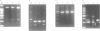Abstract
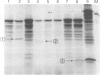
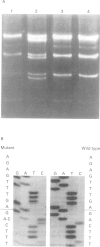
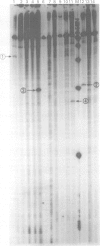
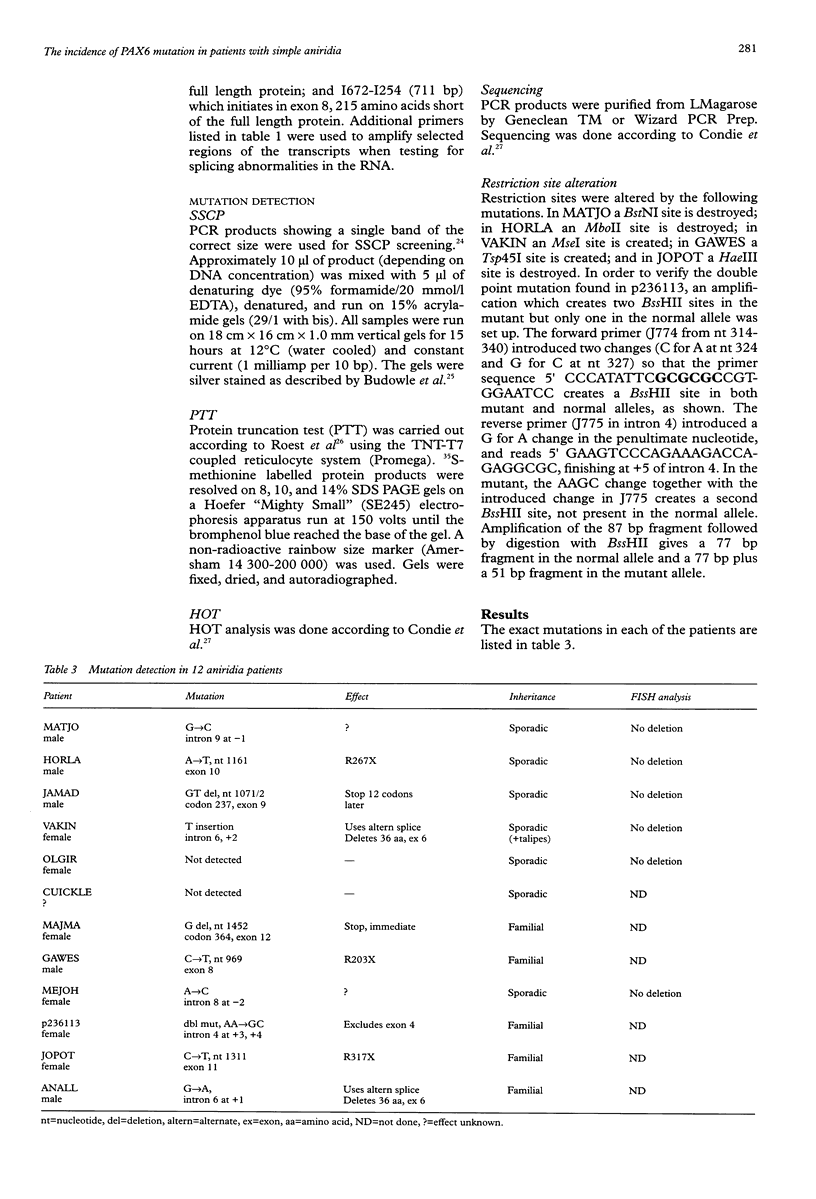
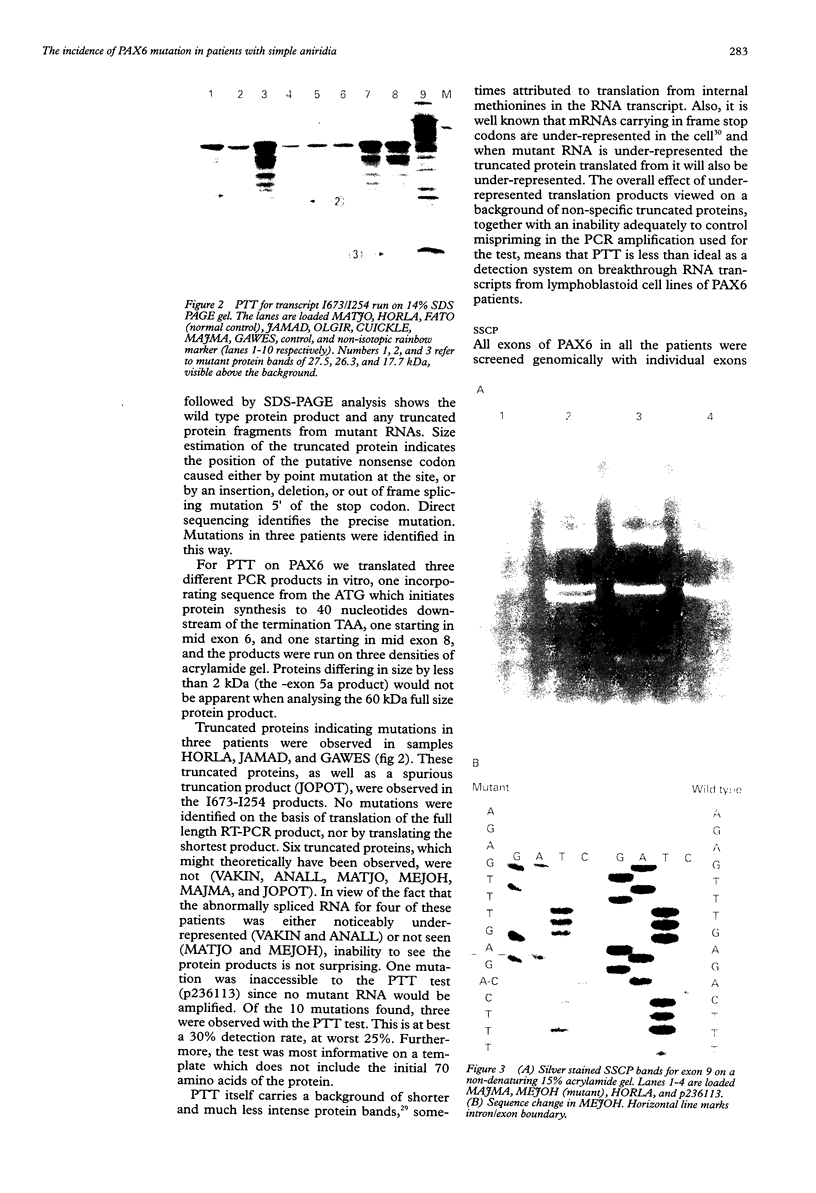
Twelve aniridia patients, five with a family history and seven presumed to be sporadic, were exhaustively screened in order to test what proportion of people with aniridia, uncomplicated by associated anomalies, carry mutations in the human PAX6 gene. Mutations were detected in 90% of the cases. Three mutation detection techniques were used to determine if one method was superior for this gene. The protein truncation test (PTT) was used on RT-PCR products, SSCP on genomic PCR amplifications, and chemical cleavage of mismatch on both RT-PCR and genomic amplifications. For RT-PCR products, only the translated portion of the gene was screened. On genomic products exons 1 to 13 (including 740 bp of the 3' untranslated sequence and all intron/exon boundaries) were screened, as was a neuroretina specific enhancer in intron 4. Ten of the possible 12 mutations in the five familial cases and five of the sporadic patients were found, all of which conformed to a functional outcome of haploinsufficiency. Five were splice site mutations (one in the donor site of intron 4, two in the donor site of intron 6, one in each of the acceptor sites of introns 8 and 9) and five were nonsense mutations in exons 8, 9, 10, 11, and 12. SSCP analysis of individually amplified exons, with which nine of the 10 mutations were seen, was the most useful detection method for PAX6.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoufouchi S., Yélamos J., Milstein C. Nonsense mutations inhibit RNA splicing in a cell-free system: recognition of mutant codon is independent of protein synthesis. Cell. 1996 May 3;85(3):415–422. doi: 10.1016/s0092-8674(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Azuma N., Nishina S., Yanagisawa H., Okuyama T., Yamada M. PAX6 missense mutation in isolated foveal hypoplasia. Nat Genet. 1996 Jun;13(2):141–142. doi: 10.1038/ng0696-141. [DOI] [PubMed] [Google Scholar]
- Budowle B., Chakraborty R., Giusti A. M., Eisenberg A. J., Allen R. C. Analysis of the VNTR locus D1S80 by the PCR followed by high-resolution PAGE. Am J Hum Genet. 1991 Jan;48(1):137–144. [PMC free article] [PubMed] [Google Scholar]
- Condie A., Eeles R., Borresen A. L., Coles C., Cooper C., Prosser J. Detection of point mutations in the p53 gene: comparison of single-strand conformation polymorphism, constant denaturant gel electrophoresis, and hydroxylamine and osmium tetroxide techniques. Hum Mutat. 1993;2(1):58–66. doi: 10.1002/humu.1380020111. [DOI] [PubMed] [Google Scholar]
