Abstract
Background
Guideline recommendations for the treatment of heart failure with mildly reduced ejection fraction (HFmrEF) derive from small subgroups in post-hoc analyses of randomized trials.
Objectives
We investigated predictors of renin–angiotensin system inhibitors/angiotensin receptor neprilysin inhibitors (RASI/ARNI) and beta-blockers use, and the associations between these medications and mortality/morbidity in a large real-world cohort with HFmrEF.
Methods and results
Patients with HFmrEF (EF 40–49%) from the Swedish HF Registry were included. The associations between medications and cardiovascular (CV) mortality/HF hospitalization (HFH), and all-cause mortality were assessed through Cox regressions in a 1:1 propensity score-matched cohort. A positive control analysis was performed in patients with EF < 40%, while a negative control outcome analysis had cancer-related hospitalization as endpoint. Of 12 421 patients with HFmrEF, 84% received RASI/ARNI and 88% beta-blockers. Shared-independent predictors of RASI/ARNI and beta-blockers use were younger age, being an outpatient, follow-up in specialty care, and hypertension. In the matched cohorts, use of both RASI/ARNI and beta-blocker use was separately associated with lower risk of CV mortality/HFH [hazard ratio (HR) = 0.90, 95% confidence interval (CI): 0.83–0.98 and HR = 0.82, 95% CI: 0.74–0.90, respectively] and of all-cause mortality (HR = 0.75, 95% CI: 0.69–0.81 and HR = 0.79, 95% CI: 0.72–0.87, respectively). Results were consistent at the positive control analysis, and there were no associations between treatment use and the negative control outcome.
Conclusions
RASI/ARNI and beta-blockers were extensively used in this large real-world cohort with HFmrEF. Their use was safe since associated with lower mortality and morbidity. Our findings confirm the real-world evidence from previous post-hoc analyses of trials, and represent a further call for implementing guideline recommendations.
Keywords: Heart failure, Mildly reduced ejection fraction, SwedeHF, Registry, Beta-blockers, Renin–angiotensin system inhibitors
Graphical Abstract
Graphical Abstract.

The overall study cohort counted on 12 421 patients with mildly reduced ejection fraction heart failure (HFmrEF). Two sensitivity outcome analyses were adopted, the first conducted on the same population with cancer hospitalization as an outcome (falsification analysis), and the second on 26 143 patients with reduced ejection fraction heart failure (HFrEF). In the upper right panel: use of RASI/ARNI and beta-blockers and percentage of target dose achievement in the overall study population with HFmrEF (n = 12 421). In the bottom panels: Kaplan–Meier curves for the association between RASI/ARNI use (left panel) and the composite outcome (cardiovascular mortality or heart failure hospitalization) and between beta-blockers use (right panel) and the composite outcome. CI, confidence interval; HR, hazard ratio; RASI/ARNI, renin–angiotensin system inhibitors/angiotensin receptor neprilysin inhibitor; TD, target dose.
Introduction
Heart failure (HF) is a global pandemic with increasing prevalence.1 Its prognosis remains poor being the leading cause of hospitalization among adults, with a 10–35% 1-year mortality.2,3
In the 2016 European Society of Cardiology (ESC) HF guidelines, a new HF subtype, i.e. HF with mid-range EF (HFmrEF; defined as EF 40–49%), was introduced.4 Although HFmrEF carries some intermediate features between HFrEF and HFpEF, it presents distinct similarities with HFrEF that supported its redefinition as HF with ‘mildly reduced’ EF (EF 41–49%) proposed in the Universal Definition and Classification of HF and in the 2021 ESC and 2022 American Heart Association (AHA) HF guidelines.5–8
No former randomized controlled trials (RCTs) specifically tested treatment efficacy HFmrEF, and the evidence supporting the use of HFrEF guideline-directed medical therapy in HFmrEF derives from subgroup or post-hoc analyses of RCTs in HFpEF that included, partially or in toto, the 41–49% EF range.9–15 This explains the low level of evidence (level C in the ESC Guidelines and B-NR in the American Guidelines) for guidelines recommendations on the treatment of HFmrEF.5,7
The potential effectiveness of HF medications in HFmrEF has never been assessed in large real-world populations, which might significantly differ from the cohorts tested in RCTs.
Thus, in this study, we sought (1) to assess the use and associated patient profiles of renin–angiotensin system/angiotensin receptor neprilysin inhibitors (RASI/ARNI) and beta-blockers in HFmrEF patients; and (2) to test the association between their use and CV mortality/HF hospitalization (HFH) and all-cause mortality in the large, contemporary, real-world HFmrEF cohort enrolled in the Swedish HF Registry.
Methods
Data sources
Data from the Swedish HF Registry (SwedeHF), linked with the National Patient Registry, the Cause of Death Registry, and Statistics Sweden, were analysed. Data sources are described in detail in the Supplemental material.
Patients and outcomes
Patients registered between 11 May 2000 and 31 December 2018, with HF duration ≥3 months (to allow treatment optimization), follow-up ≥1 day (i.e. patients who died during the hospitalization/visit linked with the registration in SwedeHF were excluded), and no missing data for RASI/ARNI and beta-blocker use were considered.
Patients with EF 40–49% represented the study population, i.e. HFmrEF, whereas patients with HFrEF (EF < 40%) were included as a positive control population where RASI/ARNI and beta-blockers have proven to improve outcomes. We excluded patients receiving RASI or beta-blockers other than those recommended by HF.5 Data on doses were reported, and target dose (TD) was defined according to guidelines (Supplementary material online, Table S1).5 For patients with multiple registrations, we considered the first one to allow longer follow-up. End of follow-up was 31 December 2019. The co-primary outcomes were (1) a composite of cardiovascular (CV) mortality and HFH and (2) all-cause mortality. Secondary outcomes were CV mortality and HFH, separately. A negative control (falsification) analysis was also performed, which consisted of testing the association between the study treatments and hospitalization for cancer in HFmrEF patients, since this association is not supposed to be plausible and therefore, whether retrieved, it might indicate the presence of significant residual confounding.
