This meta-analysis reviews studies investigating the association of rehabilitation interventions with physical capacity and quality of life in adults with post–COVID-19 condition (PCC).
Key Points
Question
Are respiratory training and exercise-based rehabilitation interventions associated with improved functional exercise capacity in adults with post–COVID-19 condition?
Findings
This systematic review, which incorporated a bayesian meta-analysis of 14 randomized clinical trials involving 1244 patients, found moderate-certainty evidence indicating that standardized rehabilitation interventions were associated with improvements in functional exercise capacity (standardized mean difference, −0.56; 95% credible interval −0.87 to −0.22) and had a 99% posterior probability of superiority compared with standard care. However, a high level of uncertainty and imprecision was observed concerning the probability of experiencing exercise-induced adverse events.
Meaning
Although respiratory training and exercise-based rehabilitation interventions might be associated with improved functional exercise capacity in patients with post–COVID-19 condition, it is recommended that health care professionals closely monitor these patients during the implementation of such interventions to ensure patient safety until more definitive evidence is available.
Abstract
Importance
Current rehabilitation guidelines for patients with post–COVID-19 condition (PCC) are primarily based on expert opinions and observational data, and there is an urgent need for evidence-based rehabilitation interventions to support patients with PCC.
Objective
To synthesize the findings of existing studies that report on physical capacity (including functional exercise capacity, muscle function, dyspnea, and respiratory function) and quality of life outcomes following rehabilitation interventions in patients with PCC.
Data Sources
A systematic electronic search was performed from January 2020 until February 2023, in MEDLINE, Scopus, CINAHL, and the Clinical Trials Registry. Key terms that were used to identify potentially relevant studies included long-covid, post-covid, sequelae, exercise therapy, rehabilitation, physical activity, physical therapy, and randomized controlled trial.
Study Selection
This study included randomized clinical trials that compared respiratory training and exercise-based rehabilitation interventions with either placebo, usual care, waiting list, or control in patients with PCC.
Data Extraction and Synthesis
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. A pairwise bayesian random-effects meta-analysis was performed using vague prior distributions. Risk of bias was assessed using the Cochrane risk of bias tool version 2, and the certainty of evidence was evaluated using the GRADE system by 2 independent researchers.
Main Outcomes and Measures
The primary outcome was functional exercise capacity, measured at the closest postintervention time point by the 6-minute walking test. Secondary outcomes were fatigue, lower limb muscle function, dyspnea, respiratory function, and quality of life. All outcomes were defined a priori. Continuous outcomes were reported as standardized mean differences (SMDs) with 95% credible intervals (CrIs) and binary outcomes were summarized as odds ratios with 95% CrIs. The between-trial heterogeneity was quantified using the between-study variance, τ2, and 95% CrIs.
Results
Of 1834 identified records, 1193 were screened, and 14 trials (1244 patients; 45% female participants; median [IQR] age, 50 [47 to 56] years) were included in the analyses. Rehabilitation interventions were associated with improvements in functional exercise capacity (SMD, −0.56; 95% CrI, −0.87 to −0.22) with moderate certainty in 7 trials (389 participants). These improvements had a 99% posterior probability of superiority when compared with current standard care. The value of τ2 (0.04; 95% CrI, 0.00 to 0.60) indicated low statistical heterogeneity. However, there was significant uncertainty and imprecision regarding the probability of experiencing exercise-induced adverse events (odds ratio, 1.68; 95% CrI, 0.32 to 9.94).
Conclusions and Relevance
The findings of this systematic review and meta-analysis suggest that rehabilitation interventions are associated with improvements in functional exercise capacity, dyspnea, and quality of life, with a high probability of improvement compared with the current standard care; the certainty of evidence was moderate for functional exercise capacity and quality of life and low for other outcomes. Given the uncertainty surrounding the safety outcomes, additional trials with enhanced monitoring of adverse events are necessary.
Introduction
The World Health Organization defines post–COVID-19 condition (PCC) as, “the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation.”1 Although COVID-19 was initially recognized as a respiratory illness, PCC symptoms can range from mild impairment to severe systemic disease, with many patients experiencing dozens of symptoms across multiple organ systems.2,3,4 At least 65 million individuals worldwide are estimated to have PCC, but the number is likely much higher due to many undocumented cases.4 The incidence is estimated at 10% to 30% of nonhospitalized cases, 50% to 70% of hospitalized cases, and 10% to 12% of vaccinated cases.2 In Canada, 14.8% of those who reported a previous positive test or a suspected infection of SARS-CoV-2 experienced symptoms at least 3 months after their infection,5 which translates into approximately 1.4 million Canadian adults or 4.6% of the Canadian population aged 18 years and older. Similarly, in the US, as of February 23, 2023, 14% of adults with a previous positive test for COVID-19 reported having experienced PCC symptoms at some point, and 6.5% reported current symptoms.6
The 5 most frequently observed symptoms include fatigue (58%), headache (44%), attention disorder (27%), hair loss (25%), and dyspnea (24%).7 Emerging evidence suggests that female sex, socioeconomic determinants of health (eg, lower income), inability to adequately rest in the early weeks after developing COVID-19, and comorbid conditions appear to be independent factors associated with increased risk for the development of PCC.4,8
According to a 2023 review,2 the existing research is insufficient to improve outcomes for people with PCC. There is an urgent need for evidence-based rehabilitation interventions to support people affected by PCC9,10,11,12 because current guidelines are primarily based on expert opinion and observational data.13,14 The latest systematic review15 with meta-analysis on rehabilitation interventions for patients with PCC included a total of 3 trials16,17,18 (233 patients with PCC) and suggested that rehabilitation interventions may be associated with improvements in functional exercise capacity, but the association of rehabilitation interventions with respiratory function was inconsistent across studies. The accuracy of these early-evidence syntheses is likely to be compromised due to the small number of included studies. Since then, 11 additional trials19,20,21,22,23,24,25,26,27,28,29,30 have emerged. The increased attention on PCC now allows us to conduct a more comprehensive, methodologically sound, and stable analysis. The purpose of this meta-analysis is to assess whether rehabilitation interventions are associated with improvements in physical capacity (functional exercise capacity, muscle function, dyspnea, and respiratory function) and quality of life in adults with PCC.
