Abstract
Obstructive sleep apnea (OSA) is a sleep disorder of significant health concern with a high prevalence in the general population. It has been found to exhibit a high incidence of comorbidity with epilepsy, the exact underlying pathophysiology of which still remains poorly understood. OSA is characterized by apnea/hypopnea spells and arousals, leading to intermittent hypoxemia and sleep deprivation. Both sleep deprivation and hypoxemia adversely affect the cortical excitability and favor epileptogenesis and worsening of pre-existing epilepsy, if any. In patients with OSA, deprivation of rapid eye movement sleep (REMS) phase (known for its strong antiepileptic influence) is relatively more than that non rapid eye movement sleep phase leading to postulation of REMS deprivation as a significant factor in the development of epilepsy as a comorbidity in patients with OSA. Furthermore, OSA and epilepsy both have shown to exercise a bidirectional influence on one another and are also likely to exacerbate each other through a positive feedback mechanism. This is especially based on the reports of improved control of epilepsy upon treatment of comorbid OSA. This brief paper attempts to present an underlying pathophysiological basis of the comorbidity of OSA and epilepsy based upon sleep deprivation and hypoxemia that are characteristic features observed in patients with OSA.
Keywords: Arousals, hypoxemia, REM sleep, seizures, sleep deprivation, seizures
Introduction
Obstructive sleep apnea (OSA) is a sleep disorder characterized by spells of apnea and hypopnea due to the collapse of the upper airways during sleep, resulting in frequent arousals and microarousals. Diagnosis of OSA is based on an apnea/hypopnea index (AHI) ≥ 5 per hour with associated symptoms such as snoring, choking, daytime sleepiness, and fatigue, or an AHI ≥ 15 per hour [1]. Obstructive sleep apnea is of significant health concern and its prevalence is increasing over the years. According to Wisconsin Sleep Cohort Study in 1993, the prevalence reported was 24% in men and 9% in women [2]. However, between 2008 and 2013 the prevalence rose to 37% in men and 50% in women [3]. This increase in prevalence is partly due to less strict criteria for diagnosing OSA i.e., AHI ≥ 5 per hour. In a recent HypnoLaus study cohort, involving 2121 participants, 49.7% of men and 23.4% of women had moderate to severe OSA (based on AHI ≥ 15 per hour) [4]. Another recent study estimated that approximately 936 million adults aged 30-69 years (both men and women) worldwide have mild to severe obstructive sleep apnea and approximately 425 million adults aged 30-69 years globally have moderate to severe obstructive sleep apnea (AHI ≥ 15) [5]. The increase in OSA prevalence could be attributed to lifestyle changes, increase in obesity [6], increased awareness and improved diagnostic facilities [3].
Epilepsy is a worldwide common neurological disorder with an incidence rate of 61.4 per 100,000 person-years and the pooled lifetime prevalence of 7.6 per 1,000 persons [7]. The International League Against Epilepsy defined epilepsy to be a disease of the brain characterized by any of the following conditions: i) At least two unprovoked (or reflex) seizures occurring > 24 hours apart; ii) One unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years; iii) Diagnosis of an epilepsy syndrome [8]. Approximately one-third of patients with epilepsy (PWE) do not respond satisfactorily to antiepileptic drugs (AEDs) and are categorized as drug-resistant or intractable epilepsy patients [9].
Obstructive sleep apnea and epilepsy: the comorbidity
Comorbidity of OSA and epilepsy has been studied for decades and several authors have reported a high incidence of this comorbidity. In a recent meta-analysis based on 26 studies, 33.4% of PWE (40% adults and 26% children) were reported to have OSA (AHI ≥ 5 and RDI ≥ 5) [10]. Among these individuals both focal as well as generalized seizures were observed, accounting for 32% and 28.2% respectively, and a substantial proportion (19.5%) of these cases were refractory seizures [10]. In a recent multicentric PSG study of 166 patients with epilepsy, the prevalence of OSA was 38% [11]. Conversely, a study on 139 young children (0-17 months of age) with OSA, reported that approximately 17% of subjects had epilepsy as a comorbidity [12]. Another study, reported that out of 480 adult patients with sleep apnea syndrome, 4% had seizures [13]; in this study group almost all types of seizures were observed with a preponderance of primary generalized tonic-clonic seizure (> 50%) [13]. On account of the higher incidence of OSA in PWE, it has been suggested that all PWE should undergo sleep studies [14] for better evaluation and management, as treatment of OSA in PWE has resulted in better control of epilepsy [15-17].
Effect of epilepsy on obstructive sleep apnea
Epilepsy is known to exert a selective and specific influence on sleep and breathing. Epilepsy has been reported to lead to central apnea as well as obstructive apnea [18]; Seizures can potentially affect the firing in nerves innervating airway muscles. Central sleep apnea has been reported to be associated with electrographic seizures [19]. In a rat model of penicillin-induced seizures, decreased activity was seen in hypoglossal and vagal nerve with intact phrenic nerve activity initially leading to obstructive apnea, followed by decreased activity in phrenic nerve leading to central apnea [20]. Vagus nerve stimulation (VNS), an established and FDA-approved adjunctive therapy for medically refractory epilepsy, can lead to OSA or worsen pre-existing OSA [21,22]. These effects of VNS may be due to peripheral recurrent laryngeal nerve mediated vocal cord adduction [23] and centrally via vagal projections to medullary and pontine reticular formation [24]. Seizures of insular origin are known to be associated with apnea and feeling of choking [25]. Conversely, in one case of medically refractory epilepsy with OSA, resolution of clinically significant OSA was observed following left frontal lobe resection leading to a near seizure-free state [26]. Two case studies have reported an association of epileptiform discharges also with apneic episodes [27,28]. One case study has reported that nocturnal frontal lobe epilepsy (NFLE) with bifrontal epileptiform discharges on EEG manifested as an OSA [27], suggesting NFLE may potentially cause OSA. Therefore, epilepsy exhibits a strong predisposition to sleep apnea or worsening of a pre-existing OSA.
