Abstract
Obesity and type 2 diabetes(T2D) lead to defects in intestinal hormones secretion, abnormalities in the composition of bile acids (BAs), increased systemic and adipose tissue inflammation, defects of branched‐chain amino acids (BCAAs) catabolism, and dysbiosis of gut microbiota. Bariatric surgery (BS) has been shown to be highly effective in the treatment of obesity and T2D, which allows us to view BS not simply as weight‐loss surgery but as a means of alleviating obesity and its comorbidities, especially T2D. In recent years, accumulating studies have focused on the mechanisms of BS to find out which metabolic parameters are affected by BS through which pathways, such as which hormones and inflammatory processes are altered. The literatures are saturated with the role of intestinal hormones and the gut‐brain axis formed by their interaction with neural networks in the remission of obesity and T2D following BS. In addition, BAs, gut microbiota and other factors are also involved in these benefits after BS. The interaction of these factors makes the mechanisms of metabolic improvement induced by BS more complicated. To date, we do not fully understand the exact mechanisms of the metabolic alterations induced by BS and its impact on the disease process of T2D itself. This review summarizes the changes of intestinal hormones, BAs, BCAAs, gut microbiota, signaling proteins, growth differentiation factor 15, exosomes, adipose tissue, brain function, and food preferences after BS, so as to fully understand the actual working mechanisms of BS and provide nonsurgical therapeutic strategies for obesity and T2D.
Keywords: bariatric surgery, diabetes, intestinal hormones, obesity, mechanism
Highlights
Controversial changes in intestinal hormones after bariatric surgery (BS) were summarized.
The changes of signaling proteins involved in insulin resistance and the newly proposed changes in growth differentiation factor 15, exosomes, and extracellular vesicles after BS were elucidated.
The interactions between bile acids and gut microbiota and the gut–brain axis play an indispensable role in BS.
摘要
肥胖和2型糖尿病(T2D)导致肠道激素分泌缺陷、胆汁酸(BA)合成异常、脂肪组织炎症增加、支链氨基酸分解代谢异常和肠道菌群失调。减重手术(BS)已被证明在治疗肥胖和2型糖尿病方面非常有效, 这使得我们不能将BS简单地视为减重手术, 而是一种减轻肥胖及其合并症, 尤其是2型糖尿病的手段。近年来, 越来越多的研究关注BS的发病机制, 试图找出BS通过哪些途径影响代谢参数, 如哪些激素和炎症过程发生改变。目前已有大量文献证明肠道激素及其与神经网络相互作用形成的脑‐肠轴在BS后肥胖和T2D缓解中的作用。此外, BS后的益处还与BAs、肠道菌群等因素有关。这些因素的相互作用使得BS引起代谢改善的机制更加复杂。迄今为止, 我们还没有完全了解BS诱导的代谢改变的确切机制及其对T2D本身疾病过程的影响。本文综述了BS后肠道激素、BAs、BCAAs、肠道菌群、信号蛋白、生长分化因子15、外泌体、脂肪组织、脑功能、食物偏好等方面的变化, 以期全面了解BS的实际作用机制, 为肥胖和T2D的非手术治疗提供策略。
Keywords: 减重手术, 糖尿病, 肠道激素, 肥胖, 机制
1. INTRODUCTION
The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 revealed that the prevalence of all metabolic diseases increased from 2000 to 2019. 1 The latest national prevalence estimates for 2015–2019 were 34.3% for overweight and 16.4% for obesity in adults (≥18 years) according to Chinese criteria. 2 According to GBD data, the absolute burden of obesity is highest and the number of deaths is highest, with a total of 5.0 million deaths in 2019, in addition to an estimated 43.8 million type 2 diabetes (T2D) patients worldwide in 2019, with 1.4 million deaths among these patients. 1 Moreover, the mortality rates of obesity and T2D did not decrease over time. 1 Obesity is a complex disease with multiple etiologies, with its own disabling capabilities and comorbidities. 3 , 4 Studies have shown that obesity is the strongest risk factor for the development of diabetes 5 and is also associated with an increased risk of cardiovascular diseases, liver diseases, and some kinds of cancers. 6 , 7 Statistically, the largest proportion of metabolic disease‐related mortality was contributed by obesity. 1 Basic treatments for obesity include low‐calorie low‐fat diets, increased physical activity, and strategies that contribute to the modifications in lifestyle. Antiobesity drugs contribute to weight loss and further improve the health risks. 7 However, existing antiobesity drugs, including sympathomimetics, GABAA receptor activators, pancreatic lipase inhibitors, a serotonin 2C receptor agonist, dopamine–norepinephrine reuptake inhibitor, opioid antagonist, and glucagon‐like peptide‐1 (GLP‐1) receptor agonists, are not as effective as desired, and they have side effects. 8 For example, topiramate may cause increased pulse and blood pressure in certain patients and increase the risk of oral clefts in infants when taken by pregnant women; liraglutide has obvious gastrointestinal side effects, such as nausea, vomiting, diarrhea, and an increased risk of pancreatitis. 8 Moreover, short‐term weight control is easily achieved by the means described, but it is prone to weight regain. 9 , 10 There are certain advantages in maintaining long‐term weight loss for bariatric surgery (BS) in severely obese patients. 7 , 11 BS, also known as metabolic surgery, has been around since the 1950s. Over the years, there have been significant changes in access and types of BS, most notably the almost exclusive adoption of laparoscopic techniques in the specialty. Other significant changes were related to the techniques: the use of vertical banded gastroplasty (VBG) declined in the late 1990s, laparoscopic adjustable gastric banding (AGB) emerged around 2012, and thereafter the utilization of biliopancreatic diversion (BPD) remained at its lowest level, maintaining a relatively large proportion of Roux‐en‐Y gastric bypass (RYGB) for a long time; as well as the utilization of laparoscopic sleeve gastrectomy (SG) has increased rapidly in recent years. 12 For decades, BS has proven successful in achieving meaningful and sustainable weight loss in a large number of patients undergoing surgery. 13 In addition, BS significantly improves comorbidities as well as reduces overall mortality by 25%–50% during long‐term follow‐up. 7 Although the benefits of BS are clear, the means by which it is achieved remain to be elucidated. Different surgical methods result in different changes in the structure of gastrointestinal tract, based on the similarities and differences in weight loss and metabolic improvement in these procedures. Clinical and animal studies have focused on the changes of intestinal hormones, gut microbiota, bile acids (BAs), and circulating immune and cytokine production following BS. At present, there are a number of controversial opinions about how these various mechanisms work and how they intersect and overlap. This review is intended to elucidate the mechanisms of benefit of BS for weight loss and diabetes remission form various aspects and thus to provide ideas for unlocking other noninvasive treatment strategies against obesity and related comorbidities that were previously unknown.
2. MECHANISMS OF BARIATRIC SURGERY
2.1. Weight loss
Metabolic changes were frequently observed soon after BS and BS is more effective than dietary control in improving diabetes, even with equivalent weight loss. For example, with the same weight loss, postprandial glucose levels decreased more and the GLP‐1 levels increased more at 1 month after gastric bypass surgery (GBP) compared with a low‐caloric diet, 14 indicating that mechanisms may be partially independent of weight loss. 15 , 16
2.2. Dietary restriction
Dietary restriction after BS appears to play a major role in weight loss. In addition to a smaller gastric pouch, reduced appetite such as the decrease in orexigenic hormones after BS and the increase in anorexic hormones contributes to a further decrease in energy intake. These hormonal changes echo the changes in brain function projected by functional magnetic resonance imaging (fMRI) described below. Specifically, patients undergoing RYGB have increased postprandial plasma GLP‐1, 17 peptide YY (PYY), 18 , 19 and oxyntomodulin (OXM), 20 which are beneficial to enhancing satiety and thus lead to a reduction in energy intake. 21 Ghrelin is a peptide of 28 amino acids originally discovered in 1999. 22 It acts as an orexigenic hormone and is mainly secreted by the stomach. 22 , 23 Ghrelin has diverse biological functions in regulating energy homeostasis, including the abilities to communicate with the hypothalamus about current peripheral nutritional status and to compensate for energy. 24 It is also associated with increased plasma levels of insulin, glucagon, and leptin. 25 Studies have shown that ghrelin impairs carbohydrate and lipid metabolism in obese patients and BS such as SG, 18 AGB, 26 and GBP 27 , 28 are associated with significantly suppressed ghrelin levels. However, there have been some controversial findings regarding ghrelin level after BS, such as studies showing that ghrelin concentrations increased to 40% above baseline levels in patients receiving BPD‐RYGB who completed 1 year of follow‐up 19 and plasma ghrelin increased at 1 year in patients undergoing AGB. 29 Another study reported that there was no significant change in fasting ghrelin levels from baseline at 1 year after RYGB, nor was there a significant reduction in ghrelin levels after the test meal. 18 The discrepancies in ghrelin levels after BS can be attributed to differences in surgical techniques among centers, including the remaining size of the gastric pouch, the handling of the vagus nerve, the length of Roux‐limp, and the timing of samplings. However, orexigenic hormones were significantly attenuate after RYGB but not after weight loss with the equivalent caloric restriction 30 and RYGB results in improved metabolic flexibility, such as more complete β‐oxidation of fatty acids and greater handling of glucose and amino acids, compared with the equivalent dietary restriction, 31 indicated that mechanisms other than energy restriction cannot be excluded.