Statistical analysis
Multiple imputations (10 imputed datasets generated–10 interactions) were used to handle missing values in variables that were covariates in multivariable models (marked by * in Supplementary material online, Table S2; the primary outcome was included in the multiple imputation model as well). Supplementary material online, Table S3 reports the number of missing records per baseline variable. Multivariable logistic regression models were fitted to investigate predictors of use/non-use of treatments.
The propensity score for use of each treatment of interest was separately calculated in each imputed dataset by a logistic regression model, including the clinically relevant variables marked by ^ in Supplementary material online, Table S2 as covariates, and then averaged across the 10 imputed datasets.16 Users and non-users for each individual study treatment were then matched 1:1 using the nearest neighbour method with caliper <0.01 and no replacement. The ability of the matching to balance baseline characteristics in treatment users vs. non-users was assessed by calculating the absolute standard differences, with a value <10% considered as not significant. We used a Cox proportional hazard model to estimate the independent association between treatment use and outcomes, with the matched pairs modelled using a frailty term. Results are presented as hazard ratio (HR) with 95% confidence interval (CI), and survival functions are visualized by the Kaplan–Meier method. Matching reduces the sample size and may limit generalizability; therefore, as a sensitivity analysis, a Cox proportional hazard model was fitted in the overall cohort adjusting, rather than matching, for the propensity score.
All the statistical analyses were performed by Stata 17.0 (StataCorp LLC, College Station, TX, USA). A P-value < 0.05 was considered statistically significant.
The data that support the findings of this study are available from the corresponding author, provided that data sharing is permitted by European Union General Data Protection Regulation regulations and appropriate ethics committees.
Results
Of 12 421 patients with HFmrEF (EF 40–49%, median HF duration 2.5 years, interquartile range 0.9–6.4), 10 419 (84%) received RASI/ARNI, of whom 151 (1.4%) received ARNI, and 10 941 (88%) received beta-blockers (Graphical Abstract). Patients treated with both RASI/ARNI and beta-blockers were 9332 (75%), 2696 (22%) received one drug, and 393 (3%) received neither drug. Mean age was 74 ± 12 years, 64% were males.
Baseline characteristics and predictors of treatments
RASI/ARNI
Twenty-eight percent of treated patients received <50% of TD, 28% patients received 50–99% of TD, and 44% the TD (Graphical Abstract). Main characteristics of treated vs. untreated patients are shown in Table 1 and Supplementary material online, Table S3. Key independent predictors of RASI/ARNI use were younger age, lower heart rate, better renal function, hyper/normokalemia, systemic hypertension, being registered as outpatient and referred for follow-up in specialty care and in nurse-led HF clinic, concomitant treatment with beta-blockers, and cardiac resynchronization therapy (CRT). Higher NT-proBNP, history of valve disease, anaemia, AF, stroke/TIA, chronic obstructive pulmonary disease (COPD), liver disease, and use of mineralocorticoid receptor antagonist (MRA) were independently associated with less use of RASI/ARNI (Figure 1).
Table 1.
Main characteristics of the overall study population and divided according to treatments use vs. non-use
| Total | RASI/ARNI | Beta-blockers | |||||
|---|---|---|---|---|---|---|---|
| Variable | population | Non-use | Use | P-value | Non-use | Use | P-value |
| N | 12 421 | 2002 (16%) | 10 419 (84%) | 1480 (12%) | 10 941 (88%) | ||
| Demographic/organizational characteristics | |||||||
| Male, n (%) | 7921 (64%) | 1125 (56%) | 6796 (65%) | <0.001 | 972 (66%) | 6949 (64%) | 0.100 |
| Age (years), mean (SD) | 74 (12) | 79 (11) | 73 (12) | <0.001 | 77 (12) | 74 (12) | <0.001 |
| Outpatient, n (%) | 7987 (64%) | 796 (40%) | 7191 (69%) | <0.001 | 805 (54%) | 7182 (66%) | <0.001 |
| F-up referral HF nurse clinic, n (%) | 6148 (52.0%) | 689 (37%) | 5459 (55%) | <0.001 | 634 (46%) | 5514 (53%) | <0.001 |
| F-up referral specialty, n (%) | <0.001 | <0.001 | |||||
| Hospital | 7132 (60%) | 838 (45%) | 6294 (62%) | 691 (49%) | 6441 (61%) | ||
| Primary care | 4493 (37%) | 931 (50%) | 3562 (35%) | 663 (47%) | 3830 (36%) | ||
