Abstract
Background and aims
Anthracyclines can cause cancer therapy-related cardiac dysfunction (CTRCD). We aimed to assess whether statins prevent decline in left ventricular ejection fraction (LVEF) in anthracycline-treated patients at increased risk for CTRCD.
Methods
In this multicenter double-blinded, placebo-controlled trial, patients with cancer at increased risk of anthracycline-related CTRCD (per ASCO guidelines) were randomly assigned to atorvastatin 40 mg or placebo once-daily. Cardiovascular magnetic resonance (CMR) imaging was performed before and within 4 weeks after anthracyclines. Blood biomarkers were measured at every cycle. The primary outcome was post-anthracycline LVEF, adjusted for baseline. CTRCD was defined as a fall in LVEF by >10% to <53%. Secondary endpoints included left ventricular (LV) volumes, CTRCD, CMR tissue characterization, high sensitivity troponin I (hsTnI), and B-type natriuretic peptide (BNP).
Results
We randomized 112 patients (56.9 ± 13.6 years, 87 female, and 73 with breast cancer): 54 to atorvastatin and 58 to placebo. Post-anthracycline CMR was performed 22 (13–27) days from last anthracycline dose. Post-anthracycline LVEF did not differ between the atorvastatin and placebo groups (57.3 ± 5.8% and 55.9 ± 7.4%, respectively) when adjusted for baseline LVEF (P = 0.34). There were no significant between-group differences in post-anthracycline LV end-diastolic (P = 0.20) or end-systolic volume (P = 0.12), CMR myocardial edema and/or fibrosis (P = 0.06–0.47), or peak hsTnI (P ≥ 0.99) and BNP (P = 0.23). CTRCD incidence was similar (4% versus 4%, P ≥ 0.99). There was no difference in adverse events.
Conclusions
In patients at increased risk of CTRCD, primary prevention with atorvastatin during anthracycline therapy did not ameliorate early LVEF decline, LV remodeling, CTRCD, change in serum cardiac biomarkers, or CMR myocardial tissue changes.
Trial registration
Keywords: Statins, Anthracycline, Cardiotoxicity, Primary prevention, Magnetic resonance imaging
Graphical Abstract
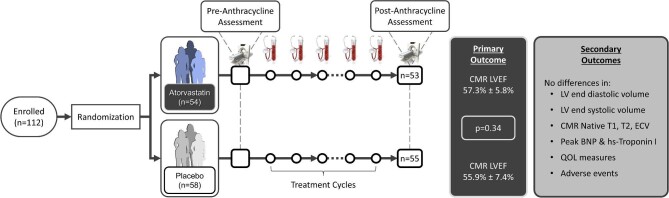
Graphical Abstract.
Summary of study enrollment, assessments, and outcomes. Randomized patients had cardiovascular magnetic resonance imaging (CMR) pre- and 72 (63–122) days post-anthracycline initiation / 22 (13–27) days post last dose of anthracycline. The stethoscopes and blood tubes reflect repeated clinical and biomarker assessment after every anthracycline cycle.
Introduction
Anthracycline chemotherapy has reduced mortality from many solid and hematological malignancies. However, anthracyclines are associated with an incidence of stage B or C heart failure of up to 21%.1,2 Contemporary practice is to initiate cardiac treatment after cancer therapy-related cardiac dysfunction (CTRCD) occurs. With this approach, many patients do not fully recover their cardiac function and go on to experience major adverse cardiac events.3,4 Hopes of detecting this problem at the preclinical stage, and intervening to prevent progression, have not yet shown definitive benefit.5 Given that anthracycline-induced cardiac injury is ubiquitous in high-risk patients, primary prevention approaches may be more effective in reducing their heart failure risk.6 However, the disadvantage of a primary prevention strategy is that many patients are treated unnecessarily, and this may be particularly difficult to reconcile with the possible development of side effects from cardioprotective medications. Several therapies have been considered for the primary prevention of CTRCD; however, their effectiveness has been limited.7–9
Statins are cardioprotective in other settings, and remarkably well tolerated. However, evidence regarding their use in cardioprotection at the time of chemotherapy is limited. Retrospective observational studies,10,11 small randomized controlled trials (RCT),12,13 and animal studies14 have suggested benefit. On this basis, the recently published European Society of Cardiology Cardio-oncology guidelines suggest that statins should be considered for primary prevention in patients at increased risk for cardiotoxicity from anthracycline therapy (Grade IIa, Level of Evidence B).15 However, the recent PREVENT trial16 of 279 patients (age 49 ± 12 years, 92% women) showed a similar, minor reduction of LVEF values with chemotherapy in the atorvastatin and placebo groups, despite > 90% adherence to therapy. Nonetheless, this trial had a number of limitations—including over one third with missing primary outcome data, the inclusion of lower-risk patients, and the lack of adjunctive blood and tissue biomarkers. Accordingly, to try to elucidate this inconsistency, we present here an RCT of atorvastatin vs. placebo in patients receiving anthracyclines for the treatment of solid or hematological malignancies who met American Society of Clinical Oncology (ASCO) criteria for being at increased risk for CTRCD.
Methods
Trial design
The Statins for the primary prevention of heart failure in patients with cancer receiving anthracycline-based chemotherapy (SPARE-HF) trial was a randomized, double blinded, placebo-controlled, parallel design, and superiority study with blinded ascertainment of outcomes (clinicaltrials.gov: NCT03186404). The study was approved provincially by Clinical Trials Ontario and at each participating institution (University Health Network, Mount Sinai Hospital, St. Michael's Hospital, Sunnybrook Health Sciences Centre). All participants provided written informed consent.
Participants
We included patients with breast cancer, lymphoma, leukemia, sarcoma, or thymoma treated with anthracyclines with curative intent, living in a geographic area conducive to repeated follow-up and meeting one of the following criteria for being at increased risk of CTRCD based on a modification of the ASCO clinical practice guidelines17:
Criteria (1) patients ≥ 60 years of age with at least one of (i) LVEF < 55% measured by echocardiography or multigated acquisition scans, or moderate left-sided valvular disease; (ii) planned cumulative doxorubicin-equivalent dose of ≥ 200 mg/m2; (iii) prior anthracycline therapy or chest/mediastinal radiation therapy; or (iv) any one of hypertension, smoking, obesity (BMI ≥ 30 kg/m2), history of cardiomyopathy, or heart failure with recovered LVEF ≥ 50%.
Criteria (2) age < 60 years of age and (i) at least 2 of the risk factors i–iv listed for Criteria 1or (ii) type 2 diabetes with age < 40 years, or (iii) type 1 diabetes with duration < 15 years.
Criteria (3) high cumulative anthracycline dose defined at ≥ 250 mg/m2 doxorubicin, ≥600 mg/m2 of epirubicin or other isoequivalent doses.
Exclusion criteria were: participating in another clinical research study that precluded participation in SPARE-HF; previous history of statin intolerance; already on a statin or known statin-indicated condition; Creatine kinase (CK) levels > 3x upper limit of normal or ALT > 2x upper limit of normal, or creatinine level > 177 µmol/L (>2 mg/dL) at baseline; on a drug that is a strong inhibitor of cytochrome P450 3A4 or may require treatment with such a drug during cancer therapy, severe regurgitant or stenotic valvular heart disease, life expectancy < 12 months, general contraindications to cardiovascular magnetic resonance (CMR), or known history of uncontrolled hypothyroidism.
