Abstract
Until recently, sheep-associated malignant catarrhal fever (SA-MCF) was diagnosed mainly on the basis of clinical presentation and histopathological changes. Using clinically diagnosed field cases, we have evaluated a seminested PCR and a competitive inhibition enzyme-linked immunosorbent assay (CI-ELISA) and compared these assays in the diagnosis of SA-MCF in cattle with histopathology as a provisional “gold standard.” Samples from 44 cattle with clinical signs suggestive of SA-MCF were examined by histopathology, PCR, and CI-ELISA. In addition, samples from healthy cattle were evaluated by PCR (n = 96) and CI-ELISA (n = 75). Based on histopathology, 38 of the 44 clinical cases were classified as SA-MCF positive, 3 were classified as inconclusive, and 3 were classified as SA-MCF negative. The sensitivity of PCR was 95 to 97%, whereas the specificity ranged between 94 and 100%. The CI-ELISA showed a sensitivity of 56 to 87% and a specificity between 91 and 100%. In the field, there is good correlation between the diagnoses of SA-MCF by histopathology, PCR, and CI-ELISA. These data also confirm the close association of ovine herpesvirus 2 with SA-MCF in Switzerland.
Malignant catarrhal fever (MCF) is a mostly fatal, although sporadic, disease of cattle and other ruminant species which is characterized by lymphoid proliferation and is often difficult to diagnose. Clinically, the most important differential diagnoses in cattle are mucosal disease, infectious bovine rhinotracheitis, foot-and-mouth disease, and rinderpest. There are two etiologically distinct forms of MCF: (i) a wildebeest-associated form, caused by alcelaphine herpesvirus 1 (AlHV-1 [10], previously AHV-1), and (ii) a sheep-associated form (SA-MCF), occurring worldwide and implicated with the putative ovine herpesvirus 2 (OvHV-2 [10], previously OHV-2).
Histopathological examination is the most widely used diagnostic procedure to confirm clinical suspicion of SA-MCF. Lymphocytic infiltration and vasculitis in the brain and other organs are the most significant lesions. In 1990, a DNA sequence with high homology to AlHV-1 was discovered in lymphoblastoid cells from SA-MCF-diseased ruminants and strongly implicated the corresponding virus, the putative OvHV-2, with the etiology of SA-MCF (2). Subsequently, Baxter et al. (1) developed a PCR protocol to demonstrate OvHV-2 DNA.
Using a monoclonal antibody (3) against a cross-reacting epitope shared by AlHV-1 and OvHV-2 but not by the highly prevalent bovine herpesvirus 4 and other common viruses in cattle, Li et al. established a competitive inhibition enzyme-linked immunosorbent assay (CI-ELISA) (4) for the detection of antibodies to MCF viruses in ruminant species.
A number of clinical MCF cases in cattle have been examined by these laboratory methods (5, 9). However, a comparative evaluation of these tests using a larger number of independent field cases has not yet been published. We have assessed PCR and CI-ELISA for the intravitam laboratory diagnosis of SA-MCF in comparison to classical histopathological examination.
MATERIALS AND METHODS
Animals.
During 1995 and 1996, samples from 44 cases of clinically suspected field SA-MCF were submitted to the Veterinary Teaching Hospital of the University of Zürich. The affected cattle belonged to several breeds (Brown Swiss, 75%; Red Holstein, 11%; Holstein-Friesian, 7%; various others, 7%) and age groups (median, 2.4 years; range, 0.04 to 17 years). Tentative diagnosis of MCF was based on typical symptoms, including nasal and ocular discharge, keratoconjunctivitis, hyperemic mucous membranes, mucosal ulceration in oral and nasal cavities, hematuria, diarrhea, and a body temperature of 40°C. Prior to euthanasia and postmortem examination, blood samples were taken for serology and PCR on buffy coat cells. Healthy cattle from farms with (n = 3) and without (n = 5) a history of SA-MCF were sampled and examined by PCR (n = 96) and CI-ELISA (n = 75).
OvHV-2 PCR. (i) Sample preparation.
EDTA-blood samples (10 ml) were centrifuged at 18°C for 35 min at 1,400 × g. Buffy coat cells were resuspended in 4 volumes of sterile 0.2% NaCl to lyse erythrocytes. After 1 min, 7.2% NaCl was added to reconstitute isotonicity. The cells were further washed in phosphate-buffered saline and stored at −20°C.
(ii) DNA extraction.
Buffy coat cells were resuspended in 10 volumes of extraction buffer (1 mM EDTA, 50 mM Tris, 0.05% Tween 20) with freshly added proteinase K (200 μg/ml) and RNase A (10 μg/ml) before being digested overnight at 50°C. After heat inactivation of the proteinase K (95°C, 10 min) and centrifugation (13,000 × g, 30 s), the supernatant was used directly in PCR.