| Other | 319 (3%) | 88 (5%) | 231 (2%) | 52 (4%) | 267 (2%) | ||
| Clinical characteristics | |||||||
| HF duration > 6 months, n (%) | 9404 (77%) | 1543 (79%) | 7861 (77%) | 0.067 | 1131 (78%) | 8273 (77%) | 0.220 |
| NYHA class, n (%) | <0.001 | 0.070 | |||||
| III-IV | 3164 (35%) | 564 (47%) | 2600 (33%) | 371 (37%) | 2793 (34%) | ||
| SBP (mmHg), mean (SD) | 128 (20) | 128 (21) | 128 (20) | 0.420 | 129 (20) | 128 (20) | 0.007 |
| DBP (mmHg), mean (SD) | 73 (12) | 72 (12) | 73 (12) | 0.004 | 72 (12) | 73 (12) | <0.001 |
| HR (b.p.m.), mean (SD) | 72 (14) | 76 (16) | 71 (14) | <0.001 | 72 (15) | 72 (14) | 0.580 |
| Laboratory measurements | |||||||
| eGFR (mL/min/1.73 m²), n (%) | <0.001 | 0.100 | |||||
| eGFR <30 mL/min/1.73 m² | 929 (8%) | 449 (23%) | 480 (5%) | 120 (8%) | 809 (8%) | ||
| eGFR 30–59 mL/min/1.73 m² | 4812 (40%) | 922 (47%) | 3890 (38%) | 608 (42%) | 4204 (39%) | ||
| eGFR ≥60 mL/min/1.73 m² | 6417 (53%) | 598 (30%) | 5819 (57%) | 733 (50%) | 5684 (53%) | ||
| NT-proBNP (ng/L), median (IQR) | 1650 (654–3820) | 3479 (1530–8311) | 1460 (584–3306) | <0.001 | 1782 (667–4536) | 1630 (653–3730) | 0.077 |
| NT-proBNP > 1650 ng/L, n (%) | 3289 (50%) | 690 (73%) | 2605 (46%) | 353 (48%) | 2936 (50%) | ||
| NT-proBNP ≤1650 ng/L, n (%) | 3289 (50%) | 251 (27%) | 3038 (54%) | 385 (52%) | 2910 (50%) | ||
| Potassium, n (%) | <0.001 | 0.240 | |||||
| Hyperkalemia (>5 mEq/L) | 382 (4%) | 64 (4%) | 318 (4%) | ||||
| Normokalemia (3.5–5.0 mEq/L) | 9195 (92%) | 1325 (89%) | 7870 (93%) | ||||
| Hypokalemia (<3.5 mEq/L) | 370 (4%) | 105 (7%) | 265 (3%) | ||||
| Medical history/comorbidities | |||||||
| BMI (kg/m²), median (IQR) | 27 (24–31) | 26 (23–30) | 27 (24–31) | <0.001 | 26 (23–30) | 27 (24–31) | <0.001 |
| BMI > 30, n (%) | 2098 (30%) | 289 (25%) | 1809 (31%) | 202 (25%) | 1896 (31%) | ||
| Diabetes, n (%) | 3642 (29%) | 626 (31%) | 3016 (29%) | 0.037 | 416 (28%) | 3226 (29%) | 0.270 |
| AF, n (%) | 7746 (62.4%) | 1407 (70%) | 6339 (61%) | <0.001 | 919 (62%) | 6827 (62%) | 0.820 |
| Ischaemic heart disease, n (%) | 7482 (60%) | 1261 (63%) | 6221 (60%) | 0.006 | 860 (58%) | 6622 (60%) | 0.075 |
| Anaemia, n (%)a | 4349 (37.4%) | 994 (51%) | 3355 (35%) | <0.001 | 622 (44%) | 3727 (36%) | <0.001 |
| Hypertension, n (%) | 8555 (69%) | 1418 (71%) | 7137 (68%) | 0.039 | 964 (65%) | 7591 (69%) | <0.001 |
| Peripheral artery disease, n (%) | 1294 (10%) | 255 (13%) | 1039 (10%) | <0.001 | 160 (11%) | 1134 (10%) | 0.600 |
| Stroke/TIA, n (%) | 2176 (17%) | 470 (23%) | 1706 (16%) | <0.001 | 286 (19%) | 1890 (17%) | 0.052 |
| Malignant cancer < 3 years, n (%) | 1610 (13%) | 267 (13%) | 2493 (24%) | 0.032 | 238 (16%) | 1372 (12%) | <0.001 |
| COPD, n (%) | 2092 (17%) | 426 (21%) | 1666 (16%) | <0.001 | 297 (20%) | 1795 (16%) | <0.001 |
| Treatments | |||||||
| RASI/ARNI, n (%) | 10 419 (84%) | - | - | - | 1087 (73%) | 9332 (85%) | <0.001 |
| Beta-blockers, n (%) | 10 941 (88%) | 1609 (80%) | 9332 (90%) | <0.001 | - | - | - |
| MRA, n (%)*^ | 4501 (36%) | 688 (34%) | 3813 (37%) | 0.057 | 464 (31%) | 4037 (37%) | <0.001 |
| Diuretics, n (%) | 9643 (78%) | 1731 (87%) | 7912 (76%) | <0.001 | 1144 (77%) | 8499 (78%) | 0.700 |
| Digoxin, n (%) | 1765 (14%) | 295 (15%) | 1470 (14%) | 0.44 | 177 (12%) | 1588 (14%) | 0.008 |
| ICD*^ | 581 (5%) | 59 (3%) | 522 (5%) | <0.001 | 21 (2%) | 560 (5%) | <0.001 |
| CRT*^ | 468 (4%) | 40 (2%) | 428 (4%) | <0.001 | 20 (1%) | 448 (4%) | <0.001 |
| Socioeconomic characteristics | |||||||
| Education, n (%) | <0.001 | 0.160 | |||||
| Compulsory school | 5393 (44.3%) | 1004 (51%) | 4389 (43%) | 657 (45%) | 4736 (44%) | ||
| Secondary school | 4751 (39.1%) | 662 (34%) | 4089 (40%) | 532 (37%) | 4219 (39%) | ||
| University | 2017 (16.6%) | 278 (14%) | 1739 (17%) | 255 (18%) | 1762 (16%) | ||
| Income, n (%) | <0.001 | 0.086 | |||||
| Low | 4289 (34%) | 792 (40%) | 3497 (33%) | 517 (35%) | 3772 (34%) | ||
| Medium | 4763 (38%) | 810 (40.%) | 3953 (38%) | 596 (40%) | 4167 (38%) | ||
| High | 3363 (27%) | 400 (20%) | 2963 (28%) | 367 (25%) | 2996 (27%) | ||
AF, atrial fibrillation; ARNI, angiotensin receptor neprilysin inhibitor; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; F-up; follow-up, HF, heart failure; HR, heart rate; ICD, implantable cardioverter defibrillator; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NT-proBNP; N terminal pro-brain natriuretic peptide, NYHA, New York Heart Association; RASI, renin–angiotensin system inhibitor; SBP, systolic blood pressure; SD, standard deviation; TIA, transient ischaemic attack.
