Abstract
Breast cancer is the most common tumor among women worldwide and still remains the leading cause of death in women in Italy. Although survival from this pathology has increased, this disease and its treatment can have lasting or delayed effects that can greatly affect a woman's quality of life. Primary and secondary prevention are currently the best strategies to combat this cancer: improved lifestyle, early adherence to screening, Breast Self-Examination (BSE), and even now the use of technology, have become among the most important tools to ensure increasingly early diagnosis of this disease, which is a major cause of suffering and premature mortality in women. Indeed, early diagnosis of the disease can lead to a good prognosis and a high survival rate. This study investigates the attitude of Italian women to perform clinical checkups aimed at cancer prevention, particularly adherence to free screening programs offered by the National Health Service (NHS) for women in the 50–69 age group. The knowledge, use and emotional approach toward BSE as a screening tool and the use of dedicated apps for this purpose are also investigated. Low adherence to screening programs, lack of BSE practice, and nonuse of dedicated apps are just some of the results observed in this study. Therefore, it becomes essential to spread the culture of prevention, cancer awareness and the importance of screening throughout life.
Keywords: Breast Cancer, Prevention, Breast Self Examination, Screening Programs, Apps for Screening
Introduction
Breast cancer is currently the most common malignant tumor in women worldwide [2, 4]. With 55,000 new diagnoses in 2020 and 12,500 deaths expected in 2021, this cancer accounts for 30% of all cancers diagnosed in Italy [1].
The risk of developing breast cancer correlates with increasing age [1] and is associated with genetic, endocrine, dietary, environmental factors, lifestyle habits, and previous breast disease, although more than half of the cases, however, cannot be attributed to any known risk factor [6, 9, 13, 14, 18, 22].
Unfortunately, despite breast cancer awareness, public attention, and advances in breast imaging for early detection, it still remains the leading cause of cancer death in women in Italy [1]. Early diagnosis, therefore, is the most important tool to intervene in time and improve the prognosis of patients. Mammography screening is a periodic secondary prevention activity aimed at women in order to make an early stage diagnosis of breast cancer and, therefore, offer less aggressive and more effective treatments, with the aim of reducing mortality from breast cancer [1]. Mammography is still considered the most effective screening test, and the organized, population-based modality is preferred over spontaneous initiative [1]. In Italy, in accordance with the guidelines concerning prevention, diagnosis, and care in oncology and in line with the standards adopted by other European countries, free mammography screening is to be offered every two years to all women aged 50–69 years [1]. In addition, in some regions, the effectiveness of screening is also being tested in a wider age range of 45 to 74 years old [1]. However, this offering is not always accepted, and geographic differences have been found to be particularly significant in terms of screening program implementation, incidence, and survival of breast cancer [16, 17]. In addition, in recent years the limitations imposed by the pandemic have not made it easier for women to move between regions, but distancing, fear of physical proximity, and unease due to protective mask wearing during an outpatient visit have also played their part. In this rather multifaceted scenario, the rise and use of smartphone Apps and e-learning courses were found to be useful and strongly recommended to educate and improve women's health beliefs and performance in breast self-examination [3, 19].
Another prevention methodology, debated but still widely recommended, is the practice of self-palpation or Breast Self-Examination (BSE), a self-examination of the breasts that any woman, starting generally in her 20’s, may choose to perform on herself monthly or occasionally to assess visible, palpable changes and report them later to her doctor. Even women who underwent breast surgery, are pregnant or breastfeeding can perform it without risk [20]. Self-palpation is the first breast cancer "prevention" tool. This simple self-assessment test allows to learn to recognize the structure and general appearance of the breast, so that early and unusual changes from the basic physiognomy of the breast can be caught, but only regular use allows to promptly detect changes and thus to best express the usefulness of BSE.
In an increasingly difficult scenario, timely adherence to screening, self-examination to detect every slightest change, and the use of technology have become among the most important tools for increasingly early diagnosis of breast cancer, which is a major public health problem, a major cause of human suffering and premature mortality among women [5]. In light of these premises, this study investigates the attitude of Italian women to perform clinical screening for the prevention of breast cancer, particularly the participation of women in the 50–69 age group in screening programs offered free of charge by the National Health Service (NHS). The knowledge, use and emotional approach of women toward breast self-examination as an investigative tool and the use of apps dedicated to it is also investigated.
Methods
Design
From March 2021 to January 2022 a survey was administered among Italian female population. 2375 subjects agreed to participate in the study. The survey was conducted by means of an anonymous questionnaire distributed on a voluntary basis. All women belonging to the Italian population, aged between 20 and 69 years and who agreed to participate in the study by signing the informed consent were included. Those who did not meet the inclusion criteria were excluded. Women with non-Italian citizenship were also not included. All sections of the questionnaire were computerized through the use of a preset form from the Google Drive platform, and the study was conducted through electronic dissemination. Facebook groups of various types and Instagram pages used for publishing computerized questionnaires were contacted. The sampling used was (virtual) snowball sampling until data saturation.
Survey Instrument
The questionnaire was constructed 'ad hoc'. It consists of 32 items divided into 4 sections: the first section (5 items) contains socio-demographic data (age, geographical area in which she lives, marital status, level of education, employment status), and is followed by a second section (8 items) in which clinical controls aimed at breast cancer prevention are investigated, with particular attention to the adherence of women invited to participate in free screening offered by the NHS. The third section (16 items) assesses women's approach to and consideration of self-palpation, and finally a fourth section (4 items) explores the knowledge and use of dedicated self-palpation apps.
Statistical Analysis
The answers to the questionnaire items of all respondents were reported using descriptive statistics. To identify items associated with differences in behavior toward breast cancer, the subjects were divided into 2 groups: The first group represents the general population (Group A, n = 2235) while the second group represents women who have or had breast cancer in the past (Group 2, n = 140).
For each question, where appropriate, respondents were also divided by age, educational level, geographic area and marital status. Continuous variables were summarized using mean and standard deviation (SD) and categorical variables using frequencies and percentages. The Mann–Whitney U-test was used for assessing difference between Groups. Possible associations between Groups (outcome) and socio-demographics data (explanatory variables) were tested by linear logistic regression. A p value < 0.05 was considered statistically significant. The statistical analyses were conducted for all qualitative and quantitative variables using Matlab software.
