Abstract
This umbrella review aims to provide a systematic and comprehensive overview of current evidence from prospective studies on the diverse health effects of cheese consumption. We searched PubMed, Embase, and Cochrane Library to identify meta-analyses/pooled analyses of prospective studies examining the association between cheese consumption and major health outcomes from inception to August 31, 2022. We reanalyzed and updated previous meta-analyses and performed de novo meta-analyses with recently published prospective studies, where appropriate. We calculated the summary effect size, 95% prediction confidence intervals, between-study heterogeneity, small-study effects, and excess significance bias for each health outcome. We identified 54 eligible articles of meta-analyses/pooled analyses. After adding newly published original articles, we performed 35 updated meta-analyses and 4 de novo meta-analyses. Together with 8 previous meta-analyses, we finally included 47 unique health outcomes. Cheese consumption was inversely associated with all-cause mortality (highest compared with lowest category: RR = 0.95; 95% CI: 0.92, 0.99), cardiovascular mortality (RR = 0.93; 95% CI: 0.88, 0.99), incident cardiovascular disease (CVD) (RR = 0.92; 95% CI: 0.89, 0.96), coronary heart disease (CHD) (RR = 0.92; 95% CI: 0.86, 0.98), stroke (RR = 0.93; 95% CI: 0.89, 0.98), estrogen receptor-negative (ER−) breast cancer (RR = 0.89; 95% CI: 0.82, 0.97), type 2 diabetes (RR = 0.93; 95% CI: 0.88, 0.98), total fracture (RR = 0.90; 95% CI: 0.86, 0.95), and dementia (RR = 0.81; 95% CI: 0.66, 0.99). Null associations were found for other outcomes. According to the NutriGrade scoring system, moderate quality of evidence was observed for inverse associations of cheese consumption with all-cause and cardiovascular mortality, incident CVD, CHD, and stroke, and for null associations with cancer mortality, incident hypertension, and prostate cancer. Our findings suggest that cheese consumption has neutral to moderate benefits for human health.
Keywords: cheese consumption, umbrella review, updated meta-analyses, all-cause mortality, cardiovascular disease, cancer, fracture, metabolic disease
Statement of significance.
This umbrella review provides a systematic and comprehensive overview of current evidence on the association of cheese consumption with 47 major health outcomes through 35 updated, 4 de novo, and 8 previous meta-analyses of prospective observational studies. Although high saturated fat and sodium in some cheeses tend to be emphasized as a health concern in dietary guidelines, cheese also provides some nutrients and bioactive compounds that may confer some benefits.
Introduction
Cheese is generally a nutrient-dense and well-tolerated fermented dairy product consumed worldwide. However, the health effects of cheese consumption remain a matter of controversy. On one hand, cheese is a rich source of high-quality protein (mainly casein), lipids, minerals (e.g., calcium, phosphorus, and magnesium), and vitamins (e.g., vitamin A, K2, B2, B12, and folate), and probiotics and bioactive molecules (e.g., bioactive peptides, lactoferrin, short-chain fatty acids, and milk fat globule membrane), which may provide various health benefits. On the other hand, cheese contains relatively high contents of saturated fat and salt, which are perceived as unfavorable dietary components for cardiovascular health [1,2]. Currently, most dietary guidelines recommend consuming dairy products as part of a healthy diet while avoiding intake of full-fat and high-sodium versions [[3], [4], [5], [6]]. Of note, the recommendation is primarily based on extrapolated benefits and harms of single nutrient contained in dairy. However, whole dairy foods are not a simple collection of isolated nutrients but have complex physical and nutritional structures (i.e., dairy matrix), which affect digestibility and nutrient bioavailability, thereby modifying the overall effects of dairy consumption on health and disease [[7], [8], [9]]. In addition, dairy products are a heterogeneous group of foods regarding the dairy matrix due to different processing methods [8]. Because various types of dairy products appear to have distinct influences on specific health outcomes [10], merging them into 1 group (i.e., total dairy consumption) may blur the true association. Thus, a separate assessment of the health effects of cheese consumption is required.
Umbrella reviews can provide a comprehensive overview of evidence from existing meta-analyses on a given topic, with unique strengths of identifying the uncertainties, biases, and knowledge gaps of the evidence [11]. Many meta-analyses on the association between cheese consumption and a range of health end points, such as all-cause and cause-specific mortality, cardiovascular diseases (CVD), cancer, metabolic diseases, bone fracture, and other diseases, have been published [[12], [13], [14], [15], [16], [17]]. An extensive summary of the breadth and validity of these associations with diverse health outcomes will help elucidate the role of cheese consumption in human health. Therefore, we conducted an umbrella review to synthesize the available evidence from meta-analyses of prospective studies to examine the various health impacts of cheese consumption. Furthermore, we contextualized the magnitude, direction, and significance of the identified associations, evaluated risk of potential biases, and assessed the credibility of the evidence.
Methods
The present umbrella review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. The protocol of this study was registered in PROSPERO (CRD42022331328).
Literature search
We systematically searched PubMed, Embase, and Cochrane Library databases to identify existing meta-analyses (including pooled analyses) of prospective studies investigating the association between cheese consumption and any health outcome from inception to August 31, 2022. The search terms were as follows: (cheese) AND (“meta analysis” OR meta-analysis OR “meta analyzed” OR meta-analyzed OR “pooled analysis” OR “systematic review”). We also extensively searched the 3 databases for recently published original prospective studies to update previous meta-analyses or derive de novo meta-analyses. Predefined search strategies for meta-analyses and primary studies are presented in Supplemental Tables 1 and 2. Two investigators (XD, MZ) independently performed a 3-step parallel screening of titles, abstracts, and full texts for all identified studies according to the inclusion and exclusion criteria. Any discrepancies were discussed and resolved by a third investigator (ZH).
Eligibility criteria
Meta-analyses of population-based prospective studies (i.e., prospective cohort studies, case-cohort studies, nested case–control studies, and randomized controlled trials) exploring the association between cheese consumption (primary or secondary exposure of interest) and major health outcomes were included in the umbrella review. Original prospective studies eligible for updated or de novo meta-analyses were also included. Conference abstracts, interviews, letters, and narrative reviews were excluded. Meta-analyses or original studies without full text, effect size [e.g., risk ratio (RR), odds ratio (OR), or hazard ratio (HR)], or not written in English were also excluded. Studies with changes in cheese consumption rather than absolute intake as exposure, using substitution analysis, or using surrogate end points (e.g., blood lipids, blood pressure, and body weight) as outcomes were removed. If more than 1 article reported the results for an identical outcome from the same study population (or cohort), only the one with the largest sample size, the longest follow-up, or the most complete information was included.
Data extraction
From each included meta-analysis, the following information was extracted and verified by three investigators (XD, MZ, ZH): the first author’s name, publication year, outcome of interest, study population (general or disease status), study design of the primary studies, type of comparison (highest compared with lowest category of cheese consumption or each increment in cheese consumption), number of included studies, number of participants and cases, and the reported summary risk estimates (RR, OR, or HR) with corresponding 95% CIs. For meta-analyses on over 1 health outcome, each outcome was recorded separately. For original studies, the extracted data covered information on the first author’s name, publication year, study design, study population characteristics, geographic location, number of participants and cases, length of follow-up (cohort study), dietary assessment method (e.g., food frequency questionnaire and 3-d 24-h dietary records), cheese type, categorization and amount of cheese consumption, adjustment factors, and effect size with 95% CIs.
Evaluation of methodological quality
The AMSTAR-2 (A Measurement Tool to Assess Systematic Reviews) tool [19] was used to evaluate the methodological quality of the included published meta-analyses and systematic reviews. It includes 16 individual items and 7 of them are identified as critical. Systematic reviews with no or 1 noncritical weakness are rated as high confidence; those with more than 1 noncritical weakness are rated as moderate confidence; those with 1 critical flaw with or without noncritical weaknesses are rated as low confidence; and those with more than 1 critical flaw with or without noncritical weaknesses are rated as critically low confidence. Two investigators (XD, MZ) implemented the evaluation independently, with disagreements reconciled by discussion and consensus.
Assessment of evidence credibility
The credibility of evidence was assessed using the NutriGrade scoring system [20]. It comprised 8 items: risk of bias of the primary studies (0–2 points), precision of the estimate (0–1 point), heterogeneity (0–1 point), directness (0–1 point), publication bias (0–1 point), funding bias (0–1 point), effect size (0–2 points), and dose–response association (0–1 point). The meta-evidence was graded as high, moderate, low, and very low if the overall score is ≥8 points, 6 to <8 points, 4 to <6 points, and <4 points, respectively. Two investigators (XD, MZ) independently performed the rating and any discrepancies were resolved by discussion.
Statistical analysis
We reanalyzed previous meta-analyses to obtain necessary information for subsequent assessment of the credibility of evidence. If the existing meta-analysis included cross-sectional, retrospective, and prospective studies, we only kept the results from prospective studies in our meta-analysis. Furthermore, we incorporated newly identified original studies into previous meta-analyses to update or derive de novo meta-analyses, where appropriate. For each outcome, we recalculated the summary risk estimates and their corresponding 95% CIs for the highest compared with the lowest category of cheese consumption and/or per 30-g/d increment in cheese consumption by using the random-effects model by DerSimonian and Laird [21]. When results from the same cohort were reported separately for different cheese types (e.g., hard cheese and cottage cheese or low-fat cheese and high-fat cheese instead of total cheese) and disease subtypes [e.g., coronary heart disease (CHD) and stroke rather than total CVD], we used a fixed-effects model to generate an overall estimate before pooling with other studies.
Heterogeneity across studies was investigated using I2 statistic. We also performed subgroup analyses according to adjustment for total energy intake in the models (adjusted and unadjusted) and geographical location (Europe, North America, and Oceania; Asia and other regions; multiregion) to explore potential sources of heterogeneity. We computed 95% prediction intervals (95% PIs) to predict the effect size range of a future original study will lie after considering both the uncertainty in the mean effect and the heterogeneity from the random-effects model [22,23]. We assessed potential small-study effects by Egger’s test [24] if ≥3 studies were available. A P value of <0.10 was interpreted as the presence of small-study effects. The excess statistical significance test was performed to evaluate whether the observed number of nominally statistically significant studies was larger than their expected number using the χ2 test [25]. A P value of <0.10 was considered statistically significant.
We further examined the nonlinear dose–response association among studies that provided risk estimates with ≥3 exposure categories using a 2-stage restricted cubic splines (3 knots at 25, 50, and 75 percentiles) analysis [26,27]. The P value for nonlinearity was calculated by testing whether the coefficient of the second spline was equal to 0 [27]. We used the median/mean of each consumption category if available or the midpoint between the lower and upper bounds of each intake category to represent the intake levels. We assumed zero as the lower bound for the open-ended lowest category and multiplied the lower bound value by 1.2 as the upper bound for the open-ended highest category. All statistical analyses were conducted using the “metafor,” “meta,” “dosresmeta,” and “forestplot” packages in R software version 4.1.0 (The R Foundation).
Results
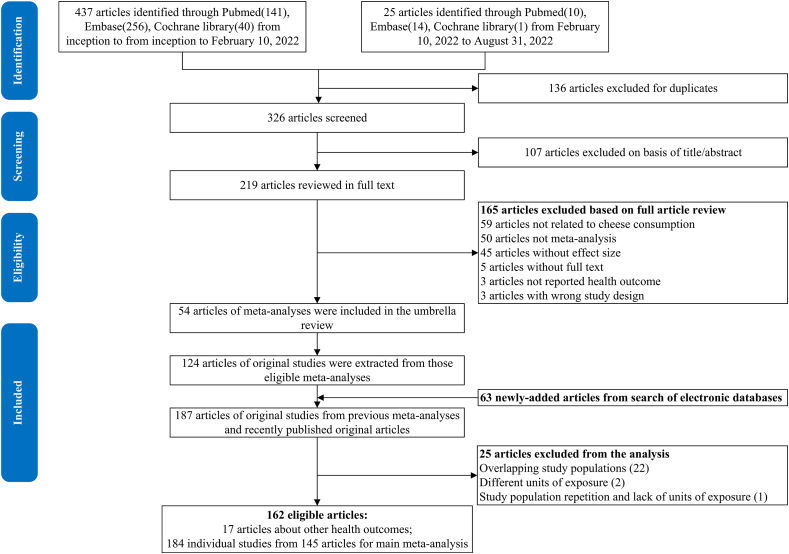
Figure 1 shows the flowchart of the study search and selection process. We identified 54 eligible articles of meta-analyses/pooled analyses on 34 health outcomes, including 11 mortality outcomes [12,[28], [29], [30], [31], [32], [33], [34]] (Supplemental Table 3), 4 CVD outcomes [13,28,[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]] (Supplemental Table 4), 13 cancer outcomes [14,32,[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66]] (Supplemental Table 5), 3 metabolic disease outcomes [15,[67], [68], [69], [70], [71], [72]] (Supplemental Table 6), and 3 aging-related outcomes [16,17,[73], [74], [75]] (Supplemental Table 7). A total of 124 articles of original studies were extracted from previous meta-analyses, which combined with 63 newly added primary articles, resulting in 187 original articles. After excluding 25 articles with overlapping study populations or without absolute intake as exposures (Supplemental Table 8), we included 162 original articles, such as 17 single articles about other health outcomes (Supplemental Table 9). A total of 184 prospective observational studies from the 145 articles were applied for 35 updated meta-analyses and 4 de novo meta-analyses on 39 health outcomes. Combined with 8 health outcomes from previous meta-analyses, we obtained 47 unique health outcomes in our umbrella review (Figures 2 and 3 and Supplemental Tables 10 and 11). For the updated and de novo meta-analyses, most of the included cohorts were conducted in North America and Europe (Supplemental Figure 1). No randomized controlled trials were eligible in this analysis; thus, all results were from prospective observational studies.
FIGURE 1.
Flow diagram of the study search and selection process.
FIGURE 2.
Association between cheese consumption (highest compared with lowest intake level) and all-cause and cause-specific mortality.
FIGURE 3.
Association between cheese consumption (highest compared with lowest intake level) and disease incidence.
Methodological quality
The evaluation of methodological quality for previous meta-analyses using AMSTAR-2 is summarized in Supplemental Table 12. A total of 11 meta-analyses [12,37,39,43,53,59,65,68,[73], [74], [75]] were of high quality, 12 meta-analyses [28,[30], [31], [32], [33],40,47,55,57,67,70,76] of moderate quality, 13 meta-analyses [16,29,34,36,38,41,42,44,49,51,54,56,69] of low quality, and the remaining 14 meta-analyses [[13], [14], [15],17,35,45,46,48,50,52,58,60,71,72] of critically low quality. Four pooled analyses [[61], [62], [63], [64]] were not suitable for the evaluation.