In addition, antiepileptic drugs can also influence symptoms of OSA [29]. Multiple AEDs are associated with weight gain, which could potentially worsen or increase the risk of OSA. AEDs causing weight gain include valproic acid, pregabalin, perampanel, and to a lesser degree, gabapentin and vigabatrin [30]. Patients with drug-resistant epilepsy on AED polytherapy may be at increased risk of obesity compared with those on monotherapy [31]. One case report documented weight gain associated with vigabatrin and the subsequent development of OSA in a patient with drug-resistant epilepsy [32]. Benzodiazepines, including clobazam and clonazepam, are often used in the treatment of drug-resistant epilepsy [33]. The use of benzodiazepines is associated with reduced upper airway muscle tone and ventilatory response to hypoxia [34]. Studies investigating the effect of benzodiazepines on OSA suggest that their use may be associated with a modest increase and prolongation of apneic events [35,36]. This highlights the importance of considering the risks and benefits of different AEDs when choosing a treatment plan for patients with epilepsy.
Effect of OSA on epilepsy
The exact underlying pathophysiology of the comorbidity of OSA and epilepsy, however, remains poorly understood. In OSA hypoxemia and sleep deprivation have been proposed to exacerbate epilepsy.
The hypoxemia theory
One of the pathophysiologic mechanisms leading to epilepsy or worsening of pre-existing epilepsy in OSA could be due to varying degrees of hypoxemia that occur intermittently during apnea/hypopnea spells [37-39]. Hypoxemic ischemic encephalopathy is one of the most common underlying causes of neonatal seizures [40]. The susceptibility to epilepsy is still higher in the immature brain as the development of the inhibitory neurotransmitter system (GABA) lags behind the excitatory neurotransmitter system [41]. In the mature brain too, hypoxemia may lead to seizure occurrence. A review of 44 studies (between 1954 and 2013) indicates that hypoxemic brain injury may lead to epilepsy even later in life [42].
Apart from direct ischemic damage, several other mechanisms have been proposed for the genesis of epilepsy due to hypoxemia. In classical experiments with dogs and cats, the seizure threshold for convulsant drugs was found to reduce after exposure to hypoxemia [43], supposedly due to the release of subcortical structures from cortical control [43]. Different brain areas have different susceptibility to damage by hypoxemia; and neocortex, which is responsible for the desynchronized activity, has been found to be relatively more susceptible [44,45]. Damage to the neocortex at moderate hypoxemia may allow subcortical structures to lead to synchronization [43], probably through thalamocortical oscillations. In humans acute anoxic episodes, requiring resuscitation, have been reported to result in epilepsy due to hypoxemic/anoxic damage of neural structures [46].
In OSA, intermittent rather than prolonged episodes of hypoxemia are generally observed. Alternating periods of intermittent hypoxemia (lasting usually for 10-40 seconds) and normoxia occur recurrently during sleep in OSA [47]. The intermittent hypoxia, a characteristic feature of OSA, has been shown to produce oxidative stress leading to increased apoptosis in the hippocampus [48]. Intermittent hypoxia has also been shown to induce a relatively more extensive neuronal damage than a similar degree of sustained hypoxia in rat pheochromocytoma PC12 neuronal cells [49]. Chronic intermittent hypoxia also has been shown to induce neural apoptosis in the hippocampus and prefrontal cortex in rats [50]. This intermittent hypoxia-induced neuronal injury is mediated via multiple pathways involving increased metabolism, induction of stress-induced proteins and apoptosis, leading to disruption of structural proteins and cellular integrity [51]. Hypoxemia in OSA may cause oxidative stress, which may in turn activate inflammatory pathway and production of interleukin-6 and tumor necrosis factor-α, that may cause seizures [52]. The brain’s unique susceptibility to oxidative stress and bioenergetic insults likely drives or at least exacerbates neuronal excitability during epileptogenesis because of high metabolic demand in hypersynchronous circuits [53].
However, several studies present contradictory evidence for the incriminatory role of hypoxemia, particularly with regard to comorbidity with OSA. The degree of desaturations and their durations observed in PWE and OSA are not severe and prolonged because apneic spells are terminated spontaneously or with brief arousals [54]. In some studies, hypoxemia was not considered the primary cause of seizures as the oxygen desaturation in PWE with OSA was not severe enough [14,16,55]. Furthermore, hypercapnia that generally accompanies hypoxemia during apneic spells in OSA can potentially protect against neuronal hyperexcitability [56]. Indeed, experimental studies in rats, macaques and humans have shown that five percent carbon dioxide inhalation has an anticonvulsant effect [57].
While hypoxemia, may exacerbate existing epilepsy through one or more mechanisms, the degree and duration of hypoxemia and along with co-occurring hypercapnia in OSA, challenge the theory of its incriminatory role in the comorbidity of OSA and epilepsy [58].
The sleep deprivation theory
The primary pathophysiologic mechanism responsible for the comorbidity of OSA and epilepsy could be due to sleep deprivation ensuing from intermittent and varying periods of arousals that occur during the apneic spells and several studies have reported an increase in seizure frequency after sleep deprivation [59,60] in PWE.
Increase in cortical excitability has been observed with total sleep deprivation in patients with juvenile myoclonic epilepsy, as determined by transcranial magnetic stimulation (TMS) [61]. In healthy subjects, the influence of sleep deprivation on cortical excitability as assessed by TMS is not in unison and the results are contradictory [62-68]. In one 120-hour sleep deprivation study [69] conducted on sixteen healthy subjects, five subjects showed an increased high voltage paroxysmal activity in their EEGs. In animal models also, sleep deprivation has been shown to increase the susceptibility to focal and generalized seizures [70,71]. Several authors have also implicated sleep deprivation and sleep fragmentation in the increased incidence of epilepsy in patients with OSA [16,72,73].
