Abstract
Introduction and importance
The occurrence of brain metastasis due to cholangiocarcinoma is an exceedingly uncommon phenomenon, documented in only a few numbers of published cases. Recent studies indicated an incidence rate of just 0.15 % for brain metastases in connection with this condition, which was also linked to a reduced survival rate.
Case presentation
A 58-year-old woman with a history of hepatobiliary cholangiocarcinoma presented with a recent onset of unsteady walking, dizziness, vomiting, and worsening occipital headaches. Her medical history included successful chemotherapy treatment for her cholangiocarcinoma. Neurological examination revealed right-sided cerebellar deficits, and imaging indicated a sizable lesion in the right cerebellar hemisphere with surrounding vasogenic edema. A PET scan revealed a liver lesion but no other significant abnormalities. The recommended approach was surgical excision of the cerebellar lesion to relieve symptoms, halt deterioration, and obtain a tissue sample for analysis. After comprehensive discussions with the patient and her family, they opted for the surgical procedure.
Clinical discussion
The major contributors to brain metastases include lung cancers, breast cancers, testicular cancers, melanomas, and renal tumors. In contrast, brain metastases originating from gastrointestinal cancers are less frequent, accounting for fewer than 4 % of cases, with notable impact on 1 % of colorectal cancers, 0.62 % of gastric cancers, and 0.33 % of pancreatic cancers. However, brain metastases are extremely rare.
Conclusion
This study underscores the significance of anticipating and identifying brain metastases in biliary tract cancers, even in the face of their low incidence and the limited amount of available literature on the subject.
Keywords: Biliary tract cancer, Cholangiocarcinoma, Brain metastases, Case report
Highlights
-
•
Cholangiocarcinoma is an infrequent tumor originating from the bile ducts, whether intrahepatic or extrahepatic.
-
•
Brain metastases stemming from cholangiocarcinoma are exceptionally uncommon.
-
•
The scarcity of metastases to the central nervous system from cholangiocarcinoma might be attributed to insufficient tumor vascularization.
-
•
Medical practitioners should maintain an open-minded approach and contemplate the possibility of brain metastases when encountering patients with cholangiocarcinoma who exhibit newly emerged neurological symptoms.
1. Introduction
Biliary tract cancers (BTCs) are extremely lethal malignancies, constituting a minority of all cancer cases worldwide, accounting for less than 1 % [1]. Typically falling under the category of adenocarcinoma, these cancers encompass types like extrahepatic and intrahepatic cholangiocarcinoma (CC), gallbladder cancer (GBC), and cancer affecting the ampulla and papilla of Vater [2,3]. The occurrence of BTC displays significant regional disparity, with the least frequent cases occurring in Western nations and the most prevalent cases found in Asia and Latin America [4]. BTC often infiltrates nearby lymph nodes and the liver, with less frequent occurrences of spreading to distant locations such as the lungs and bones. Instances of metastasis to the brain are exceedingly uncommon.
The precise incidence of brain metastases (BMs) originating from BTC remains largely uncertain. Limited information is available, with only a handful of individual case reports and two sets of cases that have detailed these BMs from BTC [[5], [6], [7], [8], [9], [10]]. According to the most recent case series, the occurrence rate of BTC-related BMs stands at 1.4 % [6]. Herein, we present a case of cholangiocarcinoma metastasized to the cerebellum, as observed within our facility. The intention behind this report is to contribute valuable insights into this uncommon clinical scenario. The case report is consistent with the SCARE 2020 guideline [11].
2. Case presentation
Our case involves a 58-year-old woman who was referred to our medical facility from a peripheral hospital to undergo a more in-depth evaluation for her chief complaints. She has been experiencing unsteady walking, accompanied by dizziness and recurrent vomiting for the past two months. Her medical history traces back to 2020 when she was diagnosed with hepatobiliary cholangiocarcinoma, confirmed through histology. Subsequently, she underwent a chemotherapy regimen consisting of cisplatin and gemcitabine, resulting in favorable responses both clinically and radiologically, leading to significant improvement in her overall health.