(iii) PCR.
The PCR protocol was adapted from Baxter et al. (1). Approximately 1 μg of DNA in 10 μl of extraction buffer was mixed with 35 μl of solution 1 to contain final concentrations of 10% dimethyl sulfoxide, 50 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, 16 mM (NH4)2SO4, 0.01% gelatin, and 1 μM (each) primers 556 (AGTCTGGGTATATGAATCCAGATGGCTCTC) and 755 (AAGATAAGCACCAGTTATGCATCTGATAAA) (1). The samples were preincubated for 5 min at 99°C and then kept at 60°C before 5 μl of solution 2 containing 2 U of SuperTaq polymerase (HT Biotechnology) and deoxynucleoside triphosphates (final concentrations of 200 μM [each] dATP, dGTP, and dCTP and 400 μM dUTP) were added to each reaction mixture. Subsequently, the thermal cycling protocol used was 30 s at 72°C, 20 s at 94°C, and 30 s at 60°C. After 39 cycles, 5% of each sample was transferred to a fresh tube containing 40 μl of solution 1 (including the seminested primer pair 556 and 555 [TTCTGGGGTAGTGGCGAGCGAAGGCTTC]) for an additional 39 cycles.
A total of 10 μl of each reaction mixture was run on a 2% agarose gel for evaluation by ethidium bromide visualization. Regularly, each sample was tested twice and contradictory results were considered inconsistent.
DNA from BJ1035, a lymphoblastoid cell line derived from a cow with SA-MCF, was kindly provided by H. W. Reid (Moredun Research Institute, Edinburgh, United Kingdom) and served as a positive control in the initial setup. Positive clinical samples were used subsequently.
CI-ELISA for antibodies to MCF viruses.
Sera were blind coded and tested for MCF virus-specific antibodies by using the original protocol of the previously described CI-ELISA (4). Both samples and controls were tested in triplicate. The cutoff values were defined as follows. On each ELISA plate, the capacity of monoclonal antibody 15A to bind to MCF virus antigen was competed by a panel of nine negative sheep sera. The means (M) and standard deviations (SD) of the absorption at 414 nm were determined. Sera which inhibited this reaction to a value not less than M of the negative panel minus 2 SD were regarded as negative. Sera reducing the value to less than M minus 3 SD were regarded as positive. Sera with an average absorption ranging from M minus 2 SD to M minus 3 SD were defined as borderline, as were all sera which reacted inconsistently (positive, negative, or borderline) in two independent ELISA runs.
Histopathology.
Histopathological examination was performed on hematoxylin-eosin-stained paraffin sections. The organs regularly examined included brain (five locations), eye, bladder, liver, kidney, lung, gut, and epithelium displaying macroscopic lesions. The histological features used for diagnosis were as follows: perivascular infiltrations with activated lymphoid cells, lymphocytic vasculitis, and a fibrinoid degeneration of vascular media (7, 8, 13). Cases in which two of these criteria were not detected or were only poorly detected were regarded as inconclusive. For the assessment of sensitivity and specificity, all healthy control animals were assumed to be negative by histopathology.
Differential diagnosis.
All cattle were tested for persistent bovine viral diarrhea virus (BVDV) infection by immunohistology (12) and/or an antigen detection ELISA (11).
RESULTS
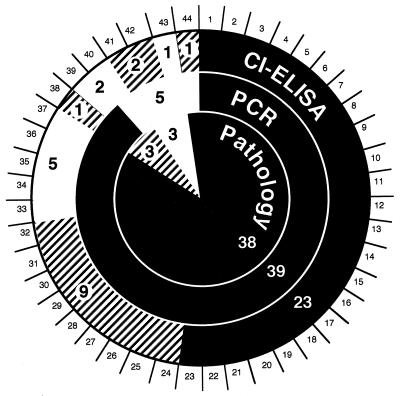
Figure 1 summarizes the results obtained from 44 independent clinical field cases in which cattle had been presented as suffering from MCF.
FIG. 1.
Comparison of test results obtained independently with samples from 44 cattle clinically suspected of suffering from MCF. Histopathology (Pathology), depicted in the innermost circle, was used as the reference test. Seminested PCR results are depicted in the adjacent circle, and CI-ELISA results are shown in the outermost circle. MCF-positive test results (black), uncertain or borderline test results (hatched), and negative test results (white) are shown. Each case is represented by a numbered segment, corresponding to the numbers used in the text. The larger numbers, within the figure, are subtotals for each category.