Methods
Our protocol was registered in PROSPERO database and is available in the eAppendix in Supplement 1. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline to report this systematic review.31
Search Strategy and Information Sources
After consulting with a librarian, a systematic electronic search of the literature was performed from January 2020 until February 2023, in MEDLINE (via Ovid), Scopus, CINAHL, and the Clinical Trials Registry. Examples of the keywords that were used to identify potentially relevant studies were long-covid, post-covid, sequelae, exercise therapy, rehabilitation, physical activity, physical therapy, and randomized controlled trials. The full research strategy is summarized in eTable 1 in Supplement 1. Additionally, we conducted a manual search of the reference lists of the included studies to identify any additional studies not retrieved in the electronic search.
Study Selection and Data Extraction
We included randomized clinical trials that compared rehabilitation interventions such as respiratory training aerobic exercises and resistance exercises with either placebo, usual care, waitlist, or control in adults with PCC.1 We did not pose any restrictions with regard to comorbidities or medication use concurrently with the rehabilitation protocol. Trials that used medication-only treatments without a rehabilitation component and nonrandomized studies were excluded.
As part of the study selection, 2 independent researchers (D.V.P. and E.S.) screened titles and abstracts of articles, reviewed the articles that met inclusion criteria in initial screening at the full text level, and extracted data from the eligible studies. We extracted authors, year of publication, participant and intervention characteristics, and outcome data.
Outcomes
The primary outcome was functional exercise capacity, measured with the 6-minute walking test. Our secondary outcomes were fatigue; functional leg strength and endurance as measured by the 30-second sit-to-stand test; dyspnea; respiratory function; and quality of life. The time point of the primary and secondary outcomes was the shortest time point available upon completion of the rehabilitation program. Respiratory function was assessed through forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC). If a study used more than 1 measure to assess dyspnea and quality of life, we planned to extract the measure reported as the primary outcome. We also assessed the safety of the rehabilitation interventions by extracting any treatment-emergent adverse events (including serious adverse events) across all studies. Safety outcomes were assessed at the last follow-up available.
Risk of Bias Assessment and Certainty of the Evidence
To assess the risk of bias, 2 independent reviewers (D.V.P. and E.S.) used the Cochrane risk of bias tool version 2.32 We assessed selection bias, performance bias, detection bias, attrition bias, and reporting bias. We used the GRADE approach33,34,35,36,37,38,39 to evaluate the certainty of evidence with regard to risk of bias, inconsistency, indirectness, imprecision, and publication bias. Discrepancies were resolved with a senior research team member (P.B.).
Statistical Analysis
We described variables with a normal distribution as mean (SD) and those with a skewed distribution as median (IQR). Categorical variables were presented as numbers (percentages).
The different rehabilitation interventions were compared against a common comparison group, termed control (ie, placebo, sham, waiting list, or usual care). We used bayesian pairwise, random-effects, meta-analysis models to summarize results across trials. We chose random-effects models to account for the anticipated clinical and methodological diversity between studies.40
For continuous outcomes, we used the normal likelihood and an identity link. For binary outcomes, we used the binomial likelihood and the logit link. All models were fitted with noninformative prior distributions. We used normal prior distributions with a mean of 0 and a large variance for treatment effects. For the main analysis, we used uniform, vague prior distributions for the between-trial variance. In sensitivity analyses, we used empirical prior distributions for the between-trial variance. We quantified the between-trial heterogeneity using the between-study variance, τ2, and 95% credible intervals (95% CrIs). In the presence of substantial heterogeneity, we performed sensitivity analysis on the basis of allocation concealment that was defined a priori as potential moderator.
For continuous outcomes, summary results were presented as standardized mean differences (SMDs) with 95% CrIs. We coined all outcomes such that negative treatment effects (SMD <0) indicate better outcomes in the intervention group compared with the comparison. We calculated the probability of rehabilitation interventions being superior to usual care (ie, probability of the true SMD <0) and the probability of rehabilitation interventions providing a treatment effect more pronounced than the between-group minimum important difference (MID) of 0.30 SD units (ie, the probability that the true SMD is ≤−0.30).
For binary outcomes, summary results were presented as odds ratios (ORs) with 95% CrIs, with an OR greater than 1 representing a higher risk of the event among patients who received the intervention than those who received the comparison.
Parameters were estimated via Markov chain Monte Carlo methods. We used a burn-in period of 50 000 iterations and 3 chains with 100 000 simulations each (300 000 in total). Summary estimates were obtained via posterior medians (the 2.5 percentile and the 97.5 percentile). We monitored model convergence via the Brooks-Gelman-Rubin R statistic and trace plots. Model diagnostics also included visual inspection of autocorrelation plots and the posterior densities.
