Abstract
Background/Objective
Ectopic Cushing syndrome can be challenging to diagnose when its presentation is atypical. Herein, we highlight features of ectopic adrenocorticotropic hormone (ACTH) syndrome in a patient with worsening hypertension, hypokalemia, ACTH-dependent hypercortisolism, and disproportionate elevation in serum androstenedione levels.
Case Report
A 59-year-old woman presented with rapidly progressing hypertension, severe hypokalemia, confusion, and weakness. Her medical history included well-controlled hypertension receiving amlodipine 5 mg/day, which worsened 3 months prior to admission requiring losartan and spironolactone therapy, with twice daily potassium supplementation. Physical examination was notable for bruising, muscle wasting, thin extremities, facial fullness, and abdominal adiposity despite body mass index 17 kg/m2. Laboratory evaluation showed potassium 2.6 mEq/L (3.5-5.3), morning cortisol >50 mcg/dL (8-25), 24-hour urine cortisol 8369 mcg/day (<50), ACTH 308 pg/mL (<46), androstenedione 398 ng/dL (20-75), dehydroepiandrosterone sulfate 48 mcg/dL (≤430), and testosterone 11 ng/dL (≤4.5) levels. A 3.8-cm carcinoid right lung tumor was identified, and resection was performed with clean margins. Cortisol, androstenedione, and potassium levels rapidly normalized postoperatively and blood pressure returned to baseline, well-controlled on amlodipine.
Discussion
Our case illustrates disproportionate elevation in androstenedione levels despite normal dehydroepiandrosterone sulfate and testosterone in a woman with ectopic ACTH syndrome. Limited reports have observed similar discordance in androgen profiles in ectopic versus pituitary ACTH hypersecretion, potentially attributable to differential activation of androgen biosynthesis.
Conclusion
Adrenal androgen assessment may help differentiate pituitary versus ectopic ACTH secretion in which androstenedione is elevated, but studies are needed to determine whether disproportionate androstenedione elevation reliably predicts the origin of ACTH excess.
Key words: ectopic Cushing syndrome, hypertension, carcinoid, androstenedione, hypokalemia
Highlights
-
•
Ectopic Cushing syndrome (ECS) is often challenging to diagnose
-
•
Timely ECS diagnosis and treatment are essential to improve disease outcomes
-
•
Resistant hypertension, hypokalemia, and muscle weakness are key presentation findings
-
•
If disproportionately elevated, serum androstenedione level can help support the diagnosis of ECS
Clinical Relevance
In an adult with ectopic ACTH syndrome presenting with muscle weakness, hypotension, and hypokalemia, ACTH-dependent hypercortisolism was accompanied by marked elevation in androstenedione level despite normal dehydroepiandrosterone sulfate and testosterone levels. This discordant androgen profile has been observed in other cases and may help determine likelihood of ectopic versus pituitary ACTH sources.
Introduction
Cushing syndrome results from prolonged exposure to supraphysiologic levels of glucocorticoids.1 The incidence of endogenous Cushing syndrome is estimated to be approximately 0.7 to 2.4 per million per year.2 The vast majority of cases are adrenocorticotropic hormone (ACTH) dependent.3 Ectopic Cushing syndrome (ECS) contributes up to 10% to 20% of all ACTH-dependent Cushing syndrome cases,2, 3, 4 with ectopic sources often arising from the neck, thoracic cavity, abdomen, and retroperitoneum.4 The most common tumor types causing ectopic ACTH production are different neuroendocrine tumors, such as small cell lung cancer, bronchial carcinoid, and thymic (neuroendocrine) tumors.4,5 Young et al4 summarized 6 published case series and identified well-differentiated neuroendocrine tumors in the bronchi as the most common thoracic tumor type causing ECS, accounting for 20% to 40% of all ectopic ACTH cases.
Recognition of Cushing syndrome in patients with ectopic ACTH syndrome can be difficult in the absence of classic symptoms, and understanding the unique presentation, including steroid biomarker profiles, may facilitate clinical recognition and management. Cunningham et al6 hypothesized that adrenal androgen secretion is not controlled by ACTH alone and suggested that variability of proopiomelanocortin processing in ectopic ACTH syndrome compared with Cushing disease may account for differences in adrenal androgen and cortisol secretion. Herein, we describe a case of ectopic ACTH syndrome from an ACTH-producing carcinoid tumor of the lung in a woman presenting with worsening hypertension, severe hypokalemia, and hypercortisolism. The patient’s androgen steroid profile was notable for a high androstenedione level without concomitant increase in dehydroepiandrosterone sulfate (DHEAS) or testosterone levels.
