukaemia, melanoma, chronic myeloid leukemia,and prostate), signaling pathways (HIF-1 and ErbB) and hepatitis B. Hypoxia inducible factor (HIF-1) is a critical transcription factor in the regulation of hypoxic responses (Samec et al. 2021). Increased levels of HIF-1 could be used as prognosis markers for metastasis, development of chemo/radio resistance as well as general poor prognosis of cancer patients (Samec et al. 2021). Quercetin inhibited HIF-1 α accumulation and protein synthesis in hypoxic conditions in cancer cell lines such as LNCAP (prostate cancer), SKBr3 (breast cancer) and CX-1 (colon cancer) in a concentration dependent manner. It is noteworthy that cycloheximide, an HIF-1 protein inhibitor showed the same effect on HIF-1 α synthesis as quercetin (Lee and Lee 2008). ErbB proteins are part of the receptor tyrosine kinases family. The over expression of ErBb proteins, mutations or amplification of their genes has been linked to the progression of cancer. Studies showing the potential phytocompounds which down regulate ErBb proteins or inhibit tyrosine kinase activity have been conducted (Filippi et al. 2017). Fridrich et al. (2008) and Huynh et al. (2003) showed that quercetin reduces the expression level of ErbB2 and ErbB3 and reduces phosphorylation of ErbB2 and ErbB3 in prostate cancer cell lines in vitro. Quercetin also prevented Epidermal Growth Factor (EGF) (one of the ligands of ErbB proteins) induced epithelial mesenchymal transition in PC-3 cells. This a process in tumor progression in which cancer cells undergo dramatic changes acquiring highly invasive properties (Bhat et al. 2014). Therefore, quercetin can possibly treat prostate cancer through various signaling pathways which require further investigations.
Network construction and hub target analysis
MAPK1, MAPK3, KRAS, TP53 and STAT3 were identified as the hub targets from the analysis of the C-D-P network and protein–protein interactions. Hub targets represent those targets with the greatest number of interactions (Kim and Kim 2021).
Molecular docking analysis
Molecular docking was performed in triplicates on the hub targets to get a better representation of the results and the binding affinities of quercetin ranged from − 5.01 to − 7.14 kcal/mol which indicates stable binding. KRAS had the lowest average binding affinity of − 7.14 kcal/mol among the five selected targets. The KRAS protein is part of the RAS/MAPK signaling pathway responsible for signal transmission from the cell exterior to its nucleus. (Cho et al. 2006) showed the mutations of codons 12 and 13 of KRAS to be found in 15/206 (7.3%) prostate adenocarcinomas evaluated. Also, constitutive activation of kinase cascade involving RAS, mitogen/extracellular signal-regulated kinase (ERK) and mitogen activated protein kinase (MAPK) are widely found in many cancers. The most essential parts of the MAPK signaling pathway include MAPK3/ERK1, MAPK1/ERK2, JNK and p38 (Yang et al. 2015). Quercetin treatment in PC-3 cells decreased ERK1/ERK2 and increased the expression of JNK, promoted cancer apoptosis and inhibited tumor growth (Kumar et al. 2011). Quercetin also caused an increase of p38-MAPK in PC-3cells enhancing their survival and proliferation (Yang et al. 2015). TP53 is also known as tumor suppressor p53/the guardian of the genome and it has been shown to induce G2cell cycle arrest and apoptosis. TP53 was increased by quercetin treatment (Hsieh and Wu 2009). Some researchers found out that quercetin increased proliferation, cell cycle arrest and p53 status (Hsieh and Wu 2009). STAT3, a member of the STAT family of transcription factors is a key player in cancer-related inflammation (Michaud-Levesque et al. 2012). Activation of STAT3 results in the expression of downstream genes that control major cellular responses such as cell proliferation and invasion as well as cancer cell survival (Alvarez and Frank 2004). Treatment of activated T cells with quercetin in vitro inhibited the interleukin-12 (IL-12)-induced phosphorylation of STAT3, JAK2, tyrosine kinase-2 and other proteins such as STAT4, which result in decreased levels of T cell propagation and Th1 variation (Muthian and Bright 2004). A previous study by Michaud-Levesque et al. (2012) found out that quercetin inhibited interleukin-6 induced STAT3 signaling pathway. These findings confirm that the mechanism of quercetin against prostate cancer and provides guidance for animal validation experiments.
