ABSTRACT
Generalized pustular psoriasis (GPP) is a severe subtype of psoriasis, commonly combined with systemic inflammation. Gene mutations have been found to be associated with GPP and vary by ethnicity. Systemic treatments are usually required for the severity and potential complications of GPP. However, there is no common consensus in China, especially among pediatric patients, whose data are scarce. Acitretin, methotrexate, and cyclosporine are widely used in pediatrics with GPP, while the adverse effects should be highlighted. The emergence of different biological agents brings us into a new era. This article discusses the genetic background of Chinese patients and demonstrates the evidence of treatment in pediatrics with GPP.
Keywords: Generalized pustular psoriasis, Pediatrics, Treatment
Generalized pustular psoriasis (GPP) is a severe inflammatory cutaneous disease presented as generalized pustular, edema erythema, and systemic inflammation, including fever, joint pains, headaches, and leukocytosis. Considering the severity of GPP, systemic treatments are required, including acitretin, methotrexate, cyclosporine, and biological agents.

INTRODUCTION
Generalized pustular psoriasis (GPP) is a severe inflammatory cutaneous disease that presents as generalized pustular, edema erythema, and systemic inflammation, including fever, joint pains, headaches, and leukocytosis (Figure 1). Patients might experience a relapse‐remission course or a persistent condition lasting for more than 3 months. 1 GPP can be primary or secondary to psoriasis vulgaris (PV), and triggered by infections, drugs, stress, or pregnancy. Primary GPP usually occurs during childhood and is caused by gene variants. Considering the severity of GPP, systemic treatments are required.
FIGURE 1.
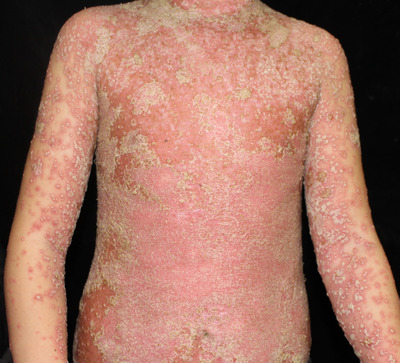
The clinical presentation of generalized pustular psoriasis.
In pediatric patients, the treatment of GPP is a definite challenge, as highlighted by the balance in efficacy and safety. In China, all systemic treatments for GPP are off‐label. According to the American National Psoriasis Foundation, acitretin, methotrexate, cyclosporine, and etanercept are regarded as first‐line therapies for childhood GPP, and adalimumab, infliximab, and ultraviolet B (UVB) phototherapy are regarded as second‐line therapy. 2 Miyachi et al. 3 identified 1516 adult patients with GPP in the Japanese national inpatient database and categorized the patients into three medication groups: biologics (tumor necrosis factor [TNF] inhibitors, interleukin [IL]‐12/IL‐23 p40 inhibitors, or IL‐17 inhibitors), oral agents without biologics (cyclosporine, retinoids, or methotrexate), and systemic corticosteroids only. The biologics group had favorable outcomes compared with the other treatments. Takeichi et al. 4 summarized the biologics, including adalimumab, ixekizumab, secukinumab, brodalumab, and guselkumab in adults, and showed that the biologics seemed to be effective and relatively well tolerated. With the introduction of biological agents targeting variable cytokines and receptors, the treatment algorithm for GPP enters a new era. However, treatment reviews for children with GPP are scarce. Here we summarize the genetic background of Chinese patients and treatment in pediatrics with GPP through a literature review. The literature search was performed in PubMed with the keywords, “generalized pustular psoriasis”, “children”, “interleukin‐36”, and “treatment”.
EPIDEMIOLOGY
Feng et al. 5 investigated the epidemiology of GPP in China based on Urban Basic Medical Insurance. In 2016, the GPP‐adjusted national prevalence is 1.108 per 100 000 person‐years, and the incidence is 0.509 per 100 000 person‐years. The prevalence of GPP in China tends to be higher than in France and Brazil, whose prevalence is 0.176 and 0.7 per 100 000 respectively. Males count a slightly higher ratio than females. The bimodal age distribution is observed, the 0‒3‐year age and the 30‒39‐year age. In the children group, the GPP crude prevalence and incidence are 0.927 and 0.742 per 100 000 person‐years in the 0‒3‐year age group, 0.471 and 0.441 per 100 000 person‐years in the 4‒9‐year age group, 0.204 and 0.158 per 100 000 person‐years in the 10‒19‐year age group. 5
GENETIC BACKGROUND
In recent years, gene mutation has been associated with GPP, including IL‐36 receptor antagonist (IL36RN), caspase recruitment domain‐containing protein 14 (CARD14), adapter protein complex 1 subunit sigma 3 (AP1S3), myeloperoxidase (MPO), serine protease inhibitor gene serpin family A member 3 (SERPINA3), and TNF alpha‐induced protein3 (TNFAIP3)‐interacting protein 1 (TNIP1). 6 , 7 , 8 , 9 , 10 , 11 The IL36RN gene mutation is the most common one, which is known as a deficiency of the interleukin‐36 receptor antagonist (DITRA).
