Abstract
This study aimed to determine the comparability of tumor-uptake indices of 18F-FDG in positron emission tomography/computed tomography (PET/CT) and positron emission tomography/magnetic resonance imaging (PET/MRI). 2-[fluorine-18]-fluoro-2-deoxy-D-glucose (FDG) PET/CT and PET/MRI were performed on 55 patients with confirmed primary malignancies. PET/CT preceded PET/MRI in all examinations. Accumulation of 18F-FDG in lesions and normal organs (brain, liver) was measured. Maximum and peak standardized uptake values (SUVs; SUVmax and SUVpeak, respectively), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) with margin thresholds of SUV of 50% (MTV50%; TLG50%, respectively) were measured as indices for comparison of measurements in tumors. Comparative indices with tumor SUVmax and liver ratio (TLRmax), brain ratio (TBRmax) were calculated. These indices were compared between PET/CT and PET/MRI examinations. The data measured using PET/CT and PET/MRI showed significant correlations for all tumor indices. The correlation was strongest for SUVpeak (r = 0.933), followed by TBRmax (r = 0.929); and the index ratio of (PET/CT)/(PET/MRI) data was close to 1.0 for TLRmax (1.00 ± 0.22) and TBRmax (1.01 ± 0.21), followed by MTV50% (0.82 ± 0.33) and TLG50% (1.18 ± 0.45). The values of all indices showed strong correlations between PET/CT and PET/MRI examinations. Among them, TLRmax, TBRmax, MTV50%, and TLG50% showed a close value and may be useful for comparison of tumor evaluation between two PET systems.
Keywords: PET/CT, PET/MRI, 18F-FDG, standardized uptake values
Introduction
The mortality rate due to cancer is high. According to the World Health Organization, in 2019, cancer was the leading or second leading cause of death among people under 70 years old in 112 of 183 countries and the third or fourth leading cause in 23 other countries [1].
Although computed tomography (CT) is useful in imaging studies, it lacks the ability to show definitive differences in physiology. Positron emission tomography (PET) has the ability to measure tissue metabolic activity, but it needs the support of high-resolution anatomical information. The combination of these two techniques provides the best of both worlds in a merged dataset, improving diagnostic accuracy and localization of many lesions [2]. Positron emission tomography/magnetic resonance imaging (PET/MRI) is considered to have advantages over PET/CT. Spatial and temporal correlations can be obtained by acquiring PET and MRI data simultaneously. Furthermore, MRI in PET/MRI hybrid imaging provides useful information about pathophysiological processes. In addition, PET/MRI may reduce patient exposure compared to PET/CT, which is particularly important in pediatric patients [3].
Highly malignant tumors generally have a higher glucose metabolism compared to normal tissues [4]. 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG-PET) plays a crucial role in the accurate diagnosis of malignant lesions. Although PET/CT and PET/MRI examinations using 18F-FDG have improved the ability to diagnose tumors, standard uptake value (SUVs) vary between facilities because not all hospitals use the same PET equipment or measurement and reconstruction methods. Therefore, comparing data measurements, such as the SUV, is difficult at different facilities.
The equivalence of quantitative indices between PET/CT and PET/MRI studies has yet to be fully reported. Significant correlations between maximum SUV (SUVmax), peak SUV (SUVpeak), metabolic tumor volume (MTV), total lesion glycolysis (TLG), and SUV accumulation ratio with the liver (maximum tumor-to-liver ratio; TLRmax) obtained from different machines have been reported [5]. MTV refers to the metabolically active volume of the tumor segmented using FDG PET. TLG of a lesion was calculated as SUVmean × MTV, which considers both the metabolic activity and tumor burden. Although the liver is considered an organ with relatively stable 18F-FDG accumulation, there are many diphosphatases (e.g., glucose-6-phosphatase) in the liver, and the intracellular hydrolysis of phosphorylated FDG-6-phosphate causes decreased 18F-FDG accumulation in the liver [6]. Increased 18F-FDG accumulation in the liver of patients with cirrhosis and chronic hepatitis has also been reported [7]. Therefore, the evaluation of tumors using TLRmax may not be sufficiently accurate in patients with liver disease.
