Abstract
There is considerably greater variation in metabolic rates between men than between women, in terms of basal, activity and total (daily) energy expenditure (EE). One possible explanation is that EE is associated with male sexual characteristics (which are known to vary more than other traits) such as musculature and athletic capacity. Such traits might be predicted to be most prominent during periods of adolescence and young adulthood, when sexual behaviour develops and peaks. We tested this hypothesis on a large dataset by comparing the amount of male variation and female variation in total EE, activity EE and basal EE, at different life stages, along with several morphological traits: height, fat free mass and fat mass. Total EE, and to some degree also activity EE, exhibit considerable greater male variation (GMV) in young adults, and then a decrease in the degree of GMV in progressively older individuals. Arguably, basal EE, and also morphometrics, do not exhibit this pattern. These findings suggest that single male sexual characteristics may not exhibit peak GMV in young adulthood, however total and perhaps also activity EE, associated with many morphological and physiological traits combined, do exhibit GMV most prominently during the reproductive life stages.
Keywords: inter-individual variation, morphometry, age, height, weight
1. Introduction
Individuals of a sexually reproducing species vary in terms of nearly every measurable characteristic, morphological, physiological and cognitive. In mammals, often the magnitude of this inter-individual variability, at least in terms of body morphometrics and, in humans, also cognition, has been reported as greater between males than between females [1,2]. This phenomenon is termed ‘greater male variability’ (GMV) [3]. For example, it has been reported that human males are more varied in their physical performance than are human females [4,5], and male chimpanzees have more varied brain structures than do female chimpanzees [6]. Given that the metabolism of an animal is arguably an emergent property influenced by the culmination of GMV in various bodily traits, in a previous study we postulated that GMV in energy expenditure could be particularly large [7]. Supporting this suggestion, we found that in adult humans, energy expenditure exhibits considerable GMV in terms of basal energy expenditure (BEE) and total (daily) expenditures (TEE), even after controlling for key morphological traits such as height, fat free mass and fat mass [7].
We also discovered that with ageing, variation between people in their TEE decreases, but this happens more rapidly in men than in women which results in the magnitude of GMV in TEE attenuating in older age groups [7]. One possible explanation for GMV is that males experiencing stronger sexual selection results in the expression of male sexual traits with greater variance than that of female sexual traits [8]. Energy expenditure is related to various traits of sexual interest to females such as cognitive capacity [9], physical endeavour, strength and muscle mass [10], and perhaps also aerobic fitness [11]. Variability in male sexual characteristics could be most prominent during the period of life associated with sexual reproduction (i.e. late adolescence and young adulthood), explaining the aforementioned decrease in GMV in energy expenditure in older people.
If indeed GMV in energy expenditure is underpinned by sexual selection, we predict there will be no GMV in humans prior to sexual maturity, and that GMV will peak during young adulthood due to an increase in male variation, which will then decrease more rapidly than female variation into old age. We tested this hypothesis by analysing extensive data on human energy expenditure for individuals of ages spanning the entire human life course.
2. Methods
We analysed data from the International Atomic Energy Agency (IAEA) DLW database v.3.7 [12]. The dataset comprised TEE measurements (MJ d−1) for 4992 females and 2626 males, and BEE measurements (MJ d−1) using indirect calorimetry for 1542 females and 934 males. Estimates of activity energy expenditure (AEE) were calculated by subtracting BEE from 0.9*TEE (TEE adjusted to account for the thermic effect to food). Ages of the participants ranged from newborns to 101 years old. We also included in our analyses the following traits: height (cm), fat-free mass (kg), fat mass (kg) and body weight (kg). Further details are provided in Halsey et al. [7].
(a) . Statistical analyses
All analyses were conducted in R v. 3.5.3 [13]. We quantified variance in male and female energy expenditure using Bayesian general linear models based on Monte-Carlo Markov chain (MCMC) models using the ‘MCMCglmm’ package [14]. Each model included one trait as the response variable (TEE, AEE or BEE) and sex as an independent variable along with age as a categorical variable, and height, fat-free mass and fat mass as continuous variables, with no intercept fitted so that the model returned separate mean estimates for males and females within each age category [15]. Age was recoded as a categorical variable with eight levels for the TEE model, and six levels for the AEE and BEE models (due to a smaller overall BEE sample size, although sample size per age category was still smaller). Using the ‘idh’ function, we allowed the residual variance to be different in males and females within each age category. Further models included height, fat-free mass or fat mass as the response variable (with only sex and age as independent variables). All models also included country as a random effect to account for the unequal sampling distribution across countries. When included as covariates, the three morphometric variables were centred so that model estimates were estimated at the centre of the distribution of the covariates [15]. To make sure that any sex differences in variance were not due to mean differences, we also standardized the variance within each sex and age category by dividing variances with the sex-specific mean estimates to obtain the coefficient of variance (CoV). From each model, we calculated the posterior mode and 95% highest posterior density credible intervals (CIs) for the CoV estimates for each age group in males and females. Treating these as 95% confidence intervals, in cases where the CIs for the relative variances of the two sexes do not overlap, the evidence of a difference in variance between the sexes is strong [16]. Finally, we calculated the male : female ratio of CoV whereby a value greater than 1 indicated GMV.