Li et al. 6 found an association between IL36RN variants and GPP in Chinese Han patients using 62 sporadic GPP, 96 healthy controls, and 174 sporadic PV patients. The patients had an age of onset of 33.10 ± 19.77 years in the GPP patients, and 29.31 ± 21.83 years in the PV patients. The IL36RN mutation was detected in 46.77% of the GPP, 6.90% of the PV patients, and 6.25% of the healthy controls. Additionally, Wang et al. 12 found the IL36RN mutation in 66.7% of children with only GPP with an average onset age of 4.27 ± 3.66 years. Our group found the IL36RN mutation in 76.67% of children with GPP with a median onset age of 2.37 years. 13 The IL36RN c.115 + 6T > C variant was the most frequent variant in Chinese patients and was reported in 63.6% of children with only GPP. 12 This differs from the European and African populations, whose dominant variants are p.Ser113Leu and p.Leu27Pro respectively. 12 , 14 , 15
Patients who carried recessive alleles (homozygous or compound heterozygous) had an earlier age of onset than those without the IL36RN mutation. 12 Wang et al. 12 reported the average age of onset in pediatric patients with only GPP and carried an IL36RN variant was 3.55 ± 3.29 years and 5.72 ± 3.55 years in pediatric patients with only GPP and no IL36RN variant. The frequency of IL36RN variants was positively correlated with GPP with acrodermatitis continua of Hallopeau (ACH) and negatively correlated with GPP with PV. 13 , 16 In our group, the IL36RN mutation was detected in 100% of children who suffered from GPP with ACH, 78.05% of children who suffered from GPP without PV and ACH, and 44.44% of children who suffered from GPP with PV. 13 Furthermore, more patients with the IL36RN mutation converted to ACH. 12
The IL36RN mutation might be associated with a more severe inflammatory reaction in GPP. Wang et al. 12 found that the incidence of confluent lakes of pustular, perianal erosion, flexural erosion, and conchal pustules was higher in patients with the IL36RN mutation. Our study discovered that patients who carried the IL36RN mutation had a longer length of hospitalization and a longer time to reach normal body temperature after treatment. 13 The IL36RN mutation seems to not affect the treatment response, but patients with IL36RN variants had a much higher half‐year recurrence rate after acitretin withdrawal.
SYSTEMIC TREATMENTS
Retinoids
Japanese guidelines recommend retinoids as a C1 degree for GPP in children, in which acitretin, the active metabolite of etretinate, is used most frequently. 17 Acitretin inhibits keratinocyte proliferation and inflammatory cytokines expression by interfering with the JAK‐STAT pathway and down‐regulating the activities of Th1 and Th17 cells and the level of interferon (IFN)‐γ. 18
Our group showed the effectiveness of acitretin in 16 Chinese children with GPP, with a mean age of 8.69 ± 2.12 years. 19 After acitretin was administrated, the average time for pustules to fade was 6 days, the temperature to normalize was 6.14 days, and the C‐reactive protein to normalize was 8.73 days. Two weeks later, 25% of the patients had a Japan Dermatology Association (JDA) severity index of 0/1, and 12 weeks later, 87.5% had a JDA severity index of 0/1. Additionally, Chen et al. 20 observed 15 Chinese children with a mean age of 3.4 ± 2.9 years treated with acitretin, including ten patients with GPP, three with palmoplantar pustulosis (PPP), and two with ACH. New pustulosis formation ceased in three days, and skin lesion remission started in 5–7 days. Fourteen patients (93.3%) achieved a good response, and one patient with ACH achieved a moderate response. Overall, acitretin was effective in about 90% of children with PP as a monotherapy or combination therapy. 20
Zhu et al. 21 analyzed 61 patients with GPP from China, discovering that IL36RN mutations did not affect the efficacy of acitretin and that maintenance therapy with low‐dose acitretin might decrease the recurrence rate. Recently, Yang et al. 22 proposed that plasma retinol might be a reliable biomarker to assess the severity of GPP and the effectiveness of acitretin monotherapy in Chinese children.
Attention must be paid to the side effects of acitretin treatment in patients with PP should be noticed. Of our 16 patients, 75% had mucocutaneous dryness, 37.5% had dyslipidemia, and 25% had abnormal liver enzymes. 19 Chen et al. 20 recorded dry skin in 66.7% of patients, itching in 26.7%, cheilitis in 20.0%, and dry mouth in 6.7%. In children, growth disturbance is a major concern, including early closure of the epiphysis and hyperostosis. Our group preliminarily found that acitretin had no significant effect on the growth and development of children. 23 We retrospectively analyzed 106 patients who were treated with oral acitretin for 1–90 months retrospectively, in which 79.2% of the patients suffered from pustular psoriasis. Of the 96 patients under 18 years, 94.8% had a normal stature, and 5.2% had short stature. Of the 83 patients receiving acitretin alone, 97.6% had a normal stature, and 2.4% had short stature. The risk of short stature was mostly associated with acitretin combined with glucocorticoid. Thirteen patients who reached near‐adult height had a normal stature. Bone age was calculated by radiology in 45 patients, the bone age minus chronological age had no significant difference after treatment, and premature closure of epiphysis was not detected in all 45 patients.
