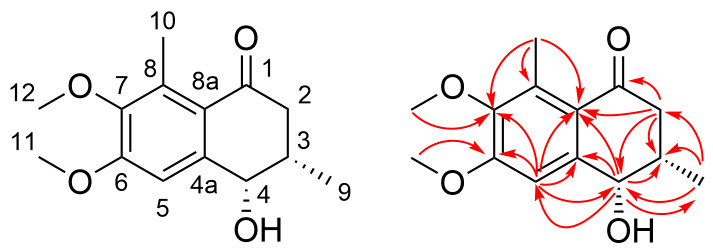
The structure of compound 1 of the original publication was misattributed and should be revised as shown in Figure 1. The error happened due to insufficient in-depth 2D NMR analysis. We reanalyzed the 2D NMR data of compound 1 in detail and finally determined the correct structure as shown in Figure 1. The revised structure of 1 is supported by the HMBC correlations of H-2/C-1, C-3, C-4, C-8a, H-4/C-3, C-4a, C-5, C-8a, C-9, H-5/C-4, C-4a, C-6, C-7, C-8a, H-9/C-2, C-3, C-4, H-10/C-7, C-8, C-8a, H-11/C-6, and H-12/C-7.
Figure 1.
Revised structure of compound 1 and key HMBC correlations.
Table 1 provides the revised 1D 1H and 13C NMR data of compound 1.
Table 1.
Revised 1H (600 MHz) and 13C NMR (150 MHz) data of compound 1 (δ in ppm, J in Hz, methanol-d4).
| No. | δH (mult, J, amount) | δC mult | No. | δH (mult, J, amount) | δC mult |
|
| |||||
| C-1 | 201.3 C | C-7 | 148.5 C | ||
| C-2 | 2.64 (dd, J = 17.2, 10.3, 1H) 2.48 (dd, J = 17.2, 4.7, 1H) |
43.7 CH2 | C-8 | 136.1 C | |
| C-3 | 2.36 (m, 1H) | 35.8 CH | C-8a | 124.5 C | |
| C-4 | 4.69 (d, J = 3.0, 1H) | 72.9 CH | C-9 | 1.09 (d, J = 6.8, 3H) | 16.3 CH3 |
| C-4a | 145.6 C | C-10 | 2.51 (s, 3H) | 14.1 CH3 | |
| C-5 | 7.02 (s, 1H) | 110.8 CH | C-11 | 3.96 (s, 3H) | 56.3 CH3 |
| C-6 | 158.0 C | C-12 | 3.73 (s, 3H) | 60.7 CH3 | |
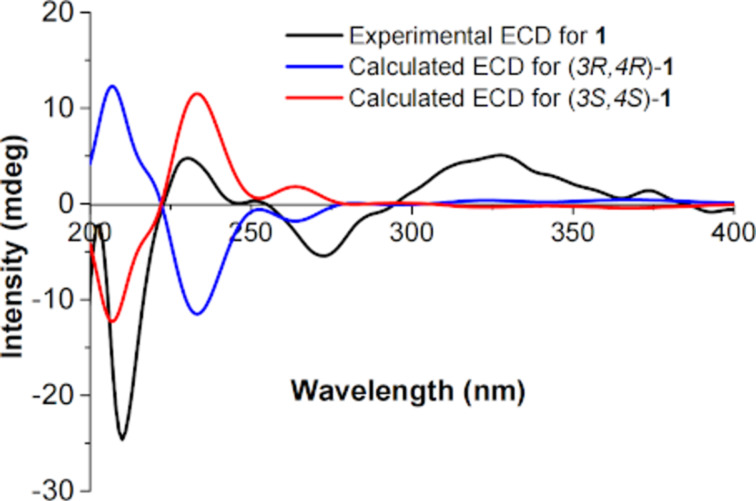
The structural revision of 1 also required recalculation of the theoretical ECD spectra of both enantiomers to determine the absolute configuration. By comparison of the recalculated and the experimental spectra, it became evident that in fact, the (3S,4S)-enantiomer rather than the (3R,4R)-enantiomer was obtained (Figure 2).
Figure 2.
Recalculated and experimental ECD spectra of compound 1.
Consequently, in the first paragraph of the Results and Discussion section in the original publication, the sentence “The HMBC correlations […] disclosed that C-10 is connected to C-5 in compound 1.” is inaccurate. Following the reanalysis of the HMBC correlations, it is now evident that C-10 is connected to C-8 in compound 1.
Contributor Information
Yong-Ming Yan, Email: yanym@szu.edu.cn.
Yong-Xian Cheng, Email: yxcheng@szu.edu.cn.