The cyclic alternating pattern (CAP) is a sequence of transient electrocortical events that recur at approximately 1-min intervals and are distinct from the tonic background activity. Phase A of CAP, characterized by synchronized EEG pattern with delta bursts and sequences of K-complexes, has been shown to correlate with interictal epileptiform discharges (IEDs) and occurrence of seizures [74-76]. Sleep deprivation increases the instability of morning recovery sleep and particularly enhances CAP A1 phases in patients with temporal lobe epilepsy (TLE) and has been proposed as a possible mechanism for the increased IED yield in sleep-deprived EEG in TLE patients [77]. Alterations in sleep micro-architecture such as increased CAP rates, duration and cycles have been reported in patients with OSA with excessive daytime sleepiness [78]. However, another study reported reduced CAP rate, longer duration of B phase and decreased A1 phase percentage in OSA subjects [79]. Therefore, the role of alterations in the microstructure of sleep in the comorbidity of OSA and epilepsy needs further exploration.
Sleep deprivation has been in vogue since long as a provocative technique for performing EEG in suspected cases of epilepsy [80,81]. The guidelines for sleep deprivation recommend total sleep deprivation for 24 hours [82]. However, in usual clinical practice, patients are advised to wake up 3-4 hours earlier than their usual waking time, which actually leads to partial sleep deprivation. The rapid eye movement sleep (REMS) distribution changes over successive sleep cycles and it gets proportionately longer during later cycles [83]. Therefore, partial sleep deprivation protocol actually leads to proportionately and relatively more of REMS deprivation than non rapid eye movement sleep. In humans, greater REMS rebound has been noticed with partial sleep deprivation compared to total sleep deprivation [84].
Selective rapid eye movement sleep deprivation theory
Rapid eye movement sleep is proposed to be inherently protective against epilepsy due to its characteristic feature, namely EEG-desynchronization, and REMS deprivation can potentially lead to epileptogenesis [85-87]. Apneic events occur more commonly during REMS due to accompanied decrease in airways muscle tone [88]; furthermore, the events are of relatively longer duration in REMS in comparison to non-rapid eye movement sleep [89,90]. Sleep architecture also alters in OSA and arousals during REMS can potentially restart sleep cycles resulting not only in shorter epochs and reduced total quantum of REMS but also fragmentation and poor quality of sleep [91]. An increase in stage N1 and N2 sleep with a corresponding decrease in REMS have been reported in adult patients with OSA [92-94], children [95] and in infants with sleep-disordered breathing (SDB) [96]. In these studies, increase with the severity of OSA was associated with reduction in REMS quanta. Another recent study reported significantly decreased REM sleep in OSA patients with comorbid epilepsy than patients with OSA without epilepsy [97]. Deprivation, fragmentation and poor quality of REMS have been reported in patients with OSA [93,98-100]. Interestingly, REMS rebound has been observed in many studies after treatment in patients with OSA indicating some degree of REMS deprivation prior to the treatment. In a meta-analysis of 14 studies evaluating the effect of continuous positive airway pressure (CPAP) on sleep architecture, 11 studies reported REMS rebound with 57% relative increase during the titration night compared to baseline sleep studies [101]. Some studies have reported increased total duration of REMS [98,99]; albeit, REMS fragmentation results in its poor quality [94]. An increase in REMS duration in such cases may be an adaptive compensatory behavior for the depth and quality of REMS. Another recent study has also highlighted the significance of REMS deprivation in the comorbidity of epilepsy and OSA [102].
In animal studies also REMS has been found to influence epileptogenesis. Rapid eye movement sleep deprivation decreased the threshold to electroconvulsive shock and nicotine-induced seizures in rats [103,104] and pentylenetetrazol-induced convulsions in mice [105]. On the other hand, enhancing REMS with carbachol resulted in decreased excitability in amygdala-kindled rats [106].
Several studies have reported an improvement in seizure control after OSA treatment in PWE [15-17]. In some studies, the improvement in seizure control was accompanied by improved sleep architecture and REMS rebound [37,107]. In one case report, there was a transient initial increase in seizures after institution of CPAP followed by a final decrease [108]. The REMS percent in that particular case initially showed a transient decrease that probably might have led to the initial increase in the seizure frequency.
Therefore, sleep deprivation and particularly REMS deprivation, appears to play a prominent role in the pathophysiology of the comorbidity of OSA and epilepsy. Further studies are required with selective REMS deprivation for exploring its role in this comorbidity.
Bidirectional relationship between OSA and epilepsy
Thus, the above evidence reveal an intimate and intrinsic association exhibiting a bidirectional relationship between OSA and epilepsy as illustrated in Figure 1. It appears that OSA and epilepsy exacerbate one another in a positive feedback manner, possibly through their actions on the neural networks involved in breathing control and sleep regulation, changes in cortical excitability or inflammatory processes, which can happen in both the conditions [61]. OSA can exacerbate epilepsy by causing sleep deprivation. Sleep deprivation can lead to increased neuronal excitability, which can enhance seizures [50-56]. Additionally, OSA can disrupt the body’s natural sleep-wake cycle, which can further increase the risk of seizures. Another way that OSA can exacerbate epilepsy is by causing hypoxemia. This can happen during apneic episodes in OSA, when the airway collapses and breathing stops for a few seconds. Hypoxemia can damage brain cells and make seizures more likely [25-27]. Epilepsy can also exacerbate OSA. Seizures (especially nocturnal) can disrupt sleep, which can lead to sleep deprivation. Additionally, seizures can weaken the muscles in the upper airway, which can make OSA worse [20]. Also anti-epileptic drugs are known to worsen OSA [29]. The positive feedback mechanisms between epilepsy and OSA has important implications for the management of both the conditions. Therefore, screening of OSA in patients with epilepsy and selection of AEDs accordingly would be a prudent approach. Treating either of the two conditions can exercise a better control and alleviation of the other disorder, which can reflect directly on the quality of life of such patients.