Methotrexate
Methotrexate (MTX) plays an anti‐inflammatory role in psoriasis. Recently, studies have found that MTX might inhibit the JAK/STAT pathway and restore the immunosuppressive function of Tregs. 24 , 25 The finding might explain the mechanism to a degree. In children, MTX is widely used for PV, and its efficacy and safety have been shown by our group. 26 However, evidence of the use of pustular psoriasis is scarce. Japanese guidelines recommend MTX as a C1 degree for GPP in children. 17
Haustein et al. 27 reviewed 157 adult patients with psoriasis, including 24 generalized pustular forms and 12 localized pustular forms, and a good response was found in 80% of the patients with generalized pustular psoriasis and 66% of the patients with localized pustular psoriasis. There are also some cases that shed light on childhood cases. 28 , 29 , 30 , 31 , 32 A two‐year‐old boy with GPP achieved remarkable pustules clearance and resolution of toxemic features after the first dose of MTX. 28 Garg et al. 29 reported on two siblings with GPP. The 7‐year‐old sister responded dramatically to MTX and showed almost 90% clearance of skin lesions after a 4‐week treatment. The 3‐year‐old brother was given combined prednisolone and MTX treatment and significantly improved. Kumar et al. 30 treated childhood psoriasis with MTX in seven patients, including two with GPP, one 14 years old and one 16 years old. Their disease was controlled at weeks 8 and 10, respectively, and they experienced disease‐free intervals for 16.8 weeks and 14.6 weeks, respectively. However, in some pediatric patients, the responses were unsatisfactory. 31 , 32 When the treatment goal is not met, combining MTX with other systemic medications, such as anti‐TNFα inhibitors and retinoids, may be a choice. 33 , 34
Cyclosporine
Cyclosporine inhibits T‐cell function, cytokine production, and MHC‐II expression by acting as an immunosuppressive agent and calcineurin inhibitor, exerting antipsoriatic effects. 35 Cyclosporine was effective in adults with GPP. 36 , 37 The efficacy is also observed in children, including a three‐month‐old infant. 38 Kiliç et al. 39 reported on three children with GPP treated with low‐dose cyclosporine (1–2 mg·kg−1·d−1) and observed the clearance of psoriatic lesions after 2–4 weeks of therapy.
Adverse events such as hypertension, nephrotoxicity, and increased risk of infection should be noted. Considering the side effects of cyclosporin are dose and treatment‐duration‐dependent, short‐term therapy might be a prospective option. 40 However, the recurrence of GPP may occur with the reduction or withdrawal of cyclosporine. 41 , 42 , 43 , 44 Thus, using cyclosporin for induction and then choosing other treatments for maintenance, such as acitretin and narrow‐band UVB (NBUVB) phototherapy, is worth consideration. 45
TNF‐α blocking agents
TNF‐α inhibitors, including infliximab, etanercept, and adalimumab, are used widely for PV. Infliximab is a chimeric anti‐TNF‐α monoclonal antibody. In data from adults, infliximab is safe and highly effective for GPP and acts as a rescue treatment that induces resolution of pustules within 1–8 days. 46 A similar effect was also observed in Chinese children. Lu et al. 47 treated two Chinese juveniles with GPP successfully, one achieved complete resolution within 2 weeks, and one had their disease controlled within 72 h. No relapse or exacerbation occurred in the two juveniles during 12 months. Pan et al. 48 described a Chinese juvenile with GPP and IL36RN c.115+6T > C homozygous mutation who had a good rapid response to short‐term infliximab and had no relapse for 21 months. However, for long‐term disease resolution, combining MTX or acitretin or switching to other biologic agents was required. 31 , 46 In contrast, infliximab also could induce GPP as a paradoxical reaction through up‐regulating Th17 and IFN‐α expression. 49
Adalimumab is a recombinant human immunoglobulin G1 monoclonal antibody. Du et al. 50 reported on seven Chinese children with GPP treated with adalimumab, and all of them had obvious improvement in skin lesions and systemic inflammatory reaction within the first week, and almost total clearance of skin lesions within one month. Du et al. 50 reviewed six Chinese pediatric patients treated with adalimumab, three with adalimumab monotherapy, two with adalimumab combined with methotrexate, and one with adalimumab combined with methotrexate and cyclosporine. All patients had complete remission within 1–15 months. In those 13 patients, no severe adverse events were recorded, and only one patient experienced flu‐like symptoms.
Etanercept is a soluble TNF receptor fusion protein. Several cases have described its efficacy in children with GPP. 51 , 52 , 53 , 54 , 55 Alvarez et al. 56 reported on a 3‐year‐old child with GPP, refractory to cyclosporine and acitretin, and experienced a relapse when infliximab was ceased, resulting in slow but sustained improvement with etanercept. Etanercept also did well in children with GPP and the IL36RN gene mutation or psoriatic arthritis. 51 , 52 Etanercept was well tolerated in those patients. Our group also observed a good response to etanercept in six Chinese patients under 4 years old. All patients achieved a JDA severity index of 1 and Generalized Pustular Psoriasis Area and Severity Index (GPPASI)‐75 after a 4‐week treatment and a JDA severity index of 0 and GPPASI‐100 after a 12‐week treatment. The treatment effect was maintained for 24 weeks. In three patients, with a median follow‐up duration of 12 months (10–12 months) after drug withdrawal, one patient relapsed at four months. No severe adverse events were recorded. This manuscript is currently being submitted.