Conclusion
This study provided a novel approach to revealing the molecular targets and potential mechanisms of quercetin against prostate cancer. Quercetin may exert its anti-prostate cancer effects through multiple targets, pathways and biological processes. The molecular docking results showed that quercetin can bind stably to key proteins namely KRAS, MAPK1, MAPK3, STAT3, and TP53 in the context of prostate cancer. However, there is need for further studies to confirm the clinical efficacy of quercetin and its mechanisms against prostate cancer.
Acknowledgements
We acknowledge the support from Dr Gerald Mboowa of the African Center of excellence in Computational Biology and Data Intensive sciences at the Infectious Diseases Institute (IDI), Makerere University, Dr. Ivan Ricardo Vega Valdez of Instituto Politecnico Nacional in Mexico, Professor Imtaiyaz Hassan of the Center for Interdisciplinary research in Basic Sciences, India, Dr. Pradeep Kumar, Miriam Nakabuye, the staff of Queen’s University and Center for Emerging and Neglected diseases at University of California, Berkeley.
Author contributions
FK conceptualised and conducted the study under the guidance and supervision of AN and GA. FK wrote the first draft of the manuscript under the guidance of AN and GA. All authors have read and approved the manuscript.
Funding
This study did not receive funding from any research body or organisation.
Data availability
Data are available upon request from the authors.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alvarez JV, Frank D. Genome-wide analysis of STAT targetgenes: elucidating the mechanism of STAT-mediatedoncogenesis. Cancer Biol. 2004;3:1045–1050. doi: 10.4161/cbt.3.11.1172. [DOI] [PubMed] [Google Scholar]
- Anywar G, Namukobe J (2020) Chapter 2 - Factors affecting the choice for plant-based products in drug discoveries. In: Phytochemicals as lead compounds for new drug discovery. Elsevier, p 15–24.10.1016/B978-0-12-817890-4.00002-0
- Bhat FA, Sharmila G, Balakrishnan S, Arunkumar R, Elumalai P, Suganya S, Singh PR, Srinivasan N, Arunakaran J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014;25(11):1132–1139. doi: 10.1016/j.jnutbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Cho NY, Choi M, Kim BH, Cho YM, Moon KC, Kang GH. BRAF and KRAS mutations in prostatic adenocarcinoma. Int J Cancer. 2006;119(8):1858–1862. doi: 10.1002/ijc.22071. [DOI] [PubMed] [Google Scholar]
- Chu LW, Ritchey J, Devesa SS, Quraishi SM, Zhang H, Hsing AW (2011) Prostate cancer incidence rates in Africa. Prostate Cancer [DOI] [PMC free article] [PubMed]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7(1):42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano WL. Pymol: An open-source molecular graphics tool. CCP4 Newsl Protein Crystallogr. 2002;40(1):82–92. [Google Scholar]
- Dibal NI, Garba SH, Jacks TW (2020) Acute toxicity of quercetin from onion skin in mice. Pharm Biomed Res
- Ebhohimen IE, Edemhanria L, Awojide S, Onyijen OH, Anywar G (2020) Chapter 3 - Advances in computer-aided drug discovery. In: Phytochemicals as lead compounds for new drug discovery. Elsevier. 10.1016/B978-0-12-817890-4.00003-2
- Filippi A, Ciolac O-A, Ganea C, Mocanu M-M (2017) ErbB proteins as molecular target of dietary phytochemicals in malignant diseases. J Oncol [DOI] [PMC free article] [PubMed]
- Fleshner N. Defining high-risk prostate cancer: current status. Can J Urol. 2005;12(Suppl 1):14–17. [PubMed] [Google Scholar]
- Fridlender M, Kapulnik Y, Koltai H. Plant derived substances with anti-cancer activity: from folklore to practice. Front Plant Sci. 2015;6:799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridrich D, Teller N, Esselen M, Pahlke G, Marko D. Comparison of delphinidin, quercetin and (–)-epigallocatechin-3-gallate as inhibitors of the EGFR and the ErbB2 receptor phosphorylation. Mol Nutr Food Res. 2008;52(7):815–822. doi: 10.1002/mnfr.200800026. [DOI] [PubMed] [Google Scholar]
- Gu Y-Y, Zhang M, Cen H, Wu Y-F, Lu Z, Lu F, Liu X-S, Lan H-Y. Quercetin as a potential treatment for COVID-19-induced acute kidney injury: Based on network pharmacology and molecular docking study. PLoS ONE. 2021;16(1):e0245209. doi: 10.1371/journal.pone.0245209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T, Wu J. Targeting CWR22Rv1 prostate cancer cell proliferation and gene expression by combinations of the phytochemicals EGCG, genistein and quercetin. Anticancer Res. 2009;29:4025–4032. [PMC free article] [PubMed] [Google Scholar]
