Abstract
A 67‐year‐old current smoker Japanese man, with no history of asthma, was diagnosed with lung adenocarcinoma. He received first‐line chemotherapy with carboplatin, pemetrexed, ipilimumab, and nivolumab in July 20XX‐1, and subsequently a maintenance therapy with nivolumab. In October 20XX, he became aware of wheezy dyspnoea, and chest computed tomography demonstrated worsening bronchial wall thickenings. Eosinophilia was noted, and a pulmonary function test showed obstructive dysfunction insufficiently responding to beta‐agonists, with 130 mL increase of forced expiratory volume in one second and high fractional exhaled nitric oxide level (85 ppb). He was clinically diagnosed with asthma and chronic obstructive pulmonary disease overlap, secondary to immune checkpoint inhibitors (ICIs). The inhibition of binding between programmed cell death‐protein‐1 (PD‐1), expressed on T cells, and programmed cell death‐ligand‐2 (PD‐L2), expressed on tumour and dendritic cells, can induce airway hyperresponsiveness. Physicians should be wary of asthmatic symptoms and chest image findings during ICIs therapy.
Keywords: adenocarcinoma, asthma, chronic obstructive pulmonary disease, immune checkpoint inhibitors
We present a case of asthma and chronic obstructive pulmonary disease overlap (ACO) induced by long‐term nivolumab maintenance therapy in a patient with lung adenocarcinoma and no history of asthma.
INTRODUCTION
The use of immune checkpoint inhibitors (ICIs) has recently expanded, becoming a standard treatment for patients with advanced‐stage lung cancer. 1 However, ICIs are associated with a variety of immune‐related adverse events (irAEs) due to the specific mechanisms of their anti‐tumour effects. 1 Among the respiratory irAEs, interstitial lung disease has a high incidence and is well‐known, whereas asthma is relatively rare. 1 , 2
The anti‐tumour mechanisms of ICIs promote eosinophilia and asthma as irAEs; these conditions are reportedly caused by the significant interaction between programmed cell death‐protein‐1 (PD‐1), expressed on T cells, and programmed cell death‐ligand‐2 (PD‐L2), expressed on tumour and dendritic cells. 3 Therefore, it is important to monitor the eosinophil count and airway wall thickening in patients with lung cancer undergoing ICI therapy, using high‐resolution computed tomography (HRCT) and pulmonary function tests.
We present a case of asthma and chronic obstructive pulmonary disease overlap (ACO) induced by long‐term nivolumab maintenance therapy in a patient with lung adenocarcinoma and no history of asthma.
CASE REPORT
A 67‐year‐old Japanese man visited our hospital in May 20XX‐1 because a routine chest radiography showed an anomalous chest shadow. He was a current smoker, though with no history of bronchial asthma and allergies or medically significant family history including asthma. A systemic computed tomography (CT) revealed a tumour 5.6 cm in diameter in the right mediastinum (Figure 1A) and an enlarged right subclavian lymph node. The laboratory findings showed increased carcinoembryonic antigen (CEA), at 4.4 ng/mL. A bronchoscopic right mediastinal tumour biopsy showed adenocarcinomatous cells, and the patient was diagnosed with lung adenocarcinoma (clinical stage IVB). First‐line chemotherapy was started in July 20XX‐1 with carboplatin, pemetrexed, ipilimumab, and nivolumab. The tumour responded with an 80% reduction in size after two courses of this chemotherapy (Figure 1B). In September 20XX‐1, ipilimumab and nivolumab maintenance therapy was started. A month after one course of maintenance therapy, he experienced malaise, and his serum sodium level was low (117 mmol/L). Other blood test results showed increased adrenocorticotropic hormone (26.7 pg/mL) and decreased cortisol (6.9 μg/dL); hence, he was diagnosed with adrenal insufficiency due to ICIs. He was treated with hydrocortisone and withdrawal of ipilimumab and nivolumab. After the start of this treatment, the symptoms and laboratory data showed an improvement, and the nivolumab maintenance therapy was resumed in November 20XX‐1. After 13 courses of nivolumab maintenance therapy, the patient noticed a wet cough and wheezy dyspnoea in October 20XX. A chest CT demonstrated bronchial wall thickenings (Figure 1C). He also had eosinophilia (Figure 2) and elevated IgE level (1372 U/mL), and the pulmonary function test showed obstructive dysfunction (forced expiratory volume in one second% [FEV1.0%] 68%) insufficiently responding to a beta‐agonist with 130 mL increase of FEV1.0 and high fractional exhaled nitric oxide level (85 ppb). He was assumed to be combined with chronic obstructive pulmonary disease before treatment due to his smoking history and obstructive dysfunction insufficiently responding to a beta‐agonist, so he was clinically diagnosed with ACO secondary to ICIs; treatment was started with a short course of systemic corticosteroids (betamethasone 8 mg per day for 3 days) and regular inhalations of fluticasone furoate/umeclidinium bromide/vilanterol trifenatate, and his chest CT findings (Figure 1D) and symptoms resolved (Figure 2).