IL‐17 pathway inhibitors
Secukinumab is a fully human immunoglobulin‐Gκ IL‐17A monoclonal antibody and decreases IL36R activation by inhibiting IL‐17A and disturbing the inflammatory loop of GPP. Several cases and case series have described the efficacy and safety of secukinumab. 19 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 Our team reviewed the efficacy and adverse events of secukinumab in 20 Chinese pediatric patients with GPP, with the ages ranging from 6 to 12 years and the age of onset ranging from 2 months to 11 years. 19 All the children had improvement in symptoms in the first week, and all patients achieved a JDA severity index of 0/1 in 3 weeks. Six children developed adverse events; two had abnormal alanine aminotransferase, two had atopic dermatitis‐like lesions, two had mild neutropenia, and one had herpes simplex. We compared these data with acitretin in 16 children with GPP and demonstrated that secukinumab had a more favorable response. Ruan et al. 58 demonstrated similar results in 18 Chinese children with GPP treated with secukinumab monotherapy and found the efficacy was maintained for 48 weeks. Wu et al. 57 described four Chinese children with DITRA aged from 5 to 11 years, and one had ACH lesions. Secukinumab monotherapy was used in one patient, co‐therapy with acitretin was used in two patients, and secukinumab with an antibiotic was used in one patient. All the children had defervescence and clearance of non‐acral pustules within 72 h, and all the participants except for the one with ACH achieved an Investigator's Global Assessment (IGA) 0/1 at week 24. Secukinumab was also effective in a 4‐week‐old infant with IL36RN and CARD14 gene mutations. 63
Ixekizumab is a humanized immunoglobulin G4 monoclonal antibody that targets IL‐17A and was approved in 2018 in Japan for patients with GPP and erythrodermic psoriasis (EP). Adult patients with GPP treated with ixekizumab had rapid and sustained improvements, and it was well tolerated. 67 , 68 Brodalumab is a human immunoglobulin G2 monoclonal antibody that blocks IL‐17 via inhibiting IL‐17R. In phase III, an open‐label multicenter study, 12 adults with GPP demonstrated that brodalumab significantly improved the symptoms throughout the 52 weeks without any new adverse events. 69 However, treatment data in children are lacking.
IL‐23 and IL‐23/IL‐12 inhibitors
Ustekinumab is a fully human L‐12/IL‐23 p40 monoclonal antibody. Case and case series have demonstrated that adults with GPP whether with or without the IL36RN gene mutation responded to ustekinumab. 70 , 71 , 72 Guselkumab is a human IL‐23 p19 subunit monoclonal antibody. A 52‐week, phase 3, multicenter, open‐label study reported the efficacy and safety in adults with GPP. 73 Risankizumab is another human IL‐23 p19 subunit monoclonal antibody. A phase 3, randomized, multicenter study in Japan studied eight adults with GPP treated with risankizumab, improvement was seen in week 4, and the patients continued to improve through week 52. 74 The improvement was maintained through week 160. 74 However, data on children are limited.
IL‐36R inhibitors
The IL‐36 pathway is central to the pathogenesis of GPP. Thus, a monoclonal antibody targeting the IL‐36 pathway is a prospective drug, theoretically. Spesolimab is a humanized monoclonal antibody that targets IL‐36R. In a phase 2 trial, 53 adults with GPP were enrolled in the placebo‐controlled trial, 35 received the spesolimab, and 18 received the placebo. 75 At week one, a pustulation subscore of 0 was achieved in 54% of the spesolimab group and 6% of the placebo group, a GPPASI score of 0/1 was achieved in 43% of the spesolimab group and 11% of the placebo group. The improvement was sustained to week 12. The improvement also was observed in the patient‐reported outcomes. 76 In the spesolimab group, two of the 35 patients reported drug reactions, one had a drug‐induced hepatic injury, and six of the 35 had infections in the first week. In the open‐label stage, 24 of the 51 patients had an infection, and 23 of the 50 detected antidrug antibodies at week 12. 75
CONCLUSIONS
In China, the adjusted national prevalence of GPP is 1.108 per 100 000 person‐years, and the incidence is 0.509 per 100 000 person‐years. The prevalence is higher than in France and Brazil. The first prevalence peak appears at the age of 0–3. IL36RN variants are associated with GPP in the Chinese population, especially in those with earlier age of onset, with ACH, without PV, severe inflammatory reactions, and likely to recurrence. Biological agents targeting different inflammatory mediators might be a prospective option for Chinese pediatrics with GPP.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Chen Y, Xiang X, Wang Z, Miao C, Xu Z. The update of treatment strategies in pediatrics with generalized pustular psoriasis in China. Pediatr Investig. 2023;7:191–198. 10.1002/ped4.12395