Figure 1.
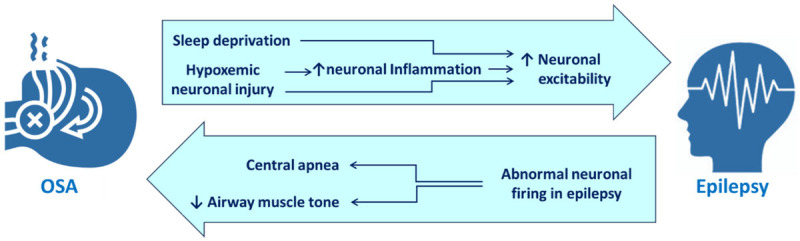
Mechanisms underlying the bidirectional relationship between OSA and epilepsy.
Conclusion
The prevalence of OSA in patients with epilepsy (PWE) and vice versa is significant, indicating a potential interplay between the two disorders. The comorbidity of obstructive sleep apnea (OSA) and epilepsy is a complex and bidirectional relationship and both can exacerbate one another in a positive feedback manner through their actions on the neural networks involved in breathing control and sleep regulation, changes in cortical excitability or inflammatory processes. The comorbidity of OSA and epilepsy poses a unique challenge for clinicians in terms of diagnosis and management. Given the bidirectional influence between the two conditions, comprehensive evaluation and treatment should be considered for patients with either disorder. Future research is needed to elucidate the specific biological, neurophysiological, and genetic factors that contribute to the bidirectional influence between the two conditions. A better understanding of the complex relationship between OSA and epilepsy will pave the way for more effective therapeutic interventions and improved patient outcomes.
Disclosure of conflict of interest
None.
References
- 1.Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-A review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311–22. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–8. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–98. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jehan S, Zizi F, Pandi-Perumal SR, Wall S, Auguste E, Myers AK, Jean-Louis G, McFarlane SI. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord. 2017;1:00019. [PMC free article] [PubMed] [Google Scholar]
- 7.Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL, Jetté N. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88:296–303. doi: 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr, Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshé SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 9.Kalilani L, Sun X, Pelgrims B, Noack-Rink M, Villanueva V. The epidemiology of drug-resistant epilepsy: a systematic review and meta-analysis. Epilepsia. 2018;59:2179–93. doi: 10.1111/epi.14596. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z, Si Q, Xiaoyi Z. Obstructive sleep apnoea in patients with epilepsy: a meta-analysis. Sleep Breath. 2017;21:263–70. doi: 10.1007/s11325-016-1391-3. [DOI] [PubMed] [Google Scholar]
- 11.Phabphal K, Sripradit M, Alan F G, Wongsritrang K, Chongsuvivatwong T, Suwanlaong K, Sithinamsuwan P. Identifying obstructive sleep apnea in patients with epilepsy: a cross-sectional multicenter study. Seizure. 2022;100:87–94. doi: 10.1016/j.seizure.2022.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Qubty WF, Mrelashvili A, Kotagal S, Lloyd RM. Comorbidities in infants with obstructive sleep apnea. J Clin Sleep Med. 2014;10:1213–6. doi: 10.5664/jcsm.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonka K, Juklícková M, Pretl M, Dostálová S, Horínek D, Nevsímalová S. Seizures in sleep apnea patients: occurrence and time distribution. Sb Lek. 2000;101:229–32. [PubMed] [Google Scholar]
- 14.Malow BA, Fromes GA, Aldrich MS. Usefulness of polysomnography in epilepsy patients. Neurology. 1997;48:1389–94. doi: 10.1212/wnl.48.5.1389. [DOI] [PubMed] [Google Scholar]
- 15.Hollinger P, Khatami R, Gugger M, Hess CW, Bassetti CL. Epilepsy and obstructive sleep apnea. Eur Neurol. 2006;55:74–9. doi: 10.1159/000092306. [DOI] [PubMed] [Google Scholar]
- 16.Vaughn BV, D’Cruz OF, Beach R, Messenheimer JA. Improvement of epileptic seizure control with treatment of obstructive sleep apnoea. Seizure. 1996;5:73–8. doi: 10.1016/s1059-1311(96)80066-5. [DOI] [PubMed] [Google Scholar]
- 17.Vendrame M, Auerbach S, Loddenkemper T, Kothare S, Montouris G. Effect of continuous positive airway pressure treatment on seizure control in patients with obstructive sleep apnea and epilepsy. Epilepsia. 2011;52:e168–71. doi: 10.1111/j.1528-1167.2011.03214.x. [DOI] [PubMed] [Google Scholar]
- 18.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131:3239–45. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz K, Lastra AC, Balabanov AJ. Obstructive and central sleep apnoea in a patient with medically intractable epilepsy. BMJ Case Rep. 2022;15:e245564. doi: 10.1136/bcr-2021-245564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St-John WM, Rudkin AH, Homes GL, Leiter JC. Changes in respiratory-modulated neural activities, consistent with obstructive and central apnea, during fictive seizures in an in situ anaesthetized rat preparation. Epilepsy Res. 2006;70:218–28. doi: 10.1016/j.eplepsyres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Gurung P, Nene Y, Sivaraman M. Vagus nerve stimulator (VNS)-induced severe obstructive sleep apnea which resolved after the VNS was turned off. Cureus. 2020;12:e6901. doi: 10.7759/cureus.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan JHM, Owens JW, Wrede JE. Case of an in-laboratory vagal nerve stimulator titration for vagal nerve stimulator-induced pediatric obstructive sleep apnea. J Clin Sleep Med. 2019;15:1539–42. doi: 10.5664/jcsm.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zambrelli E, Saibene AM, Furia F, Chiesa V, Vignoli A, Pipolo C, Felisati G, Canevini MP. Laryngeal motility alteration: a missing link between sleep apnea and vagus nerve stimulation for epilepsy. Epilepsia. 2016;57:e24–7. doi: 10.1111/epi.13252. [DOI] [PubMed] [Google Scholar]