According to the patient, her health remained stable until approximately two months ago when she began to experience bouts of vomiting and dizziness, more pronounced during midday than in the morning. These symptoms escalated in frequency and severity over the following days. Additionally, she has developed an unstable gait that has worsened over time. She has also been experiencing occipital headaches for a few months, which have recently intensified. Aside from these issues, she denied any other medical problems and is currently not taking any medications. She did mention having an unspecified allergy to a plant.
Upon initial physical assessment, she appeared well while in bed, with a Glasgow Coma Scale (GCS) score of 15/15. Her pupils responded appropriately to light stimulation. Her headache intensified with neck flexion. Cognitive functions and cranial nerve evaluations were normal, as were power and sensory assessments. In terms of cerebellar examination; She exhibited right-sided dysmetria and dysdiadokokinesia, along with an ataxic gait. Performance on the Finger-Nose test and Heel-Shin test was slightly affected on the right side. However, there was no nystagmus or tremors and the speech was normal.
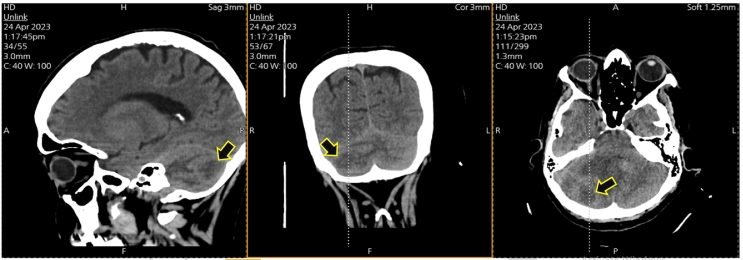
The preliminary clinical diagnosis pointed toward increased intracranial pressure with signs of right cerebellar hemisphere involvement. Radiological assessment with a non-contrast brain computed tomography (CT) scan showed a ring metastatic mass seen in the right posterior cerebellum measuring about 2.8 × 2.3 cm surrounded by vasogenic edema and with mass effect upon the posterior lower part of Pons and medulla oblongata, and with mild right tonsil seen herniated of about 4 mm, as represented in Fig. 1, magnetic resonance imaging (MRI) with IV contrast was advised. The later revealed the presence of a 3 × 2.3 × 2.5 cm lesion in the right cerebellar hemisphere, displaying heterogeneously hypointense features on T1-weighted images, hyperintense features on T2-weighted images, and peripheral irregular enhancement with gadolinium, as depicted in Fig. 2. Restricted diffusion was observed, causing substantial mass effect on the surrounding cerebellar and brainstem tissues. This resulted in compression and displacement of the fourth ventricle toward the left side, accompanied by surrounding vasogenic edema. The findings were indicative of a metastatic lesion or potentially a high-grade glioma. An official radiology report is required for a more detailed radiological diagnosis.
Fig. 1.
Is showing selected CT scans demonstrating the cerebellar lesion (arrows).
Fig. 2.
Is showing selected MRI scans with different sections and sequences illustrating the cerebellar mass (arrows).
A Positron emission tomography (PET) scan from February 2023 revealed a liver lesion but no significant abnormalities elsewhere. The patient's best course of action is deemed to be surgical excision of the lesion. This procedure is aimed at decompression, halting further deterioration, alleviating symptoms, and obtaining a tissue sample for pathological analysis. After thorough discussion with the patient and her family, they have a comprehensive understanding of the condition, the intended surgical procedure, its goals, potential outcomes, as well as associated risks and complications. Consequently, the family has decided to proceed with the surgery.
3. Operative and post-operative courses
Extra-ventricular drain (EVD) insertion on the right Kocher point, with vertical scam incision under general anesthesia, patient was placed in prone position, arterial line and 2 large pour cannulas in each forearm was applied, Electrocardiogram electrodes was applied. Covering of the eyes with gauze and paper tab, and fixation of the tube with a plaster, the stabler both the tab of the tube and the eye covering. Shaving of the hair over the occipital and suboccipital area cleaning of the site of Mayfield bins with a 10 poldine fixation of the head with Mayfield pins turn the pt to prone position. Fixation of the pt arms fixation of the Mayfield system with face locking down ward, flexed put the table in reverse Trendelenburg position scruping and draping in the usual fashion vertical incision over the posterior aspect of the suboccipital area on the right side 3 cm from the midline. The process involved incising and dissecting the muscle using a monopolar instrument. A craniotomy was performed through a single burr hole, with subsequent bone cutting; this led to the opening of the lateral transverse sinus. The dura was exposed through a cruciate incision, and the tumor was removed piece by piece. A small fragment was retained to remain attached to the sinus angle. During manipulation of the sinus, bleeding occurred, which was addressed through the application of surgical material and bone wax to achieve hemostasis. DuraGen was positioned, and the craniectomy site was covered with gel foam (Fig. 3).