Intervention
Participants were randomized to receive oral atorvastatin 40 mg once-daily or a matched placebo ≤ 10 days before the start of anthracycline. Measurements of CK, liver enzymes (AST, ALT), and CRP were performed every 2–3 weeks within 48 hours of each dose of anthracycline. Study drug was stopped if a > five-fold increase in CK or > 10 times upper limit of normal liver enzymes occurred. Study medication was continued through the duration of anthracycline therapy until the day of end-of study CMR (within 4 weeks of anthracycline completion).
Outcomes
The primary outcome was CMR-measured LVEF at end of anthracycline-based treatment. Pre-defined secondary outcomes included: (1) left ventricular end-diastolic (LVEDV) and systolic volumes (LVESV); (2) the incidence of CTRCD defined as a > 10% absolute reduction in LVEF compared to pre-anthracycline to an LVEF < 53% (in patients with preanthracycline LVEF < 53% a further > 10% reduction was required); (3) CMR-measured global longitudinal strain (GLS), global circumferential strain (GCS), T1, T2, and extracellular volume fraction (ECV) measurements; (4) peak high sensitivity troponin I and B-type natriuretic peptide (BNP) levels during treatment; and (5) interruption of study drug due to side effects.
Evaluations
Clinical
A focused clinical assessment was performed after each anthracycline cycle for cardiac symptoms and study drug side-effects. A quality-of-life (QOL) questionnaire (EQ5D-3 L) was administered pre-anthracycline, post-cycle 3, and post-anthracycline. Safety of treatment with statins was evaluated based on elevations in ALT, AST or CK, and rhabdomyolysis (clinical suspicion with acute myalgia, CK > 5x upper limit of normal, dark urine, myoglobin in the urine).
Cardiovascular magnetic resonance
All participants had a comprehensive CMR performed on a 3T imager (Biograph mMR; Siemens Healthineers, Erlangen, Germany) pre-anthracycline and within 4 weeks after the last dose of anthracycline. CMR sequences included balanced steady-state free precession images for assessment of function, T2 mapping for assessment of edema, T1 mapping for assessment of edema and fibrosis, late gadolinium enhancement (LGE) for assessment of replacement fibrosis. ECV was calculated using pre- and post-contrast T1 maps as a measure of interstitial space.18 Myocardial peak systolic global longitudinal and circumferential strain (GLS and GCS) were measured using feature tracking techniques. After de-identification of all CMR images, analysis was performed in a central laboratory using CMR using CVI42(V5.13, Circle CVI, Calgary, Canada). Details of CMR sequences, acquisition and analysis are provided in the Supplemental Methods and Supplementary material online, Table S1.
Serum biomarkers
High-sensitivity troponin I, BNP, high-sensitivity CRP, AST, ALT, and CK were measured pre-anthracycline, within 48 hours of each subsequent cycle, and at the final visit. Details in Supplemental Methods.
Study design and data management
Sample size was based on the following assumptions: baseline LVEF 60% (SD of 7%), to detect a clinically relevant absolute difference in LVEF of 5% by CMR between the placebo and statin groups using a general linear model (GLM). Assuming α = 0.05, 50 patients per arm resulted in power of > 90%. Assuming a 10% drop out we aimed to recruit 112 patients. A 5% difference in CMR–LVEF was chosen because this is a clinically relevant difference, it is just beyond the reproducibility of CMR measurements,19 we focused on a high-risk population, and a small RCT at the time of designing our study had shown a 9.2% difference in LVEF between the statin and placebo groups at follow-up.12 Randomization was stratified by hematological vs. solid malignancies and age ≥ 60 or < 60, using random permutated block sizes of 2 and 4. An independent data and safety monitoring committee met when 35% and 70% of the patients were recruited to determine the need for early termination of the study due to safety concerns. Study drug adherence was based on pill count at the post-anthracycline CMR visit (additional methods in the Supplement).
Statistical analysis
Analyses utilized an intention-to-treat approach. Baseline clinical characteristics were described using summary statistics. The primary analysis used GLMs to assess and compare the post-anthracycline LVEF between the two groups, adjusting for its pre-anthracycline value and stratification measures. The corresponding 95% confidence interval (CI) and p values were calculated based on t-statistic. Ventricular volumes, mass, and CMR tissue biomarker were also assessed using the same method. CTRCD was compared, and between-group difference was evaluated using the Cochran–Mantel–Haenszel (CMH) test.
Sensitivity, subgroup, and post-hoc analyses were performed to assess stratum-specific effects of statins, to demonstrate the effect of statin on the post-anthracycline LVEF in various clinically relevant subgroups, and to characterize serum biomarkers over the course of anthracycline treatment. For the serum biomarkers, we described group-specific time profiles of the biomarkers, their peak values, and time to peak in each group. See Supplemental Methods for details. Statistical analysis for the primary outcome assumed a significance level of 5%. All analyses were performed using R Statistical Software v4.0.3.20
Results
Patients
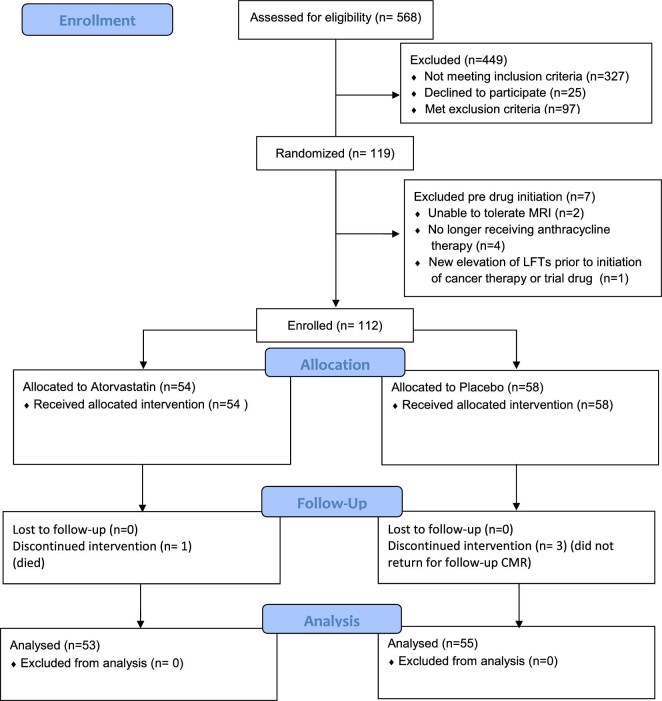
Of 568 participants screened from May 2018 to September 2021, 146 met provisional eligibility and 119 agreed to be randomized (60 in placebo and 59 in statin group) (Figure 1). Seven patients (6%) lost eligibility after initial randomization but before chemotherapy/study drug exposure resulting in 112 patients enrolled in the study. Reasons for loss of eligibility included: (1) inability to tolerate baseline CMR (2 patients), (2) anthracycline therapy no longer planned (3 patients), (3) patient declined chemotherapy (1 patient), or (4) new elevation of LFTs prior to initiation of cancer therapy or study drug (1 patient). The number of patients meeting increased risk CTRCD criteria 1– 3 were 56 (50.0%), 46 (41.0%), and 34 (30.0%), respectively (24 patients met more than one criterion). Amongst enrolled patients, four did not complete post-anthracycline CMR (3.6% dropout) as one died (statin group) and three (placebo group) allowed vital status but not CMR follow-up.
Figure 1.
Consort diagram.