All-cause mortality
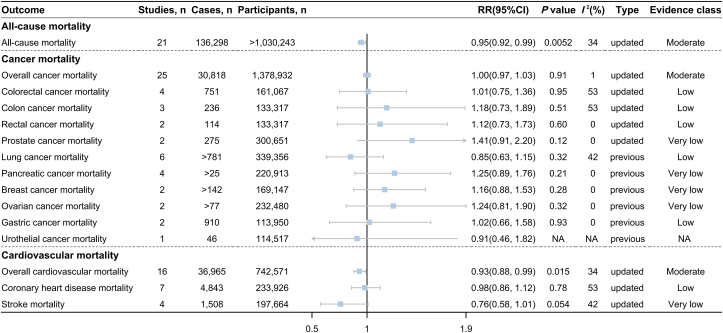
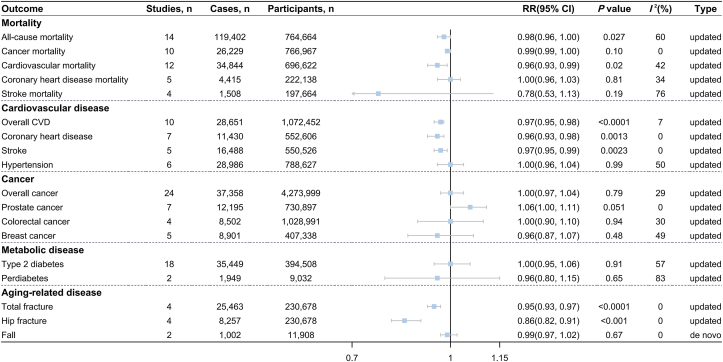
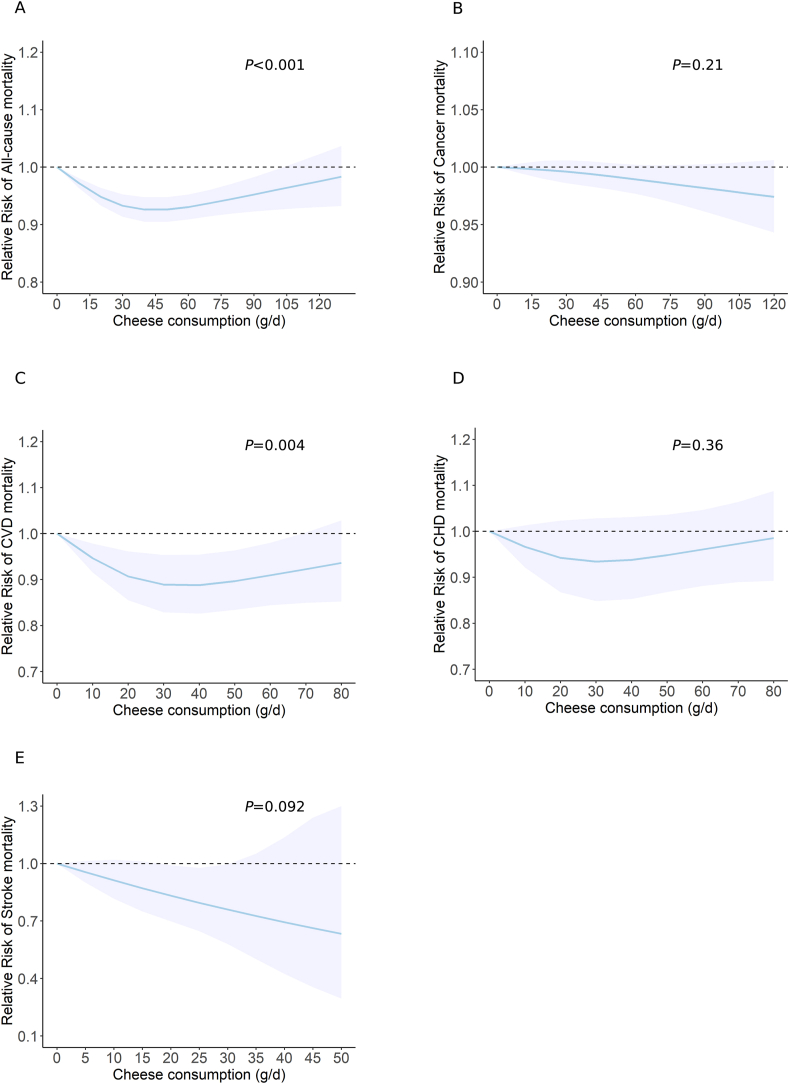
The updated meta-analysis of 21 studies [[77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94]], including 136,298 cases among >1,030,243 participants, revealed an inverse association between cheese consumption and all-cause mortality (highest compared with lowest: RR = 0.95; 95% CI: 0.92, 0.99; P = 0.0052; I2 = 34%) (Figure 2 and Supplemental Figure 2). In the dose–response meta-analysis of 14 studies [77,79,[81], [82], [83], [84], [85],[87], [88], [89], [90], [91]], each 30-g/d cheese increment was associated with a 2% lower risk of all-cause mortality (RR = 0.98; 95% CI: 0.96, 1.00; P = 0.027; I2 = 60%) (Figure 4 and Supplemental Figure 3). Nonlinear analyses further showed a U-shaped association, where the lowest risk of all-cause mortality was observed at ∼40 g/d of cheese consumption (P-nonlinearity < 0.001) (Figure 5A).
FIGURE 4.
Association between cheese consumption (per 30-g/d intake level) and mortality and multiple disease incidence.
FIGURE 5.
Dose–response association between cheese consumption and the risk of (A) all-cause mortality, (B) cancer mortality, (C) CVD mortality, (D) CHD mortality, and (E) stroke mortality. CHD, coronary heart disease; CVD, cardiovascular disease. P value was from the test of nonlinearity.
Cardiovascular mortality
The updated meta-analysis of 16 studies [77,78,81,82,84,85,87,88,90,91,[93], [94], [95]] encompassing 36,965 cases among 742,571 participants showed an inverse association between cheese consumption and cardiovascular mortality (highest compared with lowest: RR = 0.93; 95% CI: 0.88, 0.99; P = 0.015; I2 = 34%) (Figure 2 and Supplemental Figure 4). In addition, we observed a significant inverse association when cheese consumption was expressed as per 30-g/d increment in 12 studies [77,81,82,84,85,87,88,90,91,95] (RR = 0.96; 95% CI: 0.93, 0.99; P = 0.02; I2 = 42%) (Figure 4 and Supplemental Figure 5), the nonlinear dose–response meta-analysis suggested that the association between cheese consumption and cardiovascular mortality followed a U shape with the minimal risk at ∼35 g/d (P-nonlinearity = 0.004) (Figure 5C). No association was detected between cheese consumption and CHD mortality or stroke mortality (Figures 2 and 4 and Supplemental Figures 6–9).
Cancer mortality
The updated meta-analysis of 25 studies [78,[81], [82], [83],85,87,88,[96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108]] with 30,818 cases among 1,378,932 participants found no association between cheese consumption and overall cancer mortality (highest compared with lowest: RR = 1.00; 95% CI: 0.97, 1.03; P = 0.91; I2 = 1%) (Figure 2 and Supplemental Figure 10). The linear and nonlinear dose–response meta-analyses yielded similar conclusions (FIGURE 4, FIGURE 5B and Supplemental Figure 11). In addition, cheese consumption was not associated with the risk of site-specific cancer mortality (Figure 2 and Supplemental Figure 12).
Cardiovascular disease
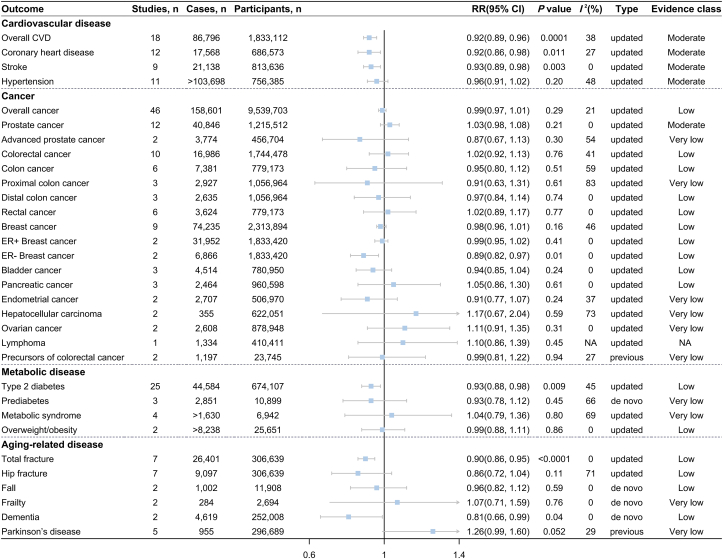
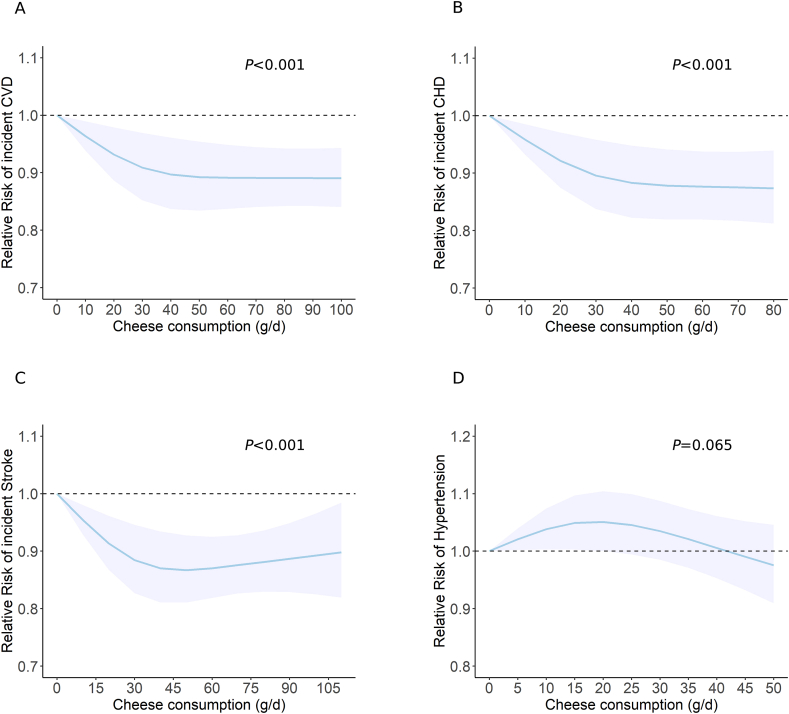
The updated meta-analyses of the highest compared with the lowest category of cheese consumption included 18 studies (86,796 cases among 1,833,112 participants) for overall CVD risk [80,89,[109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122]], 12 studies (17,568 cases among 686,573 participants) for CHD risk [89,109,[112], [113], [114], [115], [116], [117],119,120], 9 studies (21,138 cases among 813,636 participants) for stroke risk [80,111,112,115,118,119,121,122], and 11 studies (>103,698 cases among 756,385 participants) for hypertension risk [110,[123], [124], [125], [126], [127], [128], [129], [130], [131]], respectively. Higher cheese consumption was associated with reduced risk of overall CVD (RR = 0.92; 95% CI: 0.89, 0.96; P = 0.0001; I2 = 38%) (Figure 3 and Supplemental Figure 13), CHD (RR = 0.92; 95% CI: 0.86, 0.98; P = 0.0108; I2 = 27%) (Figure 3 and Supplemental Figure 15), and stroke (RR = 0.93; 95% CI: 0.89, 0.98; P = 0.003; I2 = 0%) (Figure 3 and Supplemental Figure 17). Each 30 g/d cheese intake increment was associated with approximately 3% lower risk of overall CVD (RR = 0.97; 95%CI: 0.95, 0.98; P<0.0001) (Figure 4 and Supplemental Figure 14), CHD (RR = 0.96; 95%CI: 0.93, 0.98; P = 0.0013) (Figure 4 and Supplemental Figure 16), and stroke (RR = 0.97; 95%CI: 0.95, 0.99; P = 0.0023) (Figure 4 and Supplemental Figure 18). The nonlinear dose–response meta-analysis demonstrated an L-shaped association of cheese consumption with the risk of overall CVD, CHD, and stroke, leveling off at ∼40 g/d (all P-nonlinearity<0.001) (Figure 6A–C). Cheese consumption was not associated with hypertension risk (FIGURE 3, FIGURE 4, FIGURE 6D and Supplemental Figures 19 and 20).
FIGURE 6.
Dose–response association between cheese consumption and the risk of (A) CVD, (B) CHD, (C) stroke, and (D) hypertension. CHD, coronary heart disease; CVD, cardiovascular disease. P value was from the test of nonlinearity.
Cancer
Forty-six studies [[61], [62], [63], [64],66,98,[132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169]] comprising 158,601 cases and 9,539,703 participants were included in the updated meta-analysis for overall cancer risk in the highest compared with lowest cheese intake comparison, yielding a nonsignificant summary RR (RR = 0.99; 95% CI: 0.97, 1.01; P = 0.29; I2 = 21%) (Figure 3 and Supplemental Figure 21). Meta-analyses based on 24 studies [61,66,132,136, [139], [140], [141], 143,146,149,[152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164]] also showed that each 30-g/d increment in cheese intake was not related to overall cancer risk (RR = 1.00, 95% CI: 0.97, 1.04; P = 0.79; I2 = 29%) (Figure 4 and Supplemental Figure 22). Concerning the risk of site-specific cancer and its subtypes, cheese consumption was not associated with the risk of total and advanced prostate cancer (Figure 3 and Supplemental Figure 23); colorectal cancer (FIGURE 3, FIGURE 4 and Supplemental Figures 25 and 26); total, proximal and distal colon cancer, and rectal cancer (Figure 3 and Supplemental Figure 27); total and estrogen receptor-positive (ER+) breast cancer (FIGURE 3, FIGURE 4 and Supplemental Figures 28 and 29); and other cancers (Figure 3 and Supplemental Figure 30) but was inversely associated with estrogen receptor-negative (ER−) breast cancer (RR = 0.89, 95% CI: 0.82, 0.97; P = 0.01; I2 = 0%) (Figure 3 and Supplemental Figure 28C). Linear dose–response meta-analysis of 7 studies suggested a marginally positive association between cheese consumption and prostate cancer risk (per 30-g/d increase: RR = 1.06; 95% CI: 1.00, 1.11; P = 0.051; I2 = 0%) (Figure 4 and Supplemental Figure 24). No nonlinear associations were observed between cheese consumption and the risk of overall cancer, prostate cancer, colorectal cancer, and breast cancer (Supplemental Figure 42A–D). In addition, a previous meta-analysis of 2 studies reported that cheese consumption was not associated with the risk of precursors of colorectal cancer (Figure 3).