Results
Sample Demographics and Baseline Characteristics
A total of 2375 women agreed to participate in the study. Baseline characteristics were collected and reported in section 1 of the questionnaire (Table 1). Among the respondents, 338 (14%) were over 50 years old. Most of the participants (53%, n = 1256) were from southern Italy and the Islands. The prevalence of the sample under study was unmarried women (54%, n = 1280) and students (31%, n = 746); this is in line with the prevalent young age present in the sample under study (45% of the women were under 30 years old, n = 1076).
Table 1.
Baseline characteristics and the questionnaire items of all respondents divided by the four sections. Section 2 of the Questionnaire is related to screening adhesion for all women aged 50–69 only. Possible association between Groups (outcome) and socio-demographics data (explanatory variables) such as Age, Level of education and Geographical area) were also evaluated for Sect. 2. A p value < 0.05 was considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001)
| Section 1: Baseline characteristics | N | % | |
| Age (y) | |||
| 20–29 | 1076 | 45 | |
| 30–39 | 544 | 23 | |
| 40–49 | 417 | 18 | |
| 50–59 | 249 | 10 | |
| 60–69 | 89 | 4 | |
| Geographic area | |||
| North | 607 | 26 | |
| Center | 512 | 22 | |
| South/Islands | 1256 | 53 | |
| Marital status | |||
| Married | 944 | 40 | |
| Divorced | 81 | 3 | |
| Maiden | 1280 | 54 | |
| Separate | 46 | 2 | |
| Widow | 24 | 1 | |
| Education level | |||
| Degree | 954 | 40 | |
| High school graduation | 1197 | 50 | |
| Junior high school diploma | 202 | 9 | |
| Elementary license | 17 | 1 | |
| None | 5 | 0 | |
| Employment status | |||
| Craftsman | 254 | 11 | |
| Public Administration | 624 | 26 | |
| Services/Tertiary | 356 | 15 | |
| Student | 746 | 31 | |
| Retired | 50 | 2 | |
| Unemployed | 345 | 15 | |
| Questionnaire items | N | % | |
| Section 2: Participation in screening programs (Women aged 50–69 only) | |||
| Q1. Have you ever undergone clinical screening for early detection of breast cancer? | |||
| Never | 20 | 6 | Age: < 0.001*** |
| Rarely | 45 | 13 | Education level: < 0.001*** |
| Occasionally | 17 | 5 | Geographical area: < 0.001*** |
| Often | 78 | 23 | |
| Always | 178 | 53 | |
| Q2. If yes, please indicate the frequency | |||
| I have never had a screening exam | 17 | 5 | Age: < 0.001*** |
| Every two years | 140 | 41 | Education level: < 0.001*** |
| Once a year | 164 | 49 | Geographical area: < 0.001*** |
| Every six months | 15 | 4 | |
| Once a month | 2 | 1 | |
| Q3. Have you ever taken advantage of the region's free checkups? | |||
| No | 97 | 29 | Age: < 0.001*** |
| Yes | 235 | 70 | Education level: < 0.001*** |
| I don't know what this is about | 6 | 2 | Geographical area: < 0.001*** |
| Q4. Have you ever undergone a biopsy? | |||
| No | 151 | 45 | Age: < 0.001*** |
| Yes | 56 | 17 | Education level: < 0.001*** |
| missing | 131 | 39 | Geographical area: < 0.001*** |
| Q5. Have you ever undergone mammography? | |||
| No | 6 | 2 | Age: < 0.001*** |
| Yes | 323 | 95 | Education level: < 0.001*** |
| missing | 9 | 3 | Geographical area: < 0.001*** |
| Q6. Have you ever undergone ultrasonography? | |||
| No | 51 | 15 | Age: < 0.001*** |
| Yes | 235 | 70 | Education level: < 0.001*** |
| missing | 52 | 15 | Geographical area: < 0.001*** |
| Q7. Have you ever undergone magnetic resonance imaging (MRI)? | |||
| No | 169 | 50 | Age: < 0.001*** |
| Yes | 25 | 7 | Education level: < 0.001*** |
| missing | 144 | 43 | Geographical area: < 0.001*** |
| Section 3: Approach toward self-examination (BSE) | |||
| Q8. How often do you perform breast self-examination? | |||
| Never | 445 | 19 | Age: < 0.001*** |
| Rarely | 567 | 24 | Education level: < 0.001*** |
| Occasionally | 717 | 30 | Geographical area: < 0.001*** |
| Often | 560 | 24 | |
| Always | 86 | 4 | |
| Q9. If no, state the reason | |||
| I perform it | 989 | 42 | Age: < 0.001*** |
| I perform other medical examinations | 36 | 2 | Education level: < 0.001*** |
| I don't remember to run it | 425 | 18 | Geographical area: < 0.001*** |
| Fear of ominous prognosis | 117 | 5 | |
| I don't know how to execute it correctly | 807 | 34 | |
| missing | 1 | 0 | |
| Q10. When I perform self-examination I am taking care of myself | |||
| Strongly agree | 1422 | 60 | Age: < 0.001*** |
| agree | 849 | 36 | Education level: < 0.001*** |
| uncertain | 99 | 4 | Geographical area:0.09 |
| disagree | 3 | 0 | |
| strongly disagree | 2 | 0 | |
| Q11. Self-palpation is embarrassing | |||
| Strongly agree | 32 | 2 | Age: < 0.001*** |
| agree | 93 | 4 | Education level: < 0.001*** |
| uncertain | 143 | 6 | Geographical area: < 0.05* |
| disagree | 842 | 35 | |
| strongly disagree | 1264 | 53 | |
| Q12. Self-palpation takes too long | |||