∼ Defined as Hb < 130 g/L in men and 120 g/L in women.
For complete characteristics see Supplementary material online, Table S3.
Figure 1.
Predictors of treatment with RASI/ARNI and beta-blockers in the overall HFmrEF cohort.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, heart rate; ICD, implantable cardioverter defibrillator; MAP, mean arterial pressure; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-brain natriuretic peptide; RASI/ARNI, renin–angiotensin system inhibitors/angiotensin receptor neprilysin inhibitor; TIA, transient ischaemic attack.
Beta-blockers
Among treated patients, 32% received <50% of TD, 35% received 50–99%, and 33% the TD. (Graphical Abstract). Table 1 and Supplementary material online, Table S3 summarize the main characteristics of patients treated with beta-blockers, compared with untreated. Independent predictors of beta-blockers use were younger age, female sex, higher NT-proBNP, being registered as outpatient, being referred for follow-up in specialty care, systemic hypertension, concomitant treatment with diuretics, digoxin, RASI/ARNI, CRT, and ICD. Estimated glomerular filtration rate (eGFR) 30–60 and ≥60 vs. <30 mL/min/1.73 m², anaemia and COPD, and higher education level were independently associated with less use of beta-blockers (Figure 1).
After matching, RASI/ARNI and beta-blocker users and non-users were comparable for all patient characteristics (Supplementary material online, Figure S1 and Table S4).
Outcome analysis
Median follow-up was 2.8 (IQR 1.4–5.3) years. In the overall HFmrEF population, event rates were 180 (95% CI: 176–185) patient-years for CV death/HFH, 143 (95% CI: 140–147) for all-cause death, 86 (95% CI: 83–89) for CV death, and 135 (95% CI: 132–139) for HFH. Event rates according to the treatment groups are reported in Table 2.
Table 2.
Primary and secondary study outcomes
| Overall population | Matched population | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Use n = 10 419 events/1000 pt-yr (95% CI) | Non-use n = 2002events/1000 pt-yr (95% CI) | IRR (95% CI) | HR (95% CI) | PS adjusted HR (95% CI) | Use n = 10 419 events/1000 pt-yr (95% CI) | Non-use n = 2002 | IRR (95% CI)events/1000 pts-yr (95% CI) | HR (95% CI) | |
| RASI/ARNI | |||||||||
| CV mortality/HFH | 160 (156–164) | 364 (345–385) | 0.44^ (0.41–0.47) | 0.50^ (0.47–0.53) | 0.91^ (0.85–0.97) | 314 (297–332) | 350 (331–370) | 0.89^ (0.83–0.97) | 0.90* (0.83–0.98) |
| All-cause mortality | 123 (120–127) | 316 (300–331) | 0.39^ (0.37–0.41) | 0.40^ (0.38–0.42) | 0.78^ (0.73–0.83) | 241 (228–254) | 305 (290–321) | 0.79^ (0.73–0.85) | 0.75^ (0.69–0.81) |
| CV mortality | 74 (71–76) | 192 (180–204) | 0.38^ (0.36–0.41) | 0.40^ (0.37–0.43) | 0.81^ (0.75–0.88) | 157 (147–167) | 185 (173–197) | 0.85^ (0.77–0.93) | 0.84^ (0.76–0.92) |
| HFH | 123 (119–127) | 245 (229–262) | 0.50^ (0.47–0.54) | 0.59^ (0.54–0.63) | 0.98 (0.91–1.07) | 231 (216–246) | 238 (222–254) | 0.97 (0.88–1.07) | 0.99 (0.90–1.09) |
| Beta-blockers | |||||||||
| CV mortality/HFH | 173 (169–178) | 247 (231–264) | 0.70^ (0.65–0.75 | 0.74^ (0.69–0.80) | 0.89^ (0.82–0.96) | 194 (181–208) | 246 (230–263) | 0.79^ (0.72–0.87) | 0.82^ (0.74–0.90) |
| All-cause mortality | 136 (132–139) | 214 (201–228) | 0.63^ (0.59–0.68) | 0.64^ (0.60–0.69) | 0.85^ (0.79–0.91) | 169 (158–180) | 212 (199–226) | 0.80^ (0.73–0.87) | 0.79^ (0.72–0.87) |
| CV mortality | 81 (78–84) | 130 (120–140) | 0.62^ (0.57–0.68) | 0.64^ (0.58–0.70) | 0.86^ (0.78–0.94) | 104 (96–113) | 129 (119–140) | 0.81^ (0.72–0.91) | 0.80^ (0.71–0.90) |
| HFH | 131 (127–135) | 175 (162–189) | 0.75^ (0.69–0.81) | 0.80^ (0.74–0.87) | 0.90* (0.82–0.98) | 138 (127–150) | 161 (174–188) | 0.79^ (0.71–0.89) | 0.86^ (0.76–0.96) |
CI, confidence interval; CV, cardiovascular; HFH, heart failure hospitalization; HR, hazard ratio; IRR, incidence rate ratio; PS, propensity score; RASI/ARNI, renin–angiotensin system inhibitors/angiotensin receptor neprilysin inhibitor.