Case Report
A 59-year-old woman was admitted to the hospital with acute confusion, progressive muscle weakness, and worsening hypertension. Her medical history was notable for a history of retinal detachment and hypertension, diagnosed 2 years ago, and she was treated with amlodipine 5 mg/day with good blood pressure control. Three months prior to admission, she developed worsening hypertension based on home monitoring, and amlodipine was increased to 10 mg/day, and hydrochlorothiazide 25 mg/day was added. However, she developed lower extremity edema, and amlodipine was replaced with lisinopril 10 mg/day. One month later, while traveling, she presented to an outside emergency department with rapidly progressive weakness and was found to have profound hypokalemia with serum potassium of <1.9 mEq/L (3.5-5.3). Hypokalemia was attributed to hydrochlorothiazide use, and the patient was switched to spironolactone 100 mg/day and losartan 100 mg/day, with potassium chloride 40 mEq twice daily added. However, she returned 10 days later to the same outside emergency department for ongoing weakness and was found to have persistent hypertension and hypokalemia (potassium 2.8 mEq/L) despite adherence to potassium supplementation, spironolactone, and losartan therapy. Her diagnostic hospitalization occurred 1 week later when she returned home to arrange follow-up care.
Notable physical examination findings on admission included blood pressure 161/103 mm Hg, weight 54.4 kg (baseline weight 2 years earlier was 57.2 kg), body mass index 17.2 kg/m2, thin extremities, ecchymoses over the upper extremities and abdomen, focal superficial skin breakdown, and oral thrush. Initial laboratory studies were notable for potassium 2.6 mEq/L, bicarbonate 35 mEq/L (24-33), and creatinine 0.56 mg/dL (<1.11). Computed tomography (CT) scan of the head and magnetic resonance imaging of the brain were unremarkable with normal appearing pituitary gland. Blood count showed no anemia or leukocytosis. Initial thyroid function tests showed thyroid-stimulating hormone 1.7 uIU/mL (0.4-4.5), free thyroxine 1.2 ng/dL (0.8-1.7), and total triiodothyronine 32 ng/dL (50-170). Primary hyperaldosteronism was considered, given persistent hypokalemia and hypertension, but was excluded after laboratory results showed plasma renin activity 0.34 ng/ml/h (0.25-5.82) and undetectable serum aldosterone <1 ng/dL. On further physical examination, she was noted to have muscle wasting, facial and supraclavicular fullness despite a body mass index of 17.2 kg/m2, and abdominal adiposity disproportionate to her extremities. No skin hyperpigmentation was noted. Due to muscle wasting, easy bruising, and oral thrush, a lymphoproliferative disorder or malignancy was suspected. Chest CT showed a 2.9 × 2.3 × 3.8–cm mass in the right lower lobe with mild right hilar and subcarinal adenopathy (Fig. 1). Abdomen/pelvis CT showed bilateral adrenal hyperplasia (Fig. 2). Concomitant workup results for Cushing syndrome demonstrated ACTH-dependent hypercortisolemia with morning cortisol >50 mcg/dL (8-25 μg/dL), ACTH 308 pg/mL (<46), and 24-hour urine cortisol 8369 mcg/24-h (<50). Interestingly, DHEAS 48 mcg/dL (≤430) and total testosterone 11 ng/dL (≤45) were normal, whereas androstenedione (evaluated by chromatography/mass spectrometry) was 398 ng/dL (20-75). The patient was taking spironolactone 25 mg/day at the time of these tests and had no clinical signs of androgen excess. Subsequent fluorodeoxyglucose-positron emission tomography scan identified a 3.1-cm hypermetabolic right lower lobe lung mass, hypermetabolic right hilar, and subcarinal nodes and no findings to suggest distant metastases (Fig. 3).
Fig. 1.
(A) Computed tomography scan of chest demonstrating a 3.8-cm right lower lobe lung mass. The mass is associated with compression of the right lower lobe bronchus and mild right hilar and subcarinal adenopathy. (B) Computed tomography scan of chest demonstrating right hilar adenopathy.