We used the Egger test for continuous outcomes and Peters test for binary outcomes to assess for publication bias. In the presence of large between-study heterogeneity, we used allocation concealment as a moderator. A 2-sided P < .10 was considered evidence of publication bias.41 All analyses were conducted in OpenBUGS42 statistical software version 3.2.3 (University of Cambridge) and Stata statistical software version 16 (StataCorp). Details on the prior distributions and models are provided in the eMethods in Supplement 1.
Results
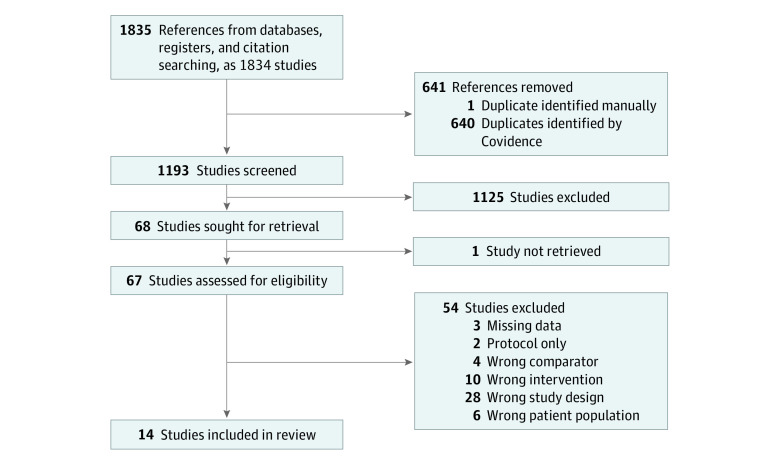
Our search identified 1834 records. After removal of duplicates, we carried out title and abstract screening of 1193 references, leaving 67 articles selected for full text review. Of these studies, 14 trials (15 records) were deemed eligible (Figure 1). Excluded references from the full text screening and the reasons for exclusion are provided in eTable 2 in Supplement 1. The characteristics of the included studies are summarized in Table 1.
Figure 1. Flowchart Showing Study Selection.
Table 1. Characteristics of the Included Studies.
| Source | Primary outcome | Population | Age, mean (SD) y | Participants, No. | Women, No. (%) | Men, No. (%) | Intervention | Control | Length of inpatient stay, d | Level of dyspnea |
|---|---|---|---|---|---|---|---|---|---|---|
| Teixeira DO Amaral et al,18 2022 | 6MWT, FEV1a and FVCa | Hospitalized patients due to COVID-19 (6.2% ICU patients) | 52.0 (10.8) | 32 | 17 (53.1) | 15 (46.9) | Clinician supported, home-based aerobic and resistance exercise program | Usual care | 6.8 | NI |
| Capin et al,20 2022 | 30-s STS, and dyspneab | Hospitalized patients due to COVID-19 (with and without ICU stay) | 53.0 (10) | 41 | 18 (44.0) | 23 (56.0) | Supervised breathing and clearance techniques, high-intensity strength training, aerobic and cardiovascular exercise, balance exercises, functional activities, stretching, coaching, and motivational interviewing | Usual care | 6.0 | Moderate to severe |
| Del Corral et al,21 2023 | 30-s STS, health-related quality of life,c FEV1,a and FVCa | Patients reporting persistent fatigue and dyspnea following PCR diagnosis of COVID-19 infection (32.0% hospital admission rate; 6.0% ICU patients) | 46.5 (10.2) | 88 | 14 (16.0) | 74 (84.0) | Supervised inspiratory muscle training and respiratory muscle training | Sham | NI | NI |
| De Souza et al,19 2021 | 30-s STS | Nonhospitalized patients | NI | 196 | NI | NI | Supervised low-intensity pulmonary rehabilitation | Usual care | NA | NI |
| Jimeno-Almazán et al,22 2023 and Jimeno-Almazán et al,23 2022 | Dyspnea,b quality of life,d FEV1,a and FVCa | Nonhospitalized patients with a PCR diagnosis of COVID-19 presenting with persistent symptoms | 45.3 (9.7) | 39 | 29 (74.3) | 10 (25.7) | Supervised resistance training combined with aerobic training | Usual care | NA | Mild and moderate |
| Li et al,16 2022 | 6MWT, quality of life,d FEV1,a and FVCa | Patients reporting persistent moderate dyspnea (mMRC >2-3) after inpatient treatment for COVID-19 | 50.6 (11.0) | 119 | 66 (55.5) | 53 (44.5) | Unsupervised breathing control and thoracic expansion, aerobic exercise, and lower limb muscle strengthening exercises | Usual care | 26.2 | Moderate |
| Liu et al,17 2020 | 6MWT | Hospitalized patients due to COVID-19 aged ≥65 y | 67.5 (7.8) | 72 | 23 (32.0) | 49 (68.0) | Supervised respiratory muscle training, cough exercise, diaphragmatic training, stretching exercise, and unsupervised home exercise | Usual care | NI | NI |
| McNarry et al,24 2022 | Dyspneae and quality of lifef | Patients reporting persistent dyspnea following COVID-19 infection | 46.6 (12.2) | 281 | 247 (88.0) | 34 (12.0) | Unsupervised inspiratory muscle training | Usual care | NI | Moderate |
| Okan et al,25 2022 | 6MWT, dyspnea,b quality of life,g FEV1,a and FVCa | Patients presenting with persistent dyspnea following COVID-19–induced pneumonia | 50.0 (12.8) | 52 | 25 (48.0) | 27 (52.0) | Supervised breathing exercise | Usual care | 9.5 | Moderate |
| Phillip et al,26 2022 | Dyspneah and quality of lifei | Patients reporting persistent dyspnea following COVID-19 infection (17.0% hospital admission rate) | 49.5 (12.0) | 150 | 26 (17.5) | 124 (82.5) | Supervised posture and breathing exercises | Usual care | NI | Mild |