Histopathological examinations revealed that 38 of the 44 diseased cattle displayed lesions typical of MCF. No conclusive histopathological diagnosis could be drawn from three cases (no. 38 to 40), whereas in three other cases (no. 41 to 43) mucosal disease was diagnosed, based on histopathology and BVDV antigen detection. These results are visualized in the innermost circle of Fig. 1. The adjoining circle presents the corresponding results by PCR. A positive signal was detected in a total of 39 cases (no. 1 to 39), including 37 positive and 2 inconclusive cases by histopathology. No signal was detected for five cases, namely, case 44, classified positive, and case 40, classified inconclusive in histopathology, as well as the three mucosal disease cases (no. 41 to 43). Interestingly, one further persistent BVDV carrier (no. 23) was detected among the 23 unanimously MCF-positive animals.
By CI-ELISA, antibodies against an MCF virus-specific epitope were detected in the sera of 23 field cases (no. 1 to 23), which had been found positive by histopathology and PCR. The sera of nine animals (no. 24 to 32) which had been found positive both by histopathology and by PCR were considered borderline. Animal 38 was borderline by serology and inconclusive by histopathology but positive by PCR. Further, two animals (no. 41 and 42) showed borderline serology, although neither histopathology nor PCR indicated MCF. Interestingly, in both animals BVDV antigen was detected by immunohistology. The sera of five animals (no. 33 to 37) reacted negatively in CI-ELISA, although PCR and histopathology indicated MCF. Contradicting results were observed with one further animal (no. 44), reacting borderline in CI-ELISA. It was negative by PCR but was judged to be positive by histopathology. Finally, the sera of three animals (no. 39, 40, and 43) gave negative serological results; two of them were inconclusive by histopathology (no. 40 and 41), whereas one animal (no. 43) was classified as negative by all three tests.
Samples from 96 clinically healthy animals were examined by PCR, and 92 of them reacted negatively. Seventy of those animals originated from farms with a history of MCF and were known to have contact with sheep. The remaining 26 animals had no known exposure to sheep. Unexpectedly, four healthy animals (all of them with sheep contact) reacted inconsistently or positively by PCR. Three of those animals were not available for further testing. However, one of them developed MCF 1 week after sampling.
Among the sera from 75 clinically healthy cattle examined by CI-ELISA, including 52 animals also tested by the PCR, 44 serum samples originated from cattle with sheep contact or an MCF history on the premises and 31 sera originated from animals with no sheep contact. None of the healthy control cattle reacted positively in any of the CI-ELISA runs, but four animals were classified as borderline.
Using the above data, the sensitivity and specificity of both PCR and CI-ELISA versus pathology, as the “gold standard,” were estimated. Taking into account the uncertainties involved in using histopathology as a gold standard, worst- and best-case scenarios were calculated for CI-ELISA and PCR by classifying borderline and uncertain results once as positive and once as negative. Under these conditions, the sensitivity for PCR ranged from 95 to 97% and the specificity was 94 to 100%. CI-ELISA gave a sensitivity of 56 to 87% and a specificity of 91 to 100%.
DISCUSSION
Taking advantage of the fact that MCF occurs quite frequently in Switzerland, we compared and evaluated histopathology, PCR, and CI-ELISA for the diagnosis of MCF by using 44 clinically diagnosed Swiss field cases and samples from over 100 clinically healthy animals.
The salient features of our findings are as follows. (i) PCR may be regarded as the method of choice for the diagnosis of clinical SA-MCF. Its superiority relative to histopathology is based not only on the fact that the diagnosis by PCR can be made intravitam but also on its sensitivity and specificity. Two cases that were inconclusive by histopathology were diagnosed as MCF by PCR. It is unlikely that the apparently healthy animals, which reacted positive by PCR, represent false-positive cases. One of those animals came down with clinical MCF only 1 week after sampling, suggesting that positive reactions may be detected during the incubation period. Furthermore, we tested by PCR several animals that had survived clinical MCF (data not shown) on multiple occasions before and after recovery. The animals remained PCR positive over time, indicating that the causative agent is not cleared from the animal even more than 2 years after clinical recovery, which is consistent with a previous report (9). (ii) Histopathology is useful for postmortem MCF diagnosis, although differentiation from other histologically similar diseases may require a wide range of organ samples. (iii) Serology with CI-ELISA is less sensitive than either histopathology or PCR. Hence, a negative CI-ELISA result must be confirmed by PCR for a valid laboratory diagnosis. Because of its many advantages, such as speed, throughput, high specificity, and the fact that the assay does not require anti-species-specific conjugates, the assay may be useful for initial screening of large sets of samples from possible reservoir species. (iv) The relatively low sensitivity of the CI-ELISA in cattle with clinical MCF may be explained in two ways: it may be attributed partially to the often rapid clinical progression, which may lead an animal to death before antibodies reach a detectable level, and partially to an individual lack of response, as some cattle with chronic or recovered MCF as well as some adult PCR-positive sheep remain antibody negative by CI-ELISA (6, 9). Whether these individuals lack humoral immune responses to the viral antigens in general or to the single 15A epitope has not been determined, but a genetic inability of some animals to respond to this epitope may indicate limitations of this assay. Thus, it will be necessary to improve the sensitivity of the CI-ELISA further if the assay is to be used for routine diagnosis of acute clinical cases.