Metabolic disease
Our updated meta-analysis of 25 studies [80,89,95,112,[170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185], [186]] enrolling 674,107 participants and 44,584 cases observed that cheese consumption was inversely associated with type 2 diabetes (T2D) risk (RR = 0.93; 95% CI: 0.88, 0.98, P = 0.009; I2 = 45%) (Figure 3 and Supplemental Figure 31) when comparing the highest with lowest levels of intake. However, the dose–response meta-analyses did not support the inverse association between cheese consumption and T2D risk (Figure 4 and Supplemental Figures 32 and 43A). The de novo meta-analysis of 3 studies [[186], [187], [188]] with 2,851 cases among 10,899 participants showed no association of cheese consumption with prediabetes risk (highest compared with lowest: RR = 0.93; 95% CI: 0.78, 1.12; P = 0.45; I2 = 66%) (FIGURE 3, FIGURE 4 and Supplemental Figures 33 and 34). Similarly, cheese consumption was not associated with metabolic syndrome risk in the updated meta-analysis of 4 studies [[123], [181], [189], [190]] (Figure 3 and Supplemental Figure 35A) and overweight/obesity risk in the meta-analysis of 2 studies [191,192] (Figure 3 and Supplemental Figure 35B).
Aging-related disease
The updated meta-analysis of 7 studies [88,[193], [194], [195], [196]] for cheese consumption (the highest compared with lowest intake) and total fracture risk yielded a summary RR of 0.90 (95% CI: 0.86, 0.95; P < 0.0001; I2 = 0%) (Figure 3 and Supplemental Figure 36). The meta-analysis of 4 studies showed that the risk of total fracture decreased by 5% for each 30-g/d cheese increment (RR = 0.95, 95% CI: 0.93, 0.97; P < 0.0001; I2 = 0%) (Figure 4 and Supplemental Figure 37). The dose–response meta-analysis showed an L-shaped association between cheese consumption and total fracture risk, where total fracture risk decreased sharply until ∼40 g/d (P-nonlinearity < 0.0001) (Supplemental Figure 43B). Null association was found between cheese consumption and hip fracture risk in the updated meta-analysis of 7 studies [88,[193], [194], [195], [196]] (RR = 0.86; 95% CI: 0.72, 1.04; P = 0.11; I2 = 71%) (Figure 3 and Supplemental Figure 38) for the highest compared with lowest intake level. However, a meta-analysis of 4 studies showed that the risk of hip fracture decreased by 14% for each 30-g/d cheese increment (RR = 0.86, 95% CI: 0.82, 0.91; P < 0.001; I2 = 0%) (Figure 4 and Supplemental Figure 39). Meanwhile, the dose–response meta-analysis revealed an L-shaped association between cheese consumption and hip fracture risk, where hip fracture risk decreased with cheese consumption and reached a plateau at ∼40 g/d (P-nonlinearity < 0.001) (Supplemental Figure 43C). The de novo meta-analysis showed no association of cheese consumption with the risk of fall (FIGURE 3, FIGURE 4 and Supplemental Figures 40A and 41) and frailty (Figure 3 and Supplemental Figure 40B). Moreover, the de novo meta-analysis of 2 studies [197,198] observed that dementia risk was 19% lower for the highest than that for lowest cheese intake (RR = 0.81; 95% CI: 0.66, 0.99; P = 0.04; I2 = 0%) (Figure 3 and Supplemental Figure 40C). In addition, cheese consumption was marginally related to an increased risk of Parkinson disease based on a previous meta-analysis of 5 studies (RR = 1.26; 95% CI: 0.99, 1.60; P = 0.052; I2 = 29%) (Figure 3).
Other health outcomes
Seventeen original articles [[199], [200], [201], [202], [203], [204], [205], [206], [207], [208], [209], [210], [211], [212], [213], [214], [215]] reported the prospective association between cheese consumption and 26 other health outcomes (Supplemental Table 9). Among them, inverse associations were found for the risk of childhood dental caries [201] (OR = 0.37; 95%CI: 0.17, 0.76), osteoporosis [205] (OR = 0.28; 95%CI: 0.12, 0.66), wheezing in infants [209] (OR = 0.51; 95%CI: 0.31, 0.85), type 1 diabetes [215] (HR = 0.55; 95%CI: 0.33, 0.94), and infant colic [210] (OR = 0.89; 95%CI: 0.79, 0.99).
Subgroup analyses
Of the 184 original studies included in the meta-analyses, 149 (81.0%) studies adjusted for total energy intake in the models, and 152 (82.6%) studies were conducted in North America, Europe, and Oceania. Among the 47 major outcomes in our study, 4 outcomes were solely based on studies without energy adjustment, 18 outcomes were only based on studies with energy adjustment, and 30 outcomes were entirely based on studies conducted in Europe, North America, and Oceania (Supplemental Tables 13–16).
Subgroup analyses indicated that the association between cheese consumption and most health outcomes remained consistent regardless of adjustment for total energy intake or geographic location (Supplemental Tables 13–16). However, heterogeneity existed in subgroups by total energy adjustment for overall and breast cancer incidence, where higher cheese consumption was linked with an increased risk of overall cancer (RR = 1.14; 95% CI: 1.01, 1.29; P = 0.0305; I2 = 0%; P-subgroup = 0.02) (Supplemental Table 14) and was marginally associated with elevated risk of breast cancer (RR = 1.43; 95% CI:0.99, 2.06; P = 0.0557; P-subgroup = 0.04) (Supplemental Table 14) in studies without adjustment for total energy intake, whereas null associations were found in studies with adjustment for total energy intake. Although heterogeneity was observed in subgroup analyses by energy adjustment for metabolic syndrome (P-subgroup = 0.03), the null association was consistent between subgroups. Meanwhile, an inverse association was detected for CHD mortality (RR: 0.67; 95% CI: 0.51, 0.89; P-subgroup = 0.03) (Supplemental Table 15) in studies conducted in Asia and other regions but not in studies conducted in North America, Europe, and Oceania.
Evidence credibility
The credibility of the identified associations with cheese consumption is summarized in Supplemental Tables 10 and 11, and the detailed NutriGrade scores for each meta-analysis are presented in Supplemental Table 17. No health outcome met the standards for high meta-evidence. Eight health outcomes (17%)—death from any cause, cancer, and CVD and incidence of overall CVD, CHD, stroke, hypertension, and prostate cancer—presented moderate meta-evidence. Twenty-two health outcomes (47%)—site-specific cancer mortality (the colorectum, colon, rectum, lung, and stomach), CHD mortality, overall and site-specific cancer (colorectum, total and distal colon, rectum, total, ER+ and ER− breast, bladder, and pancreas), T2D, overweight/obesity, total and hip fractures, fall, and dementia—presented low meta-evidence. The rest health outcomes indicated very low meta-evidence.
Discussion
This umbrella review provides a broad overview of the existing evidence on the association between cheese consumption and 47 unique outcomes through 35 updated, 4 de novo, and 8 previous meta-analyses based on 184 prospective observational studies from 145 primary articles. Moderate quality of evidence showed that cheese consumption was associated with reduced risk of all-cause mortality, CVD mortality, and incident CVD, CHD, and stroke but not related to the risk of cancer mortality, hypertension, and prostate cancer. Low quality of evidence was observed for inverse associations of cheese intake with incidence of ER− breast cancer, T2D, total fracture, and dementia and null association with site-specific cancer mortality (i.e., colorectum, colon, rectum, lung, and stomach), CHD mortality, and incidence of overall, site-specific cancer and its subtypes (i.e., colorectum, total and distal colon, rectum, total and ER+ breast, bladder, and pancreas), overweight/obesity, hip fracture, and fall. The nonlinear dose–response analyses additionally suggested a U-shaped association between cheese consumption and the risk of all-cause mortality and cardiovascular mortality and an L-shaped association with the risk of overall CVD, CHD, stroke, and total and hip fractures with the optimal intake at ∼40 g/d.
Although cheese is theorized to have detrimental effects on blood pressure and blood lipid profile based on its high sodium and saturated fat contents, a moderate quality of evidence suggest that cheese consumption does not increase the risk of cardiovascular diseases and may even have protective associations with overall CVD, CHD, and stroke incidence and cardiovascular and all-cause mortality in our updated meta-analyses. The inverse associations are in line with findings from most previous meta-analyses [13,36,37,39,40,42]. In terms of the nonlinear analysis, 1 meta-analysis in 2016 reported an L-shaped association with the risk of stroke leveling off at 25 g/d of cheese intake [41]. Still, another meta-analysis in 2017 derived a U-shaped association with the lowest CVD risk at 40 g/d, which was consistent with the findings from our study [36]. Regarding cheese intake and hypertension, a null association with moderate quality of evidence was observed in our study, which was in line with previous studies [[43], [44], [45]]. In subgroup analyses, an inverse association between cheese consumption and CHD mortality was only notable in Asian populations but not in European and American populations, which may be attributable to the differences in the amount and patterns of cheese consumption among different regions [43].
Results from pervious meta-analyses [31,57,59,60,216] and large prospective cohort studies [217,218] have raised the concern regarding high consumption of dairy products (particularly whole milk) increasing the incidence and mortality of several cancers, for example, prostate, breast, ovarian, and liver cancers and lymphoma. By contrast, cheese consumption has been reported to be inversely related to the risk of colorectal cancer, breast cancer, and prostate cancer in earlier meta-analyses including both prospective studies and case–control studies [46,51,58]. However, our latest meta-analyses of prospective observational studies found null associations between cheese consumption and overall and site-specific cancer incidence and mortality, consistent with previous meta-analyses for overall cancer incidence and mortality [14,30,31] and colorectal cancer [32,53]. Of note, total energy intake is a crucial confounder in the association between cheese consumption and cancer risk. Failure to adjust for total energy intake in the analyses could lead to spurious conclusions, such as in the case that higher cheese consumption was associated with an elevated risk of overall and breast cancer when not controlling total energy intake. The quality of evidence was moderate for null associations with total cancer mortality and incident prostate cancer and low for null associations with mortality from colorectal, colon, rectal, lung, and gastric cancers and incidence of overall, colorectal, colon (total and distal), rectal, breast (total and ER+), bladder, and pancreatic cancers. In addition, low-quality evidence revealed an inverse association between cheese intake and ER− breast cancer incidence, which was driven by a protective association of cottage/ricotta cheese consumption rather than hard cheese consumption with ER− breast cancer risk in a pooled analysis of 21 cohort studies [62]. The findings for prostate cancer incidence are also warranted to be confirmed in large-scale, long-term, prospective cohort studies because our linear dose–response meta-analysis of 7 cohort studies suggested a borderline positive association between cheese consumption and prostate cancer risk.
We found a low quality of evidence for an inverse association between cheese consumption and T2D risk in the highest compared with that of the lowest intake, which is in accordance with previous meta-analyses [67,68]. Substitution analysis demonstrated that replacing red and processed meat (per 50 g/d) with cheese (per 30 g/d) was associated with 10% decreased risk of T2D [219]. However, our linear and nonlinear dose–response analyses did not find significant associations, which was consistent with the most recent dose–response meta-analysis [220]. Inconsistently, a meta-analysis published in 2013 showed that per 50-g cheese/d was associated with 8% reduced risk of T2D, and there was a marginal nonlinear association between cheese consumption and T2D risk, with a reduction ≤50 g/d [15]. More studies are needed to clarify the discrepancy between categorized and dose–response analyses.
Dairy products are rich in calcium, magnesium, phosphorus, and protein, which are essential for good bone health [221,222]. Nevertheless, the role of dairy intake in preventing bone fractures remains debated [223]. Previous meta-analyses reported both inverse and null associations between cheese intake and the risk of hip fracture [[73], [74], [75]] and fracture at any site [73]. Our updated meta-analyses including only prospective studies supported a favorable association of cheese intake with total fracture risk in the highest compared with that of the lowest intake and with hip fracture risk per 30-g/d increase in cheese consumption. Nonlinear dose–response analyses showed an L-shaped association between cheese consumption and total and hip fracture risk, leveling off at ∼40 g/d. Given that the quality of evidence was low, further research is warranted to confirm these findings.
Additionally, low quality of evidence also showed that higher cheese intake was associated with a lower dementia risk in our de novo meta-analysis of 2 prospective cohort studies [197,198]. The beneficial association was supported by previous randomized controlled crossover trial [224] and observational studies [225,226], suggesting that cheese consumption may improve cognitive function.
The protective association of cheese consumption with mortality, CVD, bone fracture, and dementia may be attributed to the abundance of nutrients, bioactive compounds, and probiotics in cheese. Dairy products, especially cheese, are a predominant dietary source of vitamin K2 in many regions [227,228]. Vitamin K2 can improve cardiovascular health by inhibiting and reversing vascular calcification [229,230], reduce age-related bone loss through promoting the γ-carboxylation of osteocalcin and increasing osteoprotegerin [230], and maintain neurocognitive functions through contributing to the biological activation of proteins Gas6 and protein S and the synthesis of sphingolipids [231]. Probiotic bacteria in cheese may also interact with the gut microbiome [232], exerting various health enhancing functions [233]. Additionally, the cheese matrix can mitigate the harmful effects of saturated fat and sodium [[234], [235], [236], [237]]. Besides the components of cheese itself (i.e., protein or specific micronutrients), the observed inverse associations could also be owing to the fact that increased cheese intake may replace consumption of other foods (e.g., processed/red meat and refined carbohydrates) that have been consistently associated with higher risk of incidence or mortality from chronic diseases [[238], [239], [240]] because the studies adjusting for total energy intake hold calories constant, as in isocaloric intervention trials.
It is noteworthy that a borderline positive association between cheese intake and Parkinson disease risk was observed in a previous meta-analysis of 5 cohort studies, which accords with findings from the latest meta-analysis on total dairy and milk [241] and 1 recent prospective study on low-fat dairy foods (including cottage cheese and low-fat cheese) [242]. If causal, suggested mechanisms include reduction on uric acid by dairy proteins and the inhibition of calcium and phosphate in dairy products on the formation of 1,25(OH)2D3 (1,25-dihydroxy-vitamin D3 = calcitriol) because urate and 1,25(OH)2D3 may protect against Parkinson’s disease [242,243]. However, the quality of the meta-evidence was very low, and inconsistent results were observed for cheese and other fermented dairy products in some prospective studies [244,245]. Accordingly, the findings should be interpreted with caution and validated by further studies.