| Strongly agree | 12 | 1 | Age: < 0.001*** |
| agree | 53 | 2 | Education level: < 0.001*** |
| uncertain | 302 | 13 | Geographical area: < 0.001*** |
| disagree | 1136 | 48 | |
| strongly disagree | 872 | 37 | |
| Q13. It is difficult to remember to do breast checks | |||
| Strongly agree | 59 | 2 | Age: < 0.001*** |
| agree | 357 | 15 | Education level: < 0.001*** |
| uncertain | 382 | 16 | Geographical area: < 0.001*** |
| disagree | 932 | 39 | |
| strongly disagree | 645 | 27 | |
| Q14. I have no privacy to perform the breast check | |||
| Strongly agree | 10 | 0 | Age: < 0.001*** |
| agree | 85 | 4 | Education level: < 0.001*** |
| uncertain | 152 | 6 | Geographical area: < 0.05* |
| disagree | 988 | 42 | |
| strongly disagree | 1160 | 48 | |
| Q15. My breasts are too big by self-examination | |||
| Strongly agree | 23 | 1 | Age: < 0.001*** |
| agree | 80 | 3 | Education level: < 0.001*** |
| uncertain | 304 | 13 | Geographical area: < 0.001*** |
| disagree | 1006 | 42 | |
| strongly disagree | 962 | 41 | |
| Q16. I have more important problems than self-palpation | |||
| Strongly agree | 8 | 0 | Age: < 0.001*** |
| agree | 34 | 1 | Education level: < 0.001*** |
| uncertain | 113 | 5 | Geographical area: 0.06 |
| disagree | 944 | 40 | |
| strongly disagree | 1276 | 54 | |
| Q17. Can I perform self-examination correctly | |||
| Strongly agree | 299 | 13 | Age: < 0.001*** |
| agree | 965 | 41 | Education level: < 0.001*** |
| uncertain | 876 | 37 | Geographical area: < 0.001*** |
| disagree | 181 | 8 | |
| strongly disagree | 54 | 2 | |
| Q18. Would like more information about self-examination | |||
| No | 333 | 14 | Age: < 0.001*** |
| Yes | 2042 | 86 |
Education level: < 0.001*** Geographical area: < 0.001*** |
| Q19. Do you find the general practitioner useful for info on self- examination | |||
| No | 628 | 26 | Age: < 0.001*** |
| Yes | 1747 | 74 |
Education level: < 0.001*** Geographical area: < 0.001*** |
| Q20. Do you find the oncologist useful for info on self-examination | |||
| No | 459 | 19 | Age: < 0.001*** |
| Yes | 1916 | 81 |
Education level: < 0.001*** Geographical area: < 0.05* |
| Q21. Do you find the nurse useful for info on self-examination | |||
| No | 1212 | 51 | Age: < 0.001*** |
| Yes | 1162 | 49 |
Education level: < 0.001*** Geographical area: < 0.001*** |
| Q22. Do you find the psychologist useful for info on self-examination | |||
| No | 2083 | 88 | Age: < 0.001*** |
| Yes | 292 | 12 |
Education level: < 0.001*** Geographical area: < 0.05* |
| Q23. Do you find the breast specialist useful for info on self-examination | |||
| No | 67 | 3 | Age: < 0.001*** |
| Yes | 2308 | 97 |
Education level: < 0.001*** Geographical area: < 0.05* |
| SECTION 4: KNOWLEDGE AND USE OF APPS DEDICATED TO BSE | |||
| Q24. Do you know or use dedicated applications for self-palpation? | |||
| No | 2325 | 98 | Age: < 0.001*** |
| Yes | 50 | 2 |
Education level: < 0.001*** Geographical area: < 0.05* |
| Q25. Use BreastTest | |||
| I do not use any application | 273 | 11 | Age: < 0.001*** |
| Never | 775 | 33 | Education level: < 0.01** |
| Rarely | 31 | 1 | Geographical area: 0.27 |
| Occasionally | 14 | 1 | |
| Often | 3 | 0 | |
| Always | 3 | 0 | |
| missing | 1276 | 54 | |
| Q26. Do you use Igyno? | |||
| I do not use any application | 270 | 11 | Age: < 0.001*** |
| Never | 765 | 32 | Education level: < 0.01** |
| Rarely | 36 | 2 | Geographical area: 0.15 |
| Occasionally | 15 | 1 | |
| Often | 4 | 0 | |
| Always | 3 | 0 | |
| missing | 1282 | 54 | |
| Q27. Do you use Breast Cancer Indicators? | |||
| I do not use any application | 269 | 11 | Age: < 0.001*** |
| Never | 774 | 33 | Education level: < 0.001*** |
| Rarely | 27 | 1 | Geographical area: < 0.05* |
| Occasionally | 13 | 1 | |
| Often | 3 | 0 | |
| Always | 1 | 0 | |
| missing | 1288 | 54 | |
Questionnaire Items
The questionnaire items were evaluated for all respondents and data were collected (Table 1, in sections 2–4).
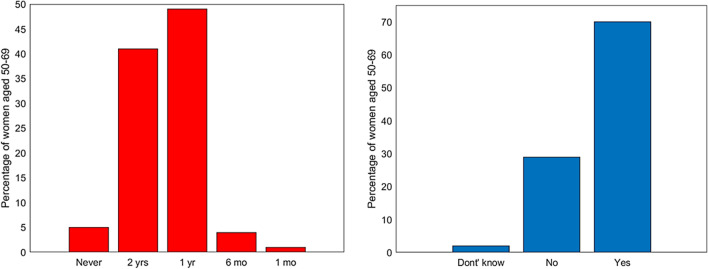
Section 2 investigates women's participation and frequency of clinical checkups performed for breast cancer prevention, with a special focus on adherence to free screenings offered by the NHS targeting women in the 50–69 age group. Surprisingly, only 53% of women over 50 say they always (n = 178) or often (23%, n = 78) participate to screenings. Specifically, 49% once a year, 41% every two years, 4% every 6 months, and 1% once a month (Fig. 1a). In addition, although almost all the women (98%) appear to be aware of the existence of free screenings offered by the NHS, 29% say they do not adhere to them (Fig. 2).
Fig. 1.
Left: frequencies of clinical controls for breast cancer for women aged 50–69 years; right: adherence to free screening program for women aged 50–69 years old
Fig. 2.
