Abstract
Background/Objective
Cystic fibrosis–related diabetes (CFRD) is one of the most common nonrespiratory complications of cystic fibrosis (CF). There is a lack of clinical research to provide guidance on optimal treatment regimens for various subtypes of CFRD.
Case Report
This case describes an 18-year-old woman, diagnosed with CF in infancy, who presented to our clinic for evaluation of possible CFRD and episodes of hypoglycemia. Subsequent testing revealed normal fasting glucose with elevated blood glucose levels on oral glucose tolerance test, consistent with the diagnosis of CFRD without fasting hyperglycemia. She was found to have large glycemic excursions after carbohydrate-containing meals, followed by delayed postprandial hypoglycemia.
Discussion
We initiated low-dose mealtime rapid-acting analog insulin and saw both a decrease in her postprandial hyperglycemia as well as resolution of her hypoglycemic episodes.
Conclusion
This case highlights the spectrum of pancreatic dysfunction and insulin dysregulation in CFRD as well as the benefit of prandial insulin alone as a treatment option.
Key words: diabetes, cystic fibrosis, insulin, CFRD
Highlights
-
•
Cystic fibrosis–related diabetes (CFRD) is linked to weight loss, decreased lung function & higher mortality in patients
-
•
Optimal insulin regimens for CFRD have not been well defined
-
•
Insulin use in patients with CFRD is known to be beneficial
-
•
Initiating prandial insulin alone addressed reactive postprandial hypoglycemia
Clinical Relevance
This manuscript highlights the spectrum of pancreatic dysfunction and insulin dysregulation that can be seen in cystic fibrosis–related diabetes (CFRD) and contributes to dialog surrounding optimal CFRD treatment. This article is significant because we found that initiating prandial insulin alone in our patient addressed both postprandial hyperglycemia as well as delayed hypoglycemic episodes.
Introduction
Approximately 29.2% of patients with cystic fibrosis (CF), age 18 and above, develop CF-related diabetes (CFRD) according to the most recent Cystic Fibrosis Foundation Registry data.1 The development of diabetes in CF is primarily attributed to CF mediated destruction of the pancreatic tissue from progressive fibrosis and fatty infiltration, leading to loss of beta cells and insulin deficiency. However, alterations in beta cell function, decrease in incretin activity, insulin resistance due to inflammation or infection, and pancreatic exocrine dysfunction leading to malabsorption can also lead to derangements in insulin and glucose dynamics.2
Risk factors for the development of diabetes mellitus in CF include female gender, poor nutrition, liver disease, high-risk genotype (delta F508), and older age.1,3,4 CFRD has also been linked to weight loss, decreased lung function, and higher mortality in patients with CF.3,5 Currently, screening recommendations include an annual oral glucose tolerance test (OGTT) starting at age 10.5,6 Diagnosis of CFRD can be categorized into CFRD with fasting hyperglycemia (FH) or without FH (Table).
Table.
Features of CFRD With and Without Fasting Hyperglycemia
| Diagnosis | Fasting plasma glucose | 2-h OGTT | HgbA1c | Additional notes |
|---|---|---|---|---|
| Normal glucose tolerance | ≤100 mg/dL | ≤140 mg/dL | ≤6.5% | |
| CFRD with fasting hyperglycemia | ≥126 mg/dL | ≥200 mg/dL | ≥6.5% (HgbA1c <6.5% does not rule out CFRD) |
Testing on 2 separate days unless unequivocal signs of hyperglycemia and/or random glucose level ≥200 mg/dL |
| CFRD without fasting hyperglycemia | ≤126 mg/dL | ≥200 mg/dL | ≥6.5% (HgbA1c <6.5% does not rule out CFRD) |
Abbreviations: CFRD = cystic fibrosis–related diabetes; HgbA1c = hemoglobin A1c; OGTT = oral glucose tolerance test.