| Rodriguez-Blanco et al,30 2023 | 6MWT, 30-s STS, dyspneah | Patients with symptoms attributed to COVID-19 by medical services | 40.7 (13.4) | 48 | 26 (54.2) | 22 (45.8) | Breathing and strength-based exercises | Usual care | NI | Mild |
| Romanet et al,27 2022 | Dyspneab and quality of lifed | Patients reporting persistent dyspnea (mMRC >1) following COVID-19–related acute respiratory distress syndrome (ICU admission) | 58.2 (12.5) | 60 | 23 (38.1) | 37 (61.9) | Supervised endurance training rehabilitation | Usual care | 26.0 | Moderate |
| Sari et al,28 2023 | 6MWT | PCC with pulmonary involvement (83.0% hospitalized, 17.0% ICU) | 56.2 (4.5) | 24 | 8 (33.3) | 16 (66.7) | Nonsupervised breathing exercises, resistance exercises, and inspiratory muscle training | Usual care | NI | Mild and moderate |
| Şahın et al,29 2023 | 6MWT, dyspnea,b FEV1,a FVC,a and quality of lifeg | Patients hospitalized with PCC (ICU and ward for >10 d) | 60.7 (8.2) | 42 | 14 (33.3) | 28 (66.7) | Clinician supported breathing exercises, strength exercises, and walking program | Usual care | 11.5 | Moderate |
Abbreviations: 6MWT, 6-minute walking-test; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; ICU, intensive care unit; mMRC, modified Medical Research Council Dyspnea Scale; NA, not applicable; NI, no information; PCC, post–COVID-19 condition; PCR, polymerase chain reaction; STS, sit-to-stand test.
Calculated as estimated percentages.
Measured with the mMRC.
Measured with the Euroqol-5 Dimension, 5-Level (EQ-5D-5L) Questionnaire.
Measured with the 12-Item Short Form Survey (SF-12) mental component score and physical component score.
Measured with the Transition Dyspnea Index.
Measured with The King’s Brief Interstitial Lung Disease Questionnaire.
Measured with the St George Respiratory Questionnaire.
Measured with the Dyspnea-12 Questionnaire.
Measured with the physical health component and mental health component of the 36-Item Short Form Survey (SF-36).
Description of the Population
The analysis included 1244 participants (median [IQR] age, 50 [47-56] years; 45% female participants). Six trials16,17,18,20,27,29 included patients who had previously been hospitalized due to a COVID-19 infection (range of intensive care unit admission, 6.2%-100%), and 3 trials19,22,23,30 (4 records) included patients who had not been hospitalized following SARS-CoV-2 infection. Five trials21,24,25,26,28 included a mixed population of both patients who had been hospitalized following initial COVID-19 infection and those who had not been hospitalized following initial COVID-19 infection. The level of dyspnea of the participants at the baseline ranged from mild to severe.
Description of the Interventions
The most common interventions in the treatment group were breathing exercises, either alone19,21,24,25,26,30 (6 trials [815 participants]), or in combination with resistance and/or aerobic training16,17,20,28,29 (5 trials [298 participants]). In 2 trials18,22,23 (3 records [71 participants]), the intervention included strengthening and aerobic exercises without a breathing exercise component, and 1 trial27 (60 participants) included only aerobic exercises. According to the TiDiER checklist,43 12 trials (86%) gave appropriate descriptions of the intervention components, 11 trials (79%) stated the dose and duration of the intervention, and 9 trials (64%) described the tailoring process. A detailed analysis of the reporting of each component for each study is summarized in eTable 3 in Supplement 1. The most common comparator was usual care in the form of respiratory training and exercise-based, self-management education16,17,18,19,20,22,23,24,25,26,27,28,29,30 (13 studies [14 records; 1156 participants]). A single study21 (88 participants) used sham or placebo comparisons in the form of a respiratory training with no resistance. Minimum exercise dose was not well defined for our population. The most common limitations of unsupervised respiratory training (ie, usual care) were wrong use of equipment and ineffective execution of the breathing techniques. Hence, all comparators will be referred to as usual care.