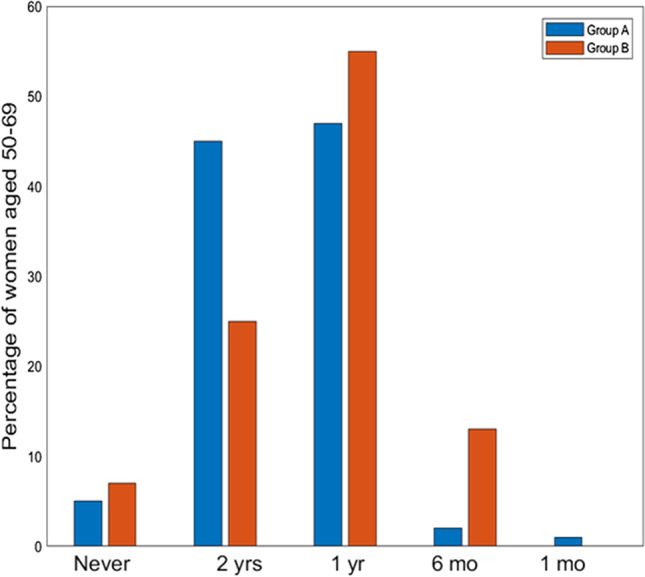
Frequencies of clinical controls for breast cancer for women aged 50–69 years divided into Group A (women of the general population) in Blue (left bars), and Group B (women already been diagnosed with breast cancer) in Orange (right bars). (yrs = years, yr = year, mo = months)
Of the examinations performed, 95% involved mammograms (n = 323) and 70% involved ultrasounds (n = 235), and this is consistent with the screening protocol that provides mammography examination for women over 50 and recommendation for ultrasound instead for younger women.
In order to test whether these data are also confirmed among women with prior breast cancer compared to other women in the general population, respondents aged 50–69 were classified into two groups: Group A including women who had not been diagnosed with breast cancer (82%, n = 278) and Group B including women who had already been diagnosed with breast cancer (18%, n = 60). As expected, looking at the participation in clinical checkups between these two groups aged 50–69 years (Table 2), significantly fewer (p < 0.05) women in Group A (51%) say they always perform preventive checkups compared to Group B (62%). Frequency in checkups is also higher in women with prior cancer, who report checking every six months/year in contrast to other women, who tend to have checkups mainly every two years (Fig. 2). This trend appears to be correlated with educational level (p = 0.001) and geographical area of origin (p = 0.001) (Table 1), with the South and Islands characterized by the lowest adherence.
Table 2.
Sect. 2 of the questionnaire responses of the adult female population aged 50–69 among subjects who had not been diagnosed with breast cancer (Group A, n = 278) and subjects who had already been diagnosed with breast cancer (Group B, n = 60). Differences in response between the two Groups were assessed. A p value < 0.05 was considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001)
| Questionnaire items | Group A Women in the general population aged 50–69 (n = 278) N (%) |
Group B Women with cancer aged 50–69 (n = 60) N (%) |
p-value |
|---|---|---|---|
| Section 2: Participation in screening programs | |||
| Have you ever undergone clinical screening for early detection of breast cancer? | |||
| Never | 16 (6%) | 4 (7%) | < 0.05* |
| Rarely | 15 (5%) | 2 (3%) | |
| Occasionally | 38 (14%) | 7 (12%) | |
| Often | 68 (24%) | 10 (17%) | |
| Always | 141 (51%) | 37 (62%) | |
| If yes, please indicate the frequency | |||
| I have never had a screening exam | 13 (5%) | 4 (7%) | 0.54 |
| Every two years | 125 (45%) | 15 (25%) | |
| Once a year | 131 (47%) | 33 (55%) | |
| Every six months | 7 (2%) | 8 (13%) | |
| Once a month | 2 (1%) | 0 | |
| Have you ever taken advantage of the region's free checkups? | |||
| No | 77 (29%) | 20 (33%) | 0.40 |
| Yes | 195 (69%) | 40 (67%) | |
| Missing | 6 (2%) | 0 | |
| Have you ever had a biopsy? | |||
| No | 138 (50%) | 13 (22%) | < 0.001*** |
| Yes | 27 (10%) | 29 (48%) | |
| Missing | 113 (40%) | 18 (30%) | |
| Have you ever had a mammogram? | |||
| No | 5 (2%) | 1 (2%) | 0.30 |
| Yes | 265 (95%) | 58 (97%) | |
| Missing | 8 (3%) | 1 (2%) | |
| Have you ever undergone ultrasound? | |||
| No | 45 (16%) | 6 (10%) | 0.09 |
| Yes | 184 (66%) | 51 (85%) | |
| Missing | 49 (18%) | 3 (5%) | |
| Have you ever undergone MRI? | |||
| No | 148 (53%) | 21 (35%) | < 0.001*** |
| Yes | 6 (2%) | 19 (33%) | |
| Missing | 124 (45%) | 20 (32%) | |
Verifying the same data in women under 50 (data not shown), it is interesting to highlight that there are many women in the 41–50 age group who undergo mammography despite not yet being called by NHS.
Women with prior cancer, especially, check themselves annually compared with their peers, and all of them, 100% of the time, report having undergone at least one ultrasound.
Section 3 of Table 1 assesses the approach of all respondents—regardless of age—to BSE. Although 96% of respondents (n = 2271) agree or strongly agree that self-examination is important for women's care, only 4% say they always perform it (n = 86) (Fig. 3). Nearly half of women also admit that they do not know how to perform self-palpation correctly or are uncertain about it (47%, n = 1111). This type of insecurity or uncertainty seems to diminish with advancing age and is more characteristic of women under 50 (86%, n = 948) and surprisingly typical of college graduates/graduates (90%, n = 995).
Fig. 3.