In this study, comparisons using SUVmax, SUVpeak, MTV, and TLG, the ratios of the tumor to the liver and brain were investigated to derive and compare various indices in tumor evaluation. By evaluating the concordance between PET/CT and PET/MRI data, we can compare tumor glucose metabolism data between different facilities and models, enabling us to correctly assess the tumor and avoid unnecessary radiation exposure to patients from repeated examinations.
Patients and methods
Patients and study design
We evaluated 123 consecutive patients who underwent single-injection dual-imaging with 18F-FDG-PET/CT followed by PET/MRI for the staging and follow-up of malignant lesions from November 2013 to July 2014. The inclusion criteria were: (a) patients who had or were highly suspected of having malignant lesions; (b) aged > 20 years; (c) not pregnant; (d) able to tolerate the long duration of image acquisition; and (e) with no contraindications for MRI examination, such as pacemakers or metal implants. All patients who met these criteria and agreed to undergo PET/MRI were included in this study. One patient who underwent PET/CT and PET/MRI was excluded because of poor image quality and elevated blood sugar level (363 mg/dL). To measure MTV50% and TLG50% as previously described by Lee et al. [5], 55 patients (24 men and 31 women; mean age, 60.3 years; range, 20-83 years) with 18F-FDG-positive tumors with SUVmax greater than 2.5 were enrolled in this study. All patients had blood glucose levels < 142 mg/dL. The patient characteristics are summarized in Table 1. Patients with the following malignancies were included: lung carcinoma (n = 14), breast carcinoma (n = 11), ovarian carcinoma (n = 5), leukemia (n = 5), esophageal carcinoma (n = 4), uterine tumor (n = 4), pancreatic carcinoma (n = 3), stomach carcinoma (n = 2), rectal carcinoma (n = 2), and other tumors (n = 5). This study was approved by the Ethics Committee of Fukushima Medical University and was conducted according to the Declaration of Helsinki. All patients provided written informed consent.
Table 1.
Patients’ characteristics and imaging protocol
| Characteristics | |
|---|---|
| Patients (n) | 55 |
| Age (years) | 60.3 ± 12.4 |
| Male:female | 24:31 |
| FDG injection dose | 182 ± 36.5 MBq (range 110-269) |
| Mean glucose level | 99.3 ± 11.4 mg/dl (range 82-142) |
| Scan time from FDG injection | PET/CT: 63 ± 8 min |
| PET/MRI: 98 ± 14 min | |
| Image reconstruction | PET/CT: OSEM + TOF + PSF |
| PET/MRI: OSEM | |
| Diseases | Lung carcinoma [n = 14: adenocarcinoma (n = 8), squamous cell carcinoma (n = 5), small cell lung cancer (n = 1)], breast carcinoma [n = 11: scirrhous carcinoma (n = 7), papillotubular carcinoma (n = 1), solid-tubular carcinoma (n = 1), unknown (n = 2)], ovarian carcinoma [n = 5: serous adenocarcinoma (n = 2), serous papillary adenocarcinoma (n = 2), mucinous adenocarcinoma (n = 1)], leukemia [n = 5: FL (n = 3), DLBCL (n = 1), MM (n = 1)], esophageal carcinoma [n = 4: squamous cell carcinoma (n = 3), basaloid carcinoma (n = 1)], uterine tumor [n = 4: carcinosarcoma (n = 2), serous adenocarcinoma (n = 1), endometrioid carcinoma (n = 1)], pancreatic carcinoma [n = 3: adenocarcinoma (n = 2), IPMC (n = 1)], stomach carcinoma [n = 2: adenocarcinoma (n = 1), neuroendocrine carcinoma (n = 1)], rectal carcinoma [n = 2: adenocarcinoma (n = 2)], and other tumors [n = 5: parotid mucoepidermoid carcinoma (n = 1), papillary thyroid cancer (n = 1), thymoma (n = 1), HCC (n = 1), intrahepatic cholangiocarcinoma (n = 1)] |
DLBCL, diffuse large B-cell lymphoma; FDG, fluorodeoxyglucose; FL, Follicular lymphoma; MM, multiple myeloma; HCC, hepatocellular carcinoma; IPMC, intraductal papillary mucinous carcinoma; PET, positron emission tomography; CT, computed tomography; MRI, magnetic resonance imaging.