2.3. The hindgut hypothesis and the foregut hypothesis
Two major hypotheses have been proposed to explain the effects of BS on T2D: the hindgut hypothesis 32 , 33 and the foregut hypothesis. 33 , 34 , 35 The former points out that remission of T2D results from faster delivery of nutrients to the distal small intestine, 36 where L‐cells are more densely distributed 37 and thus enhances the release of GLP‐1. 38 , 39 , 40 GLP‐1 stimulates not only insulin secretion but also proinsulin gene transcription and insulin biosynthesis and inhibit glucagon secretion, 41 , 42 , 43 a physiological marker of improved glucose metabolism. Some studies have shown that GLP‐1 is dispensable for the metabolic effects after BS; for example, Albaugh et al found that the glucose‐regulating effect of bile diversion to the ileum was abolished in whole‐body GLP‐1 receptor (GLP‐1R) deficient mice. 44 The effect of GLP‐1R agonists such as semaglutide and liraglutide on weight loss is comparable to that of BS, which seems to confirm the important role of GLP‐1. However, infusion of exendin‐(9–39), a GLP‐1R antagonist, 45 resulted in a slight deterioration of postprandial plasma glucose in RYGB subjects, suggesting that the resolution of T2D after RYGB may be explained by mechanisms other than enhanced GLP‐1 action. 46 Similarly, GLP‐1 played a limited role in short‐term glycemic improvement after RYGB compared with intensive lifestyle management. 47 The metabolic benefits induced by SG 48 and RYGB 49 could overcome the defects in glucose regulation due to the lack of GLP‐1 signaling in β‐cells in obese mice, further demonstrating that the hindgut hypothesis may not fully explain the benefits of BS. The foregut hypothesis, on the other hand, suggests that exclusion of the proximal small intestine from nutrient transit would reduce or suppress the secretion of anti‐incretin hormones that promote insulin resistance, thereby improving glycemic control. 50 Glucose‐dependent insulinotropic polypeptide (GIP) is an intestinal hormone that is secreted by K cells distributed in the proximal small intestine 37 and promotes the storage of both glucose and fat. 51 GIP exhibits incretin activity and increases insulin secretion in hyperglycemia. Increased GIP signaling plays an important role in adipose tissue inflammation and insulin resistance in obese mice. 52 Studies using GIP receptor knockout mice suggest that GIP action is important in fat deposition and that inhibition of GIP signaling may be a target for obesity. 51 , 53 , 54 The metabolic benefits of bypass surgery are mediated in part by surgical removal of GIP‐secreting K cells in the proximal small intestine 55 , 56 and emerging evidences have also shown that a rapid reduction in GIP levels following RYGB 57 and BPD. 58 , 59 Treatment of obese mice with GIP receptor (GIPR) antagonist leads to rapid improvement in β‐cell function and glucose tolerance through alleviation of insulin resistance, 60 but it remains to be investigated whether the effects of blocking GIP in humans are similar to those observed in mice. Some studies showed that bypassing of the jejunum or a short segment of the proximal gut is beneficial with respect to insulin‐mediated glucose disposal in obese patients, independent of effects on body weight, food intake, or hindgut nutrient delivery. 34 , 35 , 61 Interestingly, the longer the portion of the jejunal bypass during metabolic surgery was associated with greater improvement in insulin sensitivity. 61 Similarly, remission rates of T2D were higher after BPD than after RYGB in clinical trials because in RYGB, only the duodenum is bypassed, whereas in BPD, the duodenum and part of jejunum are excluded from food transport. 33 , 62 , 63 It was also found that anatomical alterations in the proximal small intestine may reduce factors associated with negative effects on insulin sensitivity, contributing to the control of diabetes after GBP in rats. 64 Interestingly, the superior efficacy of GIPR‐ GLP‐1R coagonist tirzepatide 65 in T2D has drawn attention to the role of GIP in metabolism in recent years. It is possible that both the increase and decrease of GIPR signaling reduce body weight, and further studies are needed to investigate the role of GIP in humans. However, no significant differences were found between RYGB and SG in improving glucose homeostasis; increasing insulin, GLP‐1, and PYY levels 66 ; and promoting weight loss at long‐term follow‐up, 67 , 68 , 69 , 70 which do not support the foregut hypothesis. Moreover, neither the changes in GLP‐1 plasma levels nor the changes in GIP explained the normalization of insulin sensitivity, this fact may indicate the presence of other intestinal factors.
2.4. Changes in other intestinal hormones
Some studies showed that insulin sensitivity increased more with oral glucose than with intravenous glucose after BS, suggesting the importance of intestinal hormones. The roles of ghrelin, GLP‐1, and GIP have been described earlier, and the roles of other hormonal changes in BS should be further explored. In the mid‐ to late 1980s, it was recognized that glucagon‐like peptide‐2 (GLP‐2) is specifically processed from preproglucagon in the gut 71 , 72 and is cosecreted with GLP‐1. 73 Similar to GLP‐1, GLP‐2 is also increased after RYGB to promote the proliferation of mucosal crypt cells 74 through insulin‐like growth factor‐1 receptor in intestinal epithelium, 75 which may be beneficial for the restoration of intestinal absorptive surface area, thereby limiting malabsorption and promoting long‐term weight loss in rodents and humans. 76 GLP‐2 was significantly elevated after RYGB and correlated with satiety in clinical trials, 77 as was GLP‐2 observed after SG. 78 However, the loss of GLP‐2 receptor (GLP‐2R) did not attenuate the extent of weight loss and improved glycemic control after SG in mice, demonstrating that GLP‐2R signaling is dispensable for the metabolic benefits generated after SG. 79
PYY3‐36 reduces food intake in normal‐weight subjects by modulating hypothalamic appetizing circuits; however, obese subjects have been found to have lower endogenous PYY levels and are not resistant to the anorectic effects of PYY. 80 Meanwhile, attenuated postprandial PYY secretion was observed in the early stages of T2D development. 81 Many studies have found that PYY levels increase after BS such as RYGB 18 , 82 , 83 , 84 or SG. 18 , 66 Ramracheya et al demonstrated that diabetic rats undergoing RYGB rely on PYY to restore impaired glucose‐mediated insulin and glucagon secretion. 85 Tests of human islet function before and after BS, in the presence or absence of PYY, demonstrated that PYY plays a key role in the resolution of T2D in BS. 86 However, a prospective study of severely obese individuals (25 nondiabetic and 10 diabetic) who underwent RYGB found that increased PYY levels were associated with sustained weight loss after surgery. However, there was no significant correlation between PYY and glucose tolerance in either group. 82 Regardless, the beneficial metabolic effects of BS are mediated through changes in PYY levels remain to be proven.
Gastrin and cholecystokinin (CCK) are known homologous hormone systems, which not only regulate gastric acid secretion and the growth of gastric and pancreatic cells but also participate in the development and secretion of islet cells. 87 It has been shown that resection of distal gastric mucosa significantly reduced body weight and improved glycemic control in rats, inferring that the decrease in gastrin level caused by gastric mucosa exclusion in RYGB may be the key for weight loss and T2D remission. 88 Due to structural changes in the stomach caused by surgery, gastrin level decreased after RYGB, 89 whereas others have shown that gastrin level was unchanged after AGB 90 and increased significantly after SG in rodents. 91 As for the CCK, Rhee et al found that the distribution of enteroendocrine cells underwent a lot of alterations after RYGB in obese patients with T2D, including an increased density of CCK‐positive cells. 92 It has been found that infusion of nutrients into the bypassed jejunum after the jejunal bypass stimulated CCK secretion and pancreatic growth in rats. 93 However, other studies have shown that CCK response to meals is not changed after RYGB 57 and VBG compared with presurgery, suggesting that CCK does not mediate the endocrine satiety effect of BS. 94 These controversial changes in gastrin and CCK after different bariatric surgeries indicate the need for further studies exploring their association with BS.
OXM, a postprandial peptide hormone released from the gut, activates both GLP‐1R and glucagon receptor (GCGR) to induce weight loss, 95 inhibit food intake 96 and regulate energy expenditure. 97 Some studies have shown that its analogue eliminated obesity and diabetes in mice. 98 Combined injection of OXM, GLP‐1, and PYY improved body weight and hyperglycemia compared with placebo in humans. 99 , 100 Perakakis et al found that postprandial OXM levels increased most strongly at 3 months after SG and were associated with the degree of weight loss, which serve as predictors of weight loss, presumably by regulating satiety. 101 Similarly, OXM levels were significantly increased 1 month after RYGB compared with the diet‐induced equivalent weight loss and were significantly correlated with GLP‐1 and PYY. 20 OXM is derived from the proglucagon gene and has structural similarity to glucagon. Glucagon is known to be released in the fasting state and increases blood glucose levels by promoting glycogenolysis and gluconeogenesis. There are few studies on glucagon in BS, and the present studies have shown that glucagon levels were decreased after SG 102 and RYGB. 103
Fibroblast growth factors 19 (FGF19) and 21 (FGF21) are secreted by the intestine and liver and have emerged as key regulators of energy metabolism. The biological effects of FGF21 include weight loss by reducing food intake and increasing energy expenditure, as well as lowering plasma glucose by increasing insulin sensitivity. However, FGF21 levels are elevated in obese patients and are further increased in obese patients with T2D, so obesity is proposed to be a FGF21‐resistant state. 104 Instead, circulating serum FGF19 concentrations are significantly decreased in obese and T2D patients. Many studies have yielded controversial results regarding the changes in FGF19 and FGF21 after BS. It has been shown that FGF21 levels decreased after SG 105 and GBP 106 induced weight loss, whereas they remained unchanged after RYGB. 105 Moreover, other studies have shown that FGF21 concentrations elevated after RYGB in 16 obese patients. 107 The reason why FGF21 levels are unchanged or even increased after RYGB may be related to the changes in intestinal structure that cause the rapid delivery of nutrients to the small intestine and glucose delivery to the liver or reverse FGF21‐resistance state. FGF19 inhibits gluconeogenesis and stimulates glycogen synthesis but does not increase lipogenesis. FGF19 concentrations increased after weight loss induced by SG, RYGB, and AGB 108 in obese patients, as well as after GBP 106 in obese patients with T2D. The role of FGF19 in BS is elaborated in a later section.
It has been proposed that circulating follistatin and its homologous protein, follistatin‐like 3 play an important role in glucose homeostasis and they were decreased after RYGB and SG, and were correlated with the changes of blood glucose, insulin, and glycosylated hemoglobin. 109 Circulating succinate was significantly reduced after BS and had predictive value for T2D remission proposed by Victoria et al, obese patients with T2D who with different baseline succinate levels had different responses to the type of surgery and different T2D remission rates. 110
There are also many hormones such as insulin, secretin, pancreatic polypeptide, obestatin, and so on that play certain roles in weight loss and metabolic improvement after BS, which are not discussed in this review.