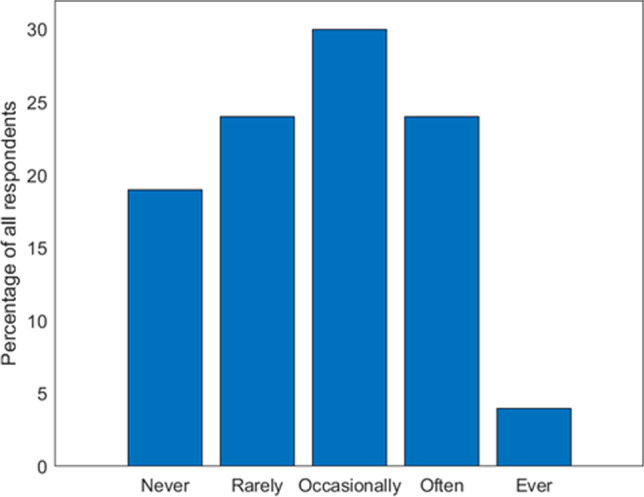
Frequencies of BSE for all respondents
Among those who say they do not perform it, 18% (n = 425) say they forget, 5% (n = 117) are afraid of an inauspicious prognosis, and 2% (n = 36) perform other medical tests.
There are also many respondents who would like more information about BSE (86% n = 2042), and the referral figures considered useful in order of preference are the breast specialist (97%, n = 2308), oncologist (81%, n = 1916), general practitioner (74%. n = 1747), nurse (49%, n = 1162) and finally the psychologist, who garners only 12% of preferences (n = 292).
In line with previous results, dividing women between Group A and Group B regardless the age of the respondents (Table 3) and evaluating the approach toward self-examination between the two surveyed groups again shows more participation by Group B (60%) than Group A (25%), (p < 0.01). Women in Group B feel more conscious about performing BSE properly than women in Group A (p < 0.05). In fact, the greatest justification for not performing BSE related precisely to the fear of not knowing how to perform it correctly (35% Group A and 14% Group B) (p = 0.01).
Table 3.
Questionnaire responses of the adult female population among subjects who had not been diagnosed with breast cancer (Group A, n = 2235) and subjects who had already been diagnosed with breast cancer (Group B, n = 140). Differences in response between the two Groups were assessed. A p value < 0.05 was considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001)
| Questionnaire items | Group A Women in the general population (n = 2235) N (%) |
Group B Women with cancer (n = 140) N (%) |
p-value |
|---|---|---|---|
| Section 2: Participation in screening programs | |||
| Q1. Have you ever undergone clinical screening for early detection of breast cancer? | |||
| Never | 1183 (53%) | 14 (10%) | < 0.01** |
| Rarely | 166 (7%) | 8 (6%) | |
| Occasionally | 272 (12%) | 13 (9%) | |
| Often | 269 (12%) | 36 (26%) | |
| Always | 345 (15%) | 69 (49%) | |
| If yes, please indicate the frequency | |||
| I have never had a screening exam | 1204 (54%) | 13 (9%) | 0.05* |
| Every two years | 390 (17%) | 23 (16%) | |
| Once a year | 585 (26%) | 78 (56%) | |
| Every six months | 52 (2%) | 24 (17%) | |
| Once a month | 4 (0%) | 2 (1%) | |
| Q2. Have you ever taken advantage of the region's free checkups? | |||
| No | 1557 (70%) | 467 (48%) | < 0.001*** |
| Yes | 419 (19%) | 71 (51%) | |
| I don't know what this is about | 259 (12%) | 2 (1%) | |
| Q3. Have you ever undergone a biopsy? | |||
| No | 1012 (45%) | 38 (27%) | < 0.001*** |
| Yes | 91 (4%) | 58 (41%) | |
| Missing | 1132 (51%) | 44 (31%) | |
| Q4. Have you ever undergone mammography? | |||
| No | 664 (30%) | 11 (8%) | < 0.001*** |
| Yes | 690 (31%) | 113 (81%) | |
| missing | 881 (39%) | 16 (11%) | |
| Q5. Have you ever undergone ultrasonography? | |||
| No | 536 (24%) | 8 (6%) | < 0.001*** |
| Yes | 858 (38%) | 121 (86%) | |
| missing | 841 (38%) | 11 (8%) | |
| Q6. Have you ever undergone MRI? | |||
| No | 1033 (46%) | 47 (34%) | < 0.001*** |
| Yes | 50 (2%) | 43 (31%) | |
| missing | 1152 (52%) | 50 (36%) | |
| Q7 | |||
| No | |||
| Yes | |||
| Missing | |||
| Section 3: Approach toward self-examination (BSE) | |||
| Q8. How often do you perform breast self- examination? | |||
| Never | 438 (20%) | 7 (5%) | < 0.01** |
| Rarely | 545 (24%) | 22 (16%) | |
| Occasionally | 690 (31%) | 27 (19%) | |
| Often | 495 (22%) | 65 (46%) | |
| Always | 67 (3%) | 19 (14%) | |
| Q9. If you do not perform it, state the reason | |||
| I perform it | 895 (40%) | 94 (67%) | 0.01** |
| I perform other medical examinations | 30 (1%) | 6 (4%) | |
| I don't remember to run it | 416 (19%) | 9 (6%) | |
| Fear of ominous prognosis | 105 (5%) | 12 (9%) | |
| I don't know how to execute it correctly | 788 (35%) | 19 (14%) | |
| Missing | 1 (0%) | 0 | |
| Q10. When I perform self-examination I am taking care of myself | |||
| Strongly agree | 1329 (59%) | 93 (66%) | 0.78 |
| agree | 806 (36%) | 43 (31%) | |
| uncertain | 95 (4%) | 4 (3%) | |
| disagree | 3 (0%) | 0 | |
| strongly disagree | 2 (0%) | 0 | |
| Q11. Self-palpation is embarrassing | |||
| Strongly agree | 30 (1%) | 3 (2%) | < 0.05* |
| agree | 86 (4%) | 7 (5%) | |
| uncertain | 140 (6%) | 3 (2%) | |
| disagree | 787 (35%) | 55 (39%) | |
| strongly disagree | 1192 (53%) | 72 (51%) | |
| Q12. Self-palpation takes too long | |||
| Strongly agree | 11 (0%) | 1 (1%) | 0.08 |
| agree | 52 (2%) | 1 (1%) | |
| uncertain | 292 (13%) | 10 (7%) | |
| disagree | 1073 (48%) | 63 (45%) | |
| strongly disagree | 807 (36%) | 65 (46%) | |
| Q13. It is difficult to remember to do breast checks | |||
| Strongly agree | 58 (3%) | 1 (1%) | < 0.05* |
| agree | 344 (15%) | 13 (9%) | |
| uncertain | 371 (17%) | 11 (8%) | |
| disagree | 880 (39%) | 52 (37%) | |
| strongly disagree | 582 (26%) | 63 (45%) | |
| Q14. I have no privacy to perform the breast check | |||
| Strongly agree | 9 (0%) | 1 (1%) | 0.05* |