REFERENCES
- 1. Navarini AA, Burden AD, Capon F, Mrowietz U, Puig L, Köks S, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1792‐1799. DOI: 10.1111/jdv.14386 [DOI] [PubMed] [Google Scholar]
- 2. Robinson A, Van Voorhees AS, Hsu S, Korman NJ, Lebwohl MG. Treatment of pustular psoriasis: From the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288. DOI: 10.1016/j.jaad.2011.01.032 [DOI] [PubMed] [Google Scholar]
- 3. Miyachi H, Konishi T, Kumazawa R, Matsui H, Shimizu S, Fushimi K, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266‐1274. DOI: 10.1016/j.jaad.2021.06.008 [DOI] [PubMed] [Google Scholar]
- 4. Takeichi T, Akiyama M. Generalized pustular psoriasis: Clinical management and update on autoinflammatory aspects. Am J Clin Dermatol. 2020;21:227‐236. DOI: 10.1007/s40257-019-00492-0 [DOI] [PubMed] [Google Scholar]
- 5. Feng JN, Guo JZ, Zhang Q, Zhuo L, Xu L, Liu LL, et al. Higher prevalence of generalized pustular psoriasis in Asia? A population‐based study using claim data in China and a systematic review. Dermatology. 2023;239:195‐205. DOI: 10.1159/000528850 [DOI] [PubMed] [Google Scholar]
- 6. Li X, Chen M, Fu X, Zhang Q, Wang Z, Yu G, et al. Mutation analysis of the IL36RN gene in Chinese patients with generalized pustular psoriasis with/without psoriasis vulgaris. J Dermatol Sci. 2014;76:132‐138. DOI: 10.1016/j.jdermsci.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 7. Qin P, Zhang Q, Chen M, Fu X, Wang C, Wang Z, et al. Variant analysis of CARD14 in a Chinese Han population with psoriasis vulgaris and generalized pustular psoriasis. J Invest Dermatol. 2014;134:2994‐2996. DOI: 10.1038/jid.2014.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frey S, Sticht H, Wilsmann‐Theis D, Gerschütz A, Wolf K, Löhr S, et al. Rare loss‐of‐function mutation in SERPINA3 in generalized pustular psoriasis. J Invest Dermatol. 2020;140:1451‐1455. DOI: 10.1016/j.jid.2019.11.024.e13 [DOI] [PubMed] [Google Scholar]
- 9. Setta‐Kaffetzi N, Simpson MA, Navarini AA, Patel VM, Lu HC, Allen MH, et al. AP1S3 mutations are associated with pustular psoriasis and impaired toll‐like receptor 3 trafficking. Am J Hum Genet. 2014;94:790‐797. DOI: 10.1016/j.ajhg.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vergnano M, Mockenhaupt M, Benzian‐Olsson N, Paulmann M, Grys K, Mahil SK, et al. Loss‐of‐function myeloperoxidase mutations are associated with increased neutrophil counts and pustular skin disease. Am J Hum Genet. 2020;107:539‐543. DOI: 10.1016/j.ajhg.2020.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han JW, Wang Y, Alateng C, Li HB, Bai YH, Lyu XX, et al. Tumor necrosis factor‐alpha induced protein 3 interacting protein 1 gene polymorphisms and pustular psoriasis in Chinese Han population. Chin Med J. 2016;129:1519‐1524. DOI: 10.4103/0366-6999.184470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Cheng R, Lu Z, Guo Y, Yan M, Liang J, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235‐240. DOI: 10.1016/j.jdermsci.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 13. Wang Z, Xiang X, Chen Y, Qi Z, Miao C, Liu Y, et al. Different clinical features of pediatric generalized pustular psoriasis in patients with or without IL36RN variants. Dermatology. 2023;239:217‐226. DOI: 10.1159/000528753 [DOI] [PubMed] [Google Scholar]
- 14. Li M, Han J, Lu Z, Li H, Zhu K, Cheng R, et al. Prevalent and rare mutations in IL‐36RN gene in Chinese patients with generalized pustular psoriasis and psoriasis vulgaris. J Invest Dermatol. 2013;133:2637‐2639. DOI: 10.1038/jid.2013.267 [DOI] [PubMed] [Google Scholar]
- 15. Wang TS, Chiu HY, Hong JB, Chan CC, Lin SJ, Tsai TF. Correlation of IL36RN mutation with different clinical features of pustular psoriasis in Chinese patients. Arch Dermatol Res. 2016;308:55‐63. DOI: 10.1007/s00403-015-1611-x [DOI] [PubMed] [Google Scholar]
- 16. Chen YL, Wang ZY, Ma L, Xu ZG. Three cases of IL36RN‐associated pustulosis: An evolution of acrodermatitis continua of Hallopeau to generalized pustular psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:562‐565. DOI: 10.4103/ijdvl.IJDVL_581_19 [DOI] [PubMed] [Google Scholar]
- 17. Fujita H, Terui T, Hayama K, Akiyama M, Ikeda S, Mabuchi T, et al. Japanese guidelines for the management and treatment of generalized pustular psoriasis: the new pathogenesis and treatment of GPP. J Dermatol. 2018;45:1235‐1270. DOI: 10.1111/1346-8138.14523 [DOI] [PubMed] [Google Scholar]
- 18. Heath MS, Sahni DR, Curry ZA, Feldman SR. Pharmacokinetics of tazarotene and acitretin in psoriasis. Expert Opin Drug Metab Toxicol. 2018;14:919‐927. DOI: 10.1080/17425255.2018.1515198 [DOI] [PubMed] [Google Scholar]