2.5. Changes in signaling proteins (adipokines, myokines, hepatokines), GDF15, exosomes, and adipose tissue
Altered adipokines levels may contribute to metabolic dysfunction in obesity. The extent of adipokines changes after BS and their impact on metabolic improvements have been explored in several studies. Adiponectin levels increase 111 , 112 , 113 and leptin levels decrease after BS, and surgery shifts the adipokines profiles of obese patients toward lean controls. 114 Specifically, in a prospective controlled Swedish Obese Subjects Study, adiponectin levels were compared between 1570 subjects undergoing BS and 1729 controls receiving usual care. The results suggest that the magnitude of weight loss after BS paralleled a significant increase in circulating high molecular weight adiponectin. 115 , 116 Patients with T2D remission after BS have higher levels of adiponectin and lower high‐sensitivity C‐reactive protein than those without remission, and elevated adiponectin is associated with enhanced β‐cells function, greater fat loss, and lower triglyceride levels, 117 which indicates that inflammation and insulin resistance may be reduced. Leptin is an anorexigenic hormone that is secreted by white adipose tissue, and despite the anorectic effect of plasma leptin, it is correlated with body fat content, suggesting that obesity is associated with a state of leptin resistance. 118 , 119 Moreover, leptin resistance may account for the decreased GLP‐1 levels in obese individuals. 120 It is reported that leptin levels decreased at 1 year after RYGB and AGB. 29 , 121
The activity of brown adipose tissue (BAT) protects against obesity and T2D. 122 Thermogenesis in BAT (both brown and beige adipocytes) plays an important role in combating the development of metabolic disorders. 123 , 124 , 125 Recently, Qian Wang et al found that interleukin‐27 (IL‐27) directly acted on BAT, stimulating uncoupling protein 1 (UCP‐1) production to increase thermogenesis, protect against obesity and ameliorate insulin resistance. 126 The serum IL‐27 levels were significantly reduced in obese individuals with T2D and were restored after RYGB, 127 indicating that BS may improves the regulation of BAT metabolism by restoring the levels of certain factors. Obesity and T2D are associated with low‐grade chronic inflammation of white adipose tissue (WAT), increased proinflammatory cytokines and local infiltration of immune cells lead to insulin resistance in obese patients. 122 Genes encoding inflammation‐related proteins in WAT continued to decline 2 and 5 years after RYGB in 38 obese patients, indicated that the metabolic effects of BS may be related in part to altered gene expression in WAT. 128 However, another study showed that elevated leukocyte infiltration and unchanged proinflammatory cytokine mRNA expression in adipose tissue at 1 month or 6 to 12 months after BS in 17 obese patients, reflect that neither short‐term nor long‐term metabolic improvement after BS significantly reduces inflammatory markers of adipose tissue. This result reveals that reduction in adipose tissue inflammation did not contribute to the metabolic benefits of BS. 129 The different results may be due to different populations, surgical centers, and follow‐up time. In addition, both myokines and hepatokines are associated with insulin resistance in obesity. Metabolic changes induced by BS appear to be related to reduction in myokines. 130 There is limited research on the role of hepatokines, including insulin‐like growth factor binding protein 2 (IGFBP2), adropin, and sex hormone binding globulin, after BS. Among them, IGFBP2 is significantly increased after RYGB in humans, rats, and mice, and deletion of IGFBP2 impairs weight loss and early improvement in insulin sensitivity induced by surgery, suggesting a potential role of circulating IGFBP2 in BS. 131 The remaining factors require further research. Growth differentiation factor 15 (GDF15), a cytokine that reduces food intake by exerting central anorexigenic effects, has anti‐inflammatory effects and increases insulin sensitivity, which may improve clinical outcomes in patients with obesity and T2D. 132 Studies have shown that the levels of GDF15 increased after SG in mice 133 and humans, 134 as well as after RYGB, 133 , 135 but lack of GDF15 signaling did not alter food intake or body weight after SG, indicating that GDF15 may not be essential for the potent effects of SG, 136 further studies are needed to explore the role of GDF15 in BS. Recent studies have highlighted the role of exosomes in mediating the crosstalk between liver, skeletal muscle and adipose tissue during the development of insulin resistance. 137 Exosomal microRNAs (miRNAs) have emerged as potential biomarkers of obesity. Exosomes derived from obese adipose contain dysregulated miRNAs associated with insulin signaling compared to lean controls, but circulating exosomes are modified following BS and associated with improved insulin resistance. 138 , 139 Extracellular vesicles (EVs) are crucial modes of intercellular communication, modulating multiple biological processes by carrying hormones, nucleic acids, and signaling molecules. 140 Obesity interferes with the function of human adipose mesenchymal stem/stromal cells (ASCs), thereby altering the size and miRNAs cargo of ASCs‐derived EVs and reducing their ability to repair damaged cells. 141 In mice experiments, the composition of intestinal EVs altered substantially after VSG and may regulate various signaling pathways. 140 Extracellular miRNAs regulate cellular metabolism by mediating intercellular communication. 142 These miRNAs are partially found in small vesicles/exosomes, and circulating miRNAs have been linked to metabolic disorders. A longitudinal study in humans revealed that 42 circulating miRNAs were differentially expressed between 6 and 12 months after RYGB. Among these, circulating miR‐15a, miR‐22, and miR‐192 were increased in nine obese individuals with T2D and positively correlated with disease severity, whereas they decreased after RYGB. 143 Circulating levels of miR‐92a were positively associated with body mass index (BMI) and impaired glucose metabolism, but decreased at 6 months following BS. 144 Thus, alterations in circulating miRNAs may partly explain the improved metabolic function after BS. However, how BS leads to changes in circulating miRNAs and how these miRNAs participate in regulating systemic metabolism require further investigation.
2.6. Changes in the concentrations and compositions of bile acids
Increasing evidence suggests that the balance of BAs synthesis pathways (between the classical pathway and the alternative pathway) may be a therapeutic target for metabolic disorders. BAs are important metabolic regulator acting through the Takeda G‐protein receptor 5 (TGR5) and the Farnesoid X receptor (FXR). BS may improve metabolism by affecting the concentrations and compositions of BAs. For example, SG improved glucose homeostasis by increasing the levels of circulating BAs and the signals of BAs via TGR5. Experiments in mice have shown that relative to TGR5 +/+ mice, the weight‐independent improvements in fasting plasma glucose, glucose tolerance, and hepatic insulin signaling following SG were attenuated in TGR5 −/− mice. 145 , 146 Similarly, notoginsenoside Ft1 was identified as a TGR5 agonist in vitro, which promoted fat browning in adipose tissue, increased lipolysis, and induced GLP‐1 secretion in obese mice, and these effects were not observed in TGR5 −/− mice. 147 The signals of BAs also act through another receptor: FXR. Ryan et al found that in the absence of FXR, the ability of SG to reduce body weight and improve glucose tolerance was greatly reduced. 148 Lili Ding et al also demonstrated that FXR knockout mice fed a high‐fat diet were resistant to the beneficial metabolic effects of SG. 149 These findings suggest that changes in the circulating BAs pool after BS play an important role in metabolic improvement through TGR5 and FXR. Treatment with the TGR5/FXR coagonist INT‐767 resulted in weight loss and improved glucose tolerance in obese mice. 150 However, the role of FXR in obesity and T2D remains controversial, with conflicting results reported in different studies. For instance, gut‐restricted FXR agonist fexaramine (Fex) was shown to enhance thermogenesis and browning of WAT, reducing obesity and insulin resistance in mice. 151 In contrast, tempol reduced obesity in mice by increasing intestinal tauro‐β‐muricholic acid, a FXR nuclear receptor antagonist. 152 This discrepancy led researchers to focus on FGF19, a downstream target gene of FXR. In ileal cells, BAs activate FXR and its downstream target, FGF19. FGF19 enters the liver through the portal venous circulation to bind to its receptor and represses BAs synthesis by inhibiting CYP7A1 (cholesterol 7a‐hydroxylase, a rate‐limiting enzyme for BAs synthesis). 153 In a study of 115 patients with T2D who underwent RYGB, those who experienced complete remission of T2D after surgery were found to have higher levels of FGF19, this suggested an important role for the FGF19‐CYP7A1‐BAs pathway in the etiology and remission of T2D after RYGB. 154 FGF19 variant M70 (NGM282) has been shown to reduce liver fat content in humans. As mentioned previously, FGF19 levels are increased after BS, suggesting that it may be a potential target for mediating the beneficial effects of BS, but the specific pathways by which it is mediated remain unclear. Studies have shown that the changes in compositions of BAs after BS such as the increase of lithocholic acid in the portal vein of mice following SG induced the production of cholic acid‐7‐sulfate (CA7S) by activating the vitamin D receptor, which acted on the TGR5 to induce GLP‐1 secretion, 155 thereby improving metabolism. 156 It has been hypothesized that the increased delivery of BAs to distal L‐cells may contribute to the increase of gut peptide secretion after BS, but research 157 showed that GLP‐1 and PYY increased rapidly after surgery, whereas BAs significantly increased at 1 year after BS, indicating that BAs do not seem to be the key regulator of the early postoperative increase of gut peptide. In addition, the changes of intestinal BAs after BS are controversial for the improvement of metabolism. For example, restoration of small intestinal BAs levels partially blocked the beneficial effects of SG in mice, 149 whereas some studies have found that in obese rats, the alterations in the gut microbiome caused by RYGB result in an increase in luminal and systemic pools of taurine‐conjugated bile acids, which induce signaling through FXR and TGR5 to improve metabolism. 158 The difference may be related to the different surgical procedures and species of BAs.
2.7. Changes of branched‐chain amino acids
The levels of circulating BCAAs (leucine, isoleucine and valine) were significantly elevated in individuals with T2D or obesity with insulin resistance. 159 , 160 , 161 BAT utilizes BCAAs in mitochondria for thermogenesis and controls BCAAs clearance through solute carrier family 25 member 44, thereby improving metabolism and in turn, defects of BCAAs catabolism in BAT were associated with obesity in mice. 159 , 162 BCAAs decreased significantly after RYGB, BPD, and SG 163 , 164 and this change lasted up to 12 months after RYGB and SG. 165 In addition, some studies have shown that although both BS (such as GBP and RYGB) and calorie restriction resulted in significant weight loss, the former induced a reduction in BCAAs levels but the latter did not, suggesting a BS‐dependent mechanism for BCAAs reduction. 166 , 167 The effects of sodium phenylbutyrate (NaPB) on metabolic health in 16 patients with T2D were evaluated in a randomized, placebo‐controlled trial. The results showed that NaPB resulted in an 8% reduction in BCAAs levels at 2 weeks, a 27% improvement in peripheral glucose disposal, and an increase in muscle mitochondrial oxidative capacity. These findings suggest that NaPB, a promoter of BCAAs catabolism, may be a promising treatment approach for T2D. 168 However, Kramer et al indicated that increased circulating BCAAs did not attenuate the benefits of SG in mice, suggesting that reductions in BCAAs were not essential for sustained weight loss and improved glucose tolerance following SG. 169 Whether the reductions of BCAAs after BS play an independent role remains to be demonstrated.