Patient characteristics are shown in Table 1. Median (interquartile range (IQR)) duration between baseline and follow-up imaging was 72 (63–122) days (75 [63–126] in the statin and 71 [63–115] days in the placebo arms). Median (IQR) duration from final anthracycline dose to CMR was 22 (13–27) days (23 [13–27] in the statin and 22 (13–27)] days in the placebo arms). Mean cumulative doxorubicin-equivalent dose was 247.7 ± 52.3 mg/m2, (245.2 ± 55.9 mg/m2 in the statin group, 250.0 ± 48.9 mg/m2 in the placebo group). The median (IQR) cumulative doxorubicin-equivalent dose was 243.0 mg/m2 (236.8–256.2 mg/m2) ([242.0 mg/m2 (236.0–254.0 mg/m2] in the statin group and [243.0 mg/m2 (238.9–259.0 mg/m2] in the placebo group). None of the patients with breast cancer received trastuzumab prior to follow-up CMR. The median (IQR) drug adherence for the statin and placebo groups were 96% (87–99%) and 97% (91–100%), respectively. There was a reduction in total cholesterol and low-density lipoprotein (LDL) levels, at the post-anthracycline visit in statin-treated patients with minimal change in the placebo group (Table 2).
Table 1 .
Comparison of baseline clinical, oncological, CMR, and blood biomarker characteristics of the patients in the statin and placebo groups
| Statin N = 54 | Placebo N = 58 | |
|---|---|---|
| Age, years | 55.2 (13.7) | 58.6 (13.4) |
| ≥60 years, n(%) | 25 (46%) | 31 (53%) |
| Female sex n (%) | 39 (72%) | 48 (83%) |
| Race, n (%)* | ||
| White | 36 (67%) | 46 (79%) |
| Black | 2 (4%) | 2 (3%) |
| Non black Hispanic | 2 (4%) | 0 (0%) |
| Asian | 13 (24%) | 9 (16%) |
| Other | 1 (2%) | 1 (2%) |
| Systolic blood pressure, mmHg | 132 (20) | 126 (16) |
| Diastolic blood pressure, mmHg | 80 (11) | 79 (9) |
| Heart rate, bpm | 78 (15) | 76 (11) |
| Diabetes, n (%) | 3 (6%) | 4 (7%) |
| Hypertension, n (%) | 11 (20%) | 18 (31%) |
| Dyslipidemia, n (%) | 2 (4%) | 3 (5%) |
| Smoking, n (%) | 25 (46%) | 26 (45%) |
| Baseline medications, n (%) | ||
| ACE inhibitor | 3 (6%) | 6 (10%) |
| Angiotensin receptor blocker | 3 (6%) | 4 (7%) |
| Betablocker | 3 (6%) | 2 (3%) |
| MRA | 1 (2%) | 1 (2%) |
| Any cardiac medication | 7 (13%) | 7 (12%) |
| Breast Cancer, n (%) | 35 (65%) | 38 (66%) |
| Breast Cancer stage, n (%) | ||
| 1 | 5 (14%) | 4 (11%) |
| 2 | 23 (66%) | 23 (61%) |
| 3 | 7 (20%) | 11 (29%) |
| Breast cancer side, n (%) | ||
| Left | 18 (51%) | 17 (45%) |
| Right | 15 (43%) | 20 (53%) |
| Bilateral | 2 (6%) | 1 (3%) |
| Lymphoma, n (%) | 12 (23%) | 12 (21%) |
| Leukemia, n (%) | 1 (2%) | 2 (3%) |
| Sarcoma, n (%) | 3 (6%) | 4 (7%) |
| Thymoma, n (%) | 3 (6%) | 2 (3%) |
| High CTRCD risk criteria* | ||
| Criterion #1 | 20 (37%) | 25 (43%) |
| Criterion #2 | 17 (31%) | 16 (28%) |
| Criterion #3 | 17 (31%) | 17 (29%) |
*Note these are mutually exclusive and represent the primary criteria used in recruitment (some patients may meet more than one high CTRCD risk criteria). All continuous data are presented as mean (SD), or median (IQR), all categorical data are presented as N (%), which represents the number of patients (percentage). ACE, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist.
Table 2.
CMR measures, blood biomarkers, and quality-of-life measures pre- and post-anthracycline summarized as mean and standard deviation (SD) and GLM-estimated treatment effects and their corresponding 95% CIs. Treatment effects with a positive value indicate higher measurement in the statin group and negative values indicate lower measurement in the statin group
| Statin (n = 53) | Placebo (n = 55) | Between Groups | ||||
|---|---|---|---|---|---|---|
| Pre-anthracycline | Post-anthracycline | Pre-anthracycline | Post-anthracycline | Treatment effect(95% CI) | P value** | |
| CMR Measures | ||||||
| LVEF, % | 60.2 (5.4) | 57.3 (5.8)* | 59.2 (6.6) | 55.9 (7.4)* | 0.79 (−0.84, 2.42) | 0.34 |
| LVEDV, mL | 125.4 (25.9) | 124.9 (25.3) | 130.2 (29.8) | 132.1 (27.7) | −3.70 (−9.40, 2.01) | 0.20 |
| LVEDV, mL /m2 | 69.8 (13.1) | 70.1 (13.4) | 71.9 (12.8) | 73.9 (13.8) | −1.84 (−5.15, 1.47) | 0.27 |
| LVESV, mL | 50.4 (13.7) | 53.8 (14.5) | 53.6 (16.9) | 58.8 (17.7)* | −2.55 (−5.79, 0.70) | 0.12 |
| LVESV, mL /m2 | 28.0 (7.3) | 30.2 (7.9) | 29.6 (8.6) | 32.8 (9.8)* | −1.17 (−3.12, 0.77) | 0.23 |