Strengths and limitations
This umbrella review provides the most recent evidence from prospective observational studies on the association between cheese intake and a wide range of health outcomes. Different from traditional umbrella reviews focusing only on published meta-analyses, we thoroughly and systematically resynthesized the available evidence by incorporating newly identified prospective studies into prospective studies included in previous meta-analyses. On one hand, we updated those outdated meta-analyses to reflect up-to-date conclusions with more statistical power. On the other hand, we performed de novo meta-analyses for specific health outcomes without previous meta-analyses despite enough published original studies to include as many potentially related health outcomes as possible. Furthermore, dose–response analyses were conducted to reveal further the linear and nonlinear association between cheese intake and multiple health outcomes, thereby determining the optimal consumption level of cheese.
There are also some limitations in our research. Given that the original studies included in this review are all observational, some of their inherent limitations could not be excluded, such as residual confounding and reverse causality. Besides, the updated meta-analyses for some health outcomes are highly heterogeneous (I2 ≥ 50%), probably due to the inclusion of original studies involving different populations. Also, caution should be taken when generalizing the conclusions to populations with different genetic backgrounds and dietary habits because most primary studies included were conducted in Europe and North America. Moreover, different types of cheese also vary a lot in dairy matrix and nutrient content like fat and sodium, which may deliver divergent health effects. However, the lack of information on cheese type deterred finer stratified analyses by cheese type. Finally, the limited number of prospective observational studies in meta-analyses for cheese consumption and specific health outcomes—such as cancer at sites other than colorectum, breast, and prostate, overweight/obesity, dementia, fall, and frailty—leads to insufficient statistical power and low credibility of evidence. Thus, further large-scale prospective studies are warranted to ascertain the association of cheese intake with these health outcomes.
Conclusions
Our results indicate that cheese consumption has neutral to moderate benefits for human health, particularly ≥40 g/d, with a moderate quality of evidence for inverse associations with all-cause and CVD mortality and overall CVD, CHD, and stroke incidences. Null associations were observed with cancer mortality, hypertension, and prostate cancer incidence. Although high saturated fat and sodium in some cheeses tend to be emphasized as a health concern in dietary guidelines, cheese also provides some nutrients and bioactive compounds, which potentially may confer some benefits. Environmental effects of cheese production should also be considered.
Author contribution
The authors’ responsibilities were as follows—AF, YW: conceived the study; AF: designed the study; MZ, XD, ZH: performed the literature search and screening and extracted the data; MZ, XD: conducted the data analyses and drafted the manuscript; AF: revised the manuscript; ELG, XL: critically reviewed the manuscript; AF, ELG: had primary responsibility for final content; and all authors: contributed substantially to the interpretation of the data and read and approved the final manuscript.
Conflicts of interest
The authors report no conflicts of interest.
Data availability
All data included in this umbrella review were extracted from publicly available systematic reviews and original studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2023.06.007.
Contributor Information
Yingyao Wang, Email: wyy@cnsoc.org.
Aiping Fang, Email: fangaip@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.He F.J., MacGregor G.A. Role of salt intake in prevention of cardiovascular disease: controversies and challenges. Nat. Rev. Cardiol. 2018;15(6):371–377. doi: 10.1038/s41569-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 2.Givens D.I. Saturated fats, dairy foods and cardiovascular health: no longer a curious paradox? Nutr. Bull. 2022;47(4):407–422. doi: 10.1111/nbu.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtenstein A.H., Appel L.J., Vadiveloo M., Hu F.B., Kris-Etherton P.M., Rebholz C.M., et al. 2021 Dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2021;144(23):e472–e487. doi: 10.1161/CIR.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 4.Phillips J.A. Dietary guidelines for Americans. Workplace Health Saf. 2021;69(8):395. doi: 10.1177/21650799211026980. 2020-2025. [DOI] [PubMed] [Google Scholar]
- 5.EFSA Panel on Dietetic Products, Nutrition, and Allergies, Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010;8(3):1461. [Google Scholar]
- 6.World Health Organization . 2020. Healthy diet.https://www.who.int/news-room/fact-sheets/detail/healthy-diet [Internet] [cited 2022 Jul 23]. Available from: [Google Scholar]
- 7.Mozaffarian D. Dairy foods, obesity, and metabolic health: the role of the food matrix compared with single nutrients. Adv. Nutr. 2019;10(5):917s–923s. doi: 10.1093/advances/nmz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorning T.K., Bertram H.C., Bonjour J.P., de Groot L., Dupont D., Feeney E., et al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am. J. Clin. Nutr. 2017;105(5):1033–1045. doi: 10.3945/ajcn.116.151548. [DOI] [PubMed] [Google Scholar]
- 9.Geiker N.A.-O., Mølgaard C.A.-O., Iuliano S., Rizzoli R., Manios Y., van Loon L.A.-O., et al. Impact of whole dairy matrix on musculoskeletal health and aging-current knowledge and research gaps. Osteoporos Int. 2020;31(4):601–615. doi: 10.1007/s00198-019-05229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godos J., Tieri M., Ghelfi F., Titta L., Marventano S., Lafranconi A., et al. Dairy foods and health: an umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020;71(2):138–151. doi: 10.1080/09637486.2019.1625035. [DOI] [PubMed] [Google Scholar]
- 11.Papatheodorou S. Umbrella reviews: what they are and why we need them. Eur. J. Epidemiol. 2019;34(6):543–546. doi: 10.1007/s10654-019-00505-6. [DOI] [PubMed] [Google Scholar]
- 12.O'Sullivan T.A., Hafekost K., Mitrou F., Lawrence D. Food sources of saturated fat and the association with mortality: a meta-analysis. Am. J. Public Health. 2013;103(9):e31–e42. doi: 10.2105/AJPH.2013.301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K., Chen X., Zhang L., Deng Z. Fermented dairy foods intake and risk of cardiovascular diseases: a meta-analysis of cohort studies. Crit. Rev. Food Sci. Nutr. 2020;60(7):1189–1194. doi: 10.1080/10408398.2018.1564019. [DOI] [PubMed] [Google Scholar]
- 14.Zhang K., Dai H., Liang W., Zhang L., Deng Z. Fermented dairy foods intake and risk of cancer. Int. J. Cancer. 2019;144(9):2099–2108. doi: 10.1002/ijc.31959. [DOI] [PubMed] [Google Scholar]
- 15.Aune D., Norat T., Romundstad P., Vatten L.J. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013;98(4):1066–1083. doi: 10.3945/ajcn.113.059030. [DOI] [PubMed] [Google Scholar]
- 16.Ong A.M., Kang K., Weiler H.A., Morin S.N. Fermented milk products and bone health in postmenopausal women: a systematic review of randomized controlled trials, prospective cohorts, and case-control studies. Adv. Nutr. 2020;11(2):251–265. doi: 10.1093/advances/nmz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang W., Ju C., Jiang H., Zhang D. Dairy foods intake and risk of Parkinson’s disease: a dose–response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2014;29(9):613–619. doi: 10.1007/s10654-014-9921-4. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 19.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017:358. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwingshackl L.A.-O., Knüppel S., Schwedhelm C., Hoffmann G., Missbach B., Stelmach-Mardas M., et al. Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv. Nutr. 2016;7(6):994–1004. doi: 10.3945/an.116.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R., Laird N. Meta-analysis in clinical trials, Control. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Schwarzer G., Carpenter J.R., Rücker G. In: Meta-Analysis With R. Schwarzer G., Carpenter J.R., Rücker G., editors. Springer International Publishing; Cham: 2015. Fixed effect and random effects meta-analysis; pp. 21–53. [Google Scholar]
- 23.Higgins J.P., Thompson S.G., Spiegelhalter D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009;172(1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannidis J.P., Trikalinos T.A. An exploratory test for an excess of significant findings. Clin. Trials. 2007;4(3):245–253. doi: 10.1177/1740774507079441. [DOI] [PubMed] [Google Scholar]
- 26.Orsini N., Li R., Wolk A., Khudyakov P., Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desquilbet L., Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010;29(9):1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 28.Guo J., Astrup A., Lovegrove J.A., Gijsbers L., Givens D.I., Soedamah-Muthu S.S. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose–response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2017;32(4):269–287. doi: 10.1007/s10654-017-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong X., Chen G.C., Zhang Z., Wei Y.L., Xu J.Y., Qin L.Q. Cheese consumption and risk of all-cause mortality: a meta-analysis of prospective studies. Nutrients. 2017;9(1):63. doi: 10.3390/nu9010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu W., Chen H., Niu Y., Wu H., Xia D., Wu Y. Dairy products intake and cancer mortality risk: a meta-analysis of 11 population-based cohort studies. Nutr. J. 2016;15(1):91. doi: 10.1186/s12937-016-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin S., Je Y. Dairy Consumption and total cancer and cancer-specific mortality: a meta-analysis of prospective cohort studies. Adv. Nutr. 2021;13(4):1063–1082. doi: 10.1093/advances/nmab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin S., Kim Y., Je Y. Dairy consumption and risks of colorectal cancer incidence and mortality: a meta-analysis of prospective cohort studies. Cancer Epidemiol. Biomarkers Prev. 2020;29(11):2309–2322. doi: 10.1158/1055-9965.EPI-20-0127. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y., Wang X., Yao Q., Qin L., Xu C. Dairy product, calcium intake and lung cancer risk: a systematic review with meta-analysis. Sci. Rep. 2016;6 doi: 10.1038/srep20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y., Lin L.J., Sang L.X., Dai C., Jiang M., Zheng C.Q. Dairy product consumption and gastric cancer risk: a meta-analysis. World J. Gastroenterol. 2014;20(42):15879–15898. doi: 10.3748/wjg.v20.i42.15879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander D.D., Bylsma L.C., Vargas A.J., Cohen S.S., Doucette A., Mohamed M., et al. Dairy consumption and CVD: a systematic review and meta-analysis. Br. J. Nutr. 2016;115(4):737–750. doi: 10.1017/S0007114515005000. [DOI] [PubMed] [Google Scholar]
- 36.Chen G.C., Wang Y., Tong X., Szeto I.M.Y., Smit G., Li Z.N., et al. Cheese consumption and risk of cardiovascular disease: a meta-analysis of prospective studies. Eur. J. Nutr. 2017;56(8):2565–2575. doi: 10.1007/s00394-016-1292-z. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z., Ahmed M., Ha V., Jefferson K., Malik V., Ribeiro P.A.B., et al. Dairy product consumption and cardiovascular health: a systematic review and meta-analysis of prospective cohort studies. Adv. Nutr. 2021;13(2):439–454. doi: 10.1093/advances/nmab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gholami F., Khoramdad M., Shakiba E., Alimohamadi Y., Shafiei J., Firouzi A. Subgroup dairy products consumption on the risk of stroke and CHD: a systematic review and meta-analysis. Med. J. Islam Repub. Iran. 2017;31:25. doi: 10.18869/mjiri.31.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jakobsen M.U., Trolle E., Outzen M., Mejborn H., Grønberg M.G., Lyndgaard C.B., et al. Intake of dairy products and associations with major atherosclerotic cardiovascular diseases: a systematic review and meta-analysis of cohort studies. Sci. Rep. 2021;11(1):1303. doi: 10.1038/s41598-020-79708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin L.Q., Xu J.Y., Han S.F., Zhang Z.L., Zhao Y.Y., Szeto I.M. Dairy consumption and risk of cardiovascular disease: an updated meta-analysis of prospective cohort studies, Asia Pac. J. Clin. Nutr. 2015;24(1):90–100. doi: 10.6133/apjcn.2015.24.1.09. [DOI] [PubMed] [Google Scholar]
- 41.de Goede J., Soedamah-Muthu S.S., Pan A., Gijsbers L., Geleijnse J.M. Dairy consumption and risk of stroke: a systematic review and updated dose-response meta-analysis of prospective cohort studies. J. Am. Heart. Assoc. 2016;5(5) doi: 10.1161/JAHA.115.002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu D., Huang J., Wang Y., Zhang D., Qu Y. Dairy foods and risk of stroke: a meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2014;24(5):460–469. doi: 10.1016/j.numecd.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Heidari Z., Rashidi Pour Fard N., Clark C.C.T., Haghighatdoost F. Dairy products consumption and the risk of hypertension in adults: an updated systematic review and dose–response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2021;31(7):1962–1975. doi: 10.1016/j.numecd.2021.02.033. [DOI] [PubMed] [Google Scholar]
- 44.Ralston R.A., Lee J.H., Truby H., Palermo C.E., Walker K.Z. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J. Hum. Hypertens. 2012;26(1):3–13. doi: 10.1038/jhh.2011.3. [DOI] [PubMed] [Google Scholar]
- 45.Soedamah-Muthu S.S., Verberne L.D.M., Ding E.L., Engberink M.F., Geleijnse J.M. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension. 2012;60(5):1131–1137. doi: 10.1161/HYPERTENSIONAHA.112.195206. [DOI] [PubMed] [Google Scholar]