PET/MRI and PET/CT protocols
Imaging protocols
All patients fasted for at least 4 h or skipped one meal before their examination. The mean glucose level at the time of injection was 99.3 ± 11.4 mg/dL (range 82-142). Patients were injected with 182 ± 36.5 MBq (range, 110-269 MBq) of 18F-FDG according to their body weight. PET/CT preceded PET/MRI for all patients. After 18F-FDG injection, PET/CT was started after a mean uptake time of 63 ± 8 min and PET/MRI after a mean uptake time of 98 ± 14 min. Following the PET/CT examination, patients were immediately moved to the PET/MRI room located next door to minimize the interval between the imaging procedures. No contrast agent was used for any study.
PET/CT
18F-FDG-PET/CT data were acquired using Biograph mCT with 128-slice CT (Siemens Healthcare, Erlangen, Germany). Acquisition of PET data started from the upper thigh and ended at the head during shallow breathing. The acquisition time was 2-3 min per bed position (BP), with 6-8 BPs (21.8 cm/BP). A matrix from 200 × 200 was used. The PET data were reconstructed using ordered subset expectation maximization (3D-OSEM) containing three iterations and 21 subsets, with the time of flight (TOF), point spread function (PSF), and a Gaussian filter of 3 mm and full width at half maximum. Attenuation correction was performed based on the data obtained from the CT scan before the PET scan.
PET/MRI
PET/MRI data were obtained using an integrated wholebody PET/MRI system (Biograph mMR; Siemens Healthcare) with a 3.0-tesla MRI scanner. The technical performances of the Biograph mMR and Biograph mCT systems were summarized in a recent paper [8]. Acquisition of the PET data began at the upper thigh and finished at the head, with shallow breathing during MRI acquisition. The PET data acquisition time was 3 min per BP, with 4-6 BPs (25.8 cm/BP). Besides the Dixon sequence for attenuation correction, axial half-Fourier acquisition single-shot turbo spin-echo (HASTE) and coronal turbo spin-echo T1-weighted (TSE-T1W) images (both with a 6 mm slice thickness) were obtained. TSE-T1WI data from the chest and upper abdominal regions were acquired with breath holding, whereas TSE-T1WI data from other regions and HASTE data were obtained with shallow breathing. The parameters for HASTE imaging were as follows: repetition time, 750 ms; echo time, 73 ms; flip angle, 120; matrix, 384 9 276; slice thickness, 6 mm; slice gap, 1.8 mm. The parameters for TSE-T1W imaging were as follows: repetition time, 500 ms; echo time, 8.2 ms; flip angle, 140; matrix, 384 9 931; slice thickness, 6 mm; slice gap, 1.8 mm. A matrix of 172 9 172 was used for PET in PET/MRI. The PET data were reconstructed using 3D-OSEM containing three iterations and 21 subsets, and a Gaussian filter of 5 mm in full width at half maximum. The PET data underwent automatic attenuation correction with attenuation maps generated from the two-point Dixon sequence.
Data analyses
To measure MTV50% and TLG50%, 18F-FDG-positive tumors with a SUVmax greater than 2.5 were selected as target lesions and measured with a spherical volume of interest (VOI). These measurements allowed us to determine the anatomical location of suspected PET-positive lesions with good reliability using both CT and MRI images (Figure 1). All primary tumors were included in the measurements when the primary tumor was identified. In cases where only tumor metastases could be identified, larger metastatic lesions were preferentially measured. As described in a previous study, when there were numerous PET-positive lesions, up to five lesions per organ or compartment were chosen to avoid bias from individual patients [9]. The measurement was performed on a dedicated workstation (EV Insite; PSP corporation).
Figure 1.
Illustration of PET data of comparable quality and of feasibility of anatomical localization by CT and MRI in a patient with lung cancer: PET/CT (A) and PET/MRI (B). (Left) maximum-intensity projection overview of findings with 18F-FDG PET. (Middle) Axial slices from both modalities showing the primary lesion, representing the primary lesion (labeled with red circles). A structural correlate of the PET lesion is detectable on CT and MRI scans. (Right) Fusion overlay of PET and CT images, and fusion overlay of PET and MRI images.