* P-value < 0.05.
^P-value < 0.01.
CV death/HFH (graphical abstract, Table 2)
RASI/ARNI
In unadjusted analyses performed in the overall population, use of RASI/ARNI was associated with a 50% lower risk of CV death/HFH (HR: 0.50, 95% CI: 0.47–0.53). Consistently, in the matched cohort, there was a 10% lower risk of CV death/HFH associated with RASI/ARNI use (HR: 0.90, 95% CI: 0.83–0.98). Adjusting rather than matching by the propensity score in the overall cohort yielded similar results with HR 0.91 (95% CI: 0.85–0.97). When the components of the primary outcome were separately analysed, use of RASI/ARNI was significantly associated with a lower risk of CV death (HR: 0.84, 95% CI: 0.76–0.92) but not of HFH (HR: 0.99, 95% CI: 0.90–1.09) in the matched cohort. Consistent results were obtained by adjusting instead of matching the propensity score.
Beta-blockers
In unadjusted analyses run in the overall population, use of beta-blockers was associated with a 26% lower risk of CV death/HFH (HR: 0.74, 95% CI: 0.69–0.80). In the matched cohort, an 18% lower risk of CV death/HFH (HR of 0.82, 95% CI: 0.74–0.90) was associated with receiving the treatment. Results were consistent when adjusting rather than matching for the propensity score in the overall population (HR: 0.89, 95% CI: 0.83–0.96). Regarding the secondary outcomes, beta-blocker use was associated with lower risk of both CV death (HR: 0.80, 95% CI: 0.71–0.90) and HFH (HR: 0.86, 95% CI: 0.76–0.96) in the matched cohort, and results were consistent in the propensity score-adjusted analysis.
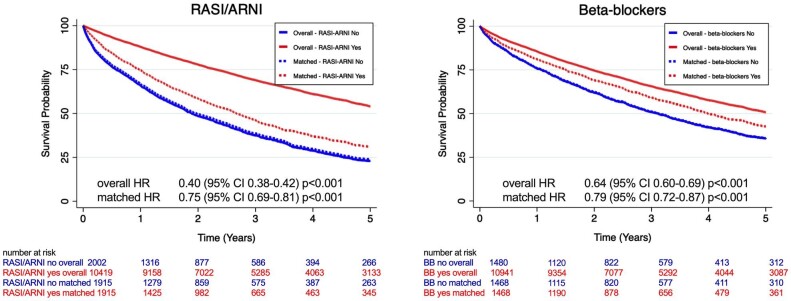
All-cause mortality (Figure 2 and Table 2)
Figure 2.
Kaplan–Meier curves for the association between RASI/ARNI use (left panel) and the composite outcome (cardiovascular mortality or heart failure hospitalization) and between beta-blockers use (right panel) and the composite outcome.
CI, confidence interval; HR, hazard ratio; RASI/ARNI, renin–angiotensin system inhibitors/angiotensin receptor neprilysin inhibitor.
RASI/ARNI
In unadjusted analyses performed in the overall population, use of RASI/ARNI was associated with a 60% lower risk of all-cause death (HR: 0.40, 95% CI: 0.38–0.42). This association persisted after matching with an HR of 0.75 (95% CI: 0.69–0.81) and a relative risk reduction of 25%. Results were consistent when adjusting rather than matching for the propensity score in the overall population (HR: 0.78, 95% CI: 0.73–0.83).
Beta-blockers
Crude HR for the association between use of beta-blockers and mortality was 0.64 (95% CI: 0.60–0.69). After matching, the HR was 0.79 (95% CI: 0.72–0.87), highlighting an independent association between use of beta-blockers and a 21% lower risk of all-cause death. Similar results were obtained when adjusting instead of matching for the propensity score in the overall population (HR 0.85, 95% CI: 0.79–0.81).
Negative control analysis (Supplementary material online, Table S5)
In the matched cohort, use of RASI/ARNI (HR: 0.86, 95% CI: 0.66–1.11) and beta-blockers (HR: 0.91, 95% CI: 0.65–1.26) was not significantly associated with the risk of cancer-related hospitalization.
Positive control analysis
Baseline characteristics of the positive control population of 26 143 patients with HFrEF (EF < 40%) are reported in Supplementary material online, Table S6; 90% were treated with RASI/ARNI and 91% with beta-blockers. In the matched population (Supplementary material online, Table S7 and Figure S2) use of RASI/ARNI was independently associated with an 18% lower risk of CV death/HFH (HR: 0.82, 95% CI: 0.77–0.87) and a 32% lower risk of all-cause mortality (HR: 0.68; 95% CI: 0.64–0.73) (Supplementary material online, Table S8). Consistent results were obtained at the propensity score-adjusted analysis. The secondary outcome analysis (CV mortality and HFH separately analysed) also reported consistent results.
Use of beta-blockers was independently associated with a 15% lower risk of CV death/HFH (HR: 0.85, 95% CI: 0.79–0.91) and a 23% lower risk of all-cause mortality (HR: 0.77, 95% CI: 0.72–0.83) after matching for the propensity score (Supplementary material online, Table S8). Results were consistent when adjusting rather than matching for the propensity score and for the secondary outcomes.
Discussion
In this large nationwide study, 84% of patients with HFmrEF received RASI/ARNI and 88% beta-blockers. Patient characteristics commonly associated with the use of these treatments, included being registered as an outpatient, referral for follow-up in specialty care, and among comorbidities, hypertension, whereas older age and other comorbidities were associated with lower probability of treatment. RASI/ARNI and beta-blockers use were associated with a lower risk of CV mortality/HFH and of all-cause mortality.