| LV mass, g | 75.5 (17.3) | 75.5 (16.0) | 74.9 (18.0) | 75.7 (18.0) | −0.98 (−3.24, 1.28) | 0.39 |
| LV mass, g/m2 | 41.7 (7.3) | 42.0 (7.3) | 41.1 (6.8) | 42.0 (7.1) | −0.28 (−1.51, 0.94) | 0.65 |
| GLS, % | 17.5 (2.1) | 16.5 (2.1)* | 17.3 (2.1) | 16.6 (2.6) | −0.09 (−0.78, 0.60) | 0.79 |
| GCS, % | 19.6 (2.5) | 18.4 (2.5) | 19.2 (2.6) | 18.1 (3.0)* | 0.20 (−0.62, 1.01) | 0.64 |
| T1, ms | 1229 (40) | 1268 (42)* | 1247 (40) | 1280 (36)* | −5.70 (−21.32, 9.92) | 0.47 |
| T2, ms | 39.9 (3.0) | 40.5 (2.4) | 40.0 (2.4) | 41.1 (2.3) | −0.52 (−1.36, 0.32) | 0.22 |
| ECV, % | 24.7 (3.0) | 27.7 (2.6)* | 25.5 (2.2) | 29.0 (2.4)* | −0.76 (−1.56, 0.04) | 0.06 |
| iECV | 9.8 (2.0) | 11.1 (2.3) * | 10.0 (2.0) | 11.6 (2.1)* | −0.29 (−0.79, 0.21) | 0.25 |
| iICV | 30.1 (5.6) | 29.0 (5.3) | 29.2 (4.9) | 28.5 (5.1) | −0.14 (−1.12, 0.84) | 0.78 |
| Blood Biomarkers | ||||||
| CK, U/L | 73 (39) | 63 (45) | 80 (44) | 54 (48)* | 10.62 (−4.87, 26.104) | 0.18 |
| Glucose, mmol/L | 5.3 (1.2) | 6.2 (1.6) | 5.6 (1.8) | 5.7 (1.2) | 0.59 (0.05, 1.12) | 0.03 |
| HbA1c, % | 5.5 (0.7) | 5.9 (1.1)* | 5.4 (0.6) | 5.8 (0.9)* | 0.03 (−0.22, 0.28) | 0.80 |
| AST, U/L | 21 (8) | 29 (24) | 21 (7) | 24 (14) | 5.02 (−2.52, 12.57) | 0.19 |
| ALT, U/L | 21 (12) | 35 (34) | 21 (14) | 29 (25)* | 5.50 (−6.16, 17.16) | 0.35 |
| TC, mmol/L | 5.00 (1.22) | 3.60 (0.96)* | 5.13 (1.06) | 4.90 (0.98) | −1.29 (−1.61, −0.97) | <0.001 |
| LDL, mmol/L | 2.89 (0.89) | 1.73 (0.69)* | 2.97 (0.95) | 2.91 (0.87) | −1.14 (−1.40, −0.88) | <0.001 |
| HDL, mmol/L | 1.37 (0.45) | 1.02 (0.33)* | 1.42 (0.42) | 1.07 (0.35)* | −0.06 (−0.17, 0.06) | 0.32 |
| TG, mmol/L | 1.61 (0.85) | 1.89 (1.09) | 1.49 (1.19) | 1.95 (0.84) | −0.12 (−0.47, 0.24) | 0.51 |
| hsCRP | 2.0 (1.0–7.0) | 5.0 (2.0–19.0) | 3 (1.0–8.0) | 7.0 (2.0–14.0) | 2.69 (−4.25, 9.62) | 0.44 |
| Troponin, ng/L | 2.0 (2.0—2.0) | 13 (7–29)* | 2.0 (2.0—2.0) | 14 (8 −34)* | −0.23 (−10.88, 10.42) | 0.97 |
| BNP pg/ mL | 16.8 (9.9—39.0) | 39.0 (26.9—81.3) | 26.0 (12.1—49.0) | 36.2 (16.4—64.8) | −12.25 (−33.11, 8.61) | 0.25 |
| Quality of Life Measures | ||||||
| EQ-VAS | 70 (19) | 69 (17) | 71 (22) | 74 (16) | −4.80 (−10.96, 1.34) | 0.12 |
| EQ Index | 0.85 (0.16) | 0.84 (0.13) | 0.85 (0.19) | 0.86 (0.17) | −0.02 (−0.07, 0.03) | 0.44 |
After Bonferroni correction, significance threshold is P < 0.001; *Denotes P < 0.001 for significance after Bonferroni Correction for inter-group comparison (i.e. pre- vs. post-anthracyclines). CMR, cardiovascular magnetic resonance; LVEF; left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; ECV, extracellular volume fraction. HbA1c, hemoglobin A1C; TC, total cholesterol; LDL, low density lipoprotein; HDL, high density lipoprotein; TG, triglycerides; hsCRP, high sensitivity c-reactive protein. Smoking, current, or former smoking. iECV, indexed extracellular volume; iICV, indexed intracellular volume; BNP, B-type natriuretic peptide; EQVAS, EuroQol visual analog scale. The EQ Index scores were calculated using a formula that weighs each quality-of-life dimension and is interpreted using normative EQ-5D-3 L index score data for Canadians.
Outcomes
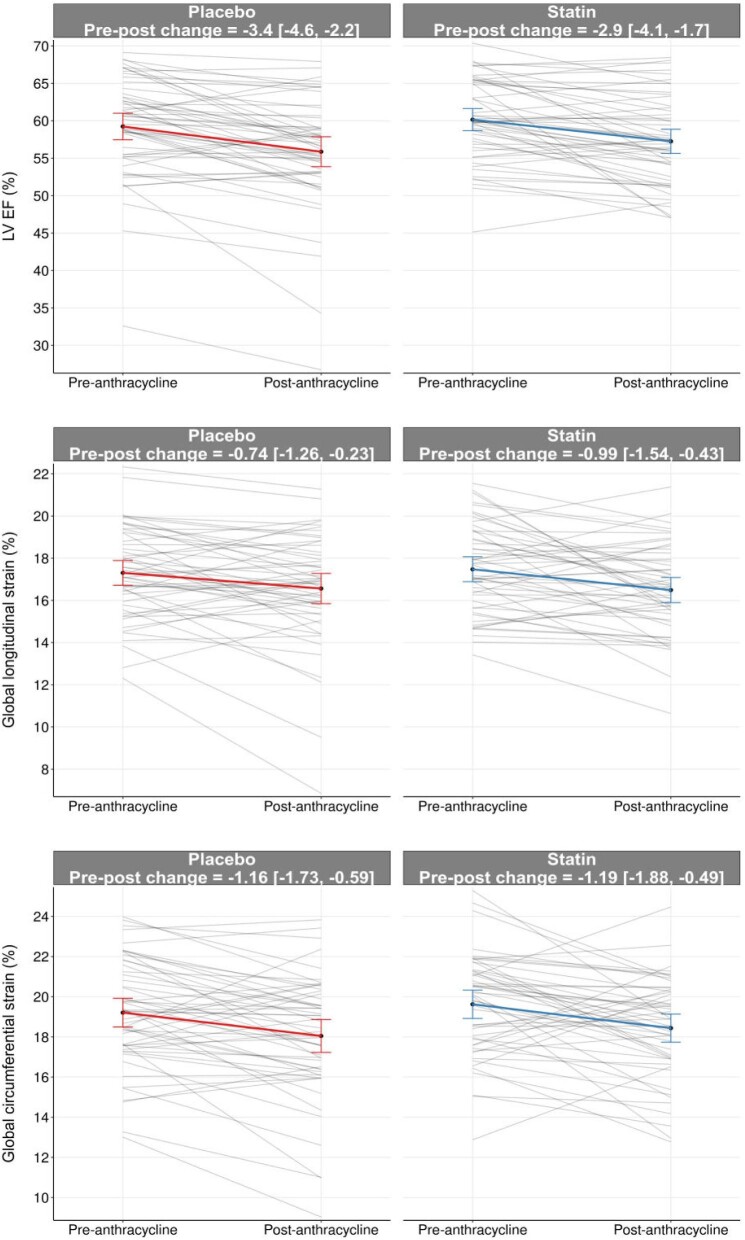
There was a decrease in LVEF and an increase in LVESV observed in both groups post-anthracycline compared to pre-anthracycline (Table 2). The post-anthracycline LVEF was 57.3 ± 5.8% in the statin group and 55.9 ± 7.4% in the placebo group (Figure 2). The between-group difference was 0.79% (95% CI: –0.84, 2.42%, P = 0.34) adjusted for pre-anthracycline LVEF. There were no significant between-group differences in left ventricular volumes or mass (P = 0.12–0.39) (Supplementary material online Fig. S1, Table 2). None of our patients developed heart failure symptoms or received heart failure therapy during anthracycline treatment. CTRCD developed in two patients (4%) in each group (P ≥ 0.99) on the follow-up CMR. Both GLS and GCS worsened post-anthracycline vs. pre-anthracycline in both groups (Table 2), but no significant between-group differences was seen (P = 0.79 and 0.64, respectively) (Figure 2).
Figure 2.
Pre- and post-anthracycline measures of LVEF, GLS, and GCS in the atorvastatin and placebo groups. Individual lines represent individual patients. The solid line and bars represent the mean and 95% CI pre- and post-anthracycline.