- 46.Boyd N.F., Martin L.J., Noffel M., Lockwood G.A., Tritchler D.L. A meta-analysis of studies of dietary fat and breast cancer risk. Br. J. Cancer. 1993;68(3):627–636. doi: 10.1038/bjc.1993.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kazemi A., Barati-Boldaji R., Soltani S., Mohammadipoor N., Esmaeilinezhad Z., Clark C.C.T., et al. Intake of various food groups and risk of breast cancer: a systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2021;12(3):809–849. doi: 10.1093/advances/nmaa147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li F., An S.L., Zhou Y., Liang Z.K., Jiao Z.J., Jing Y.M., et al. Milk and dairy consumption and risk of bladder cancer: a meta-analysis. Urology. 2011;78(6):1298–1305. doi: 10.1016/j.urology.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Ralston R.A., Truby H., Palermo C.E., Walker K.Z. Colorectal cancer and nonfermented milk, solid cheese, and fermented milk consumption: a systematic review and meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2014;54(9):1167–1179. doi: 10.1080/10408398.2011.629353. [DOI] [PubMed] [Google Scholar]
- 50.Vieira A.R., Abar L., Chan D.S.M., Vingeliene S., Polemiti E., Stevens C., et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017;28(8):1788–1802. doi: 10.1093/annonc/mdx171. [DOI] [PubMed] [Google Scholar]
- 51.Barrubés L., Babio N., Becerra-Tomás N., Rosique-Esteban N., Salas-Salvadó J. Association between dairy product consumption and colorectal cancer risk in adults: a systematic review and meta-analysis of epidemiologic studies. Adv. Nutr. 2019;10(suppl_2):S190–S211. doi: 10.1093/advances/nmy114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aune D., Lau R., Chan D.S.M., Vieira R., Greenwood D.C., Kampman E., et al. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann. Oncol. 2012;23(1):37–45. doi: 10.1093/annonc/mdr269. [DOI] [PubMed] [Google Scholar]
- 53.Liang Z., Song X., Hu J., Wu R., Li P., Dong Z., et al. Fermented dairy food intake and risk of colorectal cancer: a systematic review and meta-analysis. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.812679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X., Zhao J., Li P., Gao Y. Dairy products intake and endometrial cancer risk: a meta-analysis of observational studies. Nutrients. 2018;10(1):25. doi: 10.3390/nu10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y., Zhou J., Yang Y., Chen Z., Zheng X. Systematic review and meta-analysis: dairy consumption and hepatocellular carcinoma risk. J. Public Health. 2017;25(6):591–599. [Google Scholar]
- 56.Khodavandi A., Alizadeh F., Razis A.F.A. Association between dietary intake and risk of ovarian cancer: a systematic review and meta-analysis. Eur. J. Nutr. 2021;60(4):1707–1736. doi: 10.1007/s00394-020-02332-y. [DOI] [PubMed] [Google Scholar]
- 57.Liao M.Q., Gao X.P., Yu X.X., Zeng Y.F., Li S.N., Naicker N., et al. Effects of dairy products, calcium and vitamin D on ovarian cancer risk: a meta-analysis of twenty-nine epidemiological studies. Br. J. Nutr. 2020;124(10):1001–1112. doi: 10.1017/S0007114520001075. [DOI] [PubMed] [Google Scholar]
- 58.Aune D., Navarro Rosenblatt D.A., Chan D.S.M., Vieira A.R., Vieira R., Greenwood D.C., et al. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am. J. Clin. Nutr. 2015;101(1):87–117. doi: 10.3945/ajcn.113.067157. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Z., Wu D., Gao S., Zhou D., Zeng X., Yao Y., et al. The association between dairy products consumption and prostate cancer risk: a systematic review and meta-analysis. Br. J. Nutr. 2022:1–43. doi: 10.1017/S0007114522002380. [DOI] [PubMed] [Google Scholar]
- 60.Wang J., Li X., Zhang D. Dairy product consumption and risk of non-Hodgkin lymphoma: a meta-analysis. Nutrients. 2016;8(3):120. doi: 10.3390/nu8030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acham M., Wesselius A., van Osch F.H.M., Yu E.Y., van den Brandt P.A., White E., et al. Intake of milk and other dairy products and the risk of bladder cancer: a pooled analysis of 13 cohort studies. Eur. J. Clin. Nutr. 2020;74(1):28–35. doi: 10.1038/s41430-019-0453-6. [DOI] [PubMed] [Google Scholar]
- 62.Wu Y., Huang R., Wang M., Bernstein L., Bethea T.N., Chen C., et al. Dairy foods, calcium, and risk of breast cancer overall and for subtypes defined by estrogen receptor status: a pooled analysis of 21 cohort studies. Am. J. Clin. Nutr. 2021;114(2):450–461. doi: 10.1093/ajcn/nqab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Genkinger J.M., Hunter D.J., Spiegelman D., Anderson K.E., Arslan A., Beeson W.L., et al. Dairy products and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev. 2006;15(2):364–372. doi: 10.1158/1055-9965.EPI-05-0484. [DOI] [PubMed] [Google Scholar]
- 64.Cho E., Smith-Warner S.A., Spiegelman D., Beeson W.L., van den Brandt P.A., Colditz G.A., et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J. Natl. Cancer Inst. 2004;96(13):1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 65.Guo L.L., Li Y.T., Yao J., Wang L.S., Chen W.W., He K.Y., et al. Dairy consumption and risk of conventional and serrated precursors of colorectal cancer: a systematic review and meta-analysis of observational studies. J. Oncol. 2021;2021 doi: 10.1155/2021/9948814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arafa A., Eshak E.S., Dong J.Y., Shirai K., Muraki I., Iso H., et al. Dairy intake and the risk of pancreatic cancer: the JACC Study and meta-analysis of prospective cohort studies. Br. J. Nutr. 2021;128(6):1147–1155. doi: 10.1017/S0007114521004232. [DOI] [PubMed] [Google Scholar]
- 67.Fan M., Li Y., Wang C., Mao Z., Zhou W., Zhang L., et al. Dietary protein consumption and the risk of type 2 diabetes: a dose-response meta-analysis of prospective studies. Nutrients. 2019;11(11):2783. doi: 10.3390/nu11112783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao D., Ning N., Wang C., Wang Y., Li Q., Meng Z., et al. Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response meta-analysis. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gijsbers L., Ding E.L., Malik V.S., De Goede J., Geleijnse J.M., Soedamah-Muthu S.S. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016;103(4):1111–1124. doi: 10.3945/ajcn.115.123216. [DOI] [PubMed] [Google Scholar]
- 70.Khoramdad M., Rahimi M., Cheraghi Z., Izadi N., Alimohamadi Y., Firouzi A., et al. The effect of dairy products subgroups consumption on the risk of diabetes: a systematic review and meta-analysis. Iranian Red Crescent. Med. 2017;19(3) [Google Scholar]
- 71.Jin S., Je Y. Dairy consumption and risk of metabolic syndrome: results from korean population and meta-analysis. Nutrients. 2021;13(5):1574. doi: 10.3390/nu13051574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Babio N., Becerra-Tomás N., Nishi S.K., López-González L., Paz-Graniel I., García-Gavilán J., et al. Total dairy consumption in relation to overweight and obesity in children and adolescents: a systematic review and meta-analysis. Obes. Rev. 2022;23(S1) doi: 10.1111/obr.13400. [DOI] [PubMed] [Google Scholar]
- 73.Matía-Martín P., Torrego-Ellacuría M., Larrad-Sainz A., Fernández-Pérez C., Cuesta-Triana F., Rubio-Herrera M.A. Effects of milk and dairy products on the prevention of osteoporosis and osteoporotic fractures in Europeans and non-Hispanic Whites from North America: a systematic review and updated meta-analysis. Adv. Nutr. 2019;10(2):S120–S143. doi: 10.1093/advances/nmy097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bian S., Hu J., Zhang K., Wang Y., Yu M., Ma J. Dairy product consumption and risk of hip fracture: a systematic review and meta-analysis. BMC Public Health. 2018;18(1):165. doi: 10.1186/s12889-018-5041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hidayat K., Du X., Shi B.M., Qin L.Q. Systematic review and meta-analysis of the association between dairy consumption and the risk of hip fracture: critical interpretation of the currently available evidence. Osteoporos Int. 2020;31(8):1411–1425. doi: 10.1007/s00198-020-05383-3. [DOI] [PubMed] [Google Scholar]
- 76.Arafa A., Eshak E.S., Dong J.Y., Shirai K., Muraki I., Iso H., et al. Dairy intake and the risk of pancreatic cancer: the Japan Collaborative Cohort Study (JACC Study) and meta-analysis of prospective cohort studies. Br. J. Nutr. 2021;128(6):1147–1155. doi: 10.1017/S0007114521004232. [DOI] [PubMed] [Google Scholar]
- 77.Cruijsen E., Jacobo Cejudo M.G., Küpers L.K., Busstra M.C., Geleijnse J.M. Dairy consumption and mortality after myocardial infarction: a prospective analysis in the Alpha Omega Cohort. Am. J. Clin. Nutr. 2021;114(1):59–69. doi: 10.1093/ajcn/nqab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu Y., Sugawara Y., Matsuyama S., Fukao A., Tsuji I. Association of dairy intake with all-cause, cancer, and cardiovascular disease mortality in Japanese adults: a 25-year population-based cohort. Eur. J. Nutr. 2022;61(3):1285–1297. doi: 10.1007/s00394-021-02734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sonestedt E., Borné Y., Wirfält E., Ericson U. Dairy consumption, lactase persistence, and mortality risk in a cohort from southern Sweden. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.779034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dekker L.H., Vinke P.C., Riphagen I.J., Minović I., Eggersdorfer M.L., van den Heuvel E., et al. Cheese and healthy diet: associations with incident cardio-metabolic diseases and all-cause mortality in the general population. Front. Nutr. 2019;6:185. doi: 10.3389/fnut.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding M., Li J., Qi L., Ellervik C., Zhang X., Manson J.E., et al. Associations of dairy intake with risk of mortality in women and men: three prospective cohort studies. BMJ. 2019;367:l6204. doi: 10.1136/bmj.l6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazidi M., Mikhailidis D.P., Sattar N., Howard G., Graham I., Banach M., et al. Consumption of dairy product and its association with total and cause specific mortality—a population-based cohort study and meta-analysis. Clin. Nutr. 2019;38(6):2833–2845. doi: 10.1016/j.clnu.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 83.Pala V., Sieri S., Chiodini P., Masala G., Palli D., Mattiello A., et al. Associations of dairy product consumption with mortality in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Italy cohort. Am. J. Clin. Nutr. 2019;110(5):1220–1230. doi: 10.1093/ajcn/nqz183. [DOI] [PubMed] [Google Scholar]
- 84.Dehghan M., Mente A., Rangarajan S., Sheridan P., Mohan V., Iqbal R., et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2018;392(10161):2288–2297. doi: 10.1016/S0140-6736(18)31812-9. [DOI] [PubMed] [Google Scholar]
- 85.Farvid M.S., Malekshah A.F., Pourshams A., Poustchi H., Sepanlou S.G., Sharafkhah M., et al. Dairy food intake and all-cause, cardiovascular disease, and cancer mortality: the Golestan cohort study. Am J Epidemiol. 2017;185(8):697–711. doi: 10.1093/aje/kww139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tognon G., Nilsson L.M., Shungin D., Lissner L., Jansson J.H., Renström F., et al. Nonfermented milk and other dairy products: associations with all-cause mortality. Am. J. Clin. Nutr. 2017;105(6):1502–1511. doi: 10.3945/ajcn.116.140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Praagman J., Dalmeijer G.W., Van Der Schouw Y.T., Soedamah-Muthu S.S., Verschuren W.M.M., Bueno-de-Mesquita H.B., et al. The relationship between fermented food intake and mortality risk in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Br. J. Nutr. 2015;113(3):498–506. doi: 10.1017/S0007114514003766. [DOI] [PubMed] [Google Scholar]
- 88.Michaëlsson K., Wolk A., Langenskiöld S., Basu S., Warensjö Lemming E., Melhus H., et al. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ. 2014;349:g6015. doi: 10.1136/bmj.g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soedamah-Muthu S.S., Masset G., Verberne L., Geleijnse J.M., Brunner E.J. Consumption of dairy products and associations with incident diabetes, CHD and mortality in the Whitehall II study. Br. J. Nutr. 2013;109(4):718–726. doi: 10.1017/S0007114512001845. [DOI] [PubMed] [Google Scholar]
- 90.Goldbohm R.A., Chorus A.M.J., Garre F.G., Schouten L.J., Van Den Brandt P.A. Dairy consumption and 10-y total and cardiovascular mortality: a prospective cohort study in the Netherlands. Am. J. Clin. Nutr. 2011;93(3):615–627. doi: 10.3945/ajcn.110.000430. [DOI] [PubMed] [Google Scholar]
- 91.Bonthuis M., Hughes M.C.B., Ibiebele T.I., Green A.C., Van Der Pols J.C. Dairy consumption and patterns of mortality of Australian adults. Eur. J. Clin. Nutr. 2010;64(6):569–577. doi: 10.1038/ejcn.2010.45. [DOI] [PubMed] [Google Scholar]
- 92.Fortes C., Forastiere F., Farchi S., Rapiti E., Pastori G., Perucci C.A. Diet and overall survival in a cohort of very elderly people. Epidemiology. 2000;11(4):440–445. doi: 10.1097/00001648-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 93.Fraser G.E., Shavlik D.J. Risk factors for all-cause and coronary heart disease mortality in the oldest-old. The Adventist Health Study. Arch. Intern. Med. 1997;157(19):2249–2258. [PubMed] [Google Scholar]
- 94.Mann J.I., Appleby P.N., Key T.J., Thorogood M. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart. 1997;78(5):450–455. doi: 10.1136/hrt.78.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buziau A.M., Soedamah-Muthu S.S., Geleijnse J.M., Mishra G.D. Total fermented dairy food intake is inversely associated with cardiovascular disease risk in women. J. Nutr. 2019;149(10):1797–1804. doi: 10.1093/jn/nxz128. [DOI] [PubMed] [Google Scholar]
- 96.Liu X., Yang W., Wu K., Ogino S., Wang W., He N., et al. Postdiagnostic dairy products intake and colorectal cancer survival in US males and females. Am. J. Clin. Nutr. 2021;113(6):1636–1646. doi: 10.1093/ajcn/nqab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu Y., Zhao J., Vallis J., Shi F., Woodrow J.R., Kong Y., et al. Prediagnostic consumption of vitamin D, calcium and dairy products and colorectal cancer survival: results from the Newfoundland Colorectal Cancer Registry Cohort Study. Br. J. Nutr. 2021;128(2):290–299. doi: 10.1017/S0007114521003299. [DOI] [PubMed] [Google Scholar]