Current guidelines recommend that patients with CFRD (both with and without FH) be initiated on basal or basal-bolus insulin.5,6 Although insulin is generally utilized, basal insulin alone is often chosen as a first therapy because of the ease of once-daily administration. Although optimal insulin regimens for CFRD have not been well defined, there is agreement on the benefits of glycemic control. Insulin use in patients with CFRD is associated with better lung function, improvement in nutritional status, increase in body mass index, and reduction in pulmonary complications.3,5,7
Case Report
An 18-year-old woman, who was diagnosed with CF in infancy (with delta F508 mutation) and was on pancreatic enzymes since early childhood, presented to our clinic for evaluation of possible CFRD and symptoms concerning episodes of hypoglycemia. She reported that after large meals, especially those that were carbohydrate-rich, she felt sweaty, anxious, and weak. On 2 occasions, capillary glucose values at the time of symptoms were 58 mg/dL and 62 mg/dL. Laboratory testing revealed an hemoglobin A1c of 5.7% (reference range < 5.7%), and OGTT showed a fasting blood glucose level of 87 mg/dL, 246 mg/dL 1 hour after glucose load, and 222 mg/dL 2 hours after glucose load. Continuous glucose monitoring (CGM) was advised to investigate blood glucose patterns. Her initial CGM tracing revealed marked postprandial hyperglycemia above 250 mg/dL, followed by precipitous drops to 70 mg/dL or less (Fig. 1).
Fig. 1.
Patient’s continuous glucose monitoring tracings prior to initiation of insulin regimen, demonstrating postprandial hyperglycemia followed by episodes of symptomatic hypoglycemia.
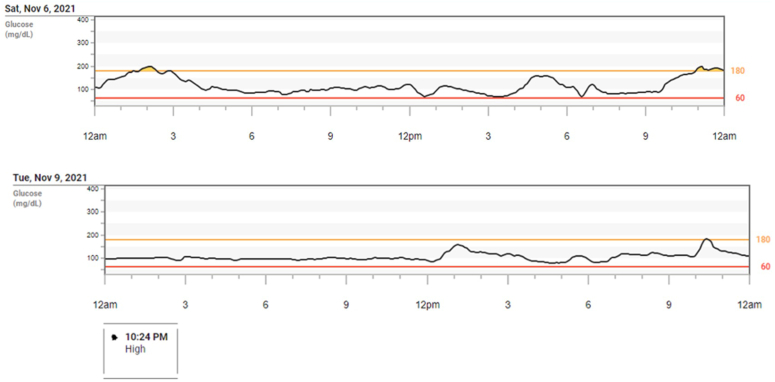
We chose to initiate our patient on low-dose rapid-acting insulin (lispro) for high carbohydrate meals or snacks and saw both a decrease in her postprandial hyperglycemia as well as resolution of her hypoglycemic episodes (Fig. 2). She only required 2 to 3 units of rapid-acting insulin to achieve this improvement. By reducing postprandial spikes in blood sugar, the steep declines in glucose resolved. Her postprandial symptoms also resolved.
Fig. 2.
Patient’s continuous glucose monitoring tracings after initiation of bolus insulin regimen with meals, showing stabilization of blood glucose measurements.