- 19. Miao C, Chen Y, Wang Z, Xiang X, Liu Y, Xu Z. Real‐world data on the use of secukinumab and acitretin in pediatric generalized pustular psoriasis. J Dermatol. 2023;50:258‐261. DOI: 10.1111/1346-8138.16551 [DOI] [PubMed] [Google Scholar]
- 20. Chen P, Li C, Xue R, Chen H, Tian X, Zeng K, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatol Treat. 2018;29:353‐363. DOI: 10.1080/09546634.2017.1395798 [DOI] [PubMed] [Google Scholar]
- 21. Zhu T, Jin H, Shu D, Li F, Wu C. Association of IL36RN mutations with clinical features, therapeutic response to acitretin, and frequency of recurrence in patients with generalized pustular psoriasis. Eur J Dermatol. 2018;28:217‐224. DOI: 10.1684/ejd.2018.3245 [DOI] [PubMed] [Google Scholar]
- 22. Yang H, Tan Q, Chen GH, Chen JS, Fu Z, Ren FL, et al. Plasma retinol as a predictive biomarker of disease activity and response to acitretin monotherapy in children with generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2020;34:e270‐e272. DOI: 10.1111/jdv.16244 [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Wang Z, Xiang X, Chen Y, Peng Y, Wu D, et al. Effect of oral acitretin on the height and bone development of children:a retrospective analysis of 106 cases (in Chinese). Chin J Dermatol. 2022;55:1073‐1077. DOI: 10.35541/cjd.20220193 [DOI] [Google Scholar]
- 24. Alqarni AM, Zeidler MP. How does methotrexate work. Biochem Soc Trans. 2020;48:559‐567. DOI: 10.1042/BST20190803 [DOI] [PubMed] [Google Scholar]
- 25. Yan K, Xu W, Huang Y, Zhang Z, Huang Q, Xin KZ, et al. Methotrexate restores the function of peripheral blood regulatory T cells in psoriasis vulgaris via the CD73/AMPK/mTOR pathway. Br J Dermatol. 2018;179:896‐905. DOI: 10.1111/bjd.16560 [DOI] [PubMed] [Google Scholar]
- 26. Wang Z, Chen Y, Xiang X, Gu Y, Zhao M, Liu Y, et al. Systemic methotrexate treatment in 42 children with severe plaque psoriasis: A retrospective study in China. Dermatology. 2022;238:919‐927. DOI: 10.1159/000521262 [DOI] [PubMed] [Google Scholar]
- 27. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years' experience with low‐dose long‐term treatment. J Eur Acad Dermatol Venereol. 2000;14:382‐388. DOI: 10.1046/j.1468-3083.2000.00058.x [DOI] [PubMed] [Google Scholar]
- 28. Dogra S, Kumaran MS, Handa S, Kanwar AJ. Methotrexate for generalized pustular psoriasis in a 2‐year‐old child. Pediatr Dermatol. 2005;22:85‐86. DOI: 10.1111/j.1525-1470.2005.22122.x [DOI] [PubMed] [Google Scholar]
- 29. Garg T, Chander R, Mittal S. Familial juvenile generalized pustular psoriasis: response to methotrexate. Skinmed. 2011;9:190‐191. [PubMed] [Google Scholar]
- 30. Kumar B, Dhar S, Handa S, Kaur I. Methotrexate in childhood psoriasis. Pediatr Dermatol. 1994;11:271‐273. DOI: 10.1111/j.1525-1470.1994.tb00602.x [DOI] [PubMed] [Google Scholar]
- 31. Skrabl‐Baumgartner A, Weger W, Salmhofer W, Jahnel J. Childhood generalized pustular psoriasis: longtime remission with combined infliximab and methotrexate treatment. Pediatr Dermatol. 2015;32:e13‐14. DOI: 10.1111/pde.12457 [DOI] [PubMed] [Google Scholar]
- 32. Fialová J, Vojáčková N, Vaňousová D, Hercogová J. Juvenile generalized pustular psoriasis treated with etanercept. Dermatol Ther. 2014;27:105‐108. DOI: 10.1111/dth.12065 [DOI] [PubMed] [Google Scholar]
- 33. Kawakami H, Maeda T, Abe N, Matsumoto Y, Mitsuhashi Y, Tsuboi R, et al. Efficacy of adalimumab and methotrexate combination therapy on generalized pustular psoriasis patients unresponsive to infliximab monotherapy due to anti‐infliximab antibody development. J Dermatol. 2015;42:94‐95. DOI: 10.1111/1346-8138.12704 [DOI] [PubMed] [Google Scholar]
- 34. Rosenbaum MM. Treatment of generalized pustular psoriasis with etretinate (Ro 10‐9359) and methotrexate. J Am Acad Dermatol. 1984;10:357‐361. DOI: 10.1016/s0190-9622(84)80007-9 [DOI] [PubMed] [Google Scholar]
- 35. Bos JD. The pathomechanisms of psoriasis; the skin immune system and cyclosporin. Br J Dermatol. 1988;118:141‐155. DOI: 10.1111/j.1365-2133.1988.tb01768.x [DOI] [PubMed] [Google Scholar]
- 36. Choon SE, Lai NM, Mohammad NA, Nanu NM, Tey KE, Chew SF. Clinical profile, morbidity, and outcome of adult‐onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676‐684. DOI: 10.1111/ijd.12070 [DOI] [PubMed] [Google Scholar]
- 37. Ozawa A, Ohkido M, Haruki Y, Kobayashi H, Ohkawara A, Ohno Y, et al. Treatments of generalized pustular psoriasis: A multicenter study in Japan. J Dermatol. 1999;26:141‐149. DOI: 10.1111/j.1346-8138.1999.tb03444.x [DOI] [PubMed] [Google Scholar]
- 38. Takahashi M, Takeuchi M, Matsunaga K. Infant with generalized pustular psoriasis who responded to cyclosporin A therapy. J Dermatol. 2015;42:911‐913. DOI: 10.1111/1346-8138.12960 [DOI] [PubMed] [Google Scholar]
- 39. Kiliç SS, Hacimustafaoğlu M, Celebi S, Karadeniz A, Ildirim I. Low dose cyclosporin A treatment in generalized pustular psoriasis. Pediatr Dermatol. 2001;18:246‐248. DOI: 10.1046/j.1525-1470.2001.018003246.x [DOI] [PubMed] [Google Scholar]