Risk of Bias
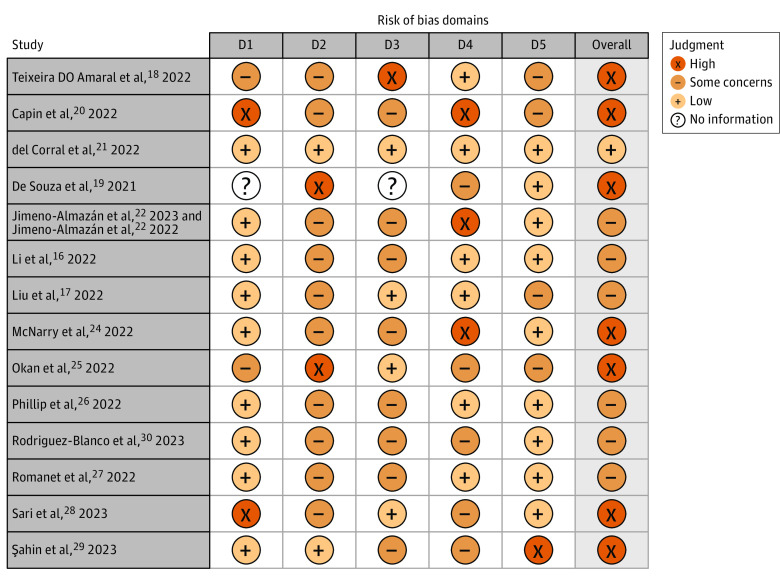
Figure 2 summarizes the risk of bias judgment for each individual trial. Overall risk of bias was deemed low in 1 study21 (7%), deemed as having some concerns in 6 trials16,17,22,23,26,27,30 (7 records [43%]), and deemed high in 7 trials18,19,20,24,25,28,29 (50%). Risk of bias arising from the randomization process and the selection of the reported outcomes was deemed low in 9 trials16,19,21,22,23,24,26,27,29,30 (10 records [64%]). Bias due to deviations of the included intervention was deemed low in 2 trials21,28 (14%). The most common sources of bias due to deviations of the included intervention were nonblinding of the patients and/or the clinicians. Bias due to missing outcome data was deemed low in 4 trials17,21,25,29 (29%). Bias arising from the measurement of the outcome was deemed low in 6 trials16,17,18,21,26,27 (43%).
Figure 2. Risk of Bias Assessment.
Domains include bias arising from the randomization process (D1), bias due to deviations from intended intervention (D2), bias due to missing outcome data (D3), bias in measurement of the outcome (D4), and bias in selection of the reported result (D5).
Primary Outcome: Functional Aerobic Capacity
A total of 7 trials16,17,18,25,28,29,30 involving 389 participants with PCC reported treatment outcomes on functional exercise capacity measured by the 6-minute walking test. The median (IQR) follow-up time for evaluating the primary outcome after randomization was 6 (5.5-7.0) weeks. Rehabilitation interventions were associated with a greater improvement in functional exercise capacity compared with usual care (SMD, −0.56; 95% CrI, −0.87 to −0.22) (Figure 3 and eFigure 1 in Supplement 1). Rehabilitation interventions demonstrated a 99.6% posterior probability of superiority and were associated with 95% probability of reaching the MID threshold when compared with controls. Specifically, patients in the intervention group were able to cover a mean (SD) of 35.84 (6.55) m more than patients in the usual care group during the 6-minute walking test (95% CI, 34.97 m to 36.71 m). The value of τ2 (0.04; 95% CrI, 0.00 to 0.60) indicated low heterogeneity for exercise capacity. The confidence in the certainty of evidence was moderate and was rated down due to high risk of bias (Table 2).
Figure 3. Treatment Outcomes of Rehabilitation Interventions vs Usual Care.
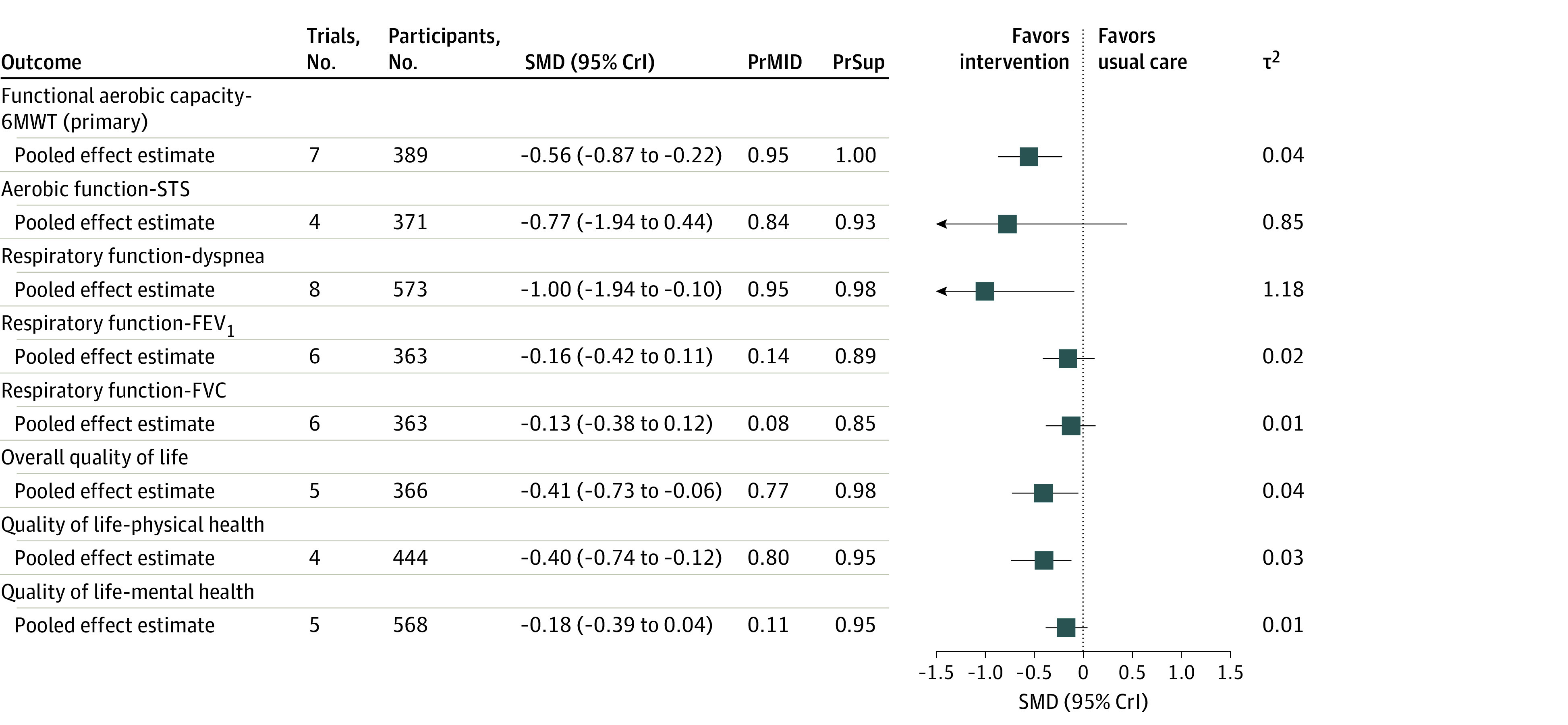
All results are based on a bayesian random-effects model. Results are reported as standardized mean differences (SMDs) and 95% credible intervals (CrIs). PrSup is the probability of the superiority of rehabilitation interventions over usual care (ie, the probability that the SMD is <0). PrMID is the probability of the true treatment effect being equal to or more exacerbated than the between-group minimal important difference (MID) threshold (ie, the probability that the SMD is ≤−0.30). 6MWT indicates 6-minute walking test; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; STS, 30-second sit-to-stand test.