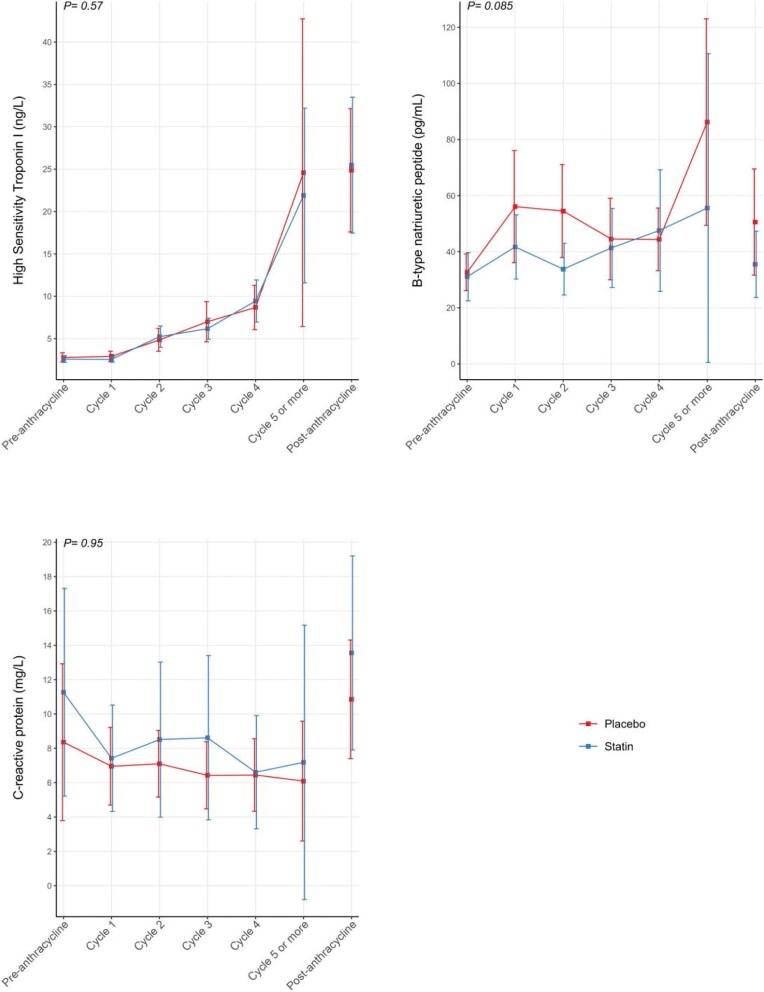
HsTnI but not BNP levels were higher post- vs. pre-anthracycline; however, there were no significant differences between groups (Table 2). The trajectories of hsTnI, BNP, and CRP measurements as measured after each anthracycline cycle are illustrated in Figure 3. There were no significant differences in the peak BNP (39 pg/mL [IQR: 27–81 pg/ mL] vs. 69 pg/ mL [IQR: 41–86 pg/ mL], P = 0.23), hsTnI (19 ng/L [7–29 ng/L] vs. 14 ng/L [8–34 ng/L], P ≥ 0.99) or CRP (13.0 mg/L [4.1–24.0 mg/L] vs. 11.0 mg/L [5.0–20.0 mg/L], P = 0.12) or post-anthracycline measures (Table 2) between the statin and placebo groups, respectively.
Figure 3.
Trajectory of high sensitivity troponin I, B-type natriuretic peptides, C-reactive proteins measured pre- and post-anthracycline, and each anthracycline cycle are summarized. The group-specific trajectories were estimated using GEE with cycles as a categorical variable, and the corresponding 95% CIs were estimated based on robust sandwich estimators. Given the variability in the number of cycles between patients and fewer patients receiving more than five cycles, we lumped all observations at Cycle 5 and all subsequent cycles into one single category. In addition, we included the estimates and the CI at the end of anthracycline treatment without connecting the trajectories to signal that not all patients received five cycles of anthracycline. Note that patient-specific data points in all cycles are shown in Supplementary material online, Fig. S2.
CMR-measured tissue biomarkers of edema with or without fibrosis post-anthracycline were increased in both groups (Table 2, Supplementary material online Fig. S3). When adjusted for pre-anthracycline values, age group and cancer type, final CMR tissue biomarker values were not significantly different between the groups (P = 0.06–0.47). A total of 12 patients (6 in each group) had myocardial replacement fibrosis measured with LGE on the pre-anthracycline CMR that was minimal, constituting 1.8% (0.8–3.4%) and 3.0% (1.4–4.5%) of the LV myocardial mass in the statin and placebo groups, respectively pre-anthracycline, and 1.6% (1.1–1.9%) and 2.4% (1.4–5.4%), respectively post-anthracycline. None of the patients developed new LGE.
EQ index score and EQVAS were similar pre- and post-anthracycline in both groups (Table 2), with no between-group differences (P = 0.44 and 0.12, respectively).
Adverse effects
Two patients in the statin group discontinued study drug. One patient discontinued study drug after 4 days due to muscle pain/cramping affecting sleep (no increase in CK or liver enzymes). A second patient stopped study drug after 2 days due to difficulty swallowing. None of the other patients discontinued study drug due to adverse effects.
There were no significant differences in the trajectory of AST, ALT, and CK between the study groups (Supplementary material online Figures 4 and 5). Eight patients had dose reduction of their anthracycline by their treating physician due to the following reasons: hospitalization for diverticulitis (n = 1), side effects or significant infection (n = 3), and good response to treatment after only four cycles (instead of six cycles) in four patients with lymphoma. Three of these patients were in the statin group and five in the placebo group. There were 11 reportable serious adverse events (SAEs, Grades 3–5) with 5 in the statin and 6 in the placebo group (Supplementary material online Table S2). There were no definitive, probable, or possible reportable SAEs associated with trial drug.
Post hoc analyses
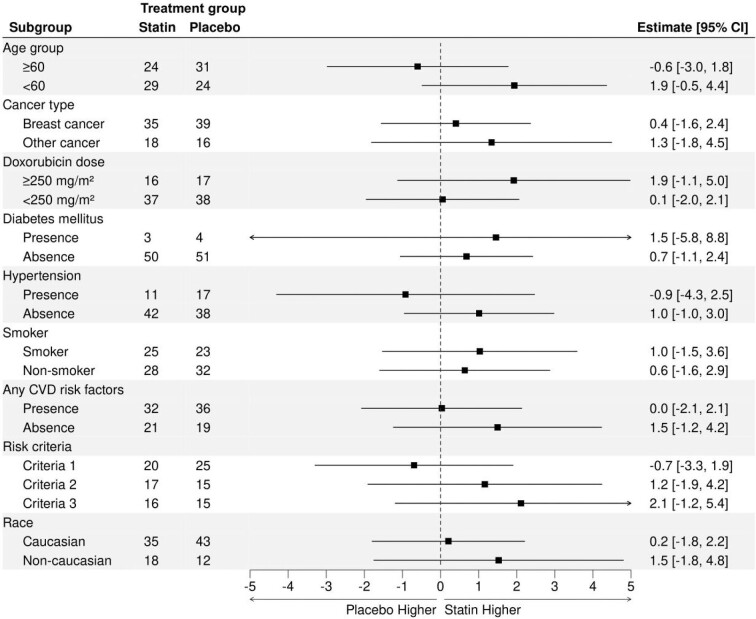
The primary outcome (LVEF) was examined in clinically relevant subgroups (Figure 4) and findings in the subgroups were consistent with the primary analysis. A > 5% absolute LVEF reduction was seen in 34 (31%) patients (19 [35%] and 15 [28%] in the placebo and statin groups respectively); >10% absolute LVEF reduction was seen in 7 (6%) patients (4 [7%] and 3 [6%]) in the placebo and statin groups, respectively). Amongst patients with a baseline LVEF ≥ 50%, (n = 8;(8%; 3 [6%] in statin and 5 [10%] in placebo group) had declines in LVEF to absolute values below 50%. The number of patients with an abnormal hsTnI (>26 ng/L in men, >16 ng/L in women) in the statin and placebo groups during anthracycline therapy was 25 (47%) and 20 (36%) respectively, P = 0.33. The number of patients with an abnormal BNP (>100 pg/mL) was 10 (19%) and 10 (18%), respectively, P ≥ 0.99. The time to peak hsTnI and BNP are shown in Supplementary material online Fig. S6 and were not significantly different between the groups.