Use and predictors of treatment use in HFmrEF
Use of RASI/ARNI and beta-blockers in HFmrEF is not supported by dedicated RCTs. However, their use in clinical practice is frequent. In large international registries, their use has been reported to be high and more similar to HFrEF than HFpEF.17–19 In our study, although treatment use was higher compared to other studies18 and overall more similar to the ESC-LT HF Registry,17 HFmrEF patients were less likely treated with RASI/ARNI or beta-blockers compared with the HFrEF patients included in our positive control analysis, i.e. 84% and 88% vs. 90% and 91%, respectively.
The characteristics of HFmrEF are more similar to HFrEF than HFpEF.20 The extensive use of HFrEF drugs reported in registry-based studies on HFmrEF has been explained by the need of treating concomitant risk factors and comorbidities such as hypertension, ischaemic heart disease, chronic kidney disease, diabetes, and AF.19,21 A further explanation is related to the proportion of patients with improved EF, which in general should not withdraw treatments since proven to be harmful.22 In our study, we identified demographic and healthcare-related organizational factors associated with a more likely use of RASI/ARNI and beta-blockers that included younger age, being registered as outpatient, referral to follow-up in specialty care, and, limited to RASI/ARNI use, referral to follow-up in nurse HF clinic. In SwedeHF, the less implemented use of medications in older patients has been reported in HFrEF.23 Follow-up in specialty care and referral to nurse HF clinics are known to be associated with higher quality of care in HF.24,25 Female sex was associated with more use of beta-blockers, which may be explained by the higher heart rate characterizing females vs. males with HF.26 Worse renal function was associated with lower probability of treatment with RASI/ARNI, but with higher probability of treatment with beta-blockers. In the CHAMP-HF registry on chronic HFrEF, a similar association was observed, which might be linked to the better safety profile for beta-blockers in patients with impaired renal function.27 However, in our analysis, use of RASI/ARNI was reduced even in eGFR ranges where use of these drugs is safe and effective. Hypokalaemia instead of normo/hyperkalaemia was associated with underuse of RASI/ARNI, which might be explained by reverse causality and thus higher potassium levels in patients on RASI/ARNI. Among comorbidities, hypertension was associated with more frequent use of RASI/ARNI and beta-blockers as both have blood pressure-lowering effects. Less use of RASI/ARNI was associated with AF, probably because treatment with beta-blockers was preferred. The association between COPD and less use of both RASI/ARNI and beta-blockers might be explained by beta-blocker use associated with higher risk of hospitalization but not overall exacerbations for COPD, and COPD being a potential cause of dyspnea beyond HF and therefore leading to question the need of RASI/ARNI in this subpopulation.28 Use of diuretics was high in our cohort, ensuring a correct diagnosis of HF and attesting the severity of symptoms and signs in this patient population. In HFmrEF where treatment guidelines recommendations have been missing for long, use of diuretics might have been considered as an HF treatment while use of RASI and BB as a therapy for the comorbidities. Additionally, our cohort might include a proportion of patients with HF and improved EF, and these patients might be symptomatic and therefore in demand of diuretics even whether EF is recovered.
Association between treatment use and outcome
The recommendations for treatment with RASI/ARNI and beta-blockers in HFmrEF introduced in most recent guidelines are supported by weak level of evidence.5,7 The HFmrEF population treated with candesartan in the CHARM Programme showed a 24% lower risk of CV death/HFH compared to those receiving placebo.11 In the PARAGON-HF trial, patients in the EF 45–57% subgroup treated with ARNI showed a 22% reduction in risk of CV death/total HFH compared to those on valsartan.29 A large meta-analysis including 11 RCTs reported a significant benefit in terms of CV death risk reduction for patients in sinus rhythm and with EF 40–49% treated with beta-blockers, but no benefit in terms of all-cause mortality or CV hospitalizations.10 In the EMPEROR-Preserved trial and in the DELIVER trial, empagliflozin and dapagliflozin reduced by 29% and 13%, respectively, the risk of CV death/HFH in the 40–49% EF range.14,15
The association between treatment with RASI/ARNI and beta-blockers and mortality/morbidity outcomes in a real-world scenario of HFmrEF lacks of dedicated studies. In our study, the incidence of all the assessed outcomes was lower in HFmrEF compared to the positive control population of HFrEF, but still higher compared to RCTs.11 Of note, the crude risk of CV death/HFH and of all-cause death was much higher in RASI/ARNI non-users compared to users, highlighting the worse underlying demographic, clinical, and socioeconomical characteristics of patients not receiving the treatment. Therefore, a propensity-score matching design was adopted to minimize the influence of confounders, leading to a reduced sample size, but still larger compared to the HFmrEF subgroups from RCTs (3830 patients in the matched RASI/ARNI analysis vs. 1322 in the CHARM and 1427 in the pooled PARADIGM HF-PARAGON HF analysis; 2936 patients in the matched beta-blockers analysis vs. 575 in the meta-analysis on beta-blockers).10–12 The results of our falsification outcome analysis support the assumption that the risk of residual confounding was well controlled. RASI/ARNI users vs. non-users showed a 10% lower risk of CV death/HFH and a 25% lower mortality risk. Compared to the results from subgroup analyses of RCTs, the magnitude of the association was lower for CV death/HFH, but higher for all-cause and CV mortality. Conversely, no statistically significant association with the risk of HFH was observed. These results could be explained by the proportionally higher risk of death in our real-world population compared to RCTs, which might reduce the exposure to the HFH outcome, i.e. risk of competing events.