Table 2. Grade Rating of Studies.
| Outcomes | Randomized clinical trials, No. | Participants, No. | Effect size estimate, (95% CrI) | Certainty of evidence (GRADE)a | Reason for downgrade |
|---|---|---|---|---|---|
| Functional exercise capacity (6MWT) | 7 | 389 | −0.56 (−0.87 to −0.22)b | Moderate | Downgraded for high risk of bias |
| Functional leg strength and endurance (STS) | 4 | 371 | −0.77 (−1.94 to 0.44)b | Very low | Downgraded for high risk of bias and imprecision |
| Dyspnea | 8 | 573 | −1.00 (−1.94 to −0.10)b | Low | Downgraded for high risk of bias and inconsistency |
| Respiratory function (FEV1, %) | 6 | 363 | −0.16 (−0.42 to 0.11)b | Low | Downgraded for high risk of bias and indirectness |
| Respiratory function (FVC, %) | 6 | 363 | −0.13 (−0.38 to 0.12)b | Low | Downgraded for high risk of bias and indirectness |
| Quality of life (overall) | 5 | 366 | −0.41 (−0.73 to −0.06)b | Moderate | Downgraded for high risk of bias |
| Quality of life (mental health) | 5 | 568 | −0.18 (−0.39 to 0.04)b | Moderate | Downgraded for high risk of bias |
| Quality of life (physical health) | 4 | 444 | −0.40 (−0.74 to −0.12)b | Moderate | Downgraded for high risk of bias |
| Adverse events | 5 | 544 | 1.68 (0.32 to 9.94)c | Low | Downgraded for high risk of bias |
Abbreviations: 6MWT, 6-minute-walking-test; CrI, credible interval; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; GRADE, grading of recommendations, assessment, development, and evaluations; STS, sit-to-stand test.
The certainty of evidence was rated using the GRADE system, with a rating of high indicating we are very confident that the true effect lies close to the effect size estimate; moderate indicating we are moderately confident in the effect size estimate and that the true effect is likely to be close to the estimate of effect size, but there is a possibility that it is substantially different; low indicating our confidence in the effect size estimate is limited and that the true effect may be substantially different from the size estimate of effect; and very low indicating we have very little confidence in the effect size estimate and that the true effect is likely to be substantially different from the size estimate of effect.
Reported as a standardized mean difference.
Reported as an odds ratio.
Fatigue
The available evidence on fatigue outcomes was limited and could not be synthesized. A single study21 measured the presence or absence of fatigue during everyday activities as a binary outcome without quantifying it. A total of 2 studies28,29 assessed only exercise-induced fatigue levels, and 3 studies (4 records)22,23,24,30 did not adequately report the circumstances under which fatigue was measured. Additionally, 2 studies20,26 recorded the presence of exercise-induced fatigue as an adverse event.
Functional Lower Limb Muscle Strength and Endurance
Four trials19,20,21,30 reported treatment outcomes on lower limb muscle function among 371 participants with PCC. The summary point estimate suggested that rehabilitation interventions could be associated with improvements in lower limb muscle function when compared with usual care. However, given that the CrI included both positive and negative values, the magnitude and direction of the association remain uncertain (SMD, −0.77; 95% CrI, −1.94 to 0.44) (Figure 3and eFigure 2 in Supplement 1). Rehabilitation interventions demonstrated a 93% posterior probability of superiority and were associated with 84% probability of reaching the MID threshold when compared with usual care. The value of τ2 (0.8; 95% CrI, 0.15 to 8.70) indicated substantial statistical heterogeneity for the 30-second sit to stand test. The confidence in the certainty of evidence of the treatment outcomes was very low and was rated down due to high risk of bias, imprecision, and indirectness (Table 2).