2.8. Metabolic alterations in the gut microbiota
Emerging evidence has shown that obese individuals have abnormal gut microbiota 170 , 171 , 172 , 173 and human microbiome influences insulin sensitivity. 160 Over the years, it identified the alterations of major gut microbiota in severe obesity, which include reduced microbial gene richness (MGR) and associated functional pathways related with metabolic deterioration. AGB and RYGB increased MGR at 1 year after surgery, improved metabolism and inflammation in 61 severe obese subjects, and was associated with changes in gut microbiota. 174 Specifically, SG resulted in an increase in the abundance of Bacteroides thetaiotaomicron in mice 156 and a decrease in serum glutamate concentration, which partially reversed obesity‐related microbial and metabolic alterations. 175 In addition, obese mice gavaged with Bacteroidetes spp. exhibited attenuated body‐weight gain 175 and improved BCAAs catabolism in BAT. 162 These results identify the links between obesity, intestinal microbiota, and circulating amino acids, suggesting that it is possible to intervene in obese individuals by targeting the Bacteroides probiotics. Cecal Prevotella copri was significantly enriched in Goto‐Kakizaki rats with spontaneous T2D after SG, and glucose homeostasis was improved through enhanced bile acid metabolism and FXR signaling. 176 However, Bacteroides vulgatus and Prevotella copri are mainly involved in the biosynthesis of BCAAs, thereby increasing the levels of BCAAs and inducing insulin resistance in mice. 160 A similar paradox is that BCAAs supplementation is beneficial for energy expenditure, but increased circulating levels of BCAAs are detrimental to metabolism, so the mechanisms of these paradoxes need to be further explored. 159 In recent years, Chaudhari et al have found that the altered gut microbiota (decreased Clostridia) following SG produced a microbial metabolism‐CA7S that increased plasma GLP‐1 level, thereby remodeling the gut‐liver axis to improve metabolism. 156 They also showed that transferring of post‐SG microbiota to germ‐free mice recreated the CA7S pathway. 156 In other words, changes in gut microbiota, Bas, and intestinal hormones resulting from alterations in gut anatomy and physiology connect the gut‐liver axis. Fecal microbiota transplantation (FMT) is generally performed in mice, and it has also been conducted in humans. Vrieze et al 177 showed that insulin sensitivity increased 6 weeks after infusion of lean donor gut microbiota in male recipients with metabolic syndrome. Allegretti et al 178 conducted a randomized trial to investigate the effects of FMT (derived from lean donors) in obese, metabolically uncompromised patients and showed that FMT did not reduce BMI in recipients but resulted in sustained changes in the gut microbiome and bile acid profile similar to lean donors. Recognized microbial changes following SG and RYGB include an increase in the relative abundance of Proteobacteria and a decrease in Firmicutes. 179 An increase in Proteobacteria has also been reported after improvement in glucose homeostasis induced by metformin treatment, suggesting that Proteobacteria may be involved in metabolic improvement. 180 Proteobacteria was increased in the fecal contents of mice in the SG group compared with the sham‐operated group. 156 Firmicutes (dominant in obese individuals) and Romboutsia were significantly decreased in individuals after BS (such as GBP, RYGB, and SG), which is associated with significant weight loss, improved insulin resistance, and decreased systemic inflammation. 181 , 182 , 183 In obese and T2D mice, the abundance of Akkermansia muciniphila in the gut is decreased, and its elevation after BS 44 has been reported to reduce fat mass, improve metabolism in mice 184 , 185 and humans, 186 increase thermogenesis by inducing UCP‐1 in BAT, and induce systemic GLP‐1 secretion in mice. 187 Recently, Munzker et al have shown that depletion of the gut microbiota largely reversed the beneficial effects of GBP and intestinal microbiota after surgery regulated metabolism by reactivating thermogenesis in BAT through the FXR‐TGR5 crosstalk. 158 Tremaroli et al found similar and durable gut microbiome changes in patients undergoing RYGB or VBG that were independent of body weight. Moreover, the surgically altered microbiome was demonstrated by FMT to promote the reduction in fat deposition, which further suggests that the gut microbiota may play a direct role in weight loss after BS. 188 A recent study revealed that absolute deficiency of bacterial biotin producers and transporters was correlated with inflammatory phenotype and metabolic disorders in obese individuals. BS increased bacterial biotin producers to improve host systemic biotin in humans and mice, thereby improving metabolism and inflammation. 189 A Chinese study found that probiotics + berberine (a natural bacteriostatic alkaloid derived from Berberis aristata and Huanglian) had better effects on lowering glycated hemoglobin in 409 participants with T2D. The result showed that berberine exerted glucose‐lowering effects through potential microbial mechanisms, further illustrating the critical role of gut microbiome in regulating host metabolism. 190 In the future, combined management using gut‐centered therapies and B vitamins, including biotin, appears to be of interest in preventing the transition of obesity and T2D to a more severe metabolic state. Samczuk et al 191 indicated that the recovery rate of T2D after SG may be related to the changes in gut microbiota composition and its effect on mitochondrial metabolism, and more investigations are still needed to explore these findings.
2.9. Changes of different brain function and food preferences
Obesity negatively affects brain function. Now, increasing studies have used fMRI to observe changes in brain activity in response to food cues in an effort to gain a deeper understanding about the mechanisms of BS. RYGB resulted in different brain responses compared with a very‐low‐calorie diets in clinical trials: RYGB resulted in a more active homeostatic appetite system, as well as reduced neural activation in response to food cues in cognitive control regions and responsiveness to food cues in the reward center of the brain, 192 resulting in favorable changes in food rewards and preferences. 10 , 193 Another study, by utilizing functional brain imaging, reported that brain responses to high‐fat milkshake cues normalized at 1 year following RYGB in obese participants. 194 In the fMRI study designed by De Silva et al, combined administration of PYY (3–36) and GLP‐1 (7–36 amide) to 15 fasted human subjects resulted in reduction in energy intake and brain activity. 195 A study designed by Farr et al revealed that elevated GIP levels were associated with deactivation of insula related to attention and reward and decreased leptin levels were associated with activation or deactivation of different brain regions. 196 GLP‐1R has also been demonstrated to be expressed in the hypothalamus, medulla oblongata, parietal cortex and so on. 197 The effects of these gut hormones on the brain highlight the importance of the gut‐brain axis in controlling reward‐based eating behavior. The pro‐opio‐melanocortin (POMC) neurons and agouti‐related protein (AGRP) neurons located in the arcuate nucleus of the hypothalamus are the cores of gut‐brain axis, regulating blood glucose and metabolism through changes in their excitability. For instance, GLP‐2 increases the excitability of POMC neurons by activating GLP‐2R‐PI3K signaling, thereby reducing hepatic glucose production. 198 However, existing studies are inconsistent with the changes of activity of POMC and AGRP neurons following BS by measuring the mRNA expression. In addition, the decrease in adipokines and inflammatory factors after BS may also be related to favorable changes in brain volume and cerebral blood flow. 199 , 200
Gustatory and olfactory function assessments in 68 participants undergoing RYGB or SG showed that BS may have positive effects on gustatory and olfactory function and eating behavior, with decreased hunger after surgery. 201 However, the results from sensory studies are variable and limited and it has been showed that gustatory changes are not associated with the surgery‐mediated alterations in major intestinal appetite‐regulating hormones. 202 Changes in food preferences and choices may contribute to the long‐term benefits of BS. Specifically, many studies have reported changes of food preferences following BS, including reductions in total fat and calorie intake and an increase in protein intake, and these changes were more common among participants who undergoing RYGB. 203 , 204 , 205 , 206 , 207 Elevated intestinal hormones such as GLP‐1, PYY, and OXM have been suggested as possible mediators of the beneficial effects of RYGB on appetite and food preferences. In addition to the changes in intestinal hormones, changes in food preferences may also be related to postoperative changes in taste sensitivity, and conditioned avoidance and related changes in feeding motivation learning after RYGB may be candidate mediators. 208 In addition, Smith et al found that the taste‐induced activation in the ventral tegmental area changed greatly after RYGB, suggesting that RYGB may more effectively reset the neural processing of reward stimulation in obese patients, thereby rescuing the blunted activation of the mesolimbic pathways, 209 but found that this effect seemed to be temporary at 1 year of follow‐up. 210 However, there are also studies reporting that RYGB and SG did not affect food preferences, much research remains to be explored in the future. 211 , 212
3. LIMITATIONS OF THE CURRENT STUDIES
As an invasive surgery, BS, coupled with potential side effects such as postoperative infection, anastomotic fistula, and malnutrition, cannot be widely applied to the population with surgical indications, and people are more inclined to choose pharmaceutical treatment. Therefore, most existing study populations on BS are limited, and future validation in larger populations is needed.
Most invasive research, such as studying changes in the composition of BAs in the portal vein after BS, has been conducted in animal models such as mice. We should be skeptical that whether the changes observed in animal studies are similar in humans. Although animal studies may provide ideas, the results should not be blindly extrapolated to humans without caution.
The study design, especially the control of confounding factors, should be determined before establishing the animal model of BS, so as to obtain convincing results. In addition, the involvement of other mechanisms should be considered comprehensively when studying one mechanism. For example, the secretion of intestinal hormones after BS is not only influenced by structural changes of the gastrointestinal tract but also by the brain. Therefore, future research should consider the comprehensive interaction among these mechanisms.
4. CONCLUSIONS
In this review, we comprehensively explore the multifactorial mechanisms underlying the beneficial effects of BS on weight loss and metabolism improvement (Figure 1). BS leads to weight loss, thereby reducing the fat content, changing the intestinal hormones, BAs, BCAAs, gut microbiota, signaling proteins (adipokines, myokines, hepatokines), GDF15, exosomes, brain function, and food preferences. However, as described in this review, none of these mechanisms appears to fully explain the beneficial effects of BS and different perspectives on the underlying mechanisms of BS remain to be elucidated. Further studies are needed to uncover the mechanisms behind BS, so as to provide new ideas for the treatment of obesity and metabolic disorders.
FIGURE 1.