- 98.Park Y., Mitrou P.N., Kipnis V., Hollenbeck A., Schatzkin A., Leitzmann M.F. Calcium, dairy foods, and risk of incident and fatal prostate cancer: the NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2007;166(11):1270–1279. doi: 10.1093/aje/kwm268. [DOI] [PubMed] [Google Scholar]
- 99.Sakauchi F., Khan M.M., Mori M., Kubo T., Fujino Y., Suzuki S., et al. Dietary habits and risk of ovarian cancer death in a large-scale cohort study (JACC study) in Japan. Nutr. Cancer. 2007;57(2):138–145. doi: 10.1080/01635580701274178. [DOI] [PubMed] [Google Scholar]
- 100.Tokui N., Yoshimura T., Fujino Y., Mizoue T., Hoshiyama Y., Yatsuya H., et al. Dietary habits and stomach cancer risk in the JACC Study. J. Epidemiol. 2005;15(Suppl 2):S98–S108. doi: 10.2188/jea.15.S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khan M.M., Goto R., Kobayashi K., Suzumura S., Nagata Y., Sonoda T., et al. Dietary habits and cancer mortality among middle aged and older Japanese living in hokkaido, Japan by cancer site and sex, Asian Pac. J. Cancer Prev. 2004;5(1):58–65. [PubMed] [Google Scholar]
- 102.Kojima M., Wakai K., Tamakoshi K., Tokudome S., Toyoshima H., Watanabe Y., et al. Diet and colorectal cancer mortality: results from the Japan Collaborative Cohort Study. Nutr. Cancer. 2004;50(1):23–32. doi: 10.1207/s15327914nc5001_4. [DOI] [PubMed] [Google Scholar]
- 103.Sakauchi F., Mori M., Washio M., Watanabe Y., Ozasa K., Hayashi K., et al. Dietary habits and risk of urothelial cancer death in a large-scale cohort study (JACC Study) in Japan. Nutr. Cancer. 2004;50(1):33–39. doi: 10.1207/s15327914nc5001_5. [DOI] [PubMed] [Google Scholar]
- 104.Ozasa K., Watanabe Y., Ito Y., Suzuki K., Tomakoshi A., Seki N., et al. Dietary habits and risk of lung cancer death in a large-scale cohort study (JACC Study) in Japan by sex and smoking habit. Jpn. J. Cancer Res. 2001;92(12):1259–1269. doi: 10.1111/j.1349-7006.2001.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Breslow R.A., Graubard B.I., Sinha R., Subar A.F. Diet and lung cancer mortality: a 1987 National Health Interview Survey cohort study. Cancer Causes Control. 2000;11(5):419–431. doi: 10.1023/a:1008996208313. [DOI] [PubMed] [Google Scholar]
- 106.Mills P.K., Annegers J.F., Phillips R.L. Animal product consumption and subsequent fatal breast cancer risk among Seventh-day Adventists. Am. J. Epidemiol. 1988;127(3):440–453. doi: 10.1093/oxfordjournals.aje.a114821. [DOI] [PubMed] [Google Scholar]
- 107.Phillips R.L., Snowdon D.A. Dietary relationships with fatal colorectal cancer among Seventh-Day Adventists. J. Natl. Cancer Inst. 1985;74(2):307–317. [PubMed] [Google Scholar]
- 108.Snowdon D.A., Phillips R.L., Choi W. Diet, obesity, and risk of fatal prostate cancer. Am. J. Epidemiol. 1984;120(2):244–250. doi: 10.1093/oxfordjournals.aje.a113886. [DOI] [PubMed] [Google Scholar]
- 109.Sellem L., Srour B., Jackson K.G., Hercberg S., Galan P., Kesse-Guyot E., et al. Consumption of dairy products and CVD risk: results from the French prospective cohort NutriNet-Santé. Br. J. Nutr. 2022;127(5):752–762. doi: 10.1017/S0007114521001422. [DOI] [PubMed] [Google Scholar]
- 110.Zhang H., Zeng Y., Yang H., Hu Y., Chen W., Ying Z., et al. Familial factors, diet, and risk of cardiovascular disease: a cohort analysis of the UK Biobank. Am. J. Clin. Nutr. 2021;114(5):1837–1846. doi: 10.1093/ajcn/nqab261. [DOI] [PubMed] [Google Scholar]
- 111.Tong T.Y.N., Appleby P.N., Key T.J., Dahm C.C., Overvad K., Olsen A., et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: a prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur. Heart J. 2020;41(28):2632–2640. doi: 10.1093/eurheartj/ehaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johansson I., Esberg A., Nilsson L.M., Jansson J.H., Wennberg P., Winkvist A. Dairy product intake and cardiometabolic diseases in Northern Sweden: a 33-year prospective cohort study. Nutrients. 2019;11(2):284. doi: 10.3390/nu11020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Key T.J., Appleby P.N., Bradbury K.E., Sweeting M., Wood A., Johansson I., et al. Consumption of meat, fish, dairy products, and eggs and risk of ischemic heart disease. Circulation. 2019;139(25):2835–2845. doi: 10.1161/CIRCULATIONAHA.118.038813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koskinen T.T., Virtanen H.E.K., Voutilainen S., Tuomainen T.P., Mursu J., Virtanen J.K. Intake of fermented and non-fermented dairy products and risk of incident CHD: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2018;120(11):1288–1297. doi: 10.1017/S0007114518002830. [DOI] [PubMed] [Google Scholar]
- 115.Praagman J., Franco O.H., Ikram M.A., Soedamah-Muthu S.S., Engberink M.F., van Rooij F.J., et al. Dairy products and the risk of stroke and coronary heart disease: the Rotterdam Study. Eur. J. Nutr. 2015;54(6):981–990. doi: 10.1007/s00394-014-0774-0. [DOI] [PubMed] [Google Scholar]
- 116.Avalos E.E., Barrett-Connor E., Kritz-Silverstein D., Wingard D.L., Bergstrom J.N., Al-Delaimy W.K. Is dairy product consumption associated with the incidence of CHD? Public Health Nutr. 2013;16(11):2055–2063. doi: 10.1017/S1368980012004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patterson E., Larsson S.C., Wolk A., Åkesson A. Association between dairy food consumption and risk of myocardial infarction in women differs by type of dairy food. J. Nutr. 2013;143(1):74–79. doi: 10.3945/jn.112.166330. [DOI] [PubMed] [Google Scholar]
- 118.Larsson S.C., Virtamo J., Wolk A. Dairy consumption and risk of stroke in swedish women and men. Stroke. 2012;43(7):1775–1780. doi: 10.1161/STROKEAHA.111.641944. [DOI] [PubMed] [Google Scholar]
- 119.Sonestedt E., Wirfält E., Wallström P., Gullberg B., Orho-Melander M., Hedblad B. Dairy products and its association with incidence of cardiovascular disease: the Malmö diet and cancer cohort. Eur. J. Epidemiol. 2011;26(8):609–618. doi: 10.1007/s10654-011-9589-y. [DOI] [PubMed] [Google Scholar]
- 120.Warensjö E., Jansson J.H., Cederholm T., Boman K., Eliasson M., Hallmans G., et al. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am. J. Clin. Nutr. 2010;92(1):194–202. doi: 10.3945/ajcn.2009.29054. [DOI] [PubMed] [Google Scholar]
- 121.Larsson S.C., Männistö S., Virtanen M.J., Kontto J., Albanes D., Virtamo J. Dairy foods and risk of stroke. Epidemiology. 2009;20(3):355–360. doi: 10.1097/EDE.0b013e3181935dd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iso H., Stampfer M.J., Manson J.E., Rexrode K., Hennekens C.H., Colditz G.A., et al. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. 1999;30(9):1772–1779. doi: 10.1161/01.str.30.9.1772. [DOI] [PubMed] [Google Scholar]
- 123.Babio N., Becerra-Tomás N., Martínez-González M., Corella D., Estruch R., Ros E., et al. Consumption of yogurt, low-fat milk, and other low-fat dairy products is associated with lower risk of metabolic syndrome incidence in an elderly Mediterranean population. J. Nutr. 2015;145(10):2308–2316. doi: 10.3945/jn.115.214593. [DOI] [PubMed] [Google Scholar]
- 124.Villaverde P., Lajous M., MacDonald C.J., Fagherazzi G., Boutron-Ruault M.C., Bonnet F. Dairy product consumption and hypertension risk in a prospective French cohort of women. Nutr. J. 2020;19(1):12. doi: 10.1186/s12937-020-0527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Buendia J.R., Li Y., Hu F.B., Cabral H.J., Loring Bradlee M., Quatromoni P.A., et al. Long-term yogurt consumption and risk of incident hypertension in adults. J. Hypertens. 2018;36(8):1671–1679. doi: 10.1097/HJH.0000000000001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johansson I., Nilsson L.M., Esberg A., Jansson J.H., Winkvist A. Dairy intake revisited – Associations between dairy intake and lifestyle related cardio-metabolic risk factors in a high milk consuming population. Nutr. J. 2018;17(1):110. doi: 10.1186/s12937-018-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mirmiran P., Golzarand M., Bahadoran Z., Ataee M., Azizi F. Paradoxical association of dairy intake between men and women with the incidence of hypertension: a three-year follow up in Tehran Lipid and Glucose Study. Nutr. Diet. 2016;73(2):153–161. [Google Scholar]
- 128.Engberink M.F., Geleijnse J.M., De Jong N, Smit H.A., Kok F.J., Verschuren W.M.M. Dairy intake, blood pressure, and incident hypertension in a general dutch population. J. Nutr. 2009;139(3):582–587. doi: 10.3945/jn.108.093088. [DOI] [PubMed] [Google Scholar]
- 129.Engberink M.F., Hendriksen M.A.H., Schouten E.G., Van Rooij F.J.A., Hofman A., Witteman J.C.M., et al. Inverse association between dairy intake and hypertension: The Rotterdam Study. Am. J. Clin. Nutr. 2009;89(6):1877–1883. doi: 10.3945/ajcn.2008.27064. [DOI] [PubMed] [Google Scholar]
- 130.Wang L., Manson J.E., Buring J.E., Lee I.M., Sesso H.D. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51(4):1073–1079. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- 131.Steffen L.M., Kroenke C.H., Yu X., Pereira M.A., Slattery M.L., Van Horn L., et al. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Clin. Nutr. 2005;82(6):1169–1177. doi: 10.1093/ajcn/82.6.1169. quiz 363-4. [DOI] [PubMed] [Google Scholar]
- 132.Kurahashi N., Inoue M., Iwasaki M., Sasazuki S. Tsugane S; Japan Public Health Center-Based Prospective Study Group, Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol. Biomarkers Prev. 2008;17(4):930–937. doi: 10.1158/1055-9965.EPI-07-2681. [DOI] [PubMed] [Google Scholar]
- 133.Larsson S.C., Andersson S.O., Johansson J.E., Wolk A. Cultured milk, yogurt, and dairy intake in relation to bladder cancer risk in a prospective study of Swedish women and men. Am. J. Clin. Nutr. 2008;88(4):1083–1087. doi: 10.1093/ajcn/88.4.1083. [DOI] [PubMed] [Google Scholar]
- 134.Larsson S.C., Bergkvist L., Rutegard J., Giovannucci E., Wolk A. Calcium and dairy food intakes are inversely associated with colorectal cancer risk in the cohort of Swedish men. Am. J. Clin. Nutr. 2006;83(3):667–729. doi: 10.1093/ajcn.83.3.667. [DOI] [PubMed] [Google Scholar]
- 135.Lin J., Zhang S.M., Cook N.R., Manson J.E., Lee I.M., Buring J.E. Intakes of calcium and vitamin D and risk of colorectal cancer in women. Am. J. Epidemiol. 2005;161(8):755–764. doi: 10.1093/aje/kwi101. [DOI] [PubMed] [Google Scholar]
- 136.Marcondes L.H., Franco O.H., Ruiter R., Ikram M.A., Mulder M., Stricker B.H., et al. Animal foods and postmenopausal breast cancer risk: a prospective cohort study. Br. J. Nutr. 2019;122(5):583–591. doi: 10.1017/S0007114519000072. [DOI] [PubMed] [Google Scholar]
- 137.Mikami K., Ozasa K., Miki T., Watanabe Y., Mori M., Kubo T., et al. Dairy products and the risk of developing prostate cancer: a large-scale cohort study (JACC Study) in Japan. Cancer Med. 2021;10(20):7298–7307. doi: 10.1002/cam4.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mills P.K., Lawrence W., Beeson, Phillips R.L., Fraser G.E. Dietary habits and breast cancer incidence among Seventh-day Adventists. Cancer. 1989;64(3):582–590. doi: 10.1002/1097-0142(19890801)64:3<582::aid-cncr2820640304>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 139.Mitrou P.N., Albanes D., Weinstein S.J., Pietinen P., Taylor P.R., Virtamo J., et al. A prospective study of dietary calcium, dairy products and prostate cancer risk (Finland) Int. J. Cancer. 2007;120(11):2466–2473. doi: 10.1002/ijc.22553. [DOI] [PubMed] [Google Scholar]
- 140.Pala V., Krogh V., Berrino F., Sieri S., Grioni S., Tjonneland A., et al. Meat, eggs, dairy products, and risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am. J. Clin. Nutr. 2009;90(3):602–612. doi: 10.3945/ajcn.2008.27173. [DOI] [PubMed] [Google Scholar]
- 141.Allen N.E., Key T.J., Appleby P.N., Travis R.C., Roddam A.W., Tjonneland A., et al. Animal foods, protein, calcium and prostate cancer risk: the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer. 2008;98(9):1574–1581. doi: 10.1038/sj.bjc.6604331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Genkinger J.M., Wang M., Li R., Albanes D., Anderson K.E., Bernstein L., et al. Dairy products and pancreatic cancer risk: a pooled analysis of 14 cohort studies. Ann. Oncol. 2014;25(6):1106–1115. doi: 10.1093/annonc/mdu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ghorbani Z., Pourshams A., Fazeltabar Malekshah A., Sharafkhah M., Poustchi H., Hekmatdoost A. Major dietary protein sources in relation to pancreatic cancer: a large prospective study. Arch. Iran Med. 2016;19(4):248–256. [PubMed] [Google Scholar]
- 144.Jarvinen R., Knekt P., Hakulinen T., Aromaa A. Prospective study on milk products, calcium and cancers of the colon and rectum. Eur. J. Clin. Nutr. 2001;55(11):1000–1007. doi: 10.1038/sj.ejcn.1601260. [DOI] [PubMed] [Google Scholar]
- 145.Key T.J., Balkwill A., Bradbury K.E., Reeves G.K., Kuan A.S., Simpson R.F., et al. Foods, macronutrients and breast cancer risk in postmenopausal women: a large UK cohort. Int. J. Epidemiol. 2019;48(2):489–500. doi: 10.1093/ije/dyy238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Park S.Y., Murphy S.P., Wilkens L.R., Stram D.O., Henderson B.E., Kolonel L.N. Calcium, vitamin D, and dairy product intake and prostate cancer risk: the multiethnic cohort study. Am. J. Epidemiol. 2007;166(11):1259–1269. doi: 10.1093/aje/kwm269. [DOI] [PubMed] [Google Scholar]
- 147.Rohrmann S., Platz E.A., Kavanaugh C.J., Thuita L., Hoffman S.C., Helzlsouer K.J. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control. 2007;18(1):41–50. doi: 10.1007/s10552-006-0082-y. [DOI] [PubMed] [Google Scholar]