Figure 4.
Post hoc analysis of the comparison of the effect of statins on post-anthracycline LVEF in clinically relevant subgroups. Regardless of the subgroup the effect remained similar to the main effect. Any CVD risk factors included diabetes, hypertension, or smoking (current or prior history). Please note that the total number of patients add to 108 given the 4 dropouts.
Discussion
In this randomized, double-blinded, placebo-controlled trial that studied the use of atorvastatin to prevent anthracycline-related decline in LVEF in at-risk patients, change between pre- and post-anthracycline LVEF or ventricular remodeling did not differ significantly between the statin and placebo groups when adjusted for pre-anthracycline values. Importantly, this RCT focused on patients at increased CTRCD risk as per current guidelines,17 and in contrast to most of the previous statin intervention studies, used CMR as the reference standard method for the measurement of ventricular volumes and LVEF rather than echocardiography.12,13 In our post hoc analysis, we did not identify benefit of statins in any specific patient subgroups. We observed no significant difference in the increase in hsTnI, BNP, or CMR tissue biomarkers between the groups during treatment suggesting that statins do not prevent myocardial injury edema and/or fibrosis.
Avoidance of CTRCD in anthracycline-treated patients21,22 by secondary prevention in patients with LV dysfunction is problematic. However, an alternative, primary prevention strategy mandates the availability of an agent that is both effective and well tolerated. Interest in the use of statins for primary prevention of CTRCD was supported by cohort studies, which suggested that statin use reduced heart failure risk.23 Although observational studies may be misleading because of the potential for bias and unmeasured confounding, their results were supported by studies with propensity-matching—most recently, a large population-based study (n = 666) of older women (≥66 years) with early-stage breast cancer, in which statin use was associated with reduced HF hospital presentations.11 These results seem to be supported by some clinical trials (Table 3). Avoidance of the decline of LVEF with doxorubicin was shown in a small non blinded randomized trial of atorvastatin 40 mg/day vs. placebo in patients receiving on average > 250 mg/m2 of doxorubicin-equivalent dose,12 as well as a more recent placebo-controlled RCT of 89 women with breast cancer treated with rosuvastatin 20 mg.13 With these data, the 2022 European Society of Cardiology (ESC) guidelines endorsed statins for primary prevention of CTRCD in high-risk anthracycline-treated patients.15
Table 3.
Summary of randomized controlled trials of statins to prevent anthracycline mediated CTRCD
| Author/Year/Imaging Technique | n | Cancer | Intervention | CTRCD | Statin | Placebo | Statin/Placebo difference |
|---|---|---|---|---|---|---|---|
| Acar et al. 2011 (echo)12 | 40 | Hematological Malignancies | Atorvastatin 40 mg (6 months) | LVEF < 50%(1/20 in statin, 5/20 in control, p = 0.18) | Baseline 61.3 ± 7.9%Post 62.6 ± 9.3%Diff + 1.3 ± 3.8% | Baseline 62.9 ± 7.0%Post 55.0 ± 9.5%Diff: −7.9 ± 8.0% | p < 0.001 |
| Nabati et al. 2019 (echo)13 | 89* | Breast Cancer | Rosuvastatin 20 mg (6 months) | LVEF < 45%(4/38 in statin, 6/39 in placebo), p = 0.963 | Baseline 55.1 ± 4.8%Post: 53.5 ± 6.7%Diff −1.5 ± 6.6 | Baseline 55.1 ± 5.1% Post 50.0 ± 6.6%Diff −5.2 ± 0.7% | p = 0.012 |
| Hundley et al. 2022 (CMR)16 | 279# | Breast and Hematological Malignancies | Atorvastatin 40 mg (24 months) | LVEF < 50%(4/100 in statin, 7/105 in placebo, no p-value) | Baseline 62.6 ± 6.4%Post: 57.7 ± 5.6%Diff 3.2 ± 0.7% | Baseline 61.7 ± 5.5%Post: 57.4 ± 6.8%Diff 3.3 ± 0.6% | p = 0.93 |
| Thavendiranathan et al. (CMR)—current study | 112 | Breast, Heme, Others | Atorvastatin 40 mg (2.5 months) | LVEF < 50%(3/53 in statin, 5/55 in placebo) | Baseline 60.2 ± 0.4%Post: 57.3 ± 5.8% | Baseline 59.2 ± 6.6%Post: 55.9 ± 7.4% | p = 0.34 |
| Overall | 520 | Statin 12/211Placebo 23/219 (p = 0.068 or 0.10 with Yates correction)‡ | OR 0.53 (0.25–1.10), p = 0.089‡ |
CTRCD, cancer therapy related cardiac dysfunction; Diff, difference; CMR, cardiovascular magnetic resonance; echo, echocardiogram; CTRCD, cancer therapy related cardiac dysfunction; OR, odds ratio *although 89 recruited, final follow-up only in 77 (39 placebo, 38 statin); #Although 279 recruited 2-year follow up only available in 205 (105 placebo, 100 statin). ‡We performed a meta-analysis using the Cochran–Mantel–Haenszel (CMH) method to estimate the pooled odds-ratio across the 4 RCTs using a random effect model) and evaluating the between-arm differences in the proportion with CTRCD with the CMH χ2 test, the OR [95% CI] was 0.51 [0.25, 1.05], P = 0.095 (see Supplementary material online Fig. S7 for forest plot).
However, the recently published PREVENT RCT of atorvastatin 40 mg once-daily vs. placebo in predominantly breast cancer patients showed no significant difference in CMR-measured LVEF between the groups at 2-year follow-up.16 However, the PREVENT trial had a number of limitations. First, 35.8% of patients in the PREVENT trial had missing primary outcome measure (vs. only 3.6% in our study). Second, the PREVENT trial involved patients across the spectrum of risk—we focused specifically on patients at increased risk of CTRCD as defined by the ASCO guidelines17 as this is the group where guidelines endorse statins for primary prevention.15 This accounts for the ∼2 fold higher incidence of CTRCD in our study compared to PREVENT (Table 3). Other factors that demonstrate the increased CTRCD risk of our patients include the high prevalence of traditional cardiovascular risk factors and the observation of a high frequency of development of abnormal hsTnI during treatment and clinically meaningful fall in CMR-measured LVEF in a substantial subgroup of patients. Finally, we supplemented routine imaging data with cardiac blood biomarker and CMR tissue biomarker data adding mechanistic information that was not presented in the PREVENT Trial or any of the previously published statin studies. Thus, the totality of published trial data with statins in anthracycline treated patients (Table 3) shows no significant effect on either EF impairment, nor reduction in CTRCD when compared as a binary variable.