- Dietrich W. F., Lander E. S., Smith J. S., Moser A. R., Gould K. A., Luongo C., Borenstein N., Dove W. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993 Nov 19;75(4):631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- Epstein J. A., Glaser T., Cai J., Jepeal L., Walton D. S., Maas R. L. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev. 1994 Sep 1;8(17):2022–2034. doi: 10.1101/gad.8.17.2022. [DOI] [PubMed] [Google Scholar]
- Fantes J., Redeker B., Breen M., Boyle S., Brown J., Fletcher J., Jones S., Bickmore W., Fukushima Y., Mannens M. Aniridia-associated cytogenetic rearrangements suggest that a position effect may cause the mutant phenotype. Hum Mol Genet. 1995 Mar;4(3):415–422. doi: 10.1093/hmg/4.3.415. [DOI] [PubMed] [Google Scholar]
- Glaser T., Jepeal L., Edwards J. G., Young S. R., Favor J., Maas R. L. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994 Aug;7(4):463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- Glaser T., Walton D. S., Maas R. L. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992 Nov;2(3):232–239. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- Halder G., Callaerts P., Gehring W. J. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995 Mar 24;267(5205):1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Hanson I. M., Fletcher J. M., Jordan T., Brown A., Taylor D., Adams R. J., Punnett H. H., van Heyningen V. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters' anomaly. Nat Genet. 1994 Feb;6(2):168–173. doi: 10.1038/ng0294-168. [DOI] [PubMed] [Google Scholar]
- Hanson I. M., Seawright A., Hardman K., Hodgson S., Zaletayev D., Fekete G., van Heyningen V. PAX6 mutations in aniridia. Hum Mol Genet. 1993 Jul;2(7):915–920. doi: 10.1093/hmg/2.7.915. [DOI] [PubMed] [Google Scholar]
- Hanson I., Van Heyningen V. Pax6: more than meets the eye. Trends Genet. 1995 Jul;11(7):268–272. doi: 10.1016/s0168-9525(00)89073-3. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Favor J., Hogan B. L., Ton C. C., Saunders G. F., Hanson I. M., Prosser J., Jordan T., Hastie N. D., van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991 Dec 19;354(6354):522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Jordan T., Hanson I., Zaletayev D., Hodgson S., Prosser J., Seawright A., Hastie N., van Heyningen V. The human PAX6 gene is mutated in two patients with aniridia. Nat Genet. 1992 Aug;1(5):328–332. doi: 10.1038/ng0892-328. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Loosli F., Kmita-Cunisse M., Gehring W. J. Isolation of a Pax-6 homolog from the ribbonworm Lineus sanguineus. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):2658–2663. doi: 10.1073/pnas.93.7.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E. When cells stop making sense: effects of nonsense codons on RNA metabolism in vertebrate cells. RNA. 1995 Jul;1(5):453–465. [PMC free article] [PubMed] [Google Scholar]