3. Results
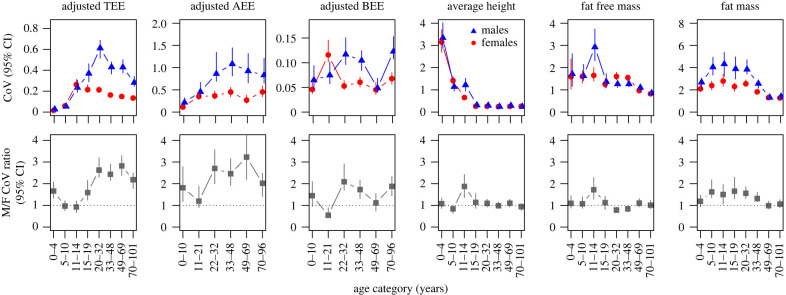
Visual interpretation of graphs of energy expenditure and morphology against age category (figure 1), using the 95% CIs for guidance, indicates that TEE and AEE exhibit increases in male variance from early childhood into young adulthood followed by decreases with further ageing. In TEE, this generates the development of GMV into young adulthood and a decrease in the elderly, while in AEE a pattern of increase and then decrease is apparent but less clear. A pattern in the data for BEE is not apparent although GMV does peak in early adulthood. Average height shows no pattern of GMV developing into young adulthood and then fading. Fat free mass also shows no such pattern. Fat mass does exhibit an increase in male variance followed by a decrease, which results in a similar pattern in GMV, however peak GMV occurs well before adulthood.
Figure 1.
Top row: male (blue triangles) and female (red circles) coefficients of variance (CoV) in measures of energy expenditure and body morphometrics as a function of age category. Error bars represent 95% credible intervals (95% CI). These data are presented in electronic supplementary material, table S1. Bottom row: the ratio of male to female (M/F) CoV with 95% CI. The dashed lines represent equal variance; values above indicate greater male variance (GMV). TEE = total energy expenditure; AEE = activity energy expenditure; BEE = basal energy expenditure.
4. Discussion
In young children, there is little difference between boys and girls in terms of between-person variance in TEE (figure 1). However, by late adolescence (15–19 years) males are exhibiting greater variation and in turn GMV. This GMV increases in magnitude into young adulthood and beyond, and then somewhat decreases by old age due to decreasing inter-male variation. This pattern through the life course bears hallmarks of a sexually selected signal, or at least a trait that is an emergent property of characteristics some or all of which are sexually selected. The qualitative pattern of increasing then decreasing inter-male variation is also apparent although less strong in AEE, and increasing then decreasing GMV is also apparent. Albeit the sample size was lower and inter-study measurement variation probably higher, the above findings together tentatively suggest that changes in AEE variance over the life course might somewhat drive the TEE variance patterns.
Patterns for BEE are less clear and might be due to, again, a lower sample size than for TEE and with greater measurement variation between studies. More data are required.
Given that body morphology is considered to harbour sexual traits, we might predict that at least some morphological measures would exhibit a pattern of GMV similar to that seen in TEE, however this was not the case. While fat mass exhibits an increase then decrease in inter-male variation over the life course, the start of peak GMV occurs before adolescence rather than during the age of peak reproduction. Average height and fat free mass both show a spike in inter-male variation, and in turn substantive GMV, for a short period in early adolescence, an observation also apparent in data for Dutch children [17]. However, this is likely generated by the variability in both magnitude and degree of growth spurt in boys at this age.
About two-thirds of the dataset are for citizens of the USA, a country that has particularly high rates of obesity (https://www.oecd.org/health/health-data.htm). Nonetheless, qualitatively the patterns in male variation, female variation and GMV through the life course are generally similar to those present in the full dataset when the USA data are removed (electronic supplementary material, figure S1 and table S2).
In conclusion, inter-individual variance over the life course for height and fat free mass do not exhibit the hypothesized patterns given that they are two morphometric variables considered to be male sexual characteristics (albeit height is a relatively fixed variable come adulthood). Fat mass is the morphometric variable that is closest to exhibiting the hypothesized patterns, despite being considered more of a female sexual characteristic. TEE and to some extent AEE present with GMV developing and peaking in young adulthood and subsequently declining, perhaps indicating that they are either male sexual characteristics or related to such characteristics. Perhaps energy expenditure most clearly presents with the hypothesized patterns of GMV over the life course because it results from the combined effects of many characteristics.