Our study has limitations. First, we assessed the benefit of atorvastatin in preventing a decrement in LVEF within 1-month post-anthracycline completion and ∼2.5 months from baseline. This differs from prior small RCTs (∼6 months from baseline)12,13 and cohort studies, which had longer follow-up. However, the mechanisms by which statins have been proposed to reduce anthracycline mediated cardiac injury are through reduction in reactive oxygen species production, impact on calcium channels, and promotion of cardiac survival.14,24 Therefore, by measuring LVEF within 1-month post-anthracyclines, we have more closely assessed the potential preventive effects of statins on anthracycline mediated injury and avoided confusion with possible statin-related prevention of other cardiovascular events, which can occur after completion of cancer treatment.25 Also assessment of this early change is clinically important as a fall in LVEF post-anthracycline is prognostically important26 and impacts the ability to administer other adjuvant cancer therapies (e.g. HER2-targeted therapy). It is also notable that despite randomization, there were small imbalances between the cohorts, which may have biased our findings. Finally, our findings do not suggest that patients who are already on statins should have their drug discontinued during chemotherapy if there are no other contraindications.
Conclusions
In patients receiving anthracycline therapy meeting ASCO criteria for increased CTRCD risk, prophylactic use of atorvastatin 40 mg once-daily did not prevent LV function decline, adverse ventricular remodeling, hsTnI or BNP elevation, or increase in CMR tissue biomarkers compared to placebo at the anthracycline doses studied and within the time frame of follow-up. These results question the recommendation for statin use in the Cardio-oncology guidelines to prevent anthracycline induced CTRCD in patients at the risk level included in our study.
Supplementary Material
Acknowledgements
We acknowledge the contribution of Rosanna Chan, Neil Spiller, and Sangkyu Moon for performing the CMR studies and Nadia Asif for study initiation support. We would also like to acknowledge members of our DSMB Dr. M. Farkouh (chair), Dr. S. Dent, Pamela Ng (pharmacist), and Fei Zuo (statistician) and thank Dr. S. Adams for support with the central illustration.
Contributor Information
Paaladinesh Thavendiranathan, Department of Medicine, Division of Cardiology, Ted Rogers Program in Cardiotoxicity Prevention, Peter Munk Cardiac Centre, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON, Canada.
Christian Houbois, Department of Medical Imaging, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada; Department of Medical Imaging, University of Toronto, Toronto, ON, Canada.
Thomas H Marwick, Baker Heart and Diabetes Institute, Melbourne, Victoria, Australia; Baker Department of Cardiometabolic Health, University of Melbourne, Melbourne, Victoria, Australia.
Tiffanie Kei, Department of Medicine, Division of Cardiology, Ted Rogers Program in Cardiotoxicity Prevention, Peter Munk Cardiac Centre, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON, Canada.
Sudipta Saha, Rogers Computational Program, Ted Rogers Centre for Heart Research, Peter Munk Cardiac Centre, University Health Network, University of Toronto, Toronto, ON, Canada.
Kyle Runeckles, Rogers Computational Program, Ted Rogers Centre for Heart Research, Peter Munk Cardiac Centre, University Health Network, University of Toronto, Toronto, ON, Canada.
Flora Huang, Department of Medicine, Division of Cardiology, Ted Rogers Program in Cardiotoxicity Prevention, Peter Munk Cardiac Centre, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON, Canada.
Tamar Shalmon, Department of Medicine, Division of Cardiology, Ted Rogers Program in Cardiotoxicity Prevention, Peter Munk Cardiac Centre, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON, Canada.
Kevin E Thorpe, Dalla Lana School of Public Health, University of Toronto and Applied Health Research Centre, Li Ka Shing Knowledge Institute, St. Michael's Hospital, Toronto, ON, Canada.
Rossanna C Pezo, Department of Medicine, Division of Medical Oncology, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
Anca Prica, Department of Medicine, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, ON, Canada.
Dawn Maze, Department of Medicine, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, ON, Canada.
Husam Abdel-Qadir, Department of Medicine, Division of Cardiology, Ted Rogers Program in Cardiotoxicity Prevention, Peter Munk Cardiac Centre, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON, Canada; Women's College Hospital (WCH), Toronto, ON, Canada.
Kim A Connelly, Keenan Research Centre, Li Ka Shing Knowledge Institute, Division of Cardiology, St. Michael's Hospital, University of Toronto, Toronto, ON, Canada.
Joyce Chan, Division of Cardiology, Peter Munk Cardiac Centre, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON, Canada.
Filio Billia, Division of Cardiology, Peter Munk Cardiac Centre, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON, Canada.
Coleen Power, Department of Medicine, Division of Cardiology, Ted Rogers Program in Cardiotoxicity Prevention, Peter Munk Cardiac Centre, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON, Canada.
Kate Hanneman, Joint Department of Medical Imaging, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON, Canada; Department of Medical Imaging, University of Toronto, Toronto, ON, Canada.
Bernd J Wintersperger, Joint Department of Medical Imaging, Toronto General Hospital, University Health Network, University of Toronto, Toronto, ON, Canada; Department of Medical Imaging, University of Toronto, Toronto, ON, Canada.
Christine Brezden-Masley, Department of Medicine, Division of Medical Oncology, Mount Sinai Hospital, University of Toronto, Toronto, ON, Canada.
Eitan Amir, Department of Medicine, Division of Medical Oncology, Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, ON, Canada.
Funding
This study was funded by an operating grant from the AHSC AFP Innovation Funds. The funders had no role in the design or analysis of the study or the interpretation of the results. Dr. Thavendiranathan (147 814) was supported by the Canadian Institutes of Health Research New Investigator Award and currently a Canada Research Chair in Cardio-oncology (950-232646). Dr. Thavendiranathan is also supported by the Canadian Cancer Society/Canadian Institutes of Health Research's W. David Hargraft Grant. Dr. Marwick is supported by an Investigator Grant (2 008 129) from the National Health and Medical Research Council, Canberra, Australia. Dr Abdel-Qadir is supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada.