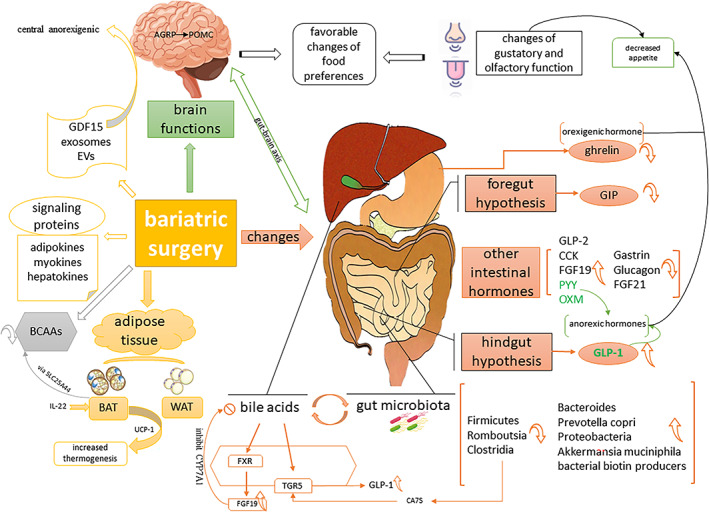
Main mechanisms associated with weight loss and remission of type 2 diabetes after bariatric surgery. The changes in gastrointestinal structure and function after bariatric surgery alter circulating hormones, gut microbiota, bile acids, thereby improving metabolism by ameliorating insulin resistance, reducing hepatic glucose output, and decreasing inflammation. In addition, the changes in adipose tissue and related factors after bariatric surgery also improve insulin resistance and reduce inflammation. At the same time, alterations in intestinal factors (through the gut‐brain axis), the central effects of certain factors such as GDF15, and the bariatric surgery itself directly or indirectly affect brain functions, thereby altering food selection, regulating appetite, and reducing energy intake. Together, these factors contribute to the remission of obesity and type 2 diabetes. AGRP, agouti‐related protein; BAT, brown adipose tissue; BCAAs, branched‐chain amino acids; CA7S, cholic acid‐7‐sulfate; CCK, cholecystokinin; CYP7A1, cholesterol 7a‐hydroxylase; EVs, extracellular vesicles; FGF‐19, fibroblast growth factor‐19; FGF‐21, fibroblast growth factor‐21; FXR, farnesoid X receptor; GDF15, growth differentiation factor 15; GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon‐like peptide‐1; GLP‐2, glucagon‐like peptide‐2; IL‐27, interleukin‐27; OXM, oxyntomodulin; POMC, pro‐opio‐melanocortin; PYY, peptide YY; SLC25A44, solute carrier family 25 member 44; TGR5, takeda G‐protein receptor 5; UCP‐1, uncoupling protein 1; WAT, white adipose tissue.
AUTHOR CONTRIBUTIONS
Mengsha Yin wrote the manuscript. Yao Wang and Mingyue Han designed the illustrations. Ruishuang Liang and Shanshan Li helped to analyze literature. Guixia Wang and Xiaokun Gang edited and revised the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
ACKNOWLEDGEMENTS
The authors' work reported herein was supported by the National Natural Science Foundation of China (Grant no. 81972372 and Grant no. 82272993).
Yin M, Wang Y, Han M, et al. Mechanisms of bariatric surgery for weight loss and diabetes remission. Journal of Diabetes. 2023;15(9):736‐752. doi: 10.1111/1753-0407.13443
Contributor Information
Guixia Wang, Email: gwang168@jlu.edu.cn.
Xiaokun Gang, Email: gangxk@jlu.edu.cn.
REFERENCES
- 1. Chew NWS, Ng CH, Tan DJH, et al. The global burden of metabolic disease: data from 2000 to 2019. Cell Metab. 2023;35(3):414‐28 e3. [DOI] [PubMed] [Google Scholar]
- 2. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373‐392. [DOI] [PubMed] [Google Scholar]
- 3. Conway B, Rene A. Obesity as a disease: no lightweight matter. Obes Rev. 2004;5(3):145‐151. [DOI] [PubMed] [Google Scholar]
- 4. Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction: novel roles of Immunometabolism in macrophage activation and inflammation. Circ Res. 2020;126(6):789‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng ACT, Delgado V, Borlaug BA, Bax JJ. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Rev Cardiol. 2021;18(4):291‐304. [DOI] [PubMed] [Google Scholar]
- 6. Marcelin G, Silveira ALM, Martins LB, Ferreira AV, Clement K. Deciphering the cellular interplays underlying obesity‐induced adipose tissue fibrosis. J Clin Invest. 2019;129(10):4032‐4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hainer V, Toplak H, Mitrakou A. Treatment modalities of obesity: what fits whom? Diabetes Care. 2008;31(Suppl 2):S269‐S277. [DOI] [PubMed] [Google Scholar]
- 8. Bessesen DH, Van Gaal LF. Progress and challenges in anti‐obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6(3):237‐248. [DOI] [PubMed] [Google Scholar]
- 9. Kassirer JP, Angell M. Losing weight – an ill‐fated new Year's resolution. N Engl J Med. 1998;338(1):52‐54. [DOI] [PubMed] [Google Scholar]
- 10. Salem V, Demetriou L, Behary P, et al. Weight loss by low‐calorie diet versus gastric bypass surgery in people with diabetes results in divergent brain activation patterns: a functional MRI study. Diabetes Care. 2021;44(8):1842‐1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Courcoulas AP, Yanovski SZ, Bonds D, et al. Long‐term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg. 2014;149(12):1323‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campos GM, Khoraki J, Browning MG, Pessoa BM, Mazzini GS, Wolfe L. Changes in utilization of bariatric surgery in the United States from 1993 to 2016. Ann Surg. 2020;271(2):201‐209. [DOI] [PubMed] [Google Scholar]
- 13. Phillips BT, Shikora SA. The history of metabolic and bariatric surgery: development of standards for patient safety and efficacy. Metabolism. 2018;79:97‐107. [DOI] [PubMed] [Google Scholar]
- 14. Laferrere B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479‐2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maggard MA, Shugarman LR, Suttorp M, et al. Meta‐analysis: surgical treatment of obesity. Ann Intern Med. 2005;142(7):547‐559. [DOI] [PubMed] [Google Scholar]
- 16. Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 2008;31(Suppl 2):S290‐S296. [DOI] [PubMed] [Google Scholar]
- 17. Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux‐en‐Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30(7):1709‐1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide‐YY levels after Roux‐en‐Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401‐407. [DOI] [PubMed] [Google Scholar]
- 19. Stratis C, Alexandrides T, Vagenas K, Kalfarentzos F. Ghrelin and peptide YY levels after a variant of biliopancreatic diversion with Roux‐en‐Y gastric bypass versus after colectomy: a prospective comparative study. Obes Surg. 2006;16(6):752‐758. [DOI] [PubMed] [Google Scholar]
- 20. Laferrère B, Swerdlow N, Bawa B, et al. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95(8):4072‐4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243(1):108‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature. 1999;402(6762):656‐660. [DOI] [PubMed] [Google Scholar]
- 23. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908‐913. [DOI] [PubMed] [Google Scholar]
- 24. Muller TD, Nogueiras R, Andermann ML, et al. Ghrelin. Mol Metab. 2015;4(6):437‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skuratovskaia D, Vulf M, Chasovskikh N, et al. The links of ghrelin to Incretins, insulin, glucagon, and leptin after bariatric surgery. Front Genet. 2021;12:612501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leonetti F, Silecchia G, Iacobellis G, et al. Different plasma ghrelin levels after laparoscopic gastric bypass and adjustable gastric banding in morbid obese subjects. J Clin Endocrinol Metab. 2003;88(9):4227‐4231. [DOI] [PubMed] [Google Scholar]
- 27. Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet‐induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623‐1630. [DOI] [PubMed] [Google Scholar]
- 28. Geloneze B, Tambascia MA, Pilla VF, Geloneze SR, Repetto EM, Pareja JC. Ghrelin: a gut‐brain hormone: effect of gastric bypass surgery. Obes Surg. 2003;13(1):17‐22. [DOI] [PubMed] [Google Scholar]
- 29. Hanusch‐Enserer U, Cauza E, Brabant G, et al. Plasma ghrelin in obesity before and after weight loss after laparoscopical adjustable gastric banding. J Clin Endocrinol Metab. 2004;89(7):3352‐3358. [DOI] [PubMed] [Google Scholar]
- 30. Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150(6):2518‐2525. [DOI] [PubMed] [Google Scholar]
- 31. Khoo CM, Muehlbauer MJ, Stevens RD, et al. Postprandial metabolite profiles reveal differential nutrient handling after bariatric surgery compared with matched caloric restriction. Ann Surg. 2014;259(4):687‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288(2):E447‐E453. [DOI] [PubMed] [Google Scholar]
- 33. Mingrone G, Castagneto‐Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab. 2009;35(6 Pt 2):518‐523. [DOI] [PubMed] [Google Scholar]
- 34. Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244(5):741‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rubino F, Marescaux J. Effect of duodenal‐jejunal exclusion in a non‐obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martinussen C, Bojsen‐Moller KN, Dirksen C, et al. Augmented GLP‐1 secretion as seen after gastric bypass may Be obtained by delaying carbohydrate digestion. J Clin Endocrinol Metab. 2019;104(8):3233‐3244. [DOI] [PubMed] [Google Scholar]
- 37. Jorsal T, Rhee NA, Pedersen J, et al. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia. 2018;61(2):284‐294. [DOI] [PubMed] [Google Scholar]
- 38. Jacobsen SH, Bojsen‐Moller KN, Dirksen C, et al. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose‐tolerant individuals. Diabetologia. 2013;56(10):2250‐2254. [DOI] [PubMed] [Google Scholar]
- 39. Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22(9):2003‐2009. [DOI] [PubMed] [Google Scholar]
- 40. Larraufie P, Roberts GP, McGavigan AK, et al. Important role of the GLP‐1 Axis for glucose homeostasis after bariatric surgery. Cell Rep. 2019;26(6):1399‐408 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fehmann HC, Habener JF. Galanin inhibits proinsulin gene expression stimulated by the insulinotropic hormone glucagon‐like peptide‐I(7‐37) in mouse insulinoma beta TC‐1 cells. Endocrinology. 1992;130(5):2890‐2896. [DOI] [PubMed] [Google Scholar]
- 42. Fehmann HC, Habener JF. Insulinotropic hormone glucagon‐like peptide‐I(7‐37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC‐1 cells. Endocrinology. 1992;130(1):159‐166. [DOI] [PubMed] [Google Scholar]
- 43. Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon‐like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987;84(10):3434‐3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Albaugh VL, Banan B, Antoun J, et al. Role of bile acids and GLP‐1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology. 2019;156(4):1041‐51 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schirra J, Sturm K, Leicht P, Arnold R, Goke B, Katschinski M. Exendin(9‐39)amide is an antagonist of glucagon‐like peptide‐1(7‐36)amide in humans. J Clin Invest. 1998;101(7):1421‐1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jimenez A, Casamitjana R, Viaplana‐Masclans J, Lacy A, Vidal J. GLP‐1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care. 2013;36(7):2062‐2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vetter ML, Wadden TA, Teff KL, et al. GLP‐1 plays a limited role in improved glycemia shortly after Roux‐en‐Y gastric bypass: a comparison with intensive lifestyle modification. Diabetes. 2015;64(2):434‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Douros JD, Lewis AG, Smith EP, et al. Enhanced glucose control following vertical sleeve gastrectomy does not require a beta‐cell glucagon‐like peptide 1 receptor. Diabetes. 2018;67(8):1504‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boland BB, Mumphrey MB, Hao Z, et al. Combined loss of GLP‐1R and Y2R does not alter progression of high‐fat diet‐induced obesity or response to RYGB surgery in mice. Mol Metab. 2019;25:64‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guidone C, Manco M, Valera‐Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55(7):2025‐2031. [DOI] [PubMed] [Google Scholar]