With these tools in hand, the accuracy of the diagnosis of MCF has now been considerably improved. Consequently, OvHV-2 has begun to be traced for its epidemiological features in both sheep and cattle (6). Its close association with SA-MCF suggests that it is the most important, if not the only, agent responsible for this condition.
ACKNOWLEDGMENTS
This work was enabled by many people, to whom we are very thankful. Felix Ehrensperger and his team from the Institute for Veterinary Pathology and many other members of staff did postmortems and immunohistology for BVDV. Mark Strasser and coworkers from the Institute of Veterinary Virology, University Berne, tested BVDV antigen in blood samples. We are indebted to Ueli Braun and his team from the Clinic of Veterinary Internal Medicine, University of Zürich, for generous support on the clinical side and to the many private veterinary practitioners who reported field cases.
This work was supported by the Swiss Federal Veterinary Office.
REFERENCES
- 1.Baxter S I, Pow I, Bridgen A, Reid H W. PCR detection of the sheep-associated agent of malignant catarrhal fever. Arch Virol. 1993;132:145–159. doi: 10.1007/BF01309849. [DOI] [PubMed] [Google Scholar]
- 2.Bridgen A, Reid H W. Derivation of a DNA clone corresponding to the viral agent of sheep-associated malignant catarrhal fever. Res Vet Sci. 1991;50:38–44. doi: 10.1016/0034-5288(91)90051-o. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Shen D T, Davis W C, Knowles D P, Gorham J R, Crawford T B. Identification and characterization of the major proteins of malignant catarrhal fever virus. J Gen Virol. 1995;76:123–129. doi: 10.1099/0022-1317-76-1-123. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Shen D T, Knowles D P, Gorham J R, Crawford T B. Competitive inhibition enzyme-linked immunosorbent assay for antibody in sheep and other ruminants to a conserved epitope of malignant catarrhal fever virus. J Clin Microbiol. 1994;32:1674–1679. doi: 10.1128/jcm.32.7.1674-1679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Shen D T, O’Toole D, Knowles D P, Gorham J R, Crawford T B. Investigation of sheep-associated malignant catarrhal fever virus infection in ruminants by PCR and competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1995;33:2048–2053. doi: 10.1128/jcm.33.8.2048-2053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Snowder G, O’Toole D, Crawford T B. Transmission of ovine herpesvirus 2 in lambs. J Clin Microbiol. 1998;36:223–226. doi: 10.1128/jcm.36.1.223-226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liggitt H D, DeMartini J C. The pathomorphology of malignant catarrhal fever. I. Generalized lymphoid vasculitis. Vet Pathol. 1980;17:58–72. doi: 10.1177/030098588001700107. [DOI] [PubMed] [Google Scholar]
- 8.Liggitt H D, DeMartini J C. The pathomorphology of malignant catarrhal fever. II. Multisystemic epithelial lesions. Vet Pathol. 1980;17:73–83. doi: 10.1177/030098588001700108. [DOI] [PubMed] [Google Scholar]
- 9.O’Toole D, Li H, Williams B, Miller D, Crawford T B. Chronic and recovered cases of sheep-associated malignant catarrhal fever in cattle. Vet Rec. 1997;140:519–524. doi: 10.1136/vr.140.20.519. [DOI] [PubMed] [Google Scholar]
- 10.Roizman B, Desrosiers R C, Fleckenstein B, Lopez C, Minson A C, Studdert M J. Family Herpesviridae. In: Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy: classification and nomenclature of viruses. Vienna, Austria: Springer-Verlag; 1995. pp. 114–127. [Google Scholar]
- 11.Strasser M, Vogt H, Pfister H, Gerber H, Peterhans E. 3rd Congress of the European Society for Veterinary Virology, Interlaken, Switzerland. 1994. Detection of bovine virus diarrhea virus (BVDV) in peripheral blood, cell cultures and tissue using a monoclonal antigen-capture ELISA; pp. 311–316. [Google Scholar]
- 12.Thür B, Zlinszky K, Ehrensperger F. Immunhistologie als zuverlässige und effiziente Methode für die Diagnose von BVDV-Infektionen. (Immunohistology as a reliable and efficient method for the diagnosis of BVDV infections) Schweiz Arch Tierheilkd. 1996;138:476–482. [PubMed] [Google Scholar]
- 13.Whiteley H E, Young S, Liggitt H D, DeMartini J C. Ocular lesions of bovine malignant catarrhal fever. Vet Pathol. 1985;22:219–225. doi: 10.1177/030098588502200304. [DOI] [PubMed] [Google Scholar]