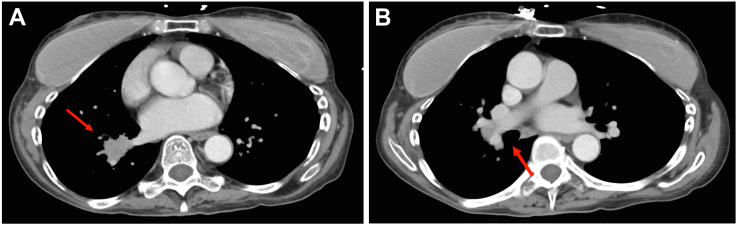
Fig. 2.
Computed tomography scan of abdomen demonstrating adrenal hyperplasia (red arrow, left adrenal).
Fig. 3.
Fluorodeoxyglucose-positron emission tomography scan showing increased metabolic activity in the right lower lobe lung mass. The red circle indicates the area of increased metabolic activity in the right lower lobe lung mass
Endobronchial ultrasound and biopsy results of her lung mass displayed an atypical carcinoid tumor that stained positive for ACTH, synaptophysin, and chromogranin. The patient underwent removal of the right middle and lower lung lobes. Surgical pathology showed a 3.9-cm atypical carcinoid tumor with lymphovascular invasion, negative surgical margins, and 5 positive regional lymph node metastases without extracapsular extension, Ki-67 ∼ 16%. The tumor was classified as stage pT2aN2M0 (American Joint Committee on Cancer Tumor, Node, Metastasis staging). Histopathology results showed tumor cells with patchy positive immunostaining for ACTH (Fig. 4).
Fig. 4.
Tumor histologic section with adrenocorticotropic hormone immunohistochemistry staining. Magnification ×400.
Postoperative day 1, her 2-am serum cortisol was 17.3 mcg/dL and ACTH 14 pg/mL. She developed changes in mental status and agitation 24 hours postsurgery, believed to be due to steroid withdrawal, and was treated with hydrocortisone 50 mg every 6 hours. Symptoms quickly resolved, and hydrocortisone was discontinued 2 days later. A random cortisol was 3.7 mcg/dL and ACTH 12 pg/ml, 24 hours from last hydrocortisone replacement. On postoperative day 10, her am cortisol was 36.5 mcg/dL with ACTH 42 pg/mL.
Three weeks postsurgery, 24-hour urine free cortisol was 26.6 mcg/24 h, and the serum androstenedione level was 62 ng/dL and remained normal a year later. Given the limited data and uncertain benefits of adjuvant chemotherapy in the setting of complete tumor resection, the patient did not undergo additional therapy. Surveillance CT imaging at 3, 6, and 12 months postoperatively showed no evidence of recurrent or residual disease. Currently, the patient remains normotensive on amlodipine 5 mg/day and normokalemic without potassium supplementation.
Discussion
Cushing syndrome due to ectopic ACTH secretion can be difficult to diagnose when the presentation lacks classic features of Cushing syndrome. In our patient with a body mass index of 17 kg/m2, ECS was initially not suspected during outside hospital visits. Similar to our case, muscle weakness is a commonly reported major symptom and, in combination with severe hypokalemia and hypertension, should prompt consideration of ectopic ACTH syndrome, particularly in the presence of weight loss.4,7 Early detection of ACTH-secreting bronchial carcinoid tumors remains difficult but reduces the risk of unnecessary adrenal surgeries and risk of metastasis.8 In our case, despite repeated presentation with weakness, hypokalemia, and hypertension over a short period of time, rapid identification of ectopic ACTH syndrome led to successful treatment.
If elevated, the serum androstenedione level may be helpful when evaluating for ectopic ACTH syndrome. Two case series have examined androstenedione levels in ACTH-dependent Cushing syndrome cases. Barbetta et al9 examined androgen levels in 36 women with ACTH-dependent Cushing syndrome and found that androstenedione levels were elevated in most ECS cases when compared with Cushing disease cases, whereas no differences were observed in the DHEAS and testosterone levels. Disproportionate androstenedione elevation in ectopic ACTH syndrome has also been reported by Cunningham et al6 and the potential role of variable proopiomelanocortin processing has been hypothesized to explain the observed dissociation of adrenal androgen and cortisol secretion. Barbetta et al9 proposed that differential activation of androgen biosynthetic pathways occurs in ectopic ACTH syndrome, resulting in markedly high androstenedione levels without concomitant elevation in the DHEAS and/or testosterone levels. This was also observed in our patient, who had high androstenedione level despite low-normal DHEAS and normal testosterone levels. However, because the early reports were published 2 to 3 decades ago, there has been little discussion regarding adrenal androgen profiles in patients with ACTH-dependent hypercortisolism. Future studies should determine whether measurement of serum androstenedione level, in addition to DHEAS and testosterone levels, may be useful in evaluating patients suspected to have ectopic ACTH syndrome.