Fig. 3.
Is showing selected intraoperative images. (B) before the opening of the dura mater. (C) after resection of the mass and achieving hemostasis (arrow). (D) the resected lesion.
The closure was conducted layer by layer, starting with the muscle using 0 vicryl sutures. The fascia was sutured with continuous stitches of 2/0 vicryl. Subcutaneous sutures were applied using 2/0 vicryl, followed by the closure of the skin using subcuticular sutures, 2/0 sutures, and staplers for the final layer. A dressing was applied, and the patient was turned on their bed to remove the pins. The estimated blood loss was around 200 cm3.
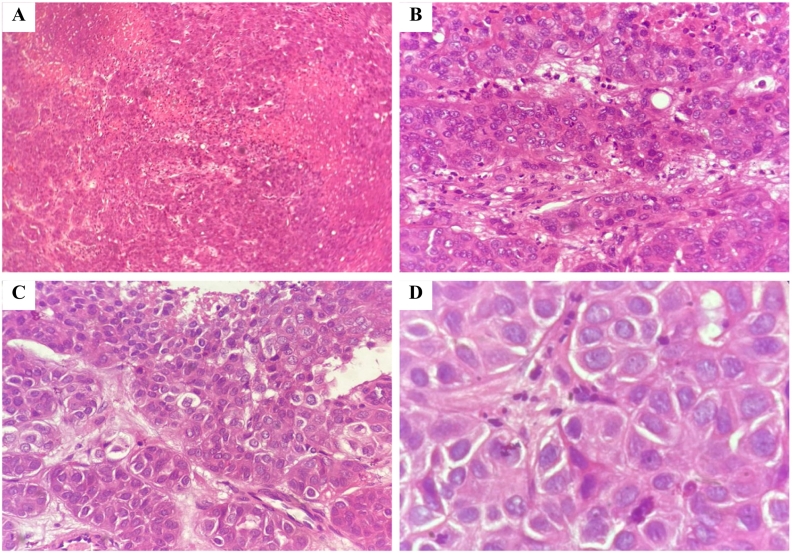
After the procedure, the patient was extubated within the operating theater and then transferred to the recovery room while maintaining a stable condition. The histopathology report of the excised cerebellar lesion revealed metastatic carcinoma with extensive necrosis mostly consistent with hepatobiliary origin as illustrated in Fig. 4.
Fig. 4.
(H&E, (A): 4×, (B): 10×, (C): 40× & (D):100×), is showing the histological findings of the resected tumor confirming the hepatobiliary origin of the cerebellar region.
The patient experienced a marked clinical improvement without any residual neurological dysfunction. She was discharged from the ICU after 5 days and transferred to the ward. The patient was followed up for 6 months with significant improvement in clinical complaints including headache and gait abnormality without any reported complications, adverse events, or re-admissions.
4. Discussion
Biliary tract cancer (BTC) pertains to a cluster of cancers affecting the biliary tract, encompassing gallbladder cancer (GBC), cholangiocarcinoma (CC), and cancers impacting the ampulla and papilla of Vater. These collectively constitute a fraction of less than 1 % among all cancer cases globally [1]. GBC occurs with a frequency twice that of CC, and within the realm of CC, the extrahepatic subtype (ECC) surpasses intrahepatic CC (ICC) in prevalence. Among ECC cases, the extrahepatic form accounts for 60–70 % of all CC occurrences and can be further subdivided into perihilar CC (or Klatskin tumor) and distal ECC. Notably, perihilar CC represents the most prevalent subtype [1,3,12,13].