- Hussain Y, Mirzaei S, Ashrafizadeh M, Zarrabi A, Hushmandi K, Khan H, Daglia M. Quercetin and its nano-scale delivery systems in prostate cancer therapy: paving the way for cancer elimination and reversing chemoresistance. Cancers. 2021;13(7):1602. doi: 10.3390/cancers13071602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh H, Nguyen TTT, Chan E, Tran E. Inhibition of ErbB-2 and ErbB-3 expression by quercetin prevents transforming growth factor alpha (TGF-α)-and epidermal growth factor (EGF)-induced human PC-3 prostate cancer cell proliferation. Int J Oncol. 2003;23(3):821–829. [PubMed] [Google Scholar]
- Ivanović V, Rančić M, Arsić B, Pavlović A. Lipinski’s rule of five, famous extensions and famous exceptions. Chemia Naissensis. 2020;3(1):171–177. doi: 10.46793/ChemN3.1.171I. [DOI] [Google Scholar]
- Jemal K (2019) Molecular docking studies of phytochemicals of Allophylus serratus against cyclooxygenase-2 enzyme. bioRxiv 866152
- Kim M, Kim YB. Uncovering quercetin’s effects against Influenza A virus using network pharmacology and molecular docking. Processes. 2021;9(9):1627. doi: 10.3390/pr9091627. [DOI] [Google Scholar]
- Kumar R, Verma V, Jain A, Jain RK, Gupta MG. Synergistic chemoprotective mechanisms of dietary phytoestrogens in a select combination against prostate cancer. J Nutr Biochem Pharmacol. 2011;22:723–731. doi: 10.1016/j.jnutbio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee YJ. Quercetin suppresses hypoxia-induced accumulation of hypoxia-inducible factor-1α (HIF-1α) through inhibiting protein synthesis. J Cell Biochem. 2008;105(2):546–553. doi: 10.1002/jcb.21851. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2):110–120. doi: 10.1016/s1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- Michaud-Levesque J, Bousquet-Gagnon N, Béliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res. 2012;318(8):925–935. doi: 10.1016/j.yexcr.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Mohammed A, Al-Numair KS, Balakrishnan A (2015) Docking studies on the interaction of flavonoids with fat mass and obesity associated protein. Pak J Pharm Sci 28(5) [PubMed]
- Munuganti RSN (2015) Identification of novel androgen receptor inhibitors for the treatment of castration resistant prostate cancer
- Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269(2):315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Muthian G, Bright JJ. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J Clin Immunol. 2004;24:542–552. doi: 10.1023/B:JOCI.0000040925.55682.a5. [DOI] [PubMed] [Google Scholar]
- Okuku F, Orem J, Holoya G, De Boer C, Thompson CL, Cooney MM. Prostate cancer burden at the Uganda cancer institute. J Glob Oncol. 2016;2(4):181–185. doi: 10.1200/JGO.2015.001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Jemal A. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev. 2014;23(6):953–966. doi: 10.1158/1055-9965.epi-14-0281. [DOI] [PubMed] [Google Scholar]
- Samec M, Liskova A, Koklesova L, Mersakova S, Strnadel J, Kajo K, Pec M, Zhai K, Smejkal K, Mirzaei S, Hushmandi K, Ashrafizadeh M, Saso L, Brockmueller A, Shakibaei M, Büsselberg D, Kubatka P. Flavonoids targeting HIF-1: implications on cancer metabolism. Cancers. 2021;13(1):130. doi: 10.3390/cancers13010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Konwar BK. Molecular docking studies of quercetin and its analogues against human inducible nitric oxide synthase. Springerplus. 2012;1(1):1–10. doi: 10.1186/2193-1801-1-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mering C, Jensen LJ, Snel B, Hooper SD, Krupp M, Foglierini M, Jouffre N, Huynen MA, Bork P (2005) STRING: known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res 33(Database issue):D433–D437. 10.1093/nar/gki005 [DOI] [PMC free article] [PubMed]
- Yang F, Song L, Wang H, Wang J, Xu Z, Xing N. Quercetin in prostate cancer: chemotherapeutic and chemopreventive effects, mechanisms and clinical application potential. Oncol Rep. 2015;33(6):2659–2668. doi: 10.3892/or.2015.3886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from the authors.