The analysis on beta-blockers showed results that were mostly consistent with RCTs.10 Use of beta-blockers was associated with a lower risk of all the study endpoints. The magnitude of the association was slightly smaller for HFH compared with CV death/HFH, all-cause mortality, and CV mortality.
In the positive control analysis, both RASI/ARNI and beta-blockers were associated with a significant lower risk of all the outcomes, including HFH, and, as expected, the magnitude of the association was greater compared with what observed in the HFmrEF cohort, which might highlight a lower treatment effect with these medications together with increasing EF. This analysis further supports the goodness of our estimates in HFmrEF.
Limitations
Despite the extensive adjustments performed by using propensity score matching, the effect of residual unmeasured/unknown confounders cannot be ruled out. HFmrEF was defined as EF 40–49% according to the 2016 ESC Guidelines on HF and not as EF 41–49% as currently recommended since EF was collected as a categorical variable (i.e. <40%, 40–49%, and ≥50%) during most of the study time period.4,5,7 The proportion of patients not receiving RASI/ARNI or beta-blockers in SwedeHF was low, which led to a great reduction of the sample size and statistical power after matching. However, our results were confirmed in the consistency analyses, where we adjusted rather than matched for the propensity score, and thus we analysed the entire SwedeHF HFmrEF population. Use of treatments was defined at baseline, and potential cross-over during follow-up might have diluted the association with outcomes. Since patients were included at their first registration in SwedeHF, some of them might have been reporting an improvement in EF over time prior to their registration, and in this subgroup of patients, the benefit from the study treatments might be higher. In a recent longitudinal study from the SwedeHF, 21% of patients with HFrEF transitioned to HFmrEF at follow-up.30 The low proportion of patients receiving TD of medications might have led to underestimating the magnitude of the association between study treatments and outcomes. Finally, the outcome analysis did not include MRAs, as they are more likely prescribed in the SwedeHF in sicker patients, thus generating a potential selection bias and confounding by indication.31 Moreover, the Food and Drug Administration has recently advised in favour of the use of MRAs in HFpEF, highlighting that the highest benefit is expected in patients in the mildly reduced range of EF, which leads to a clear support of the use of MRAs in HFmrEF.32 Sodium–glucose co-transporter 2 inhibitors were not included in the study as not implemented for the treatment of HFmrEF in clinical practice during the inclusion period.
Conclusions
In this large nationwide cohort with HFmrEF, use of RASI/ARNI and beta-blockers was high and linked with specific demographic and clinical factors, comorbidities, and concomitant therapies. The use of these treatments was associated with better outcomes, in particular with lower risk of CV and all-cause death. The magnitude of these associations was consistent with the results from subgroup/post-hoc analyses of RCTs focusing on HFmrEF, supporting guidelines recommendations on the treatment of HFmrEF and confirming the safe use of these treatments in daily clinical practice.
Supplementary Material
Contributor Information
Davide Stolfo, Division of Cardiology, Department of Medicine, Karolinska Institutet, Heart, Vascular and Neuro Theme Karolinska University Hospital, Norrbacka S3:00, Stockholm 171 76, Sweden; Cardiothoracovascular Department, Azienda Sanitaria Universitaria Giuliano Isontina (ASUGI) and Univeristy Hospital of Trieste, Trieste, Italy.
Lars H Lund, Division of Cardiology, Department of Medicine, Karolinska Institutet, Heart, Vascular and Neuro Theme Karolinska University Hospital, Norrbacka S3:00, Stockholm 171 76, Sweden; Heart, Vascular and Neuro Theme, Karolinska University Hospital, Stockholm, Sweden.
Gianfranco Sinagra, Cardiothoracovascular Department, Azienda Sanitaria Universitaria Giuliano Isontina (ASUGI) and Univeristy Hospital of Trieste, Trieste, Italy.
Felix Lindberg, Division of Cardiology, Department of Medicine, Karolinska Institutet, Heart, Vascular and Neuro Theme Karolinska University Hospital, Norrbacka S3:00, Stockholm 171 76, Sweden.
Ulf Dahlström, Department of Cardiology and Department of Health, Medicine and Caring Sciences, Linkoping University, Linkoping, Sweden.
Giuseppe Rosano, Department of Medical Sciences, IRCCS San Raffaele, Rome, Italy.
Gianluigi Savarese, Division of Cardiology, Department of Medicine, Karolinska Institutet, Heart, Vascular and Neuro Theme Karolinska University Hospital, Norrbacka S3:00, Stockholm 171 76, Sweden; Heart, Vascular and Neuro Theme, Karolinska University Hospital, Stockholm, Sweden.
Conflict of interest: D.S. reports personal fees from Novartis, Merck, Novo Nordisk, GSK, and Acceleron outside the submitted work. L.L. reports personal fees from Merck, grants and personal fees from Vifor-Fresenius, grants and personal fees from AstraZeneca, personal fees from Bayer, grants from Boston Scientific, personal fees from Pharmacosmos, personal fees from Abbott, personal fees from Medscape, personal fees from Myokardia, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from Sanofi, personal fees from Lexicon, and personal fees from Radcliffe cardiology outside the submitted work. G.F.S. reports consulting fees from Novartis, Impulse Dynamics, and Biotronik and speaker and honoraria from Novartis, Bayer, AstraZeneca, Boston Scientific, Vifor Pharma, Menarini, and Akcea Therapeutics outside the submitted work. F.L. has nothing to declare. U.D. reports grants from AstraZeneca, Vifor Pharma, Pfizer, Boehringer Ingelheim, Boston Scientific, and Roche Diagnostics and honoraria/consultancies from Amgen, Pfizer, and AstraZeneca outside the submitted work. G.R. declares no conflicts of interest related to this work. G.S. reports grants and personal fees from Vifor, personal fees from Societa´ Prodotti Antibiotici, grants and personal fees from AstraZeneca, personal fees from Roche, personal fees from Servier, grants from Novartis, personal fees from GENESIS, personal fees from Cytokinetics, personal fees from Medtronic, grants from Boston Scientific, grants from PHARMACOSMOS, grants from Merck, and grants from Bayer outside the submitted work.