- 24.Malow BA, Edwards J, Marzec M, Sagher O, Fromes G. Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology. 2000;55:1450–4. doi: 10.1212/wnl.55.10.1450. [DOI] [PubMed] [Google Scholar]
- 25.Proserpio P, Cossu M, Francione S, Tassi L, Mai R, Didato G, Castana L, Cardinale F, Sartori I, Gozzo F, Citterio A, Schiariti M, Lo Russo G, Nobili L. Insular-opercular seizures manifesting with sleep-related paroxysmal motor behaviors: a stereo-EEG study. Epilepsia. 2011;52:1781–91. doi: 10.1111/j.1528-1167.2011.03254.x. [DOI] [PubMed] [Google Scholar]
- 26.Foldvary-Schaefer N, Stephenson L, Bingaman W. Resolution of obstructive sleep apnea with epilepsy surgery? Expanding the relationship between sleep and epilepsy. Epilepsia. 2008;49:1457–9. doi: 10.1111/j.1528-1167.2008.01677.x. [DOI] [PubMed] [Google Scholar]
- 27.Cho JW, Kim DJ, Noh KH, Kim S, Lee JH, Kim JH. Nocturnal frontal lobe epilepsy presenting as obstructive type sleep apnea. J Epilepsy Res. 2011;1:74–6. doi: 10.14581/jer.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terzaghi M, Sartori I, Cremascoli R, Rustioni V, Manni R. Choking in the night due to NFLE seizures in a patient with comorbid OSA. J Clin Sleep Med. 2014;10:1149–52. doi: 10.5664/jcsm.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivathamboo S, Perucca P, Velakoulis D, Jones NC, Goldin J, Kwan P, O’Brien TJ. Sleep-disordered breathing in epilepsy: epidemiology, mechanisms, and treatment. Sleep. 2018;41 doi: 10.1093/sleep/zsy015. [DOI] [PubMed] [Google Scholar]
- 30.Pickrell WO, Lacey AS, Thomas RH, Smith PE, Rees MI. Weight change associated with antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84:796–9. doi: 10.1136/jnnp-2012-303688. [DOI] [PubMed] [Google Scholar]
- 31.Janousek J, Barber A, Goldman L, Klein P. Obesity in adults with epilepsy. Epilepsy Behav. 2013;28:391–4. doi: 10.1016/j.yebeh.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Lambert MV, Bird JM. Obstructive sleep apnoea following rapid weight gain secondary to treatment with vigabatrin (Sabril) Seizure. 1997;6:233–5. doi: 10.1016/s1059-1311(97)80011-8. [DOI] [PubMed] [Google Scholar]
- 33.Ochoa JG, Kilgo WA. The role of benzodiazepines in the treatment of epilepsy. Curr Treat Options Neurol. 2016;18:18. doi: 10.1007/s11940-016-0401-x. [DOI] [PubMed] [Google Scholar]
- 34.Hanly P, Powles P. Hypnotics should never be used in patients with sleep apnea. J Psychosom Res. 1993;37(Suppl 1):59–65. [PubMed] [Google Scholar]
- 35.Camacho ME, Morin CM. The effect of temazepam on respiration in elderly insomniacs with mild sleep apnea. Sleep. 1995;18:644–5. doi: 10.1093/sleep/18.8.644. [DOI] [PubMed] [Google Scholar]
- 36.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–4. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 37.Barthlen GM, Brown LK, Stacy C. Polysomnographic documentation of seizures in a patient with obstructive sleep apnea syndrome. Neurology. 1998;50:309–10. doi: 10.1212/wnl.50.1.309. [DOI] [PubMed] [Google Scholar]
- 38.Devinsky O, Ehrenberg B, Barthlen GM, Abramson HS, Luciano D, Epilepsy LD, Luciano D. Epilepsy and sleep apnea syndrome. Neurology. 1994;44:2060. doi: 10.1212/wnl.44.11.2060. [DOI] [PubMed] [Google Scholar]
- 39.Koh S, Ward SL, Lin M, Chen LS. Sleep apnea treatment improves seizure control in children with neurodevelopmental disorders. Pediatr Neurol. 2000;22:36–9. doi: 10.1016/s0887-8994(99)00114-9. [DOI] [PubMed] [Google Scholar]
- 40.Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, Volpe J, Bourgeois B, du Plessis AJ. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- 41.Holmes GL. Epilepsy in the developing brain: lessons from the laboratory and clinic. Epilepsia. 1997;38:12–30. doi: 10.1111/j.1528-1157.1997.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 42.Pisani F, Facini C, Pavlidis E, Spagnoli C, Boylan G. Epilepsy after neonatal seizures: literature review. Eur J Paediatr Neurol. 2015;19:6–14. doi: 10.1016/j.ejpn.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Gellhorn E, Ballin HM. Further investigations on effect of anoxia on convulsions. Am J Physiol. 1950;162:503–6. doi: 10.1152/ajplegacy.1950.162.3.503. [DOI] [PubMed] [Google Scholar]
- 44.Blennow G, Nilsson B, Siesjö BK. Influence of reduced oxygen availability on cerebral metabolic changes during bicuculline-induced seizures in rats. J Cereb Blood Flow Metab. 1985;5:439–45. doi: 10.1038/jcbfm.1985.59. [DOI] [PubMed] [Google Scholar]