- 51. Kieffer TJ. GIP or not GIP? That is the question. Trends Pharmacol Sci. 2003;24(3):110‐112. [DOI] [PubMed] [Google Scholar]
- 52. Chen S, Okahara F, Osaki N, Shimotoyodome A. Increased GIP signaling induces adipose inflammation via a HIF‐1α‐dependent pathway and impairs insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2015;308(5):E414‐E425. [DOI] [PubMed] [Google Scholar]
- 53. Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8(7):738‐742. [DOI] [PubMed] [Google Scholar]
- 54. Kanemaru Y, Harada N, Shimazu‐Kuwahara S, et al. Absence of GIP secretion alleviates age‐related obesity and insulin resistance. J Endocrinol. 2020;245(1):13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paschetta E, Hvalryg M, Musso G. Glucose‐dependent insulinotropic polypeptide: from pathophysiology to therapeutic opportunities in obesity‐associated disorders. Obes Rev. 2011;12(10):813‐828. [DOI] [PubMed] [Google Scholar]
- 56. Flatt PR. Effective surgical treatment of obesity may be mediated by ablation of the lipogenic gut hormone gastric inhibitory polypeptide (GIP): evidence and clinical opportunity for development of new obesity‐diabetes drugs? Diab Vasc Dis Res. 2007;4(2):151‐153. [DOI] [PubMed] [Google Scholar]
- 57. Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux‐en‐Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240(2):236‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First‐phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32(3):375‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mingrone G, Nolfe G, Gissey GC, et al. Circadian rhythms of GIP and GLP1 in glucose‐tolerant and in type 2 diabetic patients after biliopancreatic diversion. Diabetologia. 2009;52(5):873‐881. [DOI] [PubMed] [Google Scholar]
- 60. Gault VA, Irwin N, Green BD, et al. Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity‐related diabetes. Diabetes. 2005;54(8):2436‐2446. [DOI] [PubMed] [Google Scholar]
- 61. Angelini G, Salinari S, Castagneto‐Gissey L, et al. Small intestinal metabolism is central to whole‐body insulin resistance. Gut. 2021;70(6):1098‐1109. [DOI] [PubMed] [Google Scholar]
- 62. Mingrone G, Panunzi S, de Gaetano A, et al. Bariatric‐metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow‐up of an open‐label, single‐Centre, randomised controlled trial. Lancet. 2015;386(9997):964‐973. [DOI] [PubMed] [Google Scholar]
- 63. Mingrone G, Panunzi S, de Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10‐year follow‐up of an open‐label, single‐Centre, randomised controlled trial. Lancet. 2021;397(10271):293‐304. [DOI] [PubMed] [Google Scholar]
- 64. Salinari S, le Roux CW, Bertuzzi A, Rubino F, Mingrone G. Duodenal‐jejunal bypass and jejunectomy improve insulin sensitivity in Goto‐Kakizaki diabetic rats without changes in incretins or insulin secretion. Diabetes. 2014;63(3):1069‐1078. [DOI] [PubMed] [Google Scholar]
- 65. Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus Semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503‐515. [DOI] [PubMed] [Google Scholar]
- 66. Peterli R, Wolnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250(2):234‐241. [DOI] [PubMed] [Google Scholar]
- 67. Salminen P, Helmio M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux‐en‐Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux‐en‐Y gastric bypass on weight loss in patients with morbid obesity: the SM‐BOSS randomized clinical trial. JAMA. 2018;319(3):255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gronroos S, Helmio M, Juuti A, et al. Effect of laparoscopic sleeve gastrectomy vs Roux‐en‐Y gastric bypass on weight loss and quality of life at 7 years in patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. 2021;156(2):137‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Salminen P, Gronroos S, Helmio M, et al. Effect of laparoscopic sleeve gastrectomy vs Roux‐en‐Y gastric bypass on weight loss, comorbidities, and reflux at 10 years in adult patients with obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. 2022;157(8):656‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post‐translational processing. J Biol Chem. 1986;261(25):11880‐11889. [PubMed] [Google Scholar]
- 72. George SK, Uttenthal LO, Ghiglione M, Bloom SR. Molecular forms of glucagon‐like peptides in man. FEBS Lett. 1985;192(2):275‐278. [DOI] [PubMed] [Google Scholar]
- 73. Lee J, Koehler J, Yusta B, et al. Enteroendocrine‐derived glucagon‐like peptide‐2 controls intestinal amino acid transport. Mol Metab. 2017;6(3):245‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon‐like peptide 2. Proc Natl Acad Sci USA. 1996;93(15):7911‐7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rowland KJ, Trivedi S, Lee D, et al. Loss of glucagon‐like peptide‐2‐induced proliferation following intestinal epithelial insulin‐like growth factor‐1‐receptor deletion. Gastroenterology. 2011;141(6):2166‐75 e7. [DOI] [PubMed] [Google Scholar]
- 76. le Roux CW, Borg C, Wallis K, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon‐like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 77. Cazzo E, Pareja JC, Chaim EA, Geloneze B, Barreto MR, Magro DO. GLP‐1 and GLP‐2 levels are correlated with satiety regulation after Roux‐en‐Y gastric bypass: results of an exploratory prospective study. Obes Surg. 2017;27(3):703‐708. [DOI] [PubMed] [Google Scholar]
- 78. Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux‐En‐Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26(8):2231‐2239. [DOI] [PubMed] [Google Scholar]
- 79. Patel A, Yusta B, Matthews D, Charron MJ, Seeley RJ, Drucker DJ. GLP‐2 receptor signaling controls circulating bile acid levels but not glucose homeostasis in Gcgr(−/−) mice and is dispensable for the metabolic benefits ensuing after vertical sleeve gastrectomy. Mol Metab. 2018;16:45‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3‐36. N Engl J Med. 2003;349(10):941‐948. [DOI] [PubMed] [Google Scholar]
- 81. Viardot A, Heilbronn LK, Herzog H, Gregersen S, Campbell LV. Abnormal postprandial PYY response in insulin sensitive nondiabetic subjects with a strong family history of type 2 diabetes. Int J Obes (Lond). 2008;32(6):943‐948. [DOI] [PubMed] [Google Scholar]
- 82. Morínigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247(2):270‐275. [DOI] [PubMed] [Google Scholar]
- 83. Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux‐en‐Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90(1):359‐365. [DOI] [PubMed] [Google Scholar]
- 84. Morínigo R, Moizé V, Musri M, et al. Glucagon‐like peptide‐1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91(5):1735‐1740. [DOI] [PubMed] [Google Scholar]
- 85. Reshma DR, Laura JM, Anne C, et al. PYY‐dependent restoration of impaired insulin and glucagon secretion in type 2 diabetes following Roux‐En‐Y gastric bypass surgery. Cell Rep. 2016;15(5):944‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Claudia G, Sam DS, Michael W, et al. PYY plays a key role in the resolution of diabetes following bariatric surgery in humans. EBioMedicine. 2019;40:67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rehfeld JF. Incretin physiology beyond glucagon‐like peptide 1 and glucose‐dependent insulinotropic polypeptide: cholecystokinin and gastrin peptides. Acta Physiol (Oxf). 2011;201(4):405‐411. [DOI] [PubMed] [Google Scholar]
- 88. Dolo PR, Huang K, Widjaja J, et al. Distal gastric mucosa ablation induces significant weight loss and improved glycemic control in type 2 diabetes Sprague‐Dawley rat model. Surg Endosc. 2020;34(10):4336‐4346. [DOI] [PubMed] [Google Scholar]
- 89. Sundbom M, Holdstock C, Engstrom BE, Karlsson FA. Early changes in ghrelin following Roux‐en‐Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17(3):304‐310. [DOI] [PubMed] [Google Scholar]
- 90. Shak JR, Roper J, Perez‐Perez GI, et al. The effect of laparoscopic gastric banding surgery on plasma levels of appetite‐control, insulinotropic, and digestive hormones. Obes Surg. 2008;18(9):1089‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Grong E, Arbo IB, Thu OKF, Kuhry E, Kulseng B, Mårvik R. The effect of duodenojejunostomy and sleeve gastrectomy on type 2 diabetes mellitus and gastrin secretion in Goto‐Kakizaki rats. Surg Endosc. 2015;29(3):723‐733. [DOI] [PubMed] [Google Scholar]
- 92. Rhee NA, Wahlgren CD, Pedersen J, et al. Effect of Roux‐en‐Y gastric bypass on the distribution and hormone expression of small‐intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia. 2015;58(10):2254‐2258. [DOI] [PubMed] [Google Scholar]
- 93. Levan VH, Liddle RA, Green GM. Jejunal bypass stimulation of pancreatic growth and cholecystokinin secretion in rats: importance of luminal nutrients. Gut. 1987;28(Suppl):25‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kellum JM, Kuemmerle JF, O'Dorisio TM, et al. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211(6):763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Alessandro P. Action and therapeutic potential of oxyntomodulin. Mol Metab. 2014;3(3):241‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dakin CL, Gunn I, Small CJ, et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142(10):4244‐4250. [DOI] [PubMed] [Google Scholar]
- 97. Laurie LB, Qingling H, Theodore JB, Daniel JD. Oxyntomodulin and glucagon‐like peptide‐1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127(2):546‐558. [DOI] [PubMed] [Google Scholar]
- 98. Tao M, Su H, Bing X, et al. A novel long‐acting oxyntomodulin analogue eliminates diabetes and obesity in mice. Eur J Med Chem. 2020;203:112496. [DOI] [PubMed] [Google Scholar]
- 99. Behary P, Tharakan G, Alexiadou K, et al. Combined GLP‐1, Oxyntomodulin, and peptide YY improves body weight and Glycemia in obesity and prediabetes/type 2 diabetes: a randomized, single‐blinded, placebo‐controlled study. Diabetes Care. 2019;42(8):1446‐1453. [DOI] [PubMed] [Google Scholar]
- 100. Tan T, Behary P, Tharakan G, et al. The effect of a subcutaneous infusion of GLP‐1, OXM, and PYY on energy intake and expenditure in obese volunteers. J Clin Endocrinol Metab. 2017;102(7):2364‐2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Perakakis N, Kokkinos A, Peradze N, et al. Circulating levels of gastrointestinal hormones in response to the most common types of bariatric surgery and predictive value for weight loss over one year: evidence from two independent trials. Metabolism. 2019;101:153997. [DOI] [PubMed] [Google Scholar]
- 102. Farey JE, Preda TC, Fisher OM, et al. Effect of laparoscopic sleeve gastrectomy on fasting gastrointestinal, pancreatic, and adipose‐derived hormones and on non‐esterified fatty acids. Obes Surg. 2017;27(2):399‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Umeda LM, Silva EA, Carneiro G, Arasaki CH, Geloneze B, Zanella MT. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP‐1, and glucagon secretion in type 2 diabetic patients. Obes Surg. 2011;21(7):896‐901. [DOI] [PubMed] [Google Scholar]