Conflict of interest: P.T. has received speaker's Honorarium from Amgen, Boehringer Ingelheim, and Takeda. E.A. has received fees for expert testimony from Genentech/Roche. B.J.W. has received non financial research support from Siemens Healthineers and provides consultation to Bayer AG. B.J.W. is an inventor of the IG fitting method owned by UHN (US10314548B2) (not applied in this setting). University Health Network has a Master Research Agreement with Siemens Healthineers. H.A.Q. has received honoraria from Amgen, Astra Zeneca, and Jazz Pharmaceuticals. K.H. has received honoraria from Sanofi-Genzyme and Takeda. None of the other authors have any relevant disclosures.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Demissei BG, Fan Y, Qian Y, Cheng HG, Smith AM, Shimamoto K, Vedage N, Narayan HK, Scherrer-Crosbie M, Davatzikos C, Ky B. Left ventricular segmental strain and the prediction of cancer therapy-related cardiac dysfunction. Eur Heart J Cardiovasc Imaging 2021;22:418–426. 10.1093/ehjci/jeaa288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suerken CK, D'Agostino RB Jr., Jordan JH, Melendez GC, Vasu S, Lamar ZS, Hundley WG. Simultaneous Left Ventricular Volume and Strain Changes During Chemotherapy Associate With 2-Year Postchemotherapy Measures of Left Ventricular Ejection Fraction. J Am Heart Assoc 2020;9:e015400. 10.1161/JAHA.119.015400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. 10.1161/CIRCULATIONAHA.114.013777 [DOI] [PubMed] [Google Scholar]
- 4. Kamphuis JAM, Linschoten M, Cramer MJ, Doevendans PA, Asselbergs FW, Teske AJ. Early- and late anthracycline-induced cardiac dysfunction: echocardiographic characterization and response to heart failure therapy. Cardio-Oncology 2020;6:23. 10.1186/s40959-020-00079-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Negishi T, Thavendiranathan P, Penicka M, Lemieux J, Murbraech K, Miyazaki S, Shirazi M, Santoro C, Cho G-Y, Popescu BA, Kosmala W, Costello B, la Gerche A, Mottram P, Thomas L, Seldrum S, Hristova K, Bansal M, Kurosawa K, Fukuda N, Yamada H, Izumo M, Tajiri K, Sinski M, Vinereanu D, Shkolnik E, Banchs J, Kutty S, Negishi K, Marwick TH. Cardioprotection Using Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy: 3-Year Results of the SUCCOUR Trial. JACC: Cardiovascular Imaging 2023;16:269–278. 10.1016/j.jcmg.2022.10.010 [DOI] [PubMed] [Google Scholar]
- 6. Abdel-Qadir H, Nolan MT, Thavendiranathan P. Routine Prophylactic Cardioprotective Therapy Should Be Given to All Recipients at Risk of Cardiotoxicity From Cancer Chemotherapy. Can J Cardiol 2016;32:921–925. 10.1016/j.cjca.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 7. Vaduganathan M, Hirji SA, Qamar A, Bajaj N, Gupta A, Zaha V, Chandra A, Haykowsky M, Ky B, Moslehi J, Nohria A, Butler J, Pandey A. Efficacy of Neurohormonal Therapies in Preventing Cardiotoxicity in Patients with Cancer Undergoing Chemotherapy. JACC: CardioOncology 2019;1:54–65. 10.1016/j.jaccao.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macedo AVS, Hajjar LA, Lyon AR, Nascimento BR, Putzu A, Rossi L, Costa RB, Landoni G, Nogueira-Rodrigues A, Ribeiro ALP. Efficacy of Dexrazoxane in Preventing Anthracycline Cardiotoxicity in Breast Cancer. JACC: CardioOncology 2019;1:68–79. 10.1016/j.jaccao.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murray J, Bennett H, Bezak E, Perry R. The role of exercise in the prevention of cancer therapy-related cardiac dysfunction in breast cancer patients undergoing chemotherapy: systematic review. Eur J Prev Cardiol 2022;29:463–472. 10.1093/eurjpc/zwab006 [DOI] [PubMed] [Google Scholar]
- 10. Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol 2012;60:2384–2390. 10.1016/j.jacc.2012.07.067 [DOI] [PubMed] [Google Scholar]
- 11. Abdel-Qadir H, Bobrowski D, Zhou L, Austin PC, Calvillo-Arguelles O, Amir E, Lee DS, Thavendiranathan P.. Statin Exposure and Risk of Heart Failure After Anthracycline- or Trastuzumab-Based Chemotherapy for Early Breast Cancer: a Propensity ScoreMatched Cohort Study. J Am Heart Assoc 2021;10:e018393. 10.1161/JAHA.119.018393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Acar Z, Kale A, Turgut M, Demircan S, Durna K, Demir S, Meriç M, Ağaç MT. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol 2011;58:988–989. 10.1016/j.jacc.2011.05.025 [DOI] [PubMed] [Google Scholar]
- 13. Nabati M, Janbabai G, Esmailian J, Yazdani J. Effect of Rosuvastatin in Preventing Chemotherapy-Induced Cardiotoxicity in Women With Breast Cancer: a Randomized, Single-Blind, Placebo-Controlled Trial. J Cardiovasc Pharmacol Ther 2019;24:233–241. 10.1177/1074248418821721 [DOI] [PubMed] [Google Scholar]
- 14. Dadson K, Thavendiranathan P, Hauck L, Grothe D, Ali Azam M, Stanley-Hasnain S, Mahiny-Shahmohammady D, Si D, Bokhari M, Lai PFH, Massé S, Nanthakumar K, Billia F. Statins protect against early stages of doxorubicin-induced cardiotoxicity through the regulation of Akt signaling and SERCA2. CJC Open 2022;4:1043–1052. 10.1016/j.cjco.2022.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH; ESC Scientific Document Group . 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229–4361. 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 16. Hundley WG, D'Agostino RB, Crotts T, Craver K, Hackney MH, Jordan J, Ky B, Wagner LI, Herrington DM, Yeboah J, Reding KW, Ladd AC, Rapp SR, Russo S, O'Connell N, Weaver KE, Dressler EV, Ge Y, Melin SA, Gudena V, Lesser GJ. Statins and left ventricular ejection fraction following doxorubicin treatment. NEJM Evidence 2022;1. 10.1056/EVIDoa2200097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol 2017;35:893–911. 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 18. Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R, Dae MW, Wendland MF. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology 1999;211:698–708. 10.1148/radiology.211.3.r99jn41698 [DOI] [PubMed] [Google Scholar]
- 19. Lambert J, Lamacie M, Thampinathan B, Altaha MA, Esmaeilzadeh M, Nolan M, Fresno CU, Somerset E, Amir E, Marwick TH, Wintersperger BJ, Thavendiranathan P. Variability in echocardiography and MRI for detection of cancer therapy cardiotoxicity. Heart 2020;106:817–823. 10.1136/heartjnl-2019-316297 [DOI] [PubMed] [Google Scholar]
- 20. R Core Team . R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 21. Thavendiranathan P, Abdel-Qadir H, Fischer HD, Liu Y, Camacho X, Amir E, Austin PC, Lee DS. Risk-Imaging Mismatch in Cardiac Imaging Practices for Women Receiving Systemic Therapy for Early-Stage Breast Cancer: a Population-Based Cohort Study. J Clin Oncol 2018;36:2980–2987. 10.1200/JCO.2018.77.9736 [DOI] [PubMed] [Google Scholar]
- 22. Kang Y, Assuncao BL, Denduluri S, McCurdy S, Luger S, Lefebvre B, Carver J, Scherrer-Crosbie M. Symptomatic Heart Failure in Acute Leukemia Patients Treated With Anthracyclines. JACC: CardioOncology 2019;1:208–217. 10.1016/j.jaccao.2019.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chotenimitkhun R, D'Agostino R Jr., Lawrence JA, Hamilton CA, Jordan JH, Vasu S, Lash TL, Yeboah J, Herrington DM, Hundley WG. Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. Can J Cardiol 2015;31:302–307. 10.1016/j.cjca.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henninger C, Fritz G. Statins in anthracycline-induced cardiotoxicity: rac and Rho, and the heartbreakers. Cell Death Dis 2017;8:e2564. 10.1038/cddis.2016.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abdel-Qadir H, Thavendiranathan P, Austin PC, Lee DS, Amir E, Tu JV, Fung K, Anderson GM. The Risk of Heart Failure and Other Cardiovascular Hospitalizations After Early Stage Breast Cancer: a Matched Cohort Study. J Natl Cancer Inst 2019;111:854–862. 10.1093/jnci/djy218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE, Ewer MS, Rathi V, Fehrenbacher L, Brufsky A, Azar CA, Flynn PJ, Zapas JL, Polikoff J, Gross HM, Biggs DD, Atkins JN, Tan-Chiu E, Zheng P, Yothers G, Mamounas EP, Wolmark N. Seven-Year Follow-Up Assessment of Cardiac Function in NSABP B-31, a Randomized Trial Comparing Doxorubicin and Cyclophosphamide Followed by Paclitaxel (ACP) With ACP Plus Trastuzumab As Adjuvant Therapy for Patients With Node-Positive, Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. J Clin Oncol 2012;30:3792–3799. 10.1200/Jco.2011.40.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.