- 45.Smith ML, Auer RN, Siesjö BK. The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathol. 1984;64:319–32. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- 46.Madison D, Niedermeyer E. Epileptic seizures resulting from acute cerebral anoxia. J Neurol Neurosurg Psychiatry. 1970;33:381–6. doi: 10.1136/jnnp.33.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol (1985) 2001;90:1986–94. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- 48.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–50. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gozal E, Sachleben LR Jr, Rane MJ, Vega C, Gozal D. Mild sustained and intermittent hypoxia induce apoptosis in PC-12 cells via different mechanisms. Am J Physiol Cell Physiol. 2005;288:C535–42. doi: 10.1152/ajpcell.00270.2004. [DOI] [PubMed] [Google Scholar]
- 50.Wen ZW, Liang DS, Cai XH, Chen J. The role of AMPK/mTOR signal pathway in brain injury following chronic intermittent hypoxia in growing rats. Eur Rev Med Pharmacol Sci. 2018;22:1071–7. doi: 10.26355/eurrev_201802_14391. [DOI] [PubMed] [Google Scholar]
- 51.Gozal E, Gozal D, Pierce WM, Thongboonkerd V, Scherzer JA, Sachleben LR Jr, Brittian KR, Guo SZ, Cai J, Klein JB. Proteomic analysis of CA1 and CA3 regions of rat hippocampus and differential susceptibility to intermittent hypoxia. J Neurochem. 2002;83:331–45. doi: 10.1046/j.1471-4159.2002.01134.x. [DOI] [PubMed] [Google Scholar]
- 52.Li G, Bauer S, Nowak M, Norwood B, Tackenberg B, Rosenow F, Knake S, Oertel WH, Hamer HM. Cytokines and epilepsy. Seizure. 2011;20:249–56. doi: 10.1016/j.seizure.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Rowley S, Patel M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med. 2013;62:121–31. doi: 10.1016/j.freeradbiomed.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 55.Malow BA, Weatherwax KJ, Chervin RD, Hoban TF, Marzec ML, Martin C, Binns LA. Identification and treatment of obstructive sleep apnea in adults and children with epilepsy: a prospective pilot study. Sleep Med. 2003;4:509–15. doi: 10.1016/j.sleep.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Wang D, Thomas RJ, Yee BJ, Grunstein RR. Hypercapnia is more important than hypoxia in the neuro-outcomes of sleep-disordered breathing. J Appl Physiol (1985) 2016;120:1484. doi: 10.1152/japplphysiol.01008.2015. [DOI] [PubMed] [Google Scholar]
- 57.Tolner EA, Hochman DW, Hassinen P, Otáhal J, Gaily E, Haglund MM, Kubová H, Schuchmann S, Vanhatalo S, Kaila K. Five percent CO2 is a potent, fast-acting inhalation anticonvulsant. Epilepsia. 2011;52:104–14. doi: 10.1111/j.1528-1167.2010.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishra P, Jaseja H, Goyal M. A critical analysis of the purported role of hypoxaemia in the comorbidity of obstructive sleep apnoea and epilepsy. Clin Physiol Funct Imaging. 2021;41:4–9. doi: 10.1111/cpf.12672. [DOI] [PubMed] [Google Scholar]
- 59.Bennett DR. Sleep deprivation and major motor convulsions. Neurology. 1963;13:953–8. doi: 10.1212/wnl.13.11.953. [DOI] [PubMed] [Google Scholar]
- 60.Gunderson CH, Dunne PB, Feyer TL. Sleep deprivation seizures. Neurology. 1973;23:678–86. doi: 10.1212/wnl.23.7.678. [DOI] [PubMed] [Google Scholar]
- 61.Manganotti P, Bongiovanni LG, Fuggetta G, Zanette G, Fiaschi A. Effects of sleep deprivation on cortical excitability in patients affected by juvenile myoclonic epilepsy: a combined transcranial magnetic stimulation and EEG study. J Neurol Neurosurg Psychiatry. 2006;77:56–60. doi: 10.1136/jnnp.2004.041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Civardi C, Boccagni C, Vicentini R, Bolamperti L, Tarletti R, Varrasi C, Monaco F, Cantello R. Cortical excitability and sleep deprivation: a transcranial magnetic stimulation study. J Neurol Neurosurg Psychiatry. 2001;71:809–12. doi: 10.1136/jnnp.71.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Gennaro L, Marzano C, Veniero D, Moroni F, Fratello F, Curcio G, Ferrara M, Ferlazzo F, Novelli L, Concetta Pellicciari M, Bertini M, Rossini PM. Neurophysiological correlates of sleepiness: a combined TMS and EEG study. Neuroimage. 2007;36:1277–87. doi: 10.1016/j.neuroimage.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Huber R, Mäki H, Rosanova M, Casarotto S, Canali P, Casali AG, Tononi G, Massimini M. Human cortical excitability increases with time awake. Cereb Cortex. 2013;23:332–8. doi: 10.1093/cercor/bhs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kreuzer P, Langguth B, Popp R, Raster R, Busch V, Frank E, Hajak G, Landgrebe M. Reduced intra-cortical inhibition after sleep deprivation: a transcranial magnetic stimulation study. Neurosci Lett. 2011;493:63–6. doi: 10.1016/j.neulet.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 66.Manganotti P, Palermo A, Patuzzo S, Zanette G, Fiaschi A. Decrease in motor cortical excitability in human subjects after sleep deprivation. Neurosci Lett. 2001;304:153–6. doi: 10.1016/s0304-3940(01)01783-9. [DOI] [PubMed] [Google Scholar]