Dyspnea
Eight trials (9 records)20,22,23,24,25,26,27,29,30 involving 573 participants with PCC reported treatment outcomes on dyspnea. Rehabilitation interventions were associated with a greater improvement in functional exercise capacity compared with usual care (SMD, −1.00; 95% CrI, −1.94 to −0.10) (Figure 3 and eFigure 3 in Supplement 1). Rehabilitation interventions were associated with a 98% posterior probability of superiority when compared with usual care and were associated with a 95% probability to reach the MID threshold. The magnitude of τ2 indicated substantial statistical heterogeneity (τ2, 1.18; 95% CrI, 0.36 to 5.50). Heterogeneity was partially explained when adjusting for allocation concealment (eFigure 4 in Supplement 1). The confidence in the certainty of evidence was low and rated down due to high risk of bias and inconsistency (Table 2).
Respiratory Function: FEV1 and FVC
A total of 6 trials (7 records)16,18,21,22,23,25,29 reported treatment outcomes on FEV1 (estimated percentage) and FVC (estimated percentage) among 363 participants with PCC. We found no difference between rehabilitation interventions and usual care in either FEV1 (SMD, −0.16; 95% CrI, −0.42 to 0.11) or FVC (SMD, −0.13; 95% CrI, −0.38 to 0.12) (Figure 3 and eFigure 5 and eFigure 6 in Supplement 1). Rehabilitation interventions demonstrated 89% and 85% posterior probability of superiority when compared with usual care for FEV1 and FVC respectively. The magnitude of τ2 indicated very low statistical heterogeneity for both FEV1 (τ2, 0.02; 95% CrI, 0.00 to 0.28) and FVC (τ2, 0.01; 95% CrI, 0.00 to 0.23). The confidence in the certainty of evidence was low for both outcomes and downgraded for high risk of bias and indirectness (Table 2).
Quality of Life
Five trials21,24,25,27,29 reported treatment outcomes on overall quality of life among 366 participants with PCC. Rehabilitation interventions were associated with larger improvement in quality of life compared with the comparison group (SMD, −0.41; 95% CrI, −0.73 to −0.06) (Figure 3 and eFigure 7 in Supplement 1). Rehabilitation interventions were associated with a 98.4% posterior probability of being superior to the comparison group and were associated with a 76.9% probability to reach the MID threshold. The magnitude of τ2 indicated very low statistical heterogeneity (τ2, 0.04; 95% CrI, 0.00 to 0.63). The confidence in the certainty of evidence was moderate and rated down for high risk of bias (Table 2). Figure 3 and eFigure 8 and eFigure 9 in Supplement 1 display the association of rehabilitation interventions with physical and mental health separately.
Adverse Events
Five trials17,19,27,28,29 did not provide any information on adverse events, and 4 trials (5 records)20,22,23,25,30 reported no adverse events related to the intervention. Adverse events were reported in 5 trials.16,20,21,24,26 However, we did not find compelling evidence for a difference in the odds of adverse events occurring. The wide 95% CrI indicates a high level of uncertainty and imprecision in this estimate (OR, 1.68; 95% CrI, 0.32 to 9.94) (eTable 4 and eTable 5 in Supplement 1).
Publication Bias, Autocorrelation, and Model Convergence
We found no indication of publication bias (eTable 6 in Supplement 1). No major concerns of autocorrelation or nonconvergence were identified. The autocorrelation and the trace plots for the main outcome are presented in eFigure 10 and eFigure 11 in Supplement 1.
Sensitivity Analysis
Analyses considering empirical prior distribution for the between-trial variability rendered virtually identical results (eTable 7 in Supplement 1). Analyses incorporating a thinning of 10 are presented in eFigure 12 and eFigure 13 in Supplement 1.
Discussion
Our meta-analysis of 14 randomized clinical trials examining different rehabilitation programs for people with PCC found that the patients undergoing rehabilitation experienced larger improvement in functional exercise capacity, dyspnea, and quality of life outcomes than patients receiving usual care. The analysis consistently showed that rehabilitation interventions had a greater probability of being superior to usual care across all outcomes, with probabilities ranging between 85% and 99%. Additionally, rehabilitation interventions were associated with higher probability of reaching the predefined between-group MID threshold for functional aerobic capacity, functional lower limb strength and endurance, dyspnea, and quality of life, with probabilities ranging between 84% and 95%.
Clinical Implications
Substantial advances in vaccines and prevention strategies of acute SARS-CoV-2 infection may help reduce the burden of PCC. However, as pharmacological advances improve the prognosis of people living with comorbidities who develop an acute SARS-CoV-2 infection, the number of patients living with PCC is expected to grow. Thus, it is of high importance to develop a safe and effective strategy that will be based on high-quality evidence and will be applicable to a broad population. Given the small number of randomized clinical trials and the recent emergence of PCC, it is of no surprise that both the clinical practice and the evidence is rapidly evolving. Current treatment guidelines based on expert opinion suggest a supervised, individualized, symptom-based approach with close monitoring for adverse events such as orthostatic intolerance and postexertional symptom exacerbation.2,55 Yet, current standard care is still provided in the form of self-management recommendations in an ambulatory or home setting. The results of this study indicate that respiratory training and exercise-based rehabilitation interventions may have a greater benefit than current standard care. Inspiratory muscle training requires strict control and high adherence to achieve effective results.56,57 Research on people with chronic obstructive pulmonary disease has shown that education-based, nonsupervised rehabilitation programs are likely to be less effective than clinician-guided ones, mainly due to the wrong use of equipment, ineffective execution of the breathing techniques, and lower adherence.56,57 Additionally, we found a high level of uncertainty and imprecision in the probability of experiencing adverse events. These results further highlight the importance of supervised interventions with continuous monitoring and tailoring to ensure fidelity and patient safety until indicated otherwise.