| agree | 83 (4%) | 2 (1%) | |
| uncertain | 145 (6%) | 7 (5%) | |
| disagree | 937 (42%) | 51 (36%) | |
| strongly disagree | 1061 (47%) | 79 (56%) | |
| Q15. My breasts are too big by self- examination | |||
| Strongly agree | 21 (1%) | 2 (1%) | 0.05* |
| agree | 76 (3%) | 4 (3%) | |
| uncertain | 279 (12%) | 25 (18%) | |
| disagree | 942 (42%) | 64 (46%) | |
| strongly disagree | 917 (41%) | 45 (32%) | |
| Q16. I have more important problems than self-palpation | |||
| Strongly agree | 8 (0%) | 0 | 0.11 |
| agree | 31 (1%) | 3 (2%) | |
| uncertain | 108 (5%) | 5 (4%) | |
| disagree | 896 (40%) | 48 (34%) | |
| strongly disagree | 1192 (53%) | 84 (60%) | |
| Q17. Can I perform self-examination correctly | |||
| Strongly agree | 269 (12%) | 30 (21%) | < 0.05* |
| agree | 905 (40%) | 60 (43%) | |
| uncertain | 838 (37%) | 38 (27%) | |
| disagree | 171 (8%) | 10 (7%) | |
| strongly disagree | 52 (2%) | 2 (1%) | |
| Q18. Would like more information about self-examination | |||
| Yes | 1952 (87%) | 90 (64%) | < 0.001*** |
| No | 283 (13%) | 50 (36%) | |
| Q19. Do you find the general practitioner useful for info on self-examination | |||
| Yes | 1664 (74%) | 83 (59%) | < 0.001*** |
| No | 571 (26%) | 57 (41%) | |
| Q20. Do you find the oncologist useful for info on self-examination | |||
| Yes | 1800 (81%) | 116 (83%) | 0.58 |
| No | 435 (19%) | 24 (17%) | |
| Q21. Do you find the nurse useful for info on self-examination | |||
| Yes | 1104 (49%) | 58 (41%) | 0.081 |
| No | 1131 (51%) | 81 (59%) | |
| Q22. Do you find the psychologist useful for info on self-examination | |||
| Yes | 278 (12%) | 14 (10%) | 0.50 |
| No | 1957 (88%) | 126 (90%) | |
| Q23. Do you find the breast specialist useful for info on self-examination | |||
| Yes | 2172 (97%) | 136 (97%) | 1 |
| No | 63 (3%) | 4 (3%) | |
| SECTION 4: KNOWLEDGE AND USE OF APPS DEDICATED TO BSE | |||
| Q24. Do you know or use dedicated applications for self-palpation? | |||
| No | 2189 (98%) | 136 (97%) | 0.53 |
| Yes | 46 (2%) | 4 (3%) | |
| Q25. Use BreastTest | |||
| I do not use any application | 255 (11%) | 18 (13%) | 0.30 |
| Never | 734 (33%) | 41 (29%) | |
| Rarely | 26 (1%) | 5 (4%) | |
| Occasionally | 13 (1%) | 1 (1%) | |
| Often | 3 (0%) | 0 | |
| Always | 2 (0%) | 1 (1%) | |
| missing | 1202 (54%) | 74 (53%) | |
| Q26. Do you use Igyno? | |||
| I do not use any application | 252 (11%) | 18 (13%) | 0.60 |
| Never | 725 (32%) | 40 (29%) | |
| Rarely | 31 (1%) | 5 (4%) | |
| Occasionally | 15 (1%) | 0 | |
| Often | 4 (0%) | 0 | |
| Always | 2 (0%) | 1 (1%) | |
| missing | 1206 (54%) | 76 (54%) | |
| Q27. Do you use Breast Cancer Indicators? | |||
| I do not use any application | 251 (11%) | 18 (13%) | 0.40 |
| Never | 734 (33%) | 40 (29%) | |
| Rarely | 22 (1%) | 5 (4%) | |
| Occasionally | 13 (1%) | 0 | |
| Often | 3 (0%) | 0 | |
| Always | 0 | 1 (1%) | |
| missing | 1212 (54%) | 76 (54%) | |
Interestingly, this trend appears to be associated with age (p < 0.001), educational level (p < 0.001), and geographic area of origin (p < 0.001) (Table 1). Also associated with age, education level, and sometimes geographic area were certain concepts such as considering BSE embarrassing, time-consuming, privacy-consuming, less important than other problems, or deemed difficult because of too large breasts (Table 1).
A significant difference also relates to remembering to have checkups. While it is understandable that only a relatively small %age of women who have not had cancer remember to do checkups (26%), it is serious that only 45% of women in whom cancer was diagnosed remember to always do them, despite the risk of recurrence.
Another statistically significant difference (p < 0.001) concerns the need for clarification regarding breast self- examination. Our data show higher participation in screening and self-examination by women with previous breast cancer, while the %age of women in the general population needing further information about it is higher (87% vs 64%). Among the reference figures considered useful for the purpose of self-palpation, there is not much statistical difference between the two groups, with the exception of the general practitioner, who is considered more useful by women in Group A than those in Group B (74% vs 59%) (p < 0.001), probably due to the effectiveness of the information.
Finally, Sect. 4 investigates the knowledge and use of BSE-dedicated apps, which were used most in the pandemic period by COVID-19. Only 2% (n = 50) admit using them. However, the %age of those who say they use some apps including BreastTest, Igyno or Breast Cancer Indicators are still less than 3%. There is also no statistical difference between the two groups of women analyzed.
Discussions
The aim of the study was to investigate Italian women's attitudes toward clinical breast cancer screening. Women's knowledge, use, and emotional approach toward breast self-examination as an investigative tool and the possible use of apps dedicated to it were also investigated.