References
- 1. Taylor CJ, Ordonez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study. BMJ 2019;364:l223. 10.1136/bmj.l223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thorvaldsen T, Benson L, Dahlstrom U, Edner M, Lund LH. Use of evidence-based therapy and survival in heart failure in Sweden 2003–2012. Eur J Heart Fail 2016;18:503–511. 10.1002/ejhf.496 [DOI] [PubMed] [Google Scholar]
- 3. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. 10.1016/j.jacc.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 5. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 6. Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol 2022;19:100–116. 10.1038/s41569-021-00605-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895–e1032. 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 8. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352–380. 10.1002/ejhf.2115 [DOI] [PubMed] [Google Scholar]
- 9. Abdul-Rahim AH, Shen L, Rush CJ, Jhund PS, Lees KR, McMurray JJV. Effect of digoxin in patients with heart failure and mid-range (borderline) left ventricular ejection fraction. Eur J Heart Fail 2018;20:1139–1145. 10.1002/ejhf.1160 [DOI] [PubMed] [Google Scholar]
- 10. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J 2018;39:26–35. 10.1093/eurheartj/ehx564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018;20:1230–1239. 10.1002/ejhf.1149 [DOI] [PubMed] [Google Scholar]
- 12. Solomon SD, Vaduganathan M, LC B, Packer M, Zile M, Swedberg K. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation 2020;141:352–361. 10.1161/CIRCULATIONAHA.119.044586 [DOI] [PubMed] [Google Scholar]
- 13. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016;37:455–462. 10.1093/eurheartj/ehv464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 15. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–1098. 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 16. Mitra R, Reiter JP. A comparison of two methods of estimating propensity scores after multiple imputation. Stat Methods Med Res 2016;25:188–204. 10.1177/0962280212445945 [DOI] [PubMed] [Google Scholar]
- 17. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017;19:1574–1585. 10.1002/ejhf.813 [DOI] [PubMed] [Google Scholar]
- 18. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. Eur J Heart Fail 2017;19:1258–1269. 10.1002/ejhf.807 [DOI] [PubMed] [Google Scholar]
- 19. Koh AS, Tay WT, Teng THK, Vedin O, Benson L, Dahlstrom U. A comprehensive population-based characterization of heart failure with mid-range ejection fraction. Eur J Heart Fail 2017;19:1624–1634. 10.1002/ejhf.945 [DOI] [PubMed] [Google Scholar]
- 20. Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol 2022;19:100–116. 10.1038/s41569-021-00605-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Savarese G, Settergren C, Schrage B, Thorvaldsen T, Löfman I, Sartipy U. Comorbidities and cause-specific outcomes in heart failure across the ejection fraction spectrum: a blueprint for clinical trial design. Int J Cardiol 2020;313:76–82. 10.1016/j.ijcard.2020.04.068 [DOI] [PubMed] [Google Scholar]
- 22. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet North Am Ed 2019;393:61–73. 10.1016/S0140-6736(18)32484-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stolfo D, Lund LH, Becher PM, Orsini N, Thorvaldsen T, Benson L. Use of evidence-based therapy in heart failure with reduced ejection fraction across age strata. Eur J Heart Fail 2022;24:1047–1062. 10.1002/ejhf.2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindberg F, Lund LH, Benson L, Schrage B, Edner M, Dahlström U. Patient profile and outcomes associated with follow-up in specialty vs. primary care in heart failure. ESC Heart Fail 2022;9:822–833. 10.1002/ehf2.13848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liljeroos M, Stromberg A. Introducing nurse-led heart failure clinics in Swedish primary care settings. Eur J Heart Fail 2019;21:103–109. 10.1002/ejhf.1329 [DOI] [PubMed] [Google Scholar]
- 26. Stolfo D, Uijl A, Vedin O, Stromberg A, Faxen UL, Rosano GMC. Sex-based differences in heart failure across the ejection fraction spectrum: phenotyping, and prognostic and therapeutic implications. JACC: Heart Fail 2019;7:505–515. 10.1016/j.jchf.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 27. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol 2018;72:351–366. 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 28. Dransfield MT, Voelker H, Bhatt SP, Brenner K, Casaburi R, Come CE. Metoprolol for the prevention of acute exacerbations of COPD. N Engl J Med 2019;381:2304–2314. 10.1056/NEJMoa1908142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 30. Savarese G, Vedin O, D'Amario D, Uijl A, Dahlstrom U, Rosano G. Prevalence and prognostic implications of longitudinal ejection fraction change in heart failure. JACC: Heart Fail 2019;7:306–317. 10.1016/j.jchf.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 31. Lund LH, Svennblad B, Melhus H, Hallberg P, Dahlstrom U, Edner M. Association of spironolactone use with all-cause mortality in heart failure: a propensity scored cohort study. Circ: Heart Fail 2013;6:174–183. 10.1161/CIRCHEARTFAILURE.112.000115 [DOI] [PubMed] [Google Scholar]
- 32. Administration USFaD . Final Summary Minutes of the Cardiovascular and Renal Drugs Advisory Committee Meeting 16 December 2020. 2020. https://www.fda.gov/media/ 145548/download
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.