- 104. Fisher FM, Chui PC, Antonellis PJ, et al. Obesity is a fibroblast growth factor 21 (FGF21)‐resistant state. Diabetes. 2010;59(11):2781‐2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Javier G‐A, José MG‐E, Victoria C, et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet‐ or surgically‐induced weight loss. Clin Nutr. 2016;36(3):861‐868. [DOI] [PubMed] [Google Scholar]
- 106. Guo JY, Chen HH, Lee WJ, Chen SC, Lee SD, Chen CY. Fibroblast growth factor 19 and fibroblast growth factor 21 regulation in obese diabetics, and non‐alcoholic fatty liver disease after gastric bypass. Nutrients. 2022;14(3):645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Harris L‐ALS, Smith GI, Mittendorfer B, et al. Roux‐en‐Y gastric bypass surgery has unique effects on postprandial FGF21 but not FGF19 secretion. J Clin Endocrinol Metab. 2017;102(10):3858‐3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Thöni V, Pfister A, Melmer A, et al. Dynamics of bile acid profiles, GLP‐1, and FGF19 after laparoscopic gastric banding. J Clin Endocrinol Metab. 2017;102(8):2974‐2984. [DOI] [PubMed] [Google Scholar]
- 109. Perakakis N, Kokkinos A, Peradze N, et al. Follistatins in glucose regulation in healthy and obese individuals. Diabetes Obes Metab. 2019;21(3):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ceperuelo‐Mallafre V, Llaurado G, Keiran N, et al. Preoperative circulating succinate levels as a biomarker for diabetes remission after bariatric surgery. Diabetes Care. 2019;42(10):1956‐1965. [DOI] [PubMed] [Google Scholar]
- 111. Auguet T, Terra X, Hernandez M, et al. Clinical and adipocytokine changes after bariatric surgery in morbidly obese women. Obesity (Silver Spring). 2014;22(1):188‐194. [DOI] [PubMed] [Google Scholar]
- 112. Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high‐molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58(10):1400‐1407. [DOI] [PubMed] [Google Scholar]
- 113. Sams VG, Blackledge C, Wijayatunga N, et al. Effect of bariatric surgery on systemic and adipose tissue inflammation. Surg Endosc. 2016;30(8):3499‐3504. [DOI] [PubMed] [Google Scholar]
- 114. Wolf RM, Jaffe AE, Steele KE, et al. Cytokine, chemokine, and cytokine receptor changes are associated with metabolic improvements after bariatric surgery. J Clin Endocrinol Metab. 2019;104(3):947‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Herder C, Peltonen M, Svensson PA, et al. Adiponectin and bariatric surgery: associations with diabetes and cardiovascular disease in the Swedish obese subjects study. Diabetes Care. 2014;37(5):1401‐1409. [DOI] [PubMed] [Google Scholar]
- 116. Linscheid P, Christ‐Crain M, Stoeckli R, et al. Increase in high molecular weight adiponectin by bariatric surgery‐induced weight loss. Diabetes Obes Metab. 2008;10(12):1266‐1270. [DOI] [PubMed] [Google Scholar]
- 117. Malin SK, Bena J, Abood B, et al. Attenuated improvements in adiponectin and fat loss characterize type 2 diabetes non‐remission status after bariatric surgery. Diabetes Obes Metab. 2014;16(12):1230‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive‐leptin concentrations in normal‐weight and obese humans. N Engl J Med. 1996;334(5):292‐295. [DOI] [PubMed] [Google Scholar]
- 119. Elmquist JK, Maratos‐Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1(6):445‐450. [DOI] [PubMed] [Google Scholar]
- 120. Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon‐like peptide‐1 secretion. Diabetes. 2003;52(2):252‐259. [DOI] [PubMed] [Google Scholar]
- 121. Riedl M, Vila G, Maier C, et al. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J Clin Endocrinol Metab. 2008;93(6):2307‐2312. [DOI] [PubMed] [Google Scholar]
- 122. Villarroya F, Cereijo R, Gavalda‐Navarro A, Villarroya J, Giralt M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J Intern Med. 2018;284(5):492‐504. [DOI] [PubMed] [Google Scholar]
- 123. Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24‐36. [DOI] [PubMed] [Google Scholar]
- 124. Chouchani ET, Kajimura S. Metabolic adaptation and maladaptation in adipose tissue. Nat Metab. 2019;1(2):189‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose‐tissue plasticity in health and disease. Cell. 2022;185(3):419‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang Q, Li D, Cao G, et al. IL‐27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature. 2021;600(7888):314‐318. [DOI] [PubMed] [Google Scholar]
- 127. Katsogiannos P, Kamble PG, Pereira MJ, et al. Changes in circulating cytokines and Adipokines after RYGB in patients with and without type 2 diabetes. Obesity (Silver Spring). 2021;29(3):535‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kerr AG, Andersson DP, Ryden M, Arner P, Dahlman I. Long‐term changes in adipose tissue gene expression following bariatric surgery. J Intern Med. 2020;288(2):219‐233. [DOI] [PubMed] [Google Scholar]
- 129. Derek KH, Ilona L, Jessica NK, et al. The short‐term and long‐term effects of bariatric/metabolic surgery on subcutaneous adipose tissue inflammation in humans. Metabolism. 2017;70:12‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Faramia J, Ostinelli G, Drolet‐Labelle V, Picard F, Tchernof A. Metabolic adaptations after bariatric surgery: adipokines, myokines and hepatokines. Curr Opin Pharmacol. 2020;52:67‐74. [DOI] [PubMed] [Google Scholar]
- 131. Faramia J, Hao Z, Mumphrey MB, et al. IGFBP‐2 partly mediates the early metabolic improvements caused by bariatric surgery. Cell Rep Med. 2021;2(4):100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Eddy AC, Trask AJ. Growth differentiation factor‐15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev. 2021;57:11‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Dolo PR, Widjaja J, Yao L, Hong J, Li C, Zhu X. The effect of bariatric surgery on the expression of growth differentiation Factor‐15/macrophage‐inhibitory Cytokine‐1 (GDF‐15/MIC‐1) in rat. Surg Endosc. 2022;36(8):6205‐6213. [DOI] [PubMed] [Google Scholar]
- 134. Dolo PR, Yao L, Liu PP, et al. Effect of sleeve gastrectomy on plasma growth differentiation factor‐15 (GDF15) in human. Am J Surg. 2020;220(3):725‐730. [DOI] [PubMed] [Google Scholar]
- 135. Kleinert M, Bojsen‐Moller KN, Jorgensen NB, et al. Effect of bariatric surgery on plasma GDF15 in humans. Am J Physiol Endocrinol Metab. 2019;316(4):E615‐E621. [DOI] [PubMed] [Google Scholar]
- 136. Frikke‐Schmidt H, Hultman K, Galaske JW, Jorgensen SB, Myers MG Jr, Seeley RJ. GDF15 acts synergistically with liraglutide but is not necessary for the weight loss induced by bariatric surgery in mice. Mol Metab. 2019;21:13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mastrototaro L, Roden M. Insulin resistance and insulin sensitizing agents. Metabolism. 2021;125:154892. [DOI] [PubMed] [Google Scholar]
- 138. Bae YU, Kim Y, Lee H, et al. Bariatric surgery alters microRNA content of circulating exosomes in patients with obesity. Obesity (Silver Spring). 2019;27(2):264‐271. [DOI] [PubMed] [Google Scholar]
- 139. Hubal MJ, Nadler EP, Ferrante SC, et al. Circulating adipocyte‐derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017;25(1):102‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Myronovych A, Peck BCE, An M, et al. Intestinal extracellular vesicles are altered by vertical sleeve gastrectomy. Am J Physiol Gastrointest Liver Physiol. 2021;320(2):G153‐G165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Eirin A, Meng Y, Zhu XY, et al. The micro‐RNA cargo of extracellular vesicles released by human adipose tissue‐derived mesenchymal stem cells is modified by obesity. Front Cell Dev Biol. 2021;9:660851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Brandao BB, Lino M, Kahn CR. Extracellular miRNAs as mediators of obesity‐associated disease. J Physiol. 2022;600(5):1155‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Alkandari A, Ashrafian H, Sathyapalan T, et al. Improved physiology and metabolic flux after Roux‐en‐Y gastric bypass is associated with temporal changes in the circulating microRNAome: a longitudinal study in humans. BMC Obes. 2018;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cereijo R, Taxeras SD, Piquer‐Garcia I, et al. Elevated levels of circulating miR‐92a are associated with impaired glucose homeostasis in patients with obesity and correlate with metabolic status after bariatric surgery. Obes Surg. 2020;30(1):174‐179. [DOI] [PubMed] [Google Scholar]
- 145. McGavigan AK, Garibay D, Henseler ZM, et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. 2017;66(2):226‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ding L, Sousa KM, Jin L, et al. Vertical sleeve gastrectomy activates GPBAR‐1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology. 2016;64(3):760‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ding L, Yang Q, Zhang E, et al. Notoginsenoside Ft1 acts as a TGR5 agonist but FXR antagonist to alleviate high fat diet‐induced obesity and insulin resistance in mice. Acta Pharm Sin B. 2021;11(6):1541‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ding L, Zhang E, Yang Q, et al. Vertical sleeve gastrectomy confers metabolic improvements by reducing intestinal bile acids and lipid absorption in mice. Proc Natl Acad Sci U S A. 2021;118(6):e2019388118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Pathak P, Liu H, Boehme S, et al. Farnesoid X receptor induces Takeda G‐protein receptor 5 cross‐talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem. 2017;292(26):11055‐11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Li F, Jiang C, Krausz KW, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bozadjieva N, Heppner KM, Seeley RJ. Targeting FXR and FGF19 to treat metabolic diseases‐lessons learned from bariatric surgery. Diabetes. 2018;67(9):1720‐1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Gerhard GS, Styer AM, Wood GC, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux‐en‐Y gastric bypass. Diabetes Care. 2013;36(7):1859‐1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chaudhari SN, Harris DA, Aliakbarian H, et al. Bariatric surgery reveals a gut‐restricted TGR5 agonist with anti‐diabetic effects. Nat Chem Biol. 2020;17(1):20‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Chaudhari SN, Luo JN, Harris DA, et al. A microbial metabolite remodels the gut‐liver axis following bariatric surgery. Cell Host Microbe. 2021;29(3):408‐24 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Steinert RE, Peterli R, Keller S, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1‐year prospective randomized pilot trial. Obesity (Silver Spring). 2013;21(12):E660‐E668. [DOI] [PubMed] [Google Scholar]
- 158. Munzker J, Haase N, Till A, et al. Functional changes of the gastric bypass microbiota reactivate thermogenic adipose tissue and systemic glucose control via intestinal FXR‐TGR5 crosstalk in diet‐induced obesity. Microbiome. 2022;10(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Yoneshiro T, Wang Q, Tajima K, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572(7771):614‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Helle KP, Valborg G, Henrik BN, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nat Int Weekly J Sci. 2016;535(7612):376‐381. [DOI] [PubMed] [Google Scholar]