FIGURE 1.
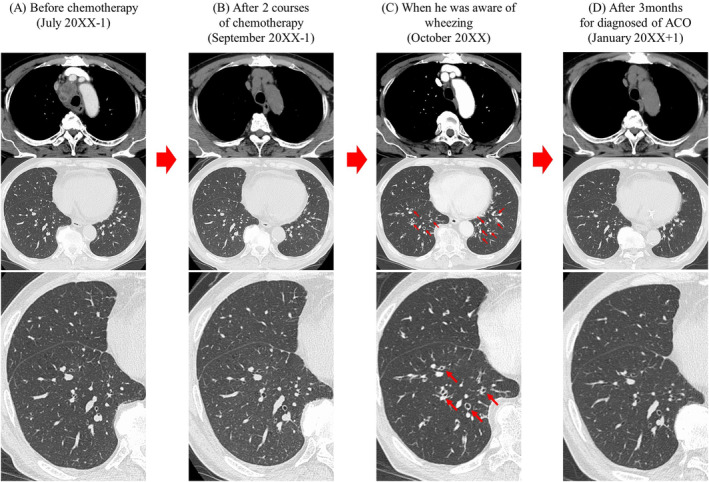
Computed tomography images. (A) Before chemotherapy (July 20XX‐1). (B) After two courses of chemotherapy, the tumour responded with an 80% reduction in size. (C) The bronchial wall of both lower lobes thickened progressively (red arrows), and the patient became symptomatic in October 20XX. (D) After treatment of ACO, the bronchial wall of both lower lobes appeared healthy (January 20XX+1). ACO, asthma and chronic obstructive pulmonary disease overlap.
FIGURE 2.
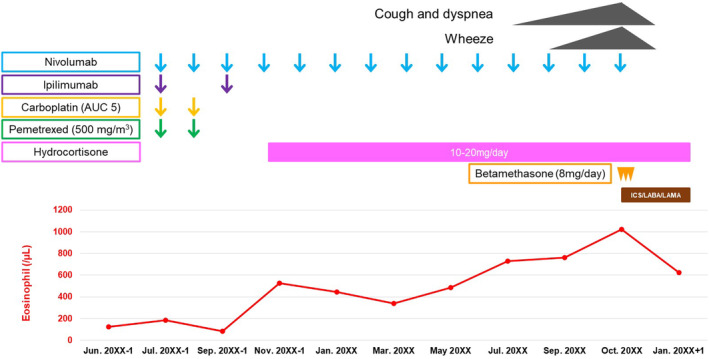
Clinical course of the patient. AUC, area under the blood concentration‐time curve; ICS/LABA/LAMA, inhaled corticosteroid/long‐acting β‐agonists/long‐acting muscarine antagonist.
DISCUSSION
We presented a case of ACO induced by long‐term ICI maintenance therapy in a patient with lung adenocarcinoma and no history of asthma.