Acknowledgements
Yvonne Schönbeck provided important information about morphometric measurements for Dutch children. A chat over dinner with Karsten Koehler, Eimear Dolan and Danny Longman brought up a number of thoughts that influenced this manuscript. The DLW database, which can be found at https://doublylabelled-waterdatabase.iaea.org/home, is hosted by the IAEA and generously supported by Taiyo Nippon Sanso and SERCON. We are grateful to the IAEA and these companies for their support and especially to Takashi Oono for his tremendous efforts at fundraising on our behalf.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The data are provided in the electronic supplementary material [18].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
L.G.H.: conceptualization, formal analysis, investigation, project administration, software, visualization, writing—original draft, writing—review and editing; V.C.: formal analysis, software, visualization, writing—review and editing; J.R.S.: data curation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
The authors also gratefully acknowledge funding from the Chinese Academy of Sciences (grant no. CAS153E11KYSB20190045) to J.R.S. and the US National Science Foundation (grant no. BCS-1824466) awarded to H.P.
References
- 1.Reinhold K, Engqvist L. 2013. The variability is in the sex chromosomes. Evolution 67, 3662-3668. ( 10.1111/evo.12224) [DOI] [PubMed] [Google Scholar]
- 2.Baye A, Monseur C. 2016. Gender differences in variability and extreme scores in an international context. Large-Scale Assess. Educ. 4, 1. ( 10.1186/s40536-015-0015-x) [DOI] [Google Scholar]
- 3.Darwin, C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 4.Lehre AC, Lehre KP, Laake P, Danbolt NC. 2009. Greater intrasex phenotype variability in males than in females is a fundamental aspect of the gender differences in humans. Dev. Psychobiol 51, 198-206. ( 10.1002/dev.20358) [DOI] [PubMed] [Google Scholar]
- 5.Wilmore JH. 1974. Alterations in strength, body composition and anthropometric measurements consequent to a 10-week weight training program. Med. Sci. Sports Exerc. 6, 133-138. [PubMed] [Google Scholar]
- 6.DeCasien AR, Sherwood CC, Schapiro SJ, Higham JP. 2020. Greater variability in chimpanzee (Pan troglodytes) brain structure among males. Proc. R. Soc. B 287, 20192858. ( 10.1098/rspb.2019.2858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halsey LG, et al. 2022. Variability in energy expenditure is much greater in males than females. J. Hum. Evol. 171, 103229. ( 10.1016/j.jhevol.2022.103229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison LM, Noble DW, Jennions MD. 2022. A meta-analysis of sex differences in animal personality: no evidence for the greater male variability hypothesis. Biol. Rev. 97, 679-707. ( 10.1111/brv.12818) [DOI] [PubMed] [Google Scholar]
- 9.Goncerzewicz A, Górkiewicz T, Dzik JM, Jędrzejewska-Szmek J, Knapska E, Konarzewski M. 2022. Brain size, gut size and cognitive abilities: the energy trade-offs tested in artificial selection experiment. Proc. R. Soc. B 289, 20212747. ( 10.1098/rspb.2021.2747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lidborg LH, Cross CP, Boothroyd LG. 2022. A meta-analysis of the association between male dimorphism and fitness outcomes in humans. Elife 11, e65031. ( 10.7554/eLife.65031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poehlman ET, Melby CL, Badylak SF, Calles J. 1989. Aerobic fitness and resting energy expenditure in young adult males. Metabolism 38, 85-90. ( 10.1016/0026-0495(89)90185-6) [DOI] [PubMed] [Google Scholar]
- 12.Speakman JR, et al. 2019. The International Atomic Energy Agency international Doubly Labelled Water Database: aims, scope and procedures. Ann. Nutr. Metab. 75, 114-118. ( 10.1159/000503668) [DOI] [PubMed] [Google Scholar]
- 13.R Development Core Team . 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 14.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1-22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 15.Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103-113. ( 10.1111/j.2041-210X.2010.00012.x) [DOI] [Google Scholar]
- 16.Belia S, Fidler F, Williams J, Cumming G. 2005. Researchers misunderstand confidence intervals and standard error bars. Psychol. Methods 10, 389. ( 10.1037/1082-989X.10.4.389) [DOI] [PubMed] [Google Scholar]
- 17.Schönbeck Y, Talma H, Van Dommelen P, Bakker B, Buitendijk SE, HiraSing RA, Van Buuren S. 2013. The world's tallest nation has stopped growing taller: the height of Dutch children from 1955 to 2009. Pediatr. Res. 73, 371-377. ( 10.1038/pr.2012.189) [DOI] [PubMed] [Google Scholar]
- 18.Halsey LG, et al. 2023. Greater male variability in daily energy expenditure develops through puberty. Figshare. ( 10.6084/m9.figshare.c.6832989) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are provided in the electronic supplementary material [18].