- 161. Christopher BN, Jie A, James RB, et al. A branched‐chain amino acid‐related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):565‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Yoshida N, Yamashita T, Osone T, et al. Bacteroides spp. promotes branched‐chain amino acid catabolism in brown fat and inhibits obesity. iScience. 2021;24(11):103342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ahlin S, Cefalo C, Bondia‐Pons I, et al. Metabolite changes after metabolic surgery – associations to parameters reflecting glucose homeostasis and lipid levels. Front Endocrinol (Lausanne). 2021;12:786952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Tan HC, Hsu JW, Kovalik JP, et al. Branched‐chain amino acid oxidation is elevated in adults with morbid obesity and decreases significantly after sleeve gastrectomy. J Nutr. 2020;150(12):3180‐3189. [DOI] [PubMed] [Google Scholar]
- 165. Tan HC, Khoo CM, Tan MZ, et al. The effects of sleeve gastrectomy and gastric bypass on branched‐chain amino acid metabolism 1 year after bariatric surgery. Obes Surg. 2016;26(8):1830‐1835. [DOI] [PubMed] [Google Scholar]
- 166. Laferrere B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3(80):80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lips MA, Van Klinken JB, van Harmelen V, et al. Roux‐en‐Y gastric bypass surgery, but not calorie restriction, reduces plasma branched‐chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Diabetes Care. 2014;37(12):3150‐3156. [DOI] [PubMed] [Google Scholar]
- 168. Vanweert F, Neinast M, Tapia EE, et al. A randomized placebo‐controlled clinical trial for pharmacological activation of BCAA catabolism in patients with type 2 diabetes. Nat Commun. 2022;13(1):3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Bozadjieva KN, Evers SS, Shin JH, et al. The role of elevated branched‐chain amino acids in the effects of vertical sleeve gastrectomy to reduce weight and improve glucose regulation. Cell Rep. 2020;33(2):108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070‐11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Selma MV, Romo‐Vaquero M, Garcia‐Villalba R, Gonzalez‐Sarrias A, Tomas‐Barberan FA, Espin JC. The human gut microbial ecology associated with overweight and obesity determines ellagic acid metabolism. Food Funct. 2016;7(4):1769‐1774. [DOI] [PubMed] [Google Scholar]
- 172. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541‐546. [DOI] [PubMed] [Google Scholar]
- 173. Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity‐related metabolic dysfunction. Gastroenterology. 2017;152(7):1671‐1678. [DOI] [PubMed] [Google Scholar]
- 174. Aron‐Wisnewsky J, Prifti E, Belda E, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68(1):70‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight‐loss intervention. Nat Med. 2017;23(7):859‐868. [DOI] [PubMed] [Google Scholar]
- 176. Péan N, Le Lay A, Brial F, et al. Dominant gut Prevotella copri in gastrectomised non‐obese diabetic Goto‐Kakizaki rats improves glucose homeostasis through enhanced FXR signalling. Diabetologia. 2020;63(6):1223‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913‐6 e7. [DOI] [PubMed] [Google Scholar]
- 178. Allegretti JR, Kassam Z, Mullish BH, et al. Effects of fecal microbiota transplantation with Oral capsules in obese patients. Clin Gastroenterol Hepatol. 2020;18(4):855‐63 e2. [DOI] [PubMed] [Google Scholar]
- 179. Ben IM, Eshel A, Cohen R, et al. Projection of gut microbiome pre‐ and post‐bariatric surgery to predict surgery outcome. mSystems. 2021;6(3):e0136720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment‐naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850‐858. [DOI] [PubMed] [Google Scholar]
- 181. Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Dang JT, Mocanu V, Park H, et al. Roux‐en‐Y gastric bypass and sleeve gastrectomy induce substantial and persistent changes in microbial communities and metabolic pathways. Gut Microbes. 2022;14(1):2050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Zeng Q, Li D, He Y, et al. Discrepant gut microbiota markers for the classification of obesity‐related metabolic abnormalities. Sci Rep. 2019;9(1):13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107‐113. [DOI] [PubMed] [Google Scholar]
- 185. Everard A, Belzer C, Geurts L, et al. Cross‐talk between Akkermansia muciniphila and intestinal epithelium controls diet‐induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066‐9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Dao MC, Everard A, Aron‐Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426‐436. [DOI] [PubMed] [Google Scholar]
- 187. Yoon HS, Cho CH, Yun MS, et al. Akkermansia muciniphila secretes a glucagon‐like peptide‐1‐inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nature . Microbiology. 2021;6(5):563‐573. [DOI] [PubMed] [Google Scholar]
- 188. Tremaroli V, Karlsson F, Werling M, et al. Roux‐en‐Y gastric bypass and vertical banded Gastroplasty induce Long‐term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22(2):228‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Belda E, Voland L, Tremaroli V, et al. Impairment of gut microbial biotin metabolism and host biotin status in severe obesity: effect of biotin and prebiotic supplementation on improved metabolism. Gut. 2022;71(12):2463‐2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zhang Y, Gu Y, Ren H, et al. Gut microbiome‐related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11(1):5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Samczuk P, Hady HR, Adamska‐Patruno E, et al. In‐and‐out molecular changes linked to the type 2 diabetes remission after bariatric surgery: An influence of gut microbes on mitochondria metabolism. Int J Mol Sci. 2018;19(12):3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Scholtz S, Miras AD, Chhina N, et al. Obese patients after gastric bypass surgery have lower brain‐hedonic responses to food than after gastric banding. Gut. 2014;63(6):891‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Aukan MI, Brandsaeter IØ, Skårvold S, et al. Changes in hedonic hunger and food reward after a similar weight loss induced by a very low‐energy diet or bariatric surgery. Obesity (Silver Spring). 2022;30(10):1963‐1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Koenis MMG, Papasavas PK, Janssen RJ, Tishler DS. Pearlson GD brain responses to anticipatory cues and milkshake taste in obesity, and their relationship to bariatric surgery outcome. Neuroimage. 2021;245:118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. De Silva A, Salem V, Long CJ, et al. The gut hormones PYY 3‐36 and GLP‐1 7‐36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14(5):700‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Farr OM, Tsoukas MA, Triantafyllou G, et al. Short‐term administration of the GLP‐1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: a randomized, placebo‐controlled, crossover study. Metabolism. 2016;65(7):945‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Farr OM, Sofopoulos M, Tsoukas MA, et al. GLP‐1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP‐1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo‐controlled trial. Diabetologia. 2016;59(5):954‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Shi X, Zhou F, Li X, et al. Central GLP‐2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab. 2013;18(1):86‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Tuulari JJ, Karlsson HK, Antikainen O, et al. Bariatric surgery induces white and Grey matter density recovery in the morbidly obese: a voxel‐based morphometric study. Hum Brain Mapp. 2016;37(11):3745‐3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Minke HCN, Debby V, Maximilian W, Edo OA, Eric JH, Amanda JK. Obesity affects brain structure and function‐ rescue by bariatric surgery? Neurosci Biobehav Rev. 2020;108(C):646‐657. [DOI] [PubMed] [Google Scholar]
- 201. Melis M, Pintus S, Mastinu M, et al. Changes of taste, smell and eating behavior in patients undergoing bariatric surgery: associations with PROP phenotypes and polymorphisms in the odorant‐binding protein OBPIIa and CD36 receptor genes. Nutrients. 2021;13(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Bernard A, Le Beyec‐Le BJ, Radoi L, et al. Orosensory perception of fat/sweet stimuli and appetite‐regulating peptides before and after sleeve gastrectomy or gastric bypass in adult women with obesity. Nutrients. 2021;13(3):878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non‐sweets eaters. Ann Surg. 1987;205(6):613‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Kenler HA, Brolin RE, Cody RP. Changes in eating behavior after horizontal gastroplasty and Roux‐en‐Y gastric bypass. Am J Clin Nutr. 1990;52(1):87‐92. [DOI] [PubMed] [Google Scholar]
- 205. Ernst B, Thurnheer M, Wilms B, Schultes B. Differential changes in dietary habits after gastric bypass versus gastric banding operations. Obes Surg. 2009;19(3):274‐280. [DOI] [PubMed] [Google Scholar]
- 206. Althumiri NA, Basyouni MH, Al‐Qahtani FS, Zamakhshary M, BinDhim NF. Food taste, dietary consumption, and food preference perception of changes following bariatric surgery in the Saudi population: a cross‐sectional study. Nutrients. 2021;13(10):3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Guyot E, Dougkas A, Nazare JA, Bagot S, Disse E, Iceta S. A systematic review and meta‐analyses of food preference modifications after bariatric surgery. Obes Rev. 2021;22(10):e13315. [DOI] [PubMed] [Google Scholar]
- 208. Kapoor N, Al‐Najim W, le Roux CW, Docherty NG. Shifts in food preferences after bariatric surgery: observational reports and proposed mechanisms. Curr Obes Rep. 2017;6(3):246‐252. [DOI] [PubMed] [Google Scholar]
- 209. Smith KR, Papantoni A, Veldhuizen MG, et al. Taste‐related reward is associated with weight loss following bariatric surgery. J Clin Invest. 2020;130(8):4370‐4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Smith KR, Aghababian A, Papantoni A, et al. One year follow‐up of taste‐related reward associations with weight loss suggests a critical time to mitigate weight regain following bariatric surgery. Nutrients. 2021;13(11):3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Nielsen MS, Christensen BJ, Ritz C, et al. Roux‐En‐Y gastric bypass and sleeve gastrectomy does not affect food preferences when assessed by an ad libitum buffet meal. Obes Surg. 2017;27(10):2599‐2605. [DOI] [PubMed] [Google Scholar]
- 212. Søndergaard NM, Rasmussen S, Just CB, et al. Bariatric surgery does not affect food preferences, but individual changes in food preferences may predict weight loss. Obesity (Silver Spring). 2018;26(12):1879‐1887. [DOI] [PubMed] [Google Scholar]