- 67.Placidi F, Zannino S, Albanese M, Romigi A, Izzi F, Marciani MG, Palmieri MG. Increased cortical excitability after selective REM sleep deprivation in healthy humans: a transcranial magnetic stimulation study. Sleep Med. 2013;14:288–92. doi: 10.1016/j.sleep.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 68.Scalise A, Desiato MT, Gigli GL, Romigi A, Tombini M, Marciani MG, Izzi F, Placidi F. Increasing cortical excitability: a possible explanation for the proconvulsant role of sleep deprivation. Sleep. 2006;29:1595–8. doi: 10.1093/sleep/29.12.1595. [DOI] [PubMed] [Google Scholar]
- 69.Rodin EA, Luby ED, Gottlieb JS. The electroencephalogram during prolonged experimental sleep deprivation. Electroencephalogr Clin Neurophysiol. 1962;14:544–51. doi: 10.1016/0013-4694(62)90060-3. [DOI] [PubMed] [Google Scholar]
- 70.Shouse MN, Sterman MB. Acute sleep deprivation reduces amygdala-kindled seizure thresholds in cats. Exp Neurol. 1982;78:716–27. doi: 10.1016/0014-4886(82)90086-3. [DOI] [PubMed] [Google Scholar]
- 71.Shouse MN. Sleep deprivation increases susceptibility to kindled and penicillin seizure events during all waking and sleep states in cats. Sleep. 1988;11:162–71. doi: 10.1093/sleep/11.2.162. [DOI] [PubMed] [Google Scholar]
- 72.Nishimura Y, Saito Y, Kondo N, Matsuda E, Fujiyama M, Morizane R, Maegaki Y. Ictal central apnea and bradycardia in temporal lobe epilepsy complicated by obstructive sleep apnea syndrome. Epilepsy Behav Case Rep. 2015;4:41–4. doi: 10.1016/j.ebcr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferini-Strambi L, Lombardi GE, Marelli S, Galbiati A. Neurological deficits in obstructive sleep apnea. Curr Treat Options Neurol. 2017;19:16. doi: 10.1007/s11940-017-0451-8. [DOI] [PubMed] [Google Scholar]
- 74.Terzano MG, Parrino L, Anelli S, Halasz P. Modulation of generalized spike-and-wave discharges during sleep by cyclic alternating pattern. Epilepsia. 1989;30:772–81. doi: 10.1111/j.1528-1157.1989.tb05337.x. [DOI] [PubMed] [Google Scholar]
- 75.Manni R, Zambrelli E, Bellazzi R, Terzaghi M. The relationship between focal seizures and sleep: an analysis of the cyclic alternating pattern. Epilepsy Res. 2005;67:73–80. doi: 10.1016/j.eplepsyres.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Parrino L, Smerieri A, Spaggiari MC, Terzano MG. Cyclic alternating pattern (CAP) and epilepsy during sleep: how a physiological rhythm modulates a pathological event. Clin Neurophysiol. 2000;111(Suppl 2):S39–46. doi: 10.1016/s1388-2457(00)00400-4. [DOI] [PubMed] [Google Scholar]
- 77.Giorgi FS, Maestri M, Guida M, Carnicelli L, Caciagli L, Ferri R, Bonuccelli U, Bonanni E. Cyclic alternating pattern and interictal epileptiform discharges during morning sleep after sleep deprivation in temporal lobe epilepsy. Epilepsy Behav. 2017;73:131–6. doi: 10.1016/j.yebeh.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Korkmaz S, Bilecenoglu NT, Aksu M, Yoldas TK. Cyclic alternating pattern in obstructive sleep apnea patients with versus without excessive sleepiness. Sleep Disord. 2018;2018:8713409. doi: 10.1155/2018/8713409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kheirandish-Gozal L, Miano S, Bruni O, Ferri R, Pagani J, Villa MP, Gozal D. Reduced NREM sleep instability in children with sleep disordered breathing. Sleep. 2007;30:450–7. doi: 10.1093/sleep/30.4.450. [DOI] [PubMed] [Google Scholar]
- 80.Díaz-Negrillo A. Influence of sleep and sleep deprivation on ictal and interictal epileptiform activity. Epilepsy Res Treat. 2013;2013:492524. doi: 10.1155/2013/492524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mattson RH, Pratt KL, Calverley JR. Electroencephalograms of epileptics following sleep deprivation. Arch Neurol. 1965;13:310–5. doi: 10.1001/archneur.1965.00470030090009. [DOI] [PubMed] [Google Scholar]
- 82.Flink R, Pedersen B, Guekht AB, Malmgren K, Michelucci R, Neville B, Pinto F, Stephani U, Ozkara C. Guidelines for the use of EEG methodology in the diagnosis of epilepsy. International League Against Epilepsy: commission report. Commission on European Affairs: Subcommission on European Guidelines. Acta Neurol Scand. 2002;106:1–7. doi: 10.1034/j.1600-0404.2002.01361.x. [DOI] [PubMed] [Google Scholar]
- 83.Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. 1957;9:673–90. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- 84.Brunner DP, Dijk DJ, Tobler I, Borbély AA. Effect of partial sleep deprivation on sleep stages and EEG power spectra: evidence for non-REM and REM sleep homeostasis. Electroencephalogr Clin Neurophysiol. 1990;75:492–9. doi: 10.1016/0013-4694(90)90136-8. [DOI] [PubMed] [Google Scholar]
- 85.Jaseja H. Purpose of REM sleep: endogenous anti-epileptogenesis in man - a hypothesis. Med Hypotheses. 2004;62:546–8. doi: 10.1016/j.mehy.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 86.Jaseja H. Mechanism of endogenous anti-epileptogenesis during rapid eye movement sleep. Med Hypotheses. 2006;66:866. doi: 10.1016/j.mehy.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 87.Jaseja H. Endogenous anti-epileptogenic purpose of REM sleep in man: corroborative clinical neurophysiological evidence. Clin Neurophysiol. 2009;120:840. doi: 10.1016/j.clinph.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 88.Ho ML, Brass SD. Obstructive sleep apnea. Neurol Int. 2011;3:e15. doi: 10.4081/ni.2011.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:682–6. doi: 10.1164/ajrccm.162.2.9908058. [DOI] [PubMed] [Google Scholar]