- 148.Sanjoaquin M.A., Appleby P.N., Thorogood M., Mann J.I., Key T.J. Nutrition, lifestyle and colorectal cancer incidence: a prospective investigation of 10998 vegetarians and non-vegetarians in the United Kingdom. Br. J. Cancer. 2004;90(1):118–121. doi: 10.1038/sj.bjc.6601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schulz M., Nothlings U., Allen N., Onland-Moret N.C., Agnoli C., Engeset D., et al. No association of consumption of animal foods with risk of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16(4):852–855. doi: 10.1158/1055-9965.EPI-07-0054. [DOI] [PubMed] [Google Scholar]
- 150.Tantamango-Bartley Y., Knutsen S.F., Jaceldo-Siegl K., Fan J., Mashchak A., Fraser G.E. Independent associations of dairy and calcium intakes with colorectal cancers in the Adventist Health Study-2 cohort. Public Health Nutr. 2017;20(14):2577–2586. doi: 10.1017/S1368980017001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tseng M., Breslow R.A., Graubard B.I., Ziegler R.G. Dairy, calcium, and vitamin D intakes and prostate cancer risk in the national health and nutrition examination epidemiologic follow-up study cohort. Am. J. Clin. Nutr. 2005;81(5):1147–1154. doi: 10.1093/ajcn/81.5.1147. [DOI] [PubMed] [Google Scholar]
- 152.Xu X. Dairy product consumption and bladder cancer risk in the prostate, lung, colorectal, and ovarian (PLCO) cohort. Front. Nutr. 2020;7:97. doi: 10.3389/fnut.2020.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bradbury K.E., Murphy N., Key T.J. Diet and colorectal cancer in UK Biobank: a prospective study. Int. J. Epidemiol. 2020;49(1):246–258. doi: 10.1093/ije/dyz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barnung R.B., Jareid M., Lukic M., Oyeyemi S.O., Rudolfsen J.H., Sovershaeva E., et al. High lactose whey cheese consumption and risk of colorectal cancer - the Norwegian Women and Cancer Study. Sci. Rep. 2019;9(1):296. doi: 10.1038/s41598-018-36445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barrubés L., Babio N., Mena-Sánchez G., Toledo E., Ramírez-Sabio J.B., Estruch R., et al. Dairy product consumption and risk of colorectal cancer in an older mediterranean population at high cardiovascular risk. Int. J. Cancer. 2018;143(6):1356–1366. doi: 10.1002/ijc.31540. [DOI] [PubMed] [Google Scholar]
- 156.Merritt M.A., Tzoulaki I., Tworoger S.S., De Vivo I., Hankinson S.E., Fernandes J., et al. Investigation of dietary factors and endometrial cancer risk using a nutrient-wide association study approach in the EPIC and nurses' health study (NHS) and NHSII. Cancer Epidemiol Biomarkers Prev. 2015;24(2):466–471. doi: 10.1158/1055-9965.EPI-14-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Duarte-Salles T., Fedirko V., Stepien M., Trichopoulou A., Bamia C., Lagiou P., et al. Dairy products and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer. 2014;135(7):1662–1672. doi: 10.1002/ijc.28812. [DOI] [PubMed] [Google Scholar]
- 158.Murphy N., Norat T., Ferrari P., Jenab M., Bueno-de-Mesquita B., Skeie G., et al. Consumption of dairy products and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0072715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Song Y., Chavarro J.E., Cao Y., Qiu W.L., Mucci L., Sesso H.D., et al. Whole milk intake is associated with prostate cancer-specific mortality among US male physicians. J. Nutr. 2013;143(2):189–196. doi: 10.3945/jn.112.168484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wirfalt E., Li C., Manjer J., Ericson U., Sonestedt E., Borgquist S., et al. Food sources of fat and sex hormone receptor status of invasive breast tumors in women of the Malmo diet and cancer cohort. Nutr. Cancer. 2011;63(5):722–733. doi: 10.1080/01635581.2011.570897. [DOI] [PubMed] [Google Scholar]
- 161.Hjartåker A., Thoresen M., Engeset D., Lund E. Dairy consumption and calcium intake and risk of breast cancer in a prospective cohort: the Norwegian Women and Cancer study. Cancer Causes Control. 2010;21(11):1875–1885. doi: 10.1007/s10552-010-9615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kesse-Guyot E., Bertrais S., Duperray B., Arnault N., Bar-Hen A., Galan P., et al. Dairy products, calcium and the risk of breast cancer: results of the S.U. French,VI.MAX prospective study. Ann. Nutr. Metab. 2007;51(2):139–145. doi: 10.1159/000103274. [DOI] [PubMed] [Google Scholar]
- 163.Kesse E., Bertrais S., Astorg P., Jaouen A., Arnault N., Galan P., et al. Dairy products, calcium and phosphorus intake, and the risk of prostate cancer: results of the French prospective SU.VI.MAX (Supplémentation en Vitamines et Minéraux Antioxydants) study. Br. J. Nutr. 2006;95(3):539–545. doi: 10.1079/bjn20051670. [DOI] [PubMed] [Google Scholar]
- 164.Schuurman A.G., Van Den Brandt P.A., Dorant E., Goldbohm R.A. Animal products, calcium and protein and prostate cancer risk in the Netherlands cohort study. Br. J. Cancer. 1999;80(7):1107–1113. doi: 10.1038/sj.bjc.6690472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fraser G.E., Jaceldo-Siegl K., Orlich M., Mashchak A., Sirirat R., Knutsen S. Dairy, soy, and risk of breast cancer: those confounded milks. Int. J. Epidemiol. 2020;49(5):1526–1537. doi: 10.1093/ije/dyaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lan T., Park Y., Colditz G.A., Liu J., Wang M., Wu K., et al. Adolescent dairy product and calcium intake in relation to later prostate cancer risk and mortality in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2020;31(10):891–904. doi: 10.1007/s10552-020-01330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nilsson L.M., Winkvist A., Esberg A., Jansson J.H., Wennberg P., van Guelpen B., et al. Dairy products and cancer risk in a northern Sweden population. Nutr. Cancer. 2020;72(3):409–420. doi: 10.1080/01635581.2019.1637441. [DOI] [PubMed] [Google Scholar]
- 168.Rohrmann S., Linseisen J., Jakobsen M.U., Overvad K., Raaschou-Nielsen O., Tjonneland A., et al. Consumption of meat and dairy and lymphoma risk in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer. 2011;128(3):623–634. doi: 10.1002/ijc.25387. [DOI] [PubMed] [Google Scholar]
- 169.Yang W., Sui J., Ma Y., Simon T.G., Chong D., Meyerhardt J.A., et al. A prospective study of dairy product intake and the risk of hepatocellular carcinoma in U.S. men and women. Int. J. Cancer. 2020;146(5):1241–1249. doi: 10.1002/ijc.32423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang J., Lim K., Shin S. Dairy product consumption and type 2 diabetes among Korean adults: a prospective cohort study based on the Health Examinees (HEXA) study. Epidemiol Health. 2022;44 doi: 10.4178/epih.e2022019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kummer K., Jensen P.N., Kratz M., Lemaitre R.N., Howard B.V., Cole S.A., et al. Full-fat dairy food intake is associated with a lower risk of incident diabetes among American Indians with low total dairy food intake. J. Nutr. 2019;149(7):1238–1244. doi: 10.1093/jn/nxz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Virtanen H.E.K., Koskinen T.T., Voutilainen S., Mursu J., Tuomainen T.P., Kokko P., et al. Intake of different dietary proteins and risk of type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2017;117(6):882–893. doi: 10.1017/S0007114517000745. [DOI] [PubMed] [Google Scholar]
- 173.Brouwer-Brolsma E.M., van Woudenbergh G.J., Oude Elferink S.J.W.H., Singh-Povel C.M., Hofman A., Dehghan A., et al. Intake of different types of dairy and its prospective association with risk of type 2 diabetes: the Rotterdam Study. Nutr. Metab. Cardiovasc. Dis. 2016;26(11):987–995. doi: 10.1016/j.numecd.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 174.Díaz-López A., Bulló M., Martínez-González M.A., Corella D., Estruch R., Fitó M., et al. Dairy product consumption and risk of type 2 diabetes in an elderly Spanish Mediterranean population at high cardiovascular risk. Eur. J. Nutr. 2016;55(1):349–360. doi: 10.1007/s00394-015-0855-8. [DOI] [PubMed] [Google Scholar]
- 175.Ericson U., Hellstrand S., Brunkwall L., Schulz C.A., Sonestedt E., Wallström P., et al. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am. J. Clin. Nutr. 2015;101(5):1065–1080. doi: 10.3945/ajcn.114.103010. [DOI] [PubMed] [Google Scholar]
- 176.Chen M., Sun Q., Giovannucci E., Willett W., Mozaffarian D., Hu F.B. Dairy consumption and risk of type 2 diabetes: 3 cohorts of us adults and an updated meta-analysis. BMC Med. 2014;12:215. doi: 10.1186/s12916-014-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O'Connor L.M., Lentjes M.A.H., Luben R.N., Khaw K.T., Wareham N.J., Forouhi N.G. Dietary dairy product intake and incident type 2 diabetes: a prospective study using dietary data from a 7-day food diary. Diabetologia. 2014;57(5):909–917. doi: 10.1007/s00125-014-3176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grantham N.M., Magliano D.J., Hodge A., Jowett J., Meikle P., Shaw J.E. The association between dairy food intake and the incidence of diabetes in Australia: the Australian Diabetes Obesity and Lifestyle Study (AusDiab) Public Health Nutr. 2013;16(2):339–345. doi: 10.1017/S1368980012001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Struijk E.A., Heraclides A., Witte D.R., Soedamah-Muthu S.S., Geleijnse J.M., Toft U., et al. Dairy product intake in relation to glucose regulation indices and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2013;23(9):822–828. doi: 10.1016/j.numecd.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 180.Sluijs I., Forouhi N.G., Beulens J.W.J., Van Der Schouw Y.T., Agnoli C., Arriola L., et al. The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct Study. Am. J. Clin. Nutr. 2012;96(2):382–390. doi: 10.3945/ajcn.111.021907. [DOI] [PubMed] [Google Scholar]
- 181.Fumeron F., Lamri A., Emery N., Bellili N., Jaziri R., Porchay-Baldérelli I., et al. Dairy products and the metabolic syndrome in a prospective study, DESIR. J. Am. Coll. Nutr. 2011;30(5):454–463. doi: 10.1080/07315724.2011.10719990. [DOI] [PubMed] [Google Scholar]
- 182.Kirii K., Mizoue T., Iso H., Takahashi Y., Kato M., Inoue M., et al. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia. 2009;52(12):2542–2550. doi: 10.1007/s00125-009-1554-x. [DOI] [PubMed] [Google Scholar]
- 183.Vang A., Singh P.N., Lee J.W., Haddad E.H., Brinegar C.H. Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: findings from Adventist Health Studies. Ann. Nutr. Metab. 2008;52(2):96–104. doi: 10.1159/000121365. [DOI] [PubMed] [Google Scholar]
- 184.Liu S., Choi H.K., Ford E., Song Y., Klevak A., Buring J.E., et al. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care. 2006;29(7):1579–1584. doi: 10.2337/dc06-0256. [DOI] [PubMed] [Google Scholar]
- 185.Choi H.K., Willett W.C., Stampfer M.J., Rimm E., Hu F.B. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch. Intern. Med. 2005;165(9):997–1003. doi: 10.1001/archinte.165.9.997. [DOI] [PubMed] [Google Scholar]
- 186.Hruby A., Ma J., Rogers G., Meigs J.B., Jacques P.F. Associations of dairy intake with incident prediabetes or diabetes in middle-aged adults vary by both dairy type and glycemic status. J. Nutr. 2017;147(9):1764–1775. doi: 10.3945/jn.117.253401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Slurink I.A.L., Voortman T., Ochoa-Rosales C., Ahmadizar F., Kavousi M., Kupper N., et al. Dairy product consumption in relation to incident prediabetes and longitudinal insulin resistance in the Rotterdam study. Nutrients. 2022;14(3):415. doi: 10.3390/nu14030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Slurink I.A.L., den Braver N.R., Rutters F., Kupper N., Smeets T., Elders P.J.M., et al. Dairy product consumption and incident prediabetes in Dutch middle-aged adults: the Hoorn Studies prospective cohort. Eur. J. Nutr. 2022;61(1):183–196. doi: 10.1007/s00394-021-02626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yuzbashian E., Nosrati-Oskouie M., Asghari G., Chan C.B., Mirmiran P., Azizi F. Associations of dairy intake with risk of incident metabolic syndrome in children and adolescents: Tehran lipid and glucose study. Acta Diabetol. 2021;58(4):447–457. doi: 10.1007/s00592-020-01651-0. [DOI] [PubMed] [Google Scholar]
- 190.Riseberg E., Lopez-Cepero A., Mangano K.M., Tucker K.L., Mattei J. Specific dietary protein sources are associated with cardiometabolic risk factors in the Boston Puerto Rican Health Study. J. Acad. Nutr. Diet. 2022;122(2):298–308.e3. doi: 10.1016/j.jand.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rautiainen S., Wang L., Lee I.M., Manson J.E., Buring J.E., Sesso H.D. Dairy consumption in association with weight change and risk of becoming overweight or obese in middle-aged and older women: a prospective cohort study. Am. J. Clin. Nutr. 2016;103(4):979–988. doi: 10.3945/ajcn.115.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Huus K., Brekke H.K., Ludvigsson J.F., Ludvigsson J. Relationship of food frequencies as reported by parents to overweight and obesity at 5 years. Acta Paediatr. 2009;98(1):139–143. doi: 10.1111/j.1651-2227.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- 193.Feart C., Lorrain S., Ginder Coupez V., Samieri C., Letenneur L., Paineau D., et al. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporos Int. 2013;24(12):3031–3041. doi: 10.1007/s00198-013-2421-7. [DOI] [PubMed] [Google Scholar]
- 194.Sahni S., Mangano K.M., Tucker K.L., Kiel D.P., Casey V.A., Hannan M.T. Protective association of milk intake on the risk of hip fracture: results from the Framingham original cohort. J. Bone Miner. Res. 2014;29(8):1756–1762. doi: 10.1002/jbmr.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Feskanich D., Meyer H.E., Fung T.T., Bischoff-Ferrari H.A., Willett W.C. Milk and other dairy foods and risk of hip fracture in men and women. Osteoporos Int. 2018;29(2):385–396. doi: 10.1007/s00198-017-4285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bergholdt H.K.M., Larsen M.K., Varbo A., Nordestgaard B.G., Ellervik C. Lactase persistence, milk intake, hip fracture and bone mineral density: a study of 97 811 Danish individuals and a meta-analysis. J. Intern. Med. 2018;284(3):254–269. doi: 10.1111/joim.12753. [DOI] [PubMed] [Google Scholar]