For volumetric indices, isoactivity-contoured VOIs were drawn for each target lesion, and the volume expressed in milliliters was defined as MTV. The mean SUV of the VOI was measured to calculate the TLG, which was defined as the mean SUV × MTV. Margin thresholds of the isoactivity contour were set as 50% of SUVmax or SUV 2.5 to determine MTV50% and TLG50%, as previously described [5].
Spherical VOIs were manually placed for normal organs, and care was taken to avoid adjacent tissues. SUVmax was measured by placing 3.0 cm3 of VOI in the right lobe of the liver, and 1.0 cm3 in the right cerebellar cortex. Similarly to the previous report by Lee et al. [5], the maximum tumor-to-liver ratio (TLRmax), and the maximum tumor-to-brain ratio (TBRmax) were calculated for PET/CT and PET/MRI as indices for comparison in this study.
Tumor 18F-FDG measurements were performed by a radiologist with 20 years of experience in diagnostic radiology and nuclear medicine, while normal tissue 18F-FDG measurements were performed by a radiologist with one year of experience in diagnostic radiology and nuclear medicine.
Statistical analyses
Percentage differences between PET/CT and PET/MRI in terms of concordance and percentage accumulation of various indices were calculated; Spearman correlation of SUVmax, SUVpeak, MTV50%, TLG50%, TLRmax, and TBRmax coefficients were calculated for PET/CT and PET/MRI. All statistical analyses were performed using IBM SPSS (version 28; IBM Corp., Chicago, Illinois, USA), and P-values less than 0.05 were considered statistically significant.
Results
A total of 149 lesions were measured in the 55 patients enrolled. None of the patients had suspected hepatic or cerebellar disorders. Table 2 summarizes the mean (SD) of PET/CT and PET/MRI measurements and how they correlate.
Table 2.
Mean values of (PET/CT measurements)/(PET/MRI measurements) and Spearman’s correlation coefficient
| PET/CT | PET/MRI | (PET/CT)/(PET/MRI) | r | p | |
|---|---|---|---|---|---|
| SUVmax | 11.7 ± 6.75 | 7.87 ± 4.38 | 1.51 ± 0.29 | 0.924 | < 0.001 |
| SUVpeak | 8.33 ± 5.07 | 6.03 ± 3.90 | 1.44 ± 0.29 | 0.933 | < 0.001 |
| MTV50% | 4612.9 ± 9720.8 | 5600.6 ± 10463.9 | 0.82 ± 0.33 | 0.913 | < 0.001 |
| TLG50% | 42468.5 ± 102887.4 | 39938.1 ± 96771.9 | 1.18 ± 0.45 | 0.932 | < 0.001 |
| TLRmax | 5.26 ± 3.20 | 5.33 ± 3.10 | 1.00 ± 0.22 | 0.924 | < 0.001 |
| TBRmax | 1.47 ± 0.91 | 1.51 ± 0.96 | 1.01 ± 0.21 | 0.929 | < 0.001 |
CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; SUV, standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; TLRmax, maximum tumor-to-liver ratio; TBRmax, maximum tumor-to-brain ratio.
The mean values of (PET/CT measurements)/(PET/MRI measurements) were close to 1 for MTV50% (0.82 ± 0.33), TLG50% (1.18 ± 0.45), TLRmax (1.00 ± 0.22), and TBRmax (1.01 ± 0.21). The mean MTV50% value was higher for PET/MRI than for PET/CT, and the PET/CT value was higher than PET/MRI for the remaining indices (Figure 2). All indices were significantly correlated with PET/CT and PET/MRI (all P < 0.001). They also showed higher r-values (all r > 0.9), particularly for TLRmax (0.924) and TBRmax (0.929), compared to other indices.
Figure 2.
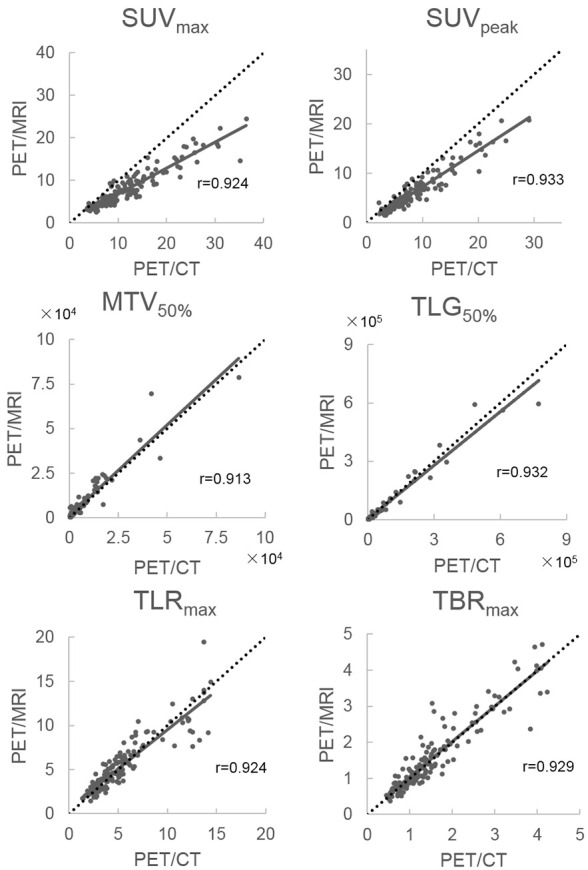
Mean (PET/CT)/(PET/MRI) and correlation coefficients for SUVmax, SUVpeak, MTV50%, TLG50%, TLRmax, and TBRmax with Y = X, added as a dotted line. PET, positron emission tomography; SUV, standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; TLRmax, tumor-to-liver ratio; TBRmax, tumor-to-brain ratio.
Discussion
In this study, except for MTV50%, the values measured by PET/CT were higher than those measured by PET/MRI. The technical differences between PET/CT and PET/MRI may have influenced these results. PET/MRI uses 3D-OSEM reconstruction, while PET/CT uses 3D-OSEM with TOF and PSF; TOF and PSF have been reported to increase SUVs [10], probably due to their improved sensitivity [11]. The Signal-to-noise ratio (SNR) was improved on images reconstructed using TOF and PSF information [12]. Alternatively, lesion SUVs may be higher with PET/CT than PET/MRI because of the different attenuation corrections used [13]. In this study, the number of iterations and number of subsets in OSEM for PET/CT and PET/MRI were the same, but when OSEM is used for tomographic reconstruction, the noise and subset-related artifacts in the images increase as the number of subsets increases [14]. Technical factors such as interscanner variability, image acquisition and reconstruction parameters, and interobserver variability, can make a difference. Biological factors such as blood glucose level, tracer uptake time, and respiratory motion, can have a substantial impact on SUV measurements as well [15]. Therefore, these factors must be taken into account when interpreting the obtained images.
Mean values of (PET/CT measurements)/(PET/MRI measurements) were SUVmax (1.51 ± 0.29), SUVpeak (1.44 ± 0.29); and the difference in values was greater between PET/CT and PET/MRI compared with MTV50%, TLG50%, TLRmax, and TBRmax. In a study by Lee et al., the ratio of PET/CT measurement value to PET/MRI measurement value in the CT precedent group was approximately 1.15 times for SUVmax and 1.1 times for SUVpeak [5]. This result may be due to differences in the performance of the PET equipment as well as the difference in acquisition start time between PET/CT and PET/MRI.
In this study, except for MTV50%, the values measured by PET/CT were higher than those measured by PET/MRI. The technical differences between PET/CT and PET/MRI may have influenced these results. PET/MRI relies on 3D-OSEM reconstruction, while PET/CT uses 3D-OSEM with TOF and PSF; TOF and PSF have been reported to increase SUVs [10], probably due to the improved sensitivity [11]. Alternatively, lesion SUVs may be higher with PET/CT than with PET/MRI because of the different attenuation corrections used [13].
Previous comparative studies have reported a higher SUVmax with PET/CT than with PET/MRI [9,13] and vice versa [16,17]. In comparative studies in which PET/CT and PET/MRI are sequentially performed on the same day, the influence of the imaging time should be considered because the uptake of 18F-FDG in malignant tumor tissues increases over time [18-20]. In our study, the time from 18F-FDG injection to the start of the PET/CT scan was approximately 30 min shorter than the PET/MRI start time, which may have resulted in higher tumor accumulation on the PET/MRI scan. However, the values of various indices of PET/CT were higher than those of PET/MRI, except for MTV50%. The impact of different reconstruction and correction methods between PET/CT and PET/MRI may have outweighed the impact of the approximately 30-min increase in uptake time.
MTV50% and TLG50% showed smaller differences in values between PET/CT and PET/MRI, and higher correlation coefficients. PET/CT and PET/MRI may show similar trends in the detection of FDG-uptake tumor volume, but the details are unknown. More volumes below the threshold (50% of SUVmax) may have been discarded in PET/CT than in PET/MRI, resulting in a larger MTV50% in the latter case. This result is consistent with the work of Lee et al. [5]. In the study by Kemin et al., the effect of TOF and PSF on TLG was relatively small [11]. These results suggest that the volumetric indices may be useful for comparison.
TLRmax and TBRmax showed smaller differences in values between PET/CT and PET/MRI and higher correlation coefficients than either tumor uptake or volumetric indices, suggesting that the comparative organ index seems to be more appropriate for comparisons when the relationship of measurements between PET devices is unknown. In addition, since TLRmax and TBRmax had nearly equivalent correlation coefficients and similar values between PET/CT and PET/MRI, using TLRmax for patients with no liver problems and TBRmax for patients with no cerebellum problems appears feasible. However, in the study by Lee et al. [5], TLRmax of PET/MRI was significantly higher in the PET/CT-first group, which is in contrast with the results of the present study. This result may be due to the difference in the performance of the PET equipment and the difference in acquisition start time between PET/CT and PET/MRI.
In the present study, there was an interval of approximately 30 minutes between the PET/CT and PET/MRI acquisition times. Liver accumulation has been reported to decrease over time, and tumor accumulation to increase over time instead [6,18-20]. If TLRmax increases linearly between one and two hours after FDG injection and rises to 2.5-fold after one hour (assuming 0.8-fold liver accumulation and 2-fold tumor accumulation), it should be approximately 1.75-fold after 30 minutes, which could have resulted in a difference of nearly 75% in TLR when compared to a PET system with the same performance.
TBRmax cannot be compared because there is no available data from the literature at present, but as with TLRmax, values may have been influenced by PET performance and may not be suitable for tumor comparison between two different PET systems. On the other hand, MTV50% and TLG50% might be better indicators, as their PET/CT and PET/MRI values were close, as previously reported by Lee et al. [5], although the conditions in that study were different from those used in the present study.
This study had several limitations. The number of female patients was greater than that of male patients (24:31). Factors that may affect 18F-FDG transport and clearance, such as renal function, were not considered. PET/CT preceded PET/MRI in all examinations, which systematically affected the tracer uptake time; having the same number of patients available for PET/CT and PET/MRI on separate days would have been ideal. Many institutions are likely to use different equipment and reconstruction methods. Although the data obtained in this study can be used clinically under our testing conditions, a multicenter study is necessary before our results can be recommended to be applied to clinical practice.
Conclusion
The values of all indices showed strong correlations between PET/CT and PET/MRI. Among them, TLRmax, TBRmax, MTV50%, and TLG50% showed the closest similarities and may be useful for comparison of tumor evaluation between the two PET systems. However, considering previous reports, the comparative organ index (TLRmax, TBRmax) may be affected by the performance of PET, and therefore volumetric indices (MTV50%, TLG50%) may be better indicators.
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Griffeth LK. Use of PET/CT scanning in cancer patients: technical and practical considerations. Proc (Bayl Univ Med Cent) 2005;18:321–330. doi: 10.1080/08998280.2005.11928089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirou I, Daisuke S, Takamitsu H, Masayuki M, Ken K, Masashi T, Kensuke K, Hiroshi I, Fumio S. Comparison of integrated whole-body PET/MR and PET/CT: is PET/MR alternative to PET/CT in routine clinical oncology? Ann Nucl Med. 2016;30:225–233. doi: 10.1007/s12149-015-1050-y. [DOI] [PubMed] [Google Scholar]
- 4.Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O’shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Paeng JC, Goo JM, Lee JM, Cheon GJ, Lee DS, Chung JK, Kang KW. Comparative characteristics of quantitative indexes for 18F-FDG uptake and metabolic volume in sequentially obtained PET/MRI and PET/CT. Nucl Med Commun. 2017;38:333–339. doi: 10.1097/MNM.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 6.Cheng G, Alavi A, Lim E, Werner TJ, del Bello CV, Akers SR. Dynamic changes of FDG uptake and clearance in normal tissues. Mol Imaging Biol. 2013;15:345–352. doi: 10.1007/s11307-012-0600-0. [DOI] [PubMed] [Google Scholar]
- 7.Vangu MDT, Momodu JI. F-18 FDG PET/CT imaging in normal variants, pitfalls, and artifacts in the abdomen and pelvis. Front Nucl Med. 2022:1. [Google Scholar]
- 8.Delso G, Fürst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, Schwaiger M, Ziegler SI. Performance measurements of the siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52:1914–1922. doi: 10.2967/jnumed.111.092726. [DOI] [PubMed] [Google Scholar]
- 9.Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Fürst S, Martinez-Möller A, Nekolla SG, Ziegler S, Ganter C, Rummeny EJ, Schwaiger M. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53:845–855. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 10.Go A, Katsuhiko M, Takafumi T, Yuji T, Shingo B, Masayuki S. Influences of point-spread function and time-of-flight reconstructions on standardized uptake value of lymph node metastases in FDG-PET. Eur J Radiol. 2014;83:226–230. doi: 10.1016/j.ejrad.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Huang K, Feng Y, Liang W, Li L. Impact of time of flight and point spread function on quantitative parameters of lung lesions in 18F-FDG PET/CT. BMC Med Imaging. 2021;21:169. doi: 10.1186/s12880-021-00699-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takafumi T, Go A, Yukiko K, Katsuhiko M, Shingo B, Yuji T, Kazuhiko H, Shohei M, Daisuke K, Masayuki S. Improvement in PET/CT image quality in overweight patients with PSF and TOF. Ann Nucl Med. 2015;29:71–77. doi: 10.1007/s12149-014-0912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiesmüller M, Quick HH, Navalpakkam B, Lell MM, Uder M, Ritt P, Schmidt D, Beck M, Kuwert T, von Gall CC. Comparison of lesion detection and quantitation of tracer uptake between PET from a simultaneously acquiring whole-body PET/MR hybrid scanner and PET from PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:12–21. doi: 10.1007/s00259-012-2249-y. [DOI] [PubMed] [Google Scholar]
- 14.Morey AM, Kadrmas DJ. Effect of varying number of OSEM subsets on PET lesion detectability. J Nucl Med Technol. 2013;41:268–273. doi: 10.2967/jnmt.113.131904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. 2010;195:310–320. doi: 10.2214/AJR.10.4923. [DOI] [PubMed] [Google Scholar]
- 16.Pace L, Nicolai E, Luongo A, Aiello M, Catalano OA, Soricelli A, Salvatore M. Comparison of whole-body PET/CT and PET/MRI in breast cancer patients: lesion detection and quantitation of 18F-deoxyglucose uptake in lesions and in normal organ tissues. Eur J Radiol. 2014;83:289–96. doi: 10.1016/j.ejrad.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Sawicki LM, Grueneisen J, Buchbender C, Schaarschmidt BM, Gomez B, Ruhlmann V, Wetter A, Umutlu L, Antoch G, Heusch P. Comparative performance of 18F-FDG PET/MRI and 18F-FDG PET/CT in detection and characterization of pulmonary lesions in 121 oncologic patients. J Nucl Med. 2016;57:582–586. doi: 10.2967/jnumed.115.167486. [DOI] [PubMed] [Google Scholar]
- 18.Matthies A, Hickeson M, Cuchiara A, Alavi A. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med. 2002;43:871–875. [PubMed] [Google Scholar]
- 19.Lodge MA, Lucas JD, Marsden PK, Cronin BF, O’Doherty MJ, Smith MA. A PET study of 18FDG uptake in soft tissue masses. Eur J Nucl Med. 1999;26:22–30. doi: 10.1007/s002590050355. [DOI] [PubMed] [Google Scholar]
- 20.Hustinx R, Smith RJ, Benard F, Rosenthal DI, Machtay M, Farber LA, Alavi A. Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med. 1999;26:1345–1348. doi: 10.1007/s002590050593. [DOI] [PubMed] [Google Scholar]