- Martha A., Ferrell R. E., Mintz-Hittner H., Lyons L. A., Saunders G. F. Paired box mutations in familial and sporadic aniridia predicts truncated aniridia proteins. Am J Hum Genet. 1994 May;54(5):801–811. [PMC free article] [PubMed] [Google Scholar]
- Mirzayans F., Pearce W. G., MacDonald I. M., Walter M. A. Mutation of the PAX6 gene in patients with autosomal dominant keratitis. Am J Hum Genet. 1995 Sep;57(3):539–548. [PMC free article] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza S., Dozier C., Langlois M. C., Saule S. Identification and characterization of a neuroretina-specific enhancer element in the quail Pax-6 (Pax-QNR) gene. Mol Cell Biol. 1995 Feb;15(2):892–903. doi: 10.1128/mcb.15.2.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer S. J., Anton-Culver H., Webster L., Noble B., Liao S., Kennedy A., Belinson J., Casey G. Detection of BRCA1 mutations by the protein truncation test. Hum Mol Genet. 1995 Oct;4(10):1989–1991. doi: 10.1093/hmg/4.10.1989. [DOI] [PubMed] [Google Scholar]
- Quiring R., Walldorf U., Kloter U., Gehring W. J. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994 Aug 5;265(5173):785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Roest P. A., Roberts R. G., Sugino S., van Ommen G. J., den Dunnen J. T. Protein truncation test (PTT) for rapid detection of translation-terminating mutations. Hum Mol Genet. 1993 Oct;2(10):1719–1721. doi: 10.1093/hmg/2.10.1719. [DOI] [PubMed] [Google Scholar]
- Shiffrar M., Li X., Lorenceau J. Motion integration across differing image features. Vision Res. 1995 Aug;35(15):2137–2146. doi: 10.1016/0042-6989(94)00299-1. [DOI] [PubMed] [Google Scholar]
- Ton C. C., Hirvonen H., Miwa H., Weil M. M., Monaghan P., Jordan T., van Heyningen V., Hastie N. D., Meijers-Heijboer H., Drechsler M. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991 Dec 20;67(6):1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- Walther C., Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991 Dec;113(4):1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Walther C., Guenet J. L., Simon D., Deutsch U., Jostes B., Goulding M. D., Plachov D., Balling R., Gruss P. Pax: a murine multigene family of paired box-containing genes. Genomics. 1991 Oct;11(2):424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]