Section 2 of the study investigated adherence to free screening programs aimed at women in the 50–69 age group and offered by the NHS. Incredibly, despite the fact that almost all over-50 women said they were aware of the existence of free mammography screenings, non-adherence is high. Participation in free screening also correlates with educational level and geographic area. Southern Italy and the islands were in fact characterized by lower participation in screening programs. The data appears to be in line with the national 2020 data, where in the South and Islands the crude rate of invitation adherence appears to be low (29%), compared to the North (58%) and the Center (44%).
According to the National Screening Observatory, from 2014 to the present, there has been a gradual decrease in the raw adherence to the invitation to free screening, also due to the pandemic in which the adherence dropped from 86 to 64% [12]. Also in this study, 30% of respondents say that screening programs were interrupted by the COVID-19 pandemic. It is certain that the pandemic affected many women's choice not to undergo breast screening and even surgery, partly because of fear of infection, as shown by the results of an Italian study, conducted by Tor Vergata University [21].
It is certainly reasonable also to note that women who had episodes of breast cancer compared with others are also getting screened at least once a year in younger age groups. The age at which screening should begin, however, is much debated. The Guidelines Development Group (GDG) of the European Commission Initiatives on Breast and Colorectal Cancer (ECIBC) suggests that mammography screening should not be implemented for women younger than 45 years of age, recommending biennial or triennial screening for them depending on the age groups [7]. However, this suggestion differs from what some American organizations such as the American College of Radiology and the National Comprehensive Cancer Network advocate, instead recommending annual screening starting at age 40 [8]. There were even some women in our study who admitted to undergo examinations every six months, especially those with previous cancer (p = 0.05). However, annual or even less than annual frequency appears to be discouraged [7]. The risk of over-diagnosis, i.e., detecting tumor formations that are treated pharmacologically—and would instead remain indolent if left untreated—carries negative psychological consequences associated with this course of ascertainment [11].
There is also significant difference between the types of examinations conducted in the A and B groups. As obvious, women in the general population undergo clinical examinations significantly less than women with prior cancer (p < 0.001). Among these examinations, mammography is believed to be the most widely used screening for breast cancer detection and contributes to reducing mortality [15]. Also in our results, mammography is ranked first among the most frequently performed examinations by women with previous cancer (81%). It is interesting to highlight that there are many women in the 41–50 age group who undergo mammography despite the fact that they are not yet called by SSN. This could result—aside from the natural tendency for greater scrutiny with increasing age—also from increased awareness by the physician and/or peer social environment, given the higher prevalence of breast cancer in young women with a family history of breast cancer [10].
Ultrasound is considered the first level of imaging to be performed especially in young women. Indeed, our data also showed that 100% of women under 30 years old with previous cancer had at least one ultrasound. In contrast, MRI is not recommended as a routine examination because of the higher rate of false-positive results and high cost. This is in line with the only 2% of women in the general population who reported undergoing MRI. In the case of women with cancer, however, 31% claimed to have undergone MRI. This %age could be traced to the use of MRI in selected cases not only for diagnostic screening purposes but also for assessment of the true tumor extent in the preoperative setting.
Section 3 of the questionnaire investigates women's emotional approaches to BSE. Although almost all respondents to the questionnaire consider BSE a useful tool to take care of themselves (96%), it is performed by only 4% of respondents and almost half claim they do not know how to perform it correctly. This insecurity is most prevalent among southern Italian women with a college or high school degree, under age 50, and seems to diminish with advancing age. Other reasons stated: forgetting to perform it (1%), fear of an inauspicious prognosis (5%), and performing other clinical tests for the purpose of possible cancer discovering (2%).
As concerns remembering to get checkups, while it is understandable that only a relatively small %age of women who have not had cancer remember to get checkups (26%), it is, on the other hand, serious that only 45% of women in whom cancer has been diagnosed remember to get checkups, despite the risk of recurrence. One must perhaps question the effectiveness of the invitation letters sent by the NHS, which should assiduously follow up with women who have already had the disease. However, in line with previous results, women with cancer prove to be more aware than other women (p < 0.05) about BSE, while Group A women say they need more information about it (86%). Useful reference figures include the senologist, oncologist, general practitioner and nurse. The psychologist garners only 12% of preferences, and this confidence does not seem to change among women with prior cancer, despite the fact that it is now clear that getting cancer is a traumatic event that also affects a person's psychological dimension.
Section 4 of our study investigated the knowledge and use of dedicated BSE apps. It was found that the %age of those using them was less than 3%, even for women with previous cancer diagnosis. Therefore, it would be recommended that health planners adopt effective educational interventions to encourage all women to perform the offered screening and practice self-examination for breast cancer [3].
The results of the study must be considered taking into account some limitations. The reference sample consisted mainly of young women under 30 years of age, and only 338 women (14%) were in the 50–69 age group so the conclusions on this group of women may be less statistically significant. This limitation is surely related to the mode of administration through the telematic medium, which is probably more used by younger women. Possible information bias may be due to a reluctant attitude to declare and therefore admit a lack of knowledge.
Conclusions
The results obtained, confirming previous studies in the literature, show poor adherence and lack of knowledge about the modalities, timing and benefits of screening programs, especially by women in the general population compared with those who had cancer before. The need to provide women with more information to ensure better access to screening has also emerged. Also on the topic of self-examination, very few women perform it and often do not know how to do it correctly, despite the fact that almost all of them consider BSE a benefit for self-care and a practice no less important than other clinical examinations. In light of all the findings of this study, there is an urgent need to improve disclosure regarding the benefits of preventive, evidence-based practices in order to increase women's confidence in screening and consequently their increased adherence. It would also be appropriate to introduce educational programs to promote the proper performance of self-examination: since this procedure can be performed as early as 20 years of age, it would also be useful to organize educational and informational projects in schools/universities that stimulate young women to become more aware of their breast health. Finally, it would be desirable to promote the use of technology and to encourage the participation of nurses in training courses that would enable them to educate women in the practice of prevention, particularly breast self-examination, fostering a better reputation of the nursing figure in the field of prevention.