- 90.Acosta-Castro P, Hirotsu C, Marti-Soler H, Marques-Vidal P, Tobback N, Andries D, Waeber G, Preisig M, Vollenweider P, Haba-Rubio J, Heinzer R. REM-associated sleep apnoea: prevalence and clinical significance in the HypnoLaus cohort. Eur Respir J. 2018;52:1702484. doi: 10.1183/13993003.02484-2017. [DOI] [PubMed] [Google Scholar]
- 91.Sullivan CE, Issa FG. Pathophysiological mechanisms in obstructive sleep apnea. Sleep. 1980;3:235–46. doi: 10.1093/sleep/3.3-4.235. [DOI] [PubMed] [Google Scholar]
- 92.Basunia M, Fahmy SA, Schmidt F, Agu C, Bhattarai B, Oke V, Enriquez D, Quist J. Relationship of symptoms with sleep-stage abnormalities in obstructive sleep apnea-hypopnea syndrome. J Community Hosp Intern Med Perspect. 2016;6:32170. doi: 10.3402/jchimp.v6.32170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Basunia RA, Fahmy S, Schmidt MF, Agu C, Bhattarai B, Oke V, Enriquez D, Quist J. Sleep architecture in obstructive sleep apnea-hypopnea syndrome (OSAHS) in adult African American population and relationship with apnea hypopnea index (AHI) and epworth sleepiness scale (ESS) Chest. 2015;148:354A. [Google Scholar]
- 94.Bianchi MT, Cash SS, Mietus J, Peng CK, Thomas R. Obstructive sleep apnea alters sleep stage transition dynamics. PLoS One. 2010;5:e11356. doi: 10.1371/journal.pone.0011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Durdik P, Sujanska A, Suroviakova S, Evangelisti M, Banovcin P, Villa MP. Sleep architecture in children with common phenotype of obstructive sleep apnea. J Clin Sleep Med. 2018;14:9–14. doi: 10.5664/jcsm.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McNamara F, Sullivan CE. Sleep-disordered breathing and its effects on sleep in infants. Sleep. 1996;19:4–12. doi: 10.1093/sleep/19.1.4. [DOI] [PubMed] [Google Scholar]
- 97.Jo H, Choi S, Kim D, Joo E. Effects of obstructive sleep apnea on epilepsy, and continuous positive airway pressure as a treatment option. J Clin Med. 2022;11:2063. doi: 10.3390/jcm11072063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu SY, Huon LK, Ruoff C, Riley RW, Strohl KP, Peng Z. Restoration of sleep architecture after maxillomandibular advancement: success beyond the apnea-hypopnea index. Int J Oral Maxillofac Surg. 2017;46:1533–8. doi: 10.1016/j.ijom.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Ng AK, Guan C. Impact of obstructive sleep apnea on sleep-wake stage ratio. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:4660–3. doi: 10.1109/EMBC.2012.6347006. [DOI] [PubMed] [Google Scholar]
- 100.Beck MC, Piccin CF, de Oliveira LCA, Scapini F, Coser Neto RF, da Silva AMV. Obstructive sleep apnea: acute effects of CPAP on polyssonographic variables. Fisioter em Mov. 2015;28:223–9. [Google Scholar]
- 101.Nigam G, Camacho M, Riaz M. Rapid eye movement (REM) rebound on initial exposure to CPAP therapy: a systematic review and meta-analysis. Sleep Sci Pract. 2017;1:13. [Google Scholar]
- 102.Goyal M, Mishra P, Jaseja H. Significance of rapid eye movement sleep in the comorbidity of obstructive sleep apnea and epilepsy. Med Hypotheses. 2020;144:109949. doi: 10.1016/j.mehy.2020.109949. [DOI] [PubMed] [Google Scholar]
- 103.Cohen HB, Dement WC. Sleep: changes in threshold to electroconvulsive shock in rats after deprivation of “paradoxical” phase. Science. 1965;150:1318–9. doi: 10.1126/science.150.3701.1318. [DOI] [PubMed] [Google Scholar]
- 104.Santos R, Carlini EA. Central responses to cholinergic drugs of REM sleep deprived rats. Pharmacol Biochem Behav. 1988;29:217–21. doi: 10.1016/0091-3057(88)90148-7. [DOI] [PubMed] [Google Scholar]
- 105.Vale NB, Leite JR. Decreased susceptibility to local anesthetics-induced convulsions after paradoxical sleep deprivation. Psychopharmacology (Berl) 1988;94:138–40. doi: 10.1007/BF00735895. [DOI] [PubMed] [Google Scholar]
- 106.Kumar P, Raju TR. Seizure susceptibility decreases with enhancement of rapid eye movement sleep. Brain Res. 2001;922:299–304. doi: 10.1016/s0006-8993(01)03174-2. [DOI] [PubMed] [Google Scholar]
- 107.Pornsriniyom D, Shinlapawittayatorn K, Fong J, Andrews ND, Foldvary-Schaefer N. Continuous positive airway pressure therapy for obstructive sleep apnea reduces interictal epileptiform discharges in adults with epilepsy. Epilepsy Behav. 2014;37:171–4. doi: 10.1016/j.yebeh.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 108.Hitomi T, Oga T, Tsuboi T, Yoshimura C, Kato T, Ikeda A, Takahashi R, Chin K. Transient increase in epileptiform discharges after the introduction of nasal continuous positive airway pressure in a patient with obstructive sleep apnea and epilepsy. Intern Med. 2012;51:2453–6. doi: 10.2169/internalmedicine.51.8042. [DOI] [PubMed] [Google Scholar]