- 40. Berth‐Jones J, Henderson CA, Munro CS, Rogers S, Chalmers RJ, Boffa MJ, et al. Treatment of psoriasis with intermittent short course cyclosporin (Neoral). A multicentre study. Br J Dermatol. 1997;136:527‐530. [PubMed] [Google Scholar]
- 41. Hong SB, Kim NI. Generalized pustular psoriasis following withdrawal of short‐term cyclosporin therapy for psoriatic arthritis. J Eur Acad Dermatol Venereol. 2005;19:522‐523. DOI: 10.1111/j.1468-3083.2005.01195.x [DOI] [PubMed] [Google Scholar]
- 42. Kamarashev J, Lor P, Forster A, Heinzerling L, Burg G, Nestle FO. Generalised pustular psoriasis induced by cyclosporin a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213‐216. DOI: 10.1159/000063919 [DOI] [PubMed] [Google Scholar]
- 43. Georgala S, Koumantaki E, Rallis E, Papadavid E. Generalized pustular psoriasis developing during withdrawal of short‐term cyclosporin therapy. Br J Dermatol. 2000;142:1057‐1058. DOI: 10.1046/j.1365-2133.2000.03503.x [DOI] [PubMed] [Google Scholar]
- 44. De Silva BD, Benton EC, Tidman MJ. Generalized pustular psoriasis following withdrawal of oral cyclosporin treatment for palmo‐plantar pustulosis. Clin Exp Dermatol. 1999;24:10‐13. DOI: 10.1046/j.1365-2230.1999.00395.x [DOI] [PubMed] [Google Scholar]
- 45. Kim HS, Kim GM, Kim SY. Two‐stage therapy for childhood generalized pustular psoriasis: low‐dose cyclosporin for induction and maintenance with acitretin/narrowband ultraviolet B phototherapy. Pediatr Dermatol. 2006;23:306‐308. DOI: 10.1111/j.1525-1470.2006.00247.x [DOI] [PubMed] [Google Scholar]
- 46. Kołt‐Kamińska M, Żychowska M, Reich A. Infliximab in combination with low‐dose acitretin in generalized pustular psoriasis: A report of two cases and review of the literature. Biologics. 2021;15:317‐327. DOI: 10.2147/BTT.S323239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu J, Li Y, Yu N, Chen F, Ding Y, Yi X. Successful treatment of juvenile generalized pustular psoriasis with infliximab therapy: two case reports. J Int Med Res. 2020;48:300060520912091. DOI: 10.1177/0300060520912091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pan J, Qiu L, Xiao T, Chen HD. Juvenile generalized pustular psoriasis with IL36RN mutation treated with short‐term infliximab. Dermatol Ther. 2016;29:164‐167. DOI: 10.1111/dth.12325 [DOI] [PubMed] [Google Scholar]
- 49. Almutairi D, Sheasgreen C, Weizman A, Alavi A. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;22:507‐510. DOI: 10.1177/1203475418758986 [DOI] [PubMed] [Google Scholar]
- 50. Du Y, Yan Q, Chen M, Dong Z, Wang F. Efficacy of adalimumab in pediatric generalized pustular psoriasis: case series and literature review. J Dermatolog Treat. 2022;33:2862‐2868. DOI: 10.1080/09546634.2022.2089327 [DOI] [PubMed] [Google Scholar]
- 51. Cuperus E, Koevoets R, van der Smagt JJ, Toonstra J, de Graaf M, Frenkel J, et al. Juvenile interleukin‐36 receptor antagonist deficiency (DITRA) with c.80T>C (p.Leu27Pro) mutation successfully treated with etanercept and acitretin. JAAD Case Rep. 2018;4:192‐195. DOI: 10.1016/j.jdcr.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mazzotta A, Saraceno R, Esposito M, Chimenti S. Etanercept, childhood and long‐term safety: A case of five years treatment. Eur J Dermatol. 2011;21:776‐777. DOI: 10.1684/ejd.2011.1432 [DOI] [PubMed] [Google Scholar]
- 53. Pereira TM, Vieira AP, Fernandes JC, Antunes H, Basto AS. Anti‐TNF‐alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350‐352. DOI: 10.1159/000096202 [DOI] [PubMed] [Google Scholar]
- 54. Papoutsaki M, Costanzo A, Mazzotta A, Gramiccia T, Soda R, Chimenti S. Etanercept for the treatment of severe childhood psoriasis. Br J Dermatol. 2006;154:181‐183. DOI: 10.1111/j.1365-2133.2005.06982.x [DOI] [PubMed] [Google Scholar]
- 55. Hawrot AC, Metry DW, Theos AJ, Levy ML. Etanercept for psoriasis in the pediatric population: experience in nine patients. Pediatr Dermatol. 2006;23:67‐71. DOI: 10.1111/j.1525-1470.2006.00174.x [DOI] [PubMed] [Google Scholar]
- 56. Alvarez AC, Rodríguez‐Nevado I, De Argila D, Rubio FP, Rovira I, Torrelo A, et al. Recalcitrant pustular psoriasis successfully treated with adalimumab. Pediatr Dermatol. 2011;28:195‐197. DOI: 10.1111/j.1525-1470.2010.01219.x [DOI] [PubMed] [Google Scholar]
- 57. Wu X, Yan T, Han C, Ma L, Zeng L, Chen X, et al. Rapid and sustained response of acute generalized pustular psoriasis of von Zumbusch to Secukinumab. J Eur Acad Dermatol Venereol. 2023;37:e338‐e341. DOI: 10.1111/jdv.18609 [DOI] [PubMed] [Google Scholar]
- 58. Ruan SF, Zhang LL, Liu Z, Lin TT, Wang HQ, Xu QY, et al. Real‐world data on the clinical use of secukinumab in pediatric generalized pustular psoriasis: a 48‐week retrospective study. J Am Acad Dermatol. 2023;88:243‐246. DOI: 10.1016/j.jaad.2022.04.064 [DOI] [PubMed] [Google Scholar]