Conclusion
ECS is a rare clinical condition that can present in both urgent and emergency care settings. Early diagnosis and management are key to optimizing clinical outcomes, given the high morbidity and mortality of both hypercortisolism and the underlying malignancy. The constellation of resistant hypertension, hypokalemia, muscle weakness in the absence of aldosterone excess, and overt signs and symptoms of Cushing syndrome should prompt consideration of ectopic ACTH syndrome.4,7 Our case supports the concept of differential activation and regulation of steroidogenic pathways by pituitary versus ectopic ACTH hypersecretion resulting in distinctive biochemical differences and the potential utility of androstenedione as an additive biomarker when ectopic ACTH syndrome is suspected.
Disclosure
The authors have no multiplicity of interest to disclose.
Acknowledgment
We thank Dr Thomas Yohannan for providing the images.
Author Contributions
All authors contributed to the conception, design, and writing of this manuscript for publication and approved the final version for publication.
Funding
No public or commercial funding.
Sources of Support
None.
Informed Patient Consent for Publication
Signed informed consent was obtained directly from the patient.
Data Availability
All available data are included in this case report publication.
References
- 1.Juszczak A., Morris D., Grossman A. In: Endotext. Feingold K.R., Anawalt B., Boyce A., et al., editors. MDText.com, Inc; 2000. Cushing’s syndrome. [Google Scholar]
- 2.Sharma S.T., Nieman L.K., Feelders R.A. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol. 2015;7:281–293. doi: 10.2147/CLEP.S44336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacroix A., Feelders R.A., Stratakis C.A., Nieman L.K. Cushing’s syndrome. Lancet. 2015;386(9996):913–927. doi: 10.1016/S0140-6736(14)61375-1. [DOI] [PubMed] [Google Scholar]
- 4.Young J., Haissaguerre M., Viera-Pinto O., Chabre O., Baudin E., Tabarin A. MANAGEMENT OF ENDOCRINE DISEASE: Cushing’s syndrome due to ectopic ACTH secretion: an expert operational opinion. Eur J Endocrinol. 2020;182(4):R29–R58. doi: 10.1530/EJE-19-0877. [DOI] [PubMed] [Google Scholar]
- 5.Fukuoka H., Shichi H., Yamamoto M., Takahashi Y. The mechanisms underlying autonomous adrenocorticotropic hormone secretion in Cushing’s disease. Int J Mol Sci. 2020;21(23):E9132. doi: 10.3390/ijms21239132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham S.K., McKenna T.J. Dissociation of adrenal androgen and cortisol secretion in Cushing’s syndrome. Clin Endocrinol (Oxf) 1994;41(6):795–800. doi: 10.1111/j.1365-2265.1994.tb02795.x. [DOI] [PubMed] [Google Scholar]
- 7.Paleń-Tytko J.E., Przybylik-Mazurek E.M., Rzepka E.J., et al. Ectopic ACTH syndrome of different origin-Diagnostic approach and clinical outcome. Experience of one Clinical Centre. PloS One. 2020;15(11) doi: 10.1371/journal.pone.0242679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boddaert G., Grand B., Le Pimpec-Barthes F., Cazes A., Bertagna X., Riquet M. Bronchial carcinoid tumors causing Cushing’s syndrome: more aggressive behavior and the need for early diagnosis. Ann Thorac Surg. 2012;94(6):1823–1829. doi: 10.1016/j.athoracsur.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Barbetta L., Dall’Asta C., Re T., Colombo P., Travaglini P., Ambrosi B. Androgen secretion in ectopic ACTH syndrome and in Cushing’s disease: modifications before and after surgery. Horm Metab Res. 2001;33(10):596–601. doi: 10.1055/s-2001-17906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All available data are included in this case report publication.