Female sex and inability to adequately rest in the early weeks after developing COVID-19 are associated with increased risk of development of PCC.4 Because women often take on societal roles such as caring and household duties, which may not allow for adequate rest in the early weeks after infection, women may face increased risk for PCC due to both biological and social factors. Yet, only 45% of the population in the included studies were women. This finding highlights an important research gap that should be addressed in future studies.
Despite research indicating that fatigue is the most frequently observed symptom among individuals with PCC, we found limited evidence quantifying the association of rehabilitation interventions with fatigue during everyday activities. This scarcity of high-quality evidence for managing fatigue has been previously underscored by de Sire et al58 in a systematic review that explored the association of rehabilitation with fatigue in patients with PCC. The review58 identified only 1 observational study and 5 intervention-based studies that lacked a control group. Evidence-based practice hinges on high-quality research, and this considerable gap in the current evidence should be addressed in future studies.
Strengths and Limitations
Although several systematic reviews on PCC have emerged,38,39,40,41,42,43,44,45,46,47,48,49,50 our findings add to the literature on PCC recovery in several ways. Some of the limitations of the currently published evidence are the lack of a meta-analysis component44,45,46,47,48,49,50,51,52,53 and the synthesis of data from both acute care patients and PCC patients in the same analysis.54 This study is the first, to our knowledge, to conduct a systematic review with a bayesian meta-analysis on rehabilitation interventions for patients with PCC. Our findings are in agreement with a previous systematic review with meta-analysis on PCC. Chen et al15 included a total of 3 trials (233 patients with PCC) and found that rehabilitation interventions were associated with a clinical important difference in favor of pulmonary rehabilitation for exercise capacity, but the outcomes on respiratory function were inconsistent across studies. Our review identified 11 additional trials (ie, a total of 14 trials with 1244 participants) and examined a full range of outcomes. To increase the robustness of our results we calculated the probability to indicate superiority of the rehabilitation interventions when compared with usual care, and we prespecified all of our analyses. The robustness and accuracy of our results are further supported by the low between-trial heterogeneity and absence of publication bias. In addition, we used an extended and comprehensive search strategy and searched all relevant sources to retrieve all the potentially eligible randomized clinical trials. We therefore believe that it is unlikely that we missed any relevant trials.
The quality of our analysis is limited by the quality of the underlying data. Although we only included randomized clinical trials, these studies were not without flaws, and the overall evidence grade was deemed to be moderate. Bias in the included studies in terms of allocation concealment, blinding, and missing data could have led to an overestimation of the treatment outcomes. Currently, there is no criterion standard measurement tool for functional aerobic capacity, fatigue, dyspnea, and quality of life in patients with PCC. Therefore, it is possible that certain outcomes did improve, but the measurement tools that were chosen by the researchers did not have the ability to measure this change, which could have led to an underestimation of the treatment outcomes. Some trials included estimates from a per-protocol analysis only, which may have also led to overestimation of the treatment outcomes. Even though female sex is associated with higher risk of PCC, only 45% of the participants in the included studies were women, which could have potentially reduced the external validity of our results. We did not pose any restriction on concurrent medication use with the rehabilitation protocol. Including studies that allowed for concurrent medications increased the chances of overestimating the treatment outcomes due to potential synergy effects. However, not allowing for concurrent medications would have made our results inapplicable to a large proportion of people living with comorbidities who depend on several medication-based treatments. Because comorbidities are associated with higher risk of development of PCC, we believe that not allowing for concurrent medication would have been unfeasible, impractical, and unethical. Our analysis was focused on the shortest time point available after the completion of the interventions, which allowed us to measure the effects of the interventions more accurately and minimize any potential impact from external trial factors that could influence treatment outcomes but prevented us from assessing any associations in the long-term follow-ups.
Conclusions
Rehabilitation interventions demonstrated an association with improved outcomes in functional exercise capacity, dyspnea, and quality of life, with a high probability of improvement compared with the current standard of care. The certainty of evidence was moderate for functional exercise capacity and quality of life and low for other outcomes. Given the uncertainty surrounding our safety outcomes, additional trials with enhanced monitoring of adverse events are necessary.
eAppendix. Study Protocol
eTable 1. Research Strategy
eMethods. Models
eTable 2. Excluded Studies–Reasons for Exclusion
eTable 3. Tidier
eFigure 1. Caterpillar Plot for Exercise Capacity
eFigure 2. Caterpillar Plot for Lower Limb Muscle Function
eFigure 3. Caterpillar Plot for Dyspnea
eFigure 4. Sensitivity Analysis for Dyspnea (Allocation Concealment)
eFigure 5. Caterpillar Plot for Respiratory Function (FEV1)
eFigure 6. Caterpillar Plot for Respiratory Function (FVC)
eFigure 7. Caterpillar Plot for Quality of Life Outcomes
eFigure 8. Caterpillar Plot of Physical Health Outcomes
eFigure 9. Caterpillar Plot for Mental Health Outcomes
eTable 4. Adverse Events Results
eTable 5. Model Fit and Model Comparison for Adverse Events
eTable 6. Publication Bias
eFigure 10. Autocorrelation
eFigure 11. Trace Plot
eTable 7. Sensitivity Analysis
eFigure 12. Sensitivity Analysis–Autocorrelation Plot
eFigure 13. Sensitivity Analysis–Trace Plot
Data Sharing Statement
References
- 1.World Health Organization . Post COVID-19