- 59. Yang Y, Xu Q, Zhang Z, Yao Z. Segmental vitiligo following acitretin treatment for infantile generalized pustular psoriasis resulting in repigmentation under secukinumab therapy. Dermatol Ther. 2022;35:e15305. DOI: 10.1111/dth.15305 [DOI] [PubMed] [Google Scholar]
- 60. Reymundo A, Vilarrasa E, Baniandrés O, Rodríguez‐Fernández‐Freire L, Feltes R, Llamas‐Velasco M, et al. Effectiveness and safety profile of secukinumab for the treatment of patients with generalized pustular psoriasis in daily practice. J Eur Acad Dermatol Venereol. 2022;36:e849‐e851. DOI: 10.1111/jdv.18317 [DOI] [PubMed] [Google Scholar]
- 61. Albela H, Begum S, Leong KF. Successful treatment of paediatric generalized pustular psoriasis with secukinumab: a case series. J Dermatol Treat. 2022;33:1769‐1773. DOI: 10.1080/09546634.2021.1899111 [DOI] [PubMed] [Google Scholar]
- 62. Zhu H, Song P, Du D, Tian H. Successful treatment of a 3‐year‐old girl with generalized pustular psoriasis using secukinumab monotherapy. Pediatr Dermatol. 2021;38:1366‐1367. DOI: 10.1111/pde.14795 [DOI] [PubMed] [Google Scholar]
- 63. López‐Sánchez C, Falla LM, Roé‐Crespo E, Arostegui JI, Mozos A, Bernal S, et al. Excellent response to secukinumab in an infant with severe generalized pustular psoriasis. J Dermatol. 2021;48:907‐910. DOI: 10.1111/1346-8138.15673 [DOI] [PubMed] [Google Scholar]
- 64. Sun ZL, Liu ZL, Xu YY, Zhang XL, Zhang CL, Guan X. Successful treatment of generalized pustular psoriasis with secukinumab: a report of two cases. Chin Med J. 2020;133:3015‐3016. DOI: 10.1097/CM9.0000000000001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nishida M, Takeichi T, Kono M, Imanishi A, Maekawa N, Akiyama M, et al. Successful secukinumab treatment of recalcitrant juvenile generalized pustular psoriasis. J Dermatol. 2020;47:e77‐e78. DOI: 10.1111/1346-8138.15228 [DOI] [PubMed] [Google Scholar]
- 66. Ho PH, Tsai TF. Successful treatment of refractory juvenile generalized pustular psoriasis with secukinumab monotherapy: A case report and review of published work. J Dermatol. 2018;45:1353‐1356. DOI: 10.1111/1346-8138.14636 [DOI] [PubMed] [Google Scholar]
- 67. Morita A, Okubo Y, Morisaki Y, Torisu‐Itakura H, Umezawa Y. Ixekizumab 80 mg every 2 weeks treatment beyond week 12 for Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis. Dermatol Ther. 2022;12:481‐494. DOI: 10.1007/s13555-021-00666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Okubo Y, Mabuchi T, Iwatsuki K, Elmaraghy H, Torisu‐Itakura H, Morisaki Y, et al. Long‐term efficacy and safety of ixekizumab in Japanese patients with erythrodermic or generalized pustular psoriasis: subgroup analyses of an open‐label, phase 3 study (UNCOVER‐J). J Eur Acad Dermatol Venereol. 2019;33:325‐332. DOI: 10.1111/jdv.15287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yamasaki K, Nakagawa H, Kubo Y, Ootaki K. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52‐week, open‐label study. Br J Dermatol. 2017;176:741‐751. DOI: 10.1111/bjd.14702 [DOI] [PubMed] [Google Scholar]
- 70. Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825‐828. DOI: 10.1001/jamadermatol.2016.0751 [DOI] [PubMed] [Google Scholar]
- 71. Daudén E, Santiago‐et‐Sánchez‐Mateos D, Sotomayor‐López E, García‐Díez A. Ustekinumab: effective in a patient with severe recalcitrant generalized pustular psoriasis. Br J Dermatol. 2010;163:1346‐1347. DOI: 10.1111/j.1365-2133.2010.09995.x [DOI] [PubMed] [Google Scholar]
- 72. Storan ER, O'Gorman SM, Markham T. Generalized pustular psoriasis treated with ustekinumab. Clin Exp Dermatol. 2016;41:689‐690. DOI: 10.1111/ced.12868 [DOI] [PubMed] [Google Scholar]
- 73. Yamamoto H, Kamiya K, Okada H, Maekawa T, Komine M, Ohtsuki M. A case of acrodermatitis continua of Hallopeau evolving into generalized pustular psoriasis successfully treated with guselkumab. Int J Dermatol. 2023;62:269‐270. DOI: 10.1111/ijd.16153 [DOI] [PubMed] [Google Scholar]
- 74. Yamanaka K, Okubo Y, Yasuda I, Saito N, Messina I, Morita A. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: Primary analysis and 180‐week follow‐up results from the phase 3, multicenter IMMspire study. J Dermatol. 2023;50:195‐202. DOI: 10.1111/1346-8138.16667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bachelez H, Choon SE, Marrakchi S, Burden AD, Tsai TF, Morita A, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431‐2440. DOI: 10.1056/NEJMoa2111563 [DOI] [PubMed] [Google Scholar]
- 76. Navarini AA, Prinz JC, Morita A, Tsai TF, Viguier MA, Li L, et al. Spesolimab improves patient‐reported outcomes in patients with generalized pustular psoriasis: Results from the Effisayil 1 study. J Eur Acad Dermatol Venereol. 2023;37:730‐736. DOI: 10.1111/jdv.18820 [DOI] [PubMed] [Google Scholar]