- 197.Dobreva I., Marston L., Mukadam N. Which components of the Mediterranean diet are associated with dementia? A UK Biobank cohort study. Geroscience. 2022;44(5):2541–2554. doi: 10.1007/s11357-022-00615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ylilauri M.P.T., Hantunen S., Lonnroos E., Salonen J.T., Tuomainen T.P., Virtanen J.K. Associations of dairy, meat, and fish intakes with risk of incident dementia and with cognitive performance: the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) Eur. J. Nutr. 2022;61(5):2531–2542. doi: 10.1007/s00394-022-02834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Camacho-Barcia L., Bullo M., Garcia-Gavilan J.F., Martinez-Gonzalez M.A., Corella D., Estruch R., et al. Dairy products intake and the risk of incident cataracts surgery in an elderly Mediterranean population: results from the PREDIMED study. Eur. J. Nutr. 2019;58(2):619–627. doi: 10.1007/s00394-018-1647-8. [DOI] [PubMed] [Google Scholar]
- 200.Miyake Y., Tanaka K., Okubo H., Sasaki S., Furukawa S., Arakawa M. Milk intake during pregnancy is inversely associated with the risk of postpartum depressive symptoms in Japan: the Kyushu Okinawa Maternal and Child Health Study. Nutr. Res. 2016;36(9):907–913. doi: 10.1016/j.nutres.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 201.Tanaka K., Miyake Y., Sasaki S., Hirota Y. Dairy products and calcium intake during pregnancy and dental caries in children. Nutr. J. 2012;11:33. doi: 10.1186/1475-2891-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yuan S., Bruzelius M., Damrauer S.M., Håkansson N., Wolk A., Åkesson A., et al. Anti-inflammatory diet and venous thromboembolism: two prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2021;31(10):2831–2838. doi: 10.1016/j.numecd.2021.06.021. [DOI] [PubMed] [Google Scholar]
- 203.Berkey C.S., Willett W.C., Tamimi R.M., Rosner B., Frazier A.L., Colditz G.A. Dairy intakes in older girls and risk of benign breast disease in young women. Cancer Epidemiol. Biomarkers Prev. 2013;22(4):670–674. doi: 10.1158/1055-9965.EPI-12-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Orta O.R., Terry K.L., Missmer S.A., Harris H.R. Dairy and related nutrient intake and risk of uterine leiomyoma: a prospective cohort study. Hum. Reprod. 2020;35(2):453–463. doi: 10.1093/humrep/dez278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Matthews V.L., Knutsen S.F., Beeson W.L., Fraser G.E. Soy milk and dairy consumption is independently associated with ultrasound attenuation of the heel bone among postmenopausal women: the Adventist Health Study-2. Nutr. Res. 2011;31(10):766–775. doi: 10.1016/j.nutres.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nicklaus S., Divaret-Chauveau A., Chardon M.L., Roduit C., Kaulek V., Ksiazek E., et al. The protective effect of cheese consumption at 18 months on allergic diseases in the first 6 years. Allergy. 2019;74(4):788–798. doi: 10.1111/all.13650. [DOI] [PubMed] [Google Scholar]
- 207.Littlejohns T.J., Neal N.L., Bradbury K.E., Heers H., Allen N.E., Turney B.W. Fluid intake and dietary factors and the risk of incident kidney stones in UK Biobank: a population-based prospective cohort study. Eur. Urol Focus. 2020;6(4):752–761. doi: 10.1016/j.euf.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 208.Opstelten J.L., Leenders M., Dik V.K., Chan S.S.M., Van Schaik F.D.M., Khaw K.T., et al. Dairy products, dietary calcium, and risk of inflammatory bowel disease: results from a European prospective cohort investigation. Inflamm. Bowel Dis. 2016;22(6):1403–1411. doi: 10.1097/MIB.0000000000000798. [DOI] [PubMed] [Google Scholar]
- 209.Miyake Y., Sasaki S., Tanaka K., Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur. Respir. J. 2010;35(6):1228–1234. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- 210.Yalçin S.S., Örün E., Mutlu B., Madendaǧ Y., Sinici I., Dursun A., et al. Why are they having infant colic? A nested case-control study. Paediatr. Perinat. Epidemiol. 2010;24(6):584–596. doi: 10.1111/j.1365-3016.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- 211.Ito M., Takamori A., Yoneda S., Shiozaki A., Tsuchida A., Matsumura K., et al. Fermented foods and preterm birth risk from a prospective large cohort study: the Japan Environment and Children's study. Environ. Health Prev. Med. 2019;24(1):25. doi: 10.1186/s12199-019-0782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Downer M.K., Batista J.L., Mucci L.A., Stampfer M.J., Epstein M.M., Håkansson N., et al. Dairy intake in relation to prostate cancer survival. Int. J. Cancer. 2017;140(9):2060–2069. doi: 10.1002/ijc.30642. [DOI] [PubMed] [Google Scholar]
- 213.Dik V.K., Murphy N., Siersema P.D., Fedirko V., Jenab M., Kong S.Y., et al. Prediagnostic intake of dairy products and dietary calcium and colorectal cancer survival-results from the EPIC cohort study. Cancer Epidemiol. Biomarkers Prev. 2014;23(9):1813–1823. doi: 10.1158/1055-9965.EPI-14-0172. [DOI] [PubMed] [Google Scholar]
- 214.Andersen J.L.M., Hansen L., Thomsen B.L.R., Christiansen L.R., Dragsted L.O., Olsen A. Pre- and post-diagnostic intake of whole grain and dairy products and breast cancer prognosis: the Danish Diet, Cancer and Health cohort. Breast Cancer Res. Treat. 2020;179(3):743–753. doi: 10.1007/s10549-019-05497-1. [DOI] [PubMed] [Google Scholar]
- 215.Niinistö S., Takkinen H.M., Uusitalo L., Rautanen J., Nevalainen J., Kenward M.G., et al. Maternal dietary fatty acid intake during pregnancy and the risk of preclinical and clinical type 1 diabetes in the offspring. Br. J. Nutr. 2014;111(5):895–903. doi: 10.1017/S0007114513003073. [DOI] [PubMed] [Google Scholar]
- 216.Zhao Q., He Y., Wang K., Wang C., Wu H., Gao L., et al. Dairy consumption and liver cancer risk: a systematic review and dose-response meta-analysis of observational studies. Nutr. Cancer. 2021;73(11–12):2821–2831. doi: 10.1080/01635581.2020.1862255. [DOI] [PubMed] [Google Scholar]
- 217.Kakkoura M.G., Du H.A.-O., Guo Y., Yu C., Yang L., Pei P., et al. Dairy consumption and risks of total and site-specific cancers in Chinese adults: an 11-year prospective study of 0.5 million people. BMC Med. 2022;20(1):134. doi: 10.1186/s12916-022-02330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kaluza J., Komatsu S., Lauriola M., Harris H.R., Bergkvist L., Michaëlsson K., et al. Long-term consumption of non-fermented and fermented dairy products and risk of breast cancer by estrogen receptor status—population-based prospective cohort study. Clin. Nutr. 2021;40(4):1966–1973. doi: 10.1016/j.clnu.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 219.Ibsen D.B., Steur M., Imamura F., Overvad K., Schulze M.B., Bendinelli B., et al. Replacement of red and processed meat with other food sources of protein and the risk of type 2 diabetes in European populations: the EPIC-InterAct Study. Diabetes Care. 2020;43(11):2660–2667. doi: 10.2337/dc20-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Feng Y.F., Zhao Y., Liu J., Huang Z.L., Yang X.J., Qin P., et al. Consumption of dairy products and the risk of overweight or obesity, hypertension, and type 2 diabetes mellitus: a dose-response meta-analysis and systematic review of cohort studies. Adv. Nutr. 2022;13(6):2165–2179. doi: 10.1093/advances/nmac096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Palacios C. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006;46(8):621–628. doi: 10.1080/10408390500466174. [DOI] [PubMed] [Google Scholar]
- 222.Rizzoli R., Biver E., Brennan-Speranza T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021;9(9):606–621. doi: 10.1016/S2213-8587(21)00119-4. [DOI] [PubMed] [Google Scholar]
- 223.Wallace T.C., Bailey R.L., Lappe J., O'Brien K.O., Wang D.D., Sahni S., et al. Dairy intake and bone health across the lifespan: a systematic review and expert narrative. Crit. Rev. Food Sci. Nutr. 2021;61(21):3661–3707. doi: 10.1080/10408398.2020.1810624. [DOI] [PubMed] [Google Scholar]
- 224.Suzuki T., Kojima N., Osuka Y., Tokui Y., Takasugi S., Kawashima A., et al. The effects of mold-fermented cheese on brain-derived neurotrophic factor in community-dwelling older Japanese women with mild cognitive impairment: a randomized, controlled, crossover trial. J. Am. Med. Dir. Assoc. 2019;20(12):1509–1514.e2. doi: 10.1016/j.jamda.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 225.de Goeij L.C., van de Rest O.A.-O., Feskens E.J.M., de Groot L.A.-O., Brouwer-Brolsma E.A.-O. Associations between the intake of different types of dairy and cognitive performance in Dutch older adults: the B-PROOF study. Nutrients. 2020;12(2):468. doi: 10.3390/nu12020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Klinedinst B.S., Le S.T., Larsen B., Pappas C., Hoth N.J., Pollpeter A., et al. Genetic factors of Alzheimer's disease modulate how diet is associated with long-term cognitive trajectories: a UK Biobank study. J. Alzheimers Dis. 2020;78(3):1245–1257. doi: 10.3233/JAD-201058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fu X., Harshman S.G., Shen X., Haytowitz D.B., Karl J.P., Wolfe B.E., et al. Multiple vitamin K forms exist in dairy foods. Curr. Dev. Nutr. 2017;1(6) doi: 10.3945/cdn.117.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Walther B., Karl J.P., Booth S.L., Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv. Nutr. 2013;4(4):463–473. doi: 10.3945/an.113.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hariri E., Kassis N.A.-O., Iskandar J.P., Schurgers L.J., Saad A., Abdelfattah O., et al. Vitamin K(2)-a neglected player in cardiovascular health: a narrative review. Open Heart. 2021;8(2) doi: 10.1136/openhrt-2021-001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Villa J.K.D., Diaz M.A.N., Pizziolo V.R., Martino H.S.D. Effect of vitamin K in bone metabolism and vascular calcification: a review of mechanisms of action and evidences. Crit. Rev. Food Sci. Nutr. 2017;57(18):3959–3970. doi: 10.1080/10408398.2016.1211616. [DOI] [PubMed] [Google Scholar]
- 231.Ferland G. Vitamin K and the nervous system: an overview of its actions. Adv. Nutr. 2012;3(2):204–212. doi: 10.3945/an.111.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mozaffarian D., Wu J.H.Y. Flavonoids, dairy foods, and cardiovascular and metabolic health a review of emerging biologic pathways. Circ. Res. 2018;122(2):369–384. doi: 10.1161/CIRCRESAHA.117.309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Leeuwendaal N.K., Stanton C., O'Toole P.W., Beresford T.P. Fermented foods, health and the gut microbiome. Nutrients. 2022;14(7):1527. doi: 10.3390/nu14071527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Drouin-Chartier J.P., Brassard D., Tessier-Grenier M., Cote J.A., Labonte M.E., Desroches S., et al. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv. Nutr. 2016;7(6):1026–1040. doi: 10.3945/an.115.011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Machlik M.L., Hopstock L.A., Wilsgaard T., Hansson P. Associations between intake of fermented dairy products and blood lipid concentrations are affected by fat content and dairy matrix—the Tromso study: Tromso7. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.773468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Steur M., Johnson L., Sharp S.J., Imamura F., Sluijs I., Key T.J., et al. Dietary fatty acids, macronutrient substitutions, food sources and incidence of coronary heart disease: findings from the EPIC-CVD case-cohort study across nine European countries. J. Am. Heart Assoc. 2021;10(23) doi: 10.1161/JAHA.120.019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Stanhewicz A.E., Alba B.K., Kenney W.L., Alexander L.M. Dairy cheese consumption ameliorates single-meal sodium-induced cutaneous microvascular dysfunction by reducing ascorbate-sensitive oxidants in healthy older adults. Br. J. Nutr. 2016;116(4):658–665. doi: 10.1017/S0007114516002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang P.L., Song M.Y., Eliassen A.H., Wang M.L., Fung T.T., Clinton S.K., et al. Optimal dietary patterns for prevention of chronic disease. Nat. Med. 2023;29(3):719–728. doi: 10.1038/s41591-023-02235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Micha R., Wallace S.K., Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus a systematic review and meta-analysis. Circulation. 2010;121(21):2271–2783. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Office of Disease Prevention and Health Promotion . 2015. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines of Americans 2015 to the Secretary of Agriculture and the Secretary of Health and Human Services.https://health.gov/dietaryguidelines/2015-scientific-report/ [Internet] [cited 2022 Jul 23]. Available from: [Google Scholar]
- 241.Talebi S., Asoudeh F., Naeini F., Sadeghi E., Travica N., Mohammadi H. Association between animal protein sources and risk of neurodegenerative diseases: a systematic review and dose-response meta-analysis. Nutr. Rev. 2023:nuac114. doi: 10.1093/nutrit/nuac114. [DOI] [PubMed] [Google Scholar]
- 242.Hughes K.C., Gao X., Kim I.Y., Wang M.L., Weisskopf M.G., Schwarzschild M.A., et al. Intake of dairy foods and risk of Parkinson disease. Neurology. 2017;89(1):46–52. doi: 10.1212/WNL.0000000000004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lang F., Ma K., Leibrock C.B., Salker M.S., Singh Y. The putative role of 1,25(OH)(2)D(3) in the association of milk consumption and Parkinson's disease. Neurosignals. 2020;28(1):14–24. [Google Scholar]
- 244.Kyrozis A., Ghika A., Stathopoulos P., Vassilopoulos D., Trichopoulos D., Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson's disease in Greece. Eur. J. Epidemiol. 2013;28(1):67–77. doi: 10.1007/s10654-012-9760-0. [DOI] [PubMed] [Google Scholar]
- 245.Olsson E., Byberg L., Hoijer J., Kilander L., Larsson S.C. Milk and fermented milk intake and Parkinson's disease: Cohort study. Nutrients. 2020;12(9):2763. doi: 10.3390/nu12092763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this umbrella review were extracted from publicly available systematic reviews and original studies.