Nivolumab, administered to this patient, is an anti‐PD‐1 antibody that inhibits the binding of PD‐1 (expressed on T cells) to programmed cell death‐ligand‐1 and PD‐L2 (expressed on tumour cells), resulting in an antitumoral effect. 1 ICIs, such as nivolumab, often cause irAEs during treatment. 1 IrAEs are diverse, including skin disorders, colitis, drug‐induced pneumonia, and endocrine disorders; however, the occurrence of asthma or eosinophilia, as in this patient, is rare. 1 , 2 Eosinophilia and asthma as irAEs occur in a relatively short period of time, between 2 and 12 months after ICIs administration. 2 , 4 This patient did not present asthma or eosinophilia prior to treatment, though he became aware of asthmatic symptoms 1 year after starting treatment, suggesting that nivolumab played a role in inducing these disorders. In addition to wheezing symptoms, this patient showed worsening bronchial wall thickenings after ICI treatment and was ultimately diagnosed with ICI‐related ACO.
PD‐1 binding inhibition and allergy are closely related. The expression of PD‐1 in T cells and IgE production are negatively correlated; hence, it has been suggested that anti‐PD‐1 antibodies may lead to increased IgE production. 5 In this patient, the IgE level was markedly elevated possibly due to the influence of nivolumab, an anti‐PD‐1 antibody. PD‐1, expressed in T cells, binds to PD‐L2, expressed in dendritic cells, to suppress airway hypersensitivity. 3 Therefore, the inhibition of PD‐1 and PD‐L2 binding, caused by nivolumab, may promote airway hyperresponsiveness. 3 Eosinophilia and asthma as irAEs were previously reported only with the use of anti‐PD‐1 antibodies, similar to this patient treatment with ICIs, 4 and the reduced binding of PD‐1 and PD‐L2 might strongly influence airway hyperresponsiveness.
Herein, we present the case of lung adenocarcinoma complicated with ACO induced by long‐term nivolumab maintenance treatment. If a patient with lung cancer undergoing ICI treatment develops eosinophilia or bronchial wall thickening, visible on HRCT, the onset or exacerbation of ICI‐induced asthma needs to be considered.
AUTHOR CONTRIBUTIONS
Yuki Hayakawa and Takako Kawaguchi wrote the draft of the manuscript. Kei Yamasaki and Kazuhiro Yatera revised it critically. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
ACKNOWLEDGMENTS
We thank Editage (www.editage.com) for English language editing.
Hayakawa Y, Kawaguchi T, Yamasaki K, Endo M, Komatsu M, Ishiguro Y, et al. Immune checkpoint inhibitor‐induced asthma and chronic obstructive pulmonary disease overlap in patient with adenocarcinoma. Respirology Case Reports. 2023;11:e01222. 10.1002/rcr2.1222
Associate Editor: James C. M. Ho
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated.
REFERENCES
- 1. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel‐Vinay S, et al. Immune‐related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 2. Maeno K, Fukuda S, Oguri T, Niimi A. Nivolumab‐induced asthma in a patient with non‐small‐cell lung cancer. Ann Oncol. 2017;28:2891. 10.1093/annonc/mdx455 [DOI] [PubMed] [Google Scholar]
- 3. Akbari O, Stock P, Singh AK, Lombardi V, Lee W‐L, Freeman GJ, et al. PD‐L1 and PD‐L2 modulate airway inflammation and iNKT‐cell‐dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3:81–91. 10.1038/mi.2009.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harada M, Naoi H, Yasuda K, Ito Y, Kagoo N, Kubota T, et al. Programmed cell death‐1 blockade in kidney carcinoma may induce eosinophilic granulomatosis with polyangiitis: a case report. BMC Pulm Med. 2021;21:6. 10.1186/s12890-020-01375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bratke K, Fritz L, Nokodian F, Geißler K, Garbe K, Lommatzsch M, et al. Differential regulation of PD‐1 and its ligands in allergic asthma. Clin Exp Allergy. 2017;47:1417–1425. 10.1111/cea.13017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable—no new data generated.