Since the therapeutic approach to BTC can encompass various methods, it's essential that patients are expeditiously referred to a multidisciplinary team at a specialized medical center [14]. For initial treatment, chemotherapy involving a combination of platinum and gemcitabine is a well-established option, but its efficacy diminishes in subsequent treatment stages [15,16]. However, complete surgical removal remains the lone potentially curative intervention [17]. The efficacy of supplementary strategies in addressing this condition remains uncertain [[18], [19], [20]]. Unfortunately, the majority of patients are diagnosed with advanced-stage disease, resulting in a bleak prognosis. Long-term survival rates over five years stand at 5–10 % for GBC and 10–40 % for CC [21]. At autopsy, more than 90 % of patients display distant metastases, primarily affecting the liver, lymph nodes, and lungs. While brain metastases from CC are rarely documented [1,3,12,13,21,22].
Metastatic brain tumors stand as the prevailing intracranial growths in adults, afflicting nearly 8 % of individuals with cancer [23,24]. Among the prominent contributors to brain metastases are lung cancers (50 %), breast cancers (12 %), testicular cancers (10–15 %), melanomas (6–10 %), and renal tumors [[24], [25], [26]]. Brain metastases arising from gastrointestinal cancers are less common, occurring in less than 4 % of cases, notably affecting 1 % of colorectal cancers, 0.62 % of gastric cancers, and 0.33 % of pancreatic cancers [[25], [26], [27]]. Notably, brain metastases stemming from BTC are infrequent, and the precise occurrence rate remains uncertain. Existing literature is limited to a handful of case reports and series, reflecting an incidence rate estimated at approximately 0.15 % [5,[7], [8], [9]].
In their study [28], D'Andrea MR and colleagues arrived at the conclusion that the prevailing symptoms upon the manifestation of brain metastases (BM) encompassed seizure (33.3 %), dizziness (33.3 %), altered mental status (33.3 %), weakness (33.3 %), headache (22.2 %), and cranial neuropathy (11.1 %). The median age at the diagnosis of BM was 63.7 years, with a range of 48.8 to 72.4 years. The median duration between the initial diagnosis and the diagnosis of BM was 16.7 months, spanning from 0.7 to 66.7 months. Among the involved patients, six patients displayed a solitary brain metastasis (BM), one patient exhibited two BMs, and two patients (22.2 %) showed three or more BMs during the initial imaging assessment. In terms of the location of the lesions, five patients had lesions in the supratentorial region, one patient had an infratentorial lesion, and three other patients presented with both supratentorial and infratentorial lesions. The most frequent affected parenchymal lobe was the frontal lobe (n = 5, 55.6 %), followed by the parietal lobe (n = 4, 44.4 %), cerebellar region (n = 4, 44.4 %), temporal lobe (n = 2, 22.2 %), and occipital lobe (n = 1, 11.1 %). None of the scans indicated hydrocephalus, while vasogenic edema was evident on the T2 FLAIR sequence in all cases. The mean cross-sectional area of the tumors was measured at 6.9 cm2 ± 7.1.
As far as we are aware, there have been no attempts to comprehensively define the genetic makeup of brain metastases (BMs) originating from BTC. This scarcity of data could be attributed to the rarity of BMs stemming from BTC. Additionally, patients afflicted with BMs often do not meet the criteria for participation in clinical trials, contributing significantly to the scarcity of insights into appropriate treatment methods for these individuals. Given the discouraging prognosis associated with BTC-related BMs, it becomes crucial to explore the genetic mechanisms that facilitate the spread of cancer to the brain. This pursuit could potentially lead to the discovery of more advanced therapeutic alternatives.
Since there has been no comprehensive genomic profiling of brain metastases (BMs) derived from BTC, the significance of targeted therapies in addressing these BMs remains uncertain. Nonetheless, there exists evidence that supports the potential benefits of targeting known genetic mutations in individuals with BMs originating from other primary cancers. Examples include the utilization of EGFR inhibitors for lung cancer [[29], [30], [31], [32], [33]], PI3K inhibitors for BMs arising from breast cancer [26], BRAF inhibitors for BMs from melanoma [34], and vascular endothelial growth factor receptor tyrosine kinase inhibitors for BMs stemming from renal cell carcinoma [35].
The feasibility of targeting specific genetic alterations in patients with BMs is currently being investigated through a national cooperative group phase II clinical trial (Alliance A071701; NCT03994796) [36]. This study aims to elucidate the role of targeted therapies in individuals affected by BMs, shedding light on potential treatment strategies.