Appendix 1
Author Contribution
Conceptualization: RL, LC, MCC, and GDN; methodology: RL, LC, MCC, and GDN; statistical analysis: LC, and GDN; investigation: MM, AL, EV, AC, MC, and IR; writing original draft: LC, RL, GDN; supervision: GDN, and MF. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by Università del Salento within the CRUI-CARE Agreement.
Data Availability
Dataset can be accessed by contacting the Corresponding author.
Declarations
Ethical Considerations
The ethical characteristics of the study were set out in the questionnaire presentation. The questionnaire was designed in accordance with the principles of the Italian data protection authority (DPA). It was emphasized that participation was voluntary and that the participant could refuse participation in the protocol whenever she wished. Those who were interested in participating were given an informed consent form, which recalled the voluntary nature of participation, as well as the confidentiality and anonymous nature of the information. In addition, to ensure that the questionnaires were anonymous, participants' responses were deidentified.
Competing Interests
Authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luana Conte and Giorgio De Nunzio have contributed equally to this work.
References
- 1.AIOM-AIRTUM Working Group et al. (2021) I numeri del cancro in Italia 2021. Intermedia Ed., Brescia,Italy
- 2.Amuta AO, Mkuu RS, Jacobs W, Ejembi AZ. Influence of cancer worry on four cancer related health protective behaviors among a nationally representative sample: implications for health promotion efforts. J Cancer Educ: Off J Am Assoc Cancer Educ. 2018;33(5):1002–1010. doi: 10.1007/s13187-017-1195-6. [DOI] [PubMed] [Google Scholar]
- 3.Bashirian S, Barati M, Mohammadi Y, MoaddabShoar L, Dogonchi M. Evaluation of an intervention program for promoting breast self-examination behavior in employed women in Iran. Breast Cancer: Basic Clin Res. 2021;15:1178223421989657. doi: 10.1177/1178223421989657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SS. Epidemiology of breast cancer in women. Adv Exp Med Biol. 2019;1152:9–29. doi: 10.1007/978-3-030-20301-6_2. [DOI] [PubMed] [Google Scholar]
- 6.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 7.European Commission (2022) Screening ages and frequencies. https://healthcare-quality.jrc.ec.europa.eu/ecibc/european-breast-cancer-guidelines/screening-ages-and-frequencies. Access date: June 2023.
- 8.Houghton SC, Hankinson SE. Cancer progress and priorities: breast cancer. Cancer Epidemiol, Biomarkers Prev: Publ Am Assoc Cancer Res, Cosponsored Am Soc Prevent Oncol. 2021;30(5):822–844. doi: 10.1158/1055-9965.EPI-20-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulka BS, Moorman PG. Breast cancer: hormones and other risk factors. Maturitas. 2001;38(1):103–113. doi: 10.1016/s0378-5122(00)00196-1. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RH, Anders CK, Litton JK, Ruddy KJ, Bleyer A. Breast cancer in adolescents and young adults. Pediatr Blood Cancer. 2018;65(12):e27397. doi: 10.1002/pbc.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working Group Breast-cancer screening–viewpoint of the IARC Working Group. N Engl J Med. 2015;372(24):2353–2358. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 12.National screening observatory 2020 report (2021) Cited 2022 Jul 24. Available from: https://www.osservatorionazionalescreening.it/content/rapporto. Access date: June 2023.
- 13.Nichols HB, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Holmes MD, Bersch AJ, Holick CN, Hampton JM, Stampfer MJ, Willett WC, Newcomb PA. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomark Prev. 2009;18(5):1403–1409. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parida S, Sharma D. Microbial alterations and risk factors of breast cancer: connections and mechanistic insights. Cells. 2020;9(5):1091. doi: 10.3390/cells9051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pattacini P, Nitrosi A, Giorgi Rossi P, Duffy SW, Iotti V, Ginocchi V, Ravaioli S, Vacondio R, Mancuso P, Ragazzi M, Campari C, RETomo Working Group A randomized trial comparing breast cancer incidence and interval cancers after tomosynthesis plus mammography versus mammography alone. Radiology. 2022;303(2):256–266. doi: 10.1148/radiol.211132. [DOI] [PubMed] [Google Scholar]
- 16.Petrelli A, di Napoli A, Sebastiani G, Rossi A, Giorgi Rossi P, Demuru E, Costa G, Zengarini N, Alicandro G, Marchetti S, Marmot M, and Frova L (n.d.) Italian Atlas of mortality inequalities by education level. Epidemiol Prev 43(1S1):1–120. 10.19191/EP19.1.S1.002 [DOI] [PubMed]
- 17.Petrelli A, Giorgi Rossi P, Francovich L, Giordani B, di Napoli A, Zappa M, Mirisola C, Gargiulo L. Geographical and socioeconomic differences in uptake of Pap test and mammography in Italy: results from the National Health Interview Survey. BMJ Open. 2018;8(9):e021653. doi: 10.1136/bmjopen-2018-021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice S, Whitehead SA. Phytoestrogens and breast cancer –promoters or protectors? Endocr Relat Cancer. 2006;13(4):995–1015. doi: 10.1677/erc.1.01159. [DOI] [PubMed] [Google Scholar]
- 19.Shakery M, Mehrabi M, Khademian Z. The effect of a smartphone application on women’s performance and health beliefs about breast self-examination: a quasi-experimental study. BMC Med Inform Decis Mak. 2021;21(1):248. doi: 10.1186/s12911-021-01609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith RA, Manassaram-Baptiste D, Brooks D, Cokkinides V, Doroshenk M, Saslow D, Wender RC, Brawley OW. Cancer screening in the United States, 2014: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: Cancer J Clin. 2014;64(1):30–51. doi: 10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 21.Vanni G, Materazzo M, Pellicciaro M, Ingallinella S, Rho M, Santori F, Cotesta M, Caspi J, Makarova A, Pistolese CA, Buonomo OC. Breast cancer and covid-19: the effect of fear on patients’ decision-making process. In Vivo (Athens, Greece) 2020;34(3 Suppl):1651–1659. doi: 10.21873/invivo.11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willett WC. Diet and cancer. Oncologist. 2000;5(5):393–404. doi: 10.1634/theoncologist.5-5-393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Dataset can be accessed by contacting the Corresponding author.