Discussion
Our patient’s OGTT and CGM revealed rapid and marked hyperglycemia postmeal, with normoglycemia in the fasting state, alongside a hemoglobin A1c of 5.7%. This fulfills diagnostic criteria for CFRD, noting that A1c is not necessary for diagnosis. Although optimal insulin regimens for CFRD have not been well defined, there is agreement on the benefits of insulin use for improved glycemic control on CF-related outcomes.3,5,7 Basal insulin alone is often chosen as a first therapy because of the ease of once-daily administration. A study by Moran et al8 found that prandial insulin in CFRD without FH led to long-term positive effects, such as increase in patient body mass index. Clinical Practice consensus guidelines also reinforce the importance of insulin as an anabolic agent and recommend that prandial insulin alone be considered in patients with CFRD without FH.9 Although prandial insulin is a more complex and labor-intensive regimen with multiple-daily injections required compared to once-daily basal insulin, in this case, we did not choose basal insulin because of the absence of FH and the risk of causing hypoglycemia in the fasting state. Additionally, our patient had the abnormality of delayed postprandial hypoglycemia. Postprandial hypoglycemia in patients with CFRD is likely because of impaired early insulin secretion with late-mismatched insulin release and diminished glucagon secretion in the setting of alpha cell failure.10 The use of CGM technology helped us identify these patterns and establish a treatment plan. Given that the patient already had an OGTT diagnostic of CFRD without FH, her clinical picture, capillary glucose, and CGM data, we felt her diagnosis was apparent and did not pursue further glucose tolerance challenges or laboratory testing of insulin at times of hypoglycemia. We elected to start treatment with the aid of CGM monitoring. We chose to initiate our patient on low-dose rapid-acting insulin of 1 to 3 units, as needed, only for higher carbohydrate content meals or snacks (over 30 g), to target the large glycemic excursions. This helped to significantly reduce the amplitude of hyperglycemia as seen on CGM (Fig. 2). She was referred for medical nutrition therapy to obtain education on carbohydrate counting and high glycemic index foods. We did not advise a carbohydrate-restrictive diet, as maximizing nutritional status is a foundation of CF treatment. She required only low doses of insulin, indicating insulin sensitivity. By reducing the marked postprandial spikes in blood sugar, the delayed insulin response that was likely leading to her hypoglycemia was now suppressed. Paradoxically, insulin treatment prevented hypoglycemia in this case.
Conclusion
Although there is a lack of research around initiating prandial insulin alone for CFRD, we believe that it is an important and appropriate first-line treatment for patients with CFRD without FH and for patients experiencing reactive postprandial hypoglycemia.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Cystic fibrosis foundation annual patient registry 2021. Cystic Fibrosis Foundation. https://www.cff.org/medical-professionals/patient-registry
- 2.Granados A., Chan C.L., Ode K.L., Moheet A., Moran A., Holl R. Cystic fibrosis related diabetes: pathophysiology, screening and diagnosis. J Cyst Fibros. 2019;18(Supplement 2):S3–S9. doi: 10.1016/j.jcf.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Moran A., Dunitz J., Nathan B., Saeed A., Holme B., Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diab Care. 2009;32(9):1626–1631. doi: 10.2337/dc09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaghue K, Robinson PD. Cystic fibrosis-related diabetes mellitus. In: Wolfsdorf JI, Mallory GB, & Hoppin AG, (Eds). UpToDate. Accessed August 1, 2023. https://www.uptodate.com/contents/cystic-fibrosis-related-diabetes-mellitus
- 5.Moran A., Brunzell C., Cohen R.C., et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diab Care. 2010;33(12):2697–2708. doi: 10.2337/dc10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ElSayed N.A., Aleppo G., Aroda V.R., et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diab Care. 2023;46(Supplement 1):S19–S40. doi: 10.2337/dc23-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohan K., Israel K.L., Miller H., Grainger R., Ledson M.J., Walshaw M.J. Long-term effect of insulin treatment in cystic fibrosis-related diabetes. Respiration. 2008;76(2):181–186. doi: 10.1159/000110206. [DOI] [PubMed] [Google Scholar]
- 8.Moran A., Pekow P., Grover P., et al. Insulin therapy to improve BMI in cystic fibrosis–related diabetes without fasting hyperglycemia: results of the cystic fibrosis related diabetes therapy trial. Diab Care. 2009;32(10):1783–1788. doi: 10.2337/dc09-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moran A., Pillay K., Becker D., Granados A., Hameed S., Acerini C.L. ISPAD Clinical Practice Consensus Guidelines 2018: management of cystic fibrosis-related diabetes in children and adolescents. Pediatr Diabetes. 2018;19(27):64–74. doi: 10.1111/pedi.12732. [DOI] [PubMed] [Google Scholar]
- 10.Kilberg M.J., Sheikh S., Stefanovski D., et al. Dysregulated insulin in pancreatic insufficient cystic fibrosis with post-prandial hypoglycemia. J Cyst Fibros. 2020;19(2):310–315. doi: 10.1016/j.jcf.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]