According to D'Andrea MR et al. research [28], four patients (44.4 %) underwent definitive surgical resection, while seven patients (77.8 %) underwent brain metastasis (BM) radiation therapy. The radiation approaches encompassed stereotactic radiosurgery (SRS) for two patients, whole-brain radiation therapy (WBRT) for three patients, and a combination of both SRS and WBRT for three patients. The median overall survival time from the point of BM diagnosis stood at 3.8 months (95 % CI 0.1–16.9). Notably, patients who received BM radiotherapy displayed a notably improved overall survival period of 10.2 months (95 % CI 3.8–16.9) in comparison to 0.8 months (95 % CI 0.1–1.5) for those who didn't receive radiotherapy (p = .0039, log-rank; relative risk = 0.38; p = .01, chi-square).
A notable constraint in treating brain metastases (BMs) arises from the limited effectiveness of systemic therapies penetrating the blood-brain barrier (BBB). This is primarily attributed to factors such as low passive permeability and the presence of efflux pumps. Nonetheless, the altered conditions associated with advanced cancer might modify BBB permeability, potentially rendering certain targeted agents effective [37]. Additionally, it's worth noting that BBB permeability is not uniform and is connected to vascular remodeling [38]. Consequently, there is a need for further investigation to delve into the capacity of targeted agents to traverse the BBB and to comprehend their potential in managing BMs arising from BTC.
5. Conclusion
To summarize, while brain metastases originating from cholangiocarcinoma are a highly uncommon occurrence, they do exist. The identification of brain metastases is indicative of a particularly grim prognosis. Hence, medical practitioners should maintain a vigilant approach and contemplate the possibility of brain metastases in patients diagnosed with cholangiocarcinoma and presenting with newly developed neurological symptoms.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical approval
Our institution has exempted this study from ethical review.
Funding
No funding or grant support was received for this study.
CRediT authorship contribution statement
Writing the manuscript: Oadi N. Shrateh.
Imaging description: Oadi N. Shrateh.
Reviewing & editing the manuscript: Shadi Abu Saa.
Guarantor
Oadi N. Shrateh.
Research registration number
-
•
Name of the registry: None.
-
•
Unique Identifying number or registration ID: None.
-
•
Hyperlink to your specific registration (must be publicly accessible and will be checked): None.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Ghosn M., et al. Optimum chemotherapy for the management of advanced biliary tract cancer. World J Gastroenterol: WJG. 2015;21(14):4121. doi: 10.3748/wjg.v21.i14.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Groen P.C., et al. Biliary tract cancers. N. Engl. J. Med. 1999;341(18):1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki T., et al. Current status of chemotherapy for the treatment of advanced biliary tract cancer. Korean J. Intern. Med. 2013;28(5):515. doi: 10.3904/kjim.2013.28.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randi G., et al. Epidemiology of biliary tract cancers: an update. Ann. Oncol. 2009;20(1):146–159. doi: 10.1093/annonc/mdn533. [DOI] [PubMed] [Google Scholar]
- 5.Chindaprasirt J., et al. Brain metastases from cholangiocarcinoma: a first case series in Thailand. Asian Pac. J. Cancer Prev. 2012;13(5):1995–1997. doi: 10.7314/apjcp.2012.13.5.1995. [DOI] [PubMed] [Google Scholar]
- 6.Frega G., et al. Brain metastases from biliary tract cancer: a monocentric retrospective analysis of 450 patients. Oncology. 2018;94(1):7–11. doi: 10.1159/000479929. [DOI] [PubMed] [Google Scholar]
- 7.Kilbourn K.J., Aferzon J., Menon M. Isolated brain metastasis in cholangiocarcinoma: a case report and review of literature. Conn. Med. 2014;78(3) [PubMed] [Google Scholar]
- 8.Mirrakhimov A.E., et al. Cholangiocarcinoma and brain lesions: an extremely rare finding. Case Rep. 2013;2013 doi: 10.1136/bcr-2013-009235. (p. bcr2013009235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.William B.M., Grem J.L. Brain metastasis and leptomeningeal carcinomatosis in a patient with cholangiocarcinoma. Gastrointest. Cancer Res.: GCR. 2011;4(4):144. [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Z., Xu J., Wang J. Isolated brain metastases prior to locoregional recurrence in hilar cholangiocarcinoma. Mol. Clin. Oncol. 2017;6(6):899–902. doi: 10.3892/mco.2017.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agha R.A., et al. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Noel M.S., Hezel A.F. New and emerging treatment options for biliary tract cancer. Onco. Targets. Ther. 2013:1545–1552. doi: 10.2147/OTT.S32545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J.O., et al. Gemcitabine plus cisplatin for advanced biliary tract cancer: a systematic review. Cancer Res. Treat. 2015;47(3):343–361. doi: 10.4143/crt.2014.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandi G., et al. Cholangiocarcinoma: current opinion on clinical practice diagnostic and therapeutic algorithms: a review of the literature and a long-standing experience of a referral center. Dig. Liver Dis. 2016;48(3):231–241. doi: 10.1016/j.dld.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Valle J., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 16.Fornaro L., et al. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br. J. Cancer. 2014;110(9):2165–2169. doi: 10.1038/bjc.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckel F., Brunner T., Jelic S. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2011;22 doi: 10.1093/annonc/mdr375. (p. vi40-vi44) [DOI] [PubMed] [Google Scholar]
- 18.Brandi G., et al. Membrane localization of human equilibrative nucleoside transporter 1 in tumor cells may predict response to adjuvant gemcitabine in resected cholangiocarcinoma patients. Oncologist. 2016;21(5):600–607. doi: 10.1634/theoncologist.2015-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edeline J., et al. Gemox versus surveillance following surgery of localized biliary tract cancer: results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. Proc. Am. Soc. Clin. Oncol. 2017 225225. [Google Scholar]
- 20.Horgan A.M., et al. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet] 2012. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. [DOI] [PubMed] [Google Scholar]
- 21.De Groen P.C., et al. Biliary tract cancers. N. Engl. J. Med. 1999;341(18):1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 22.Brandi G., et al. Asbestos: a hidden player behind the cholangiocarcinoma increase? Findings from a case–control analysis. Cancer Causes Control. 2013;24:911–918. doi: 10.1007/s10552-013-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabouret E., et al. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662. [PubMed] [Google Scholar]
- 24.Schouten L.J., et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 25.Go P.H., et al. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer. 2011;117(16):3630–3640. doi: 10.1002/cncr.25940. [DOI] [PubMed] [Google Scholar]
- 26.Farnell G.F., et al. Brain metastases from colorectal carcinoma: the long term survivors. Cancer. 1996;78(4):711–716. doi: 10.1002/(SICI)1097-0142(19960815)78:4<711::AID-CNCR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Bartelt S., et al. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: prognostic factors and survival. World J Gastroenterol: WJG. 2004;10(22):3345. doi: 10.3748/wjg.v10.i22.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Andrea M.R., et al. Brain metastases from biliary tract cancers: a case series and review of the literature in the genomic era. Oncologist. 2020;25(5):447–453. doi: 10.1634/theoncologist.2019-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosell R., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 30.Soria J.-C., et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N. Engl. J. Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 31.Mok T.S., et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 32.Sequist L.V., et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 33.Lee C.K., et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: individual patient data meta-analysis of overall survival. J. Natl. Cancer Inst. 2017;109(6) doi: 10.1093/jnci/djw279. [DOI] [PubMed] [Google Scholar]
- 34.Davies M.A., et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873. doi: 10.1016/S1470-2045(17)30429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juloori A., et al. Overall survival and response to radiation and targeted therapies among patients with renal cell carcinoma brain metastases. J. Neurosurg. 2019:1–9. doi: 10.3171/2018.8.JNS182100. [DOI] [PubMed] [Google Scholar]
- 36.Oncology A.f.C.T.i. Genetic testing in guiding treatment for patients with brain metastases. 2023. https://classic.clinicaltrials.gov/ct2/show/NCT03994796 23/08/2023. Available from:
- 37.Chacko A.M., et al. Targeted delivery of antibody-based therapeutic and imaging agents to CNS tumors: crossing the blood-brain barrier divide. Expert Opin. Drug Deliv. 2013;10(7):907–926. doi: 10.1517/17425247.2013.808184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lockman P.R., et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 2010;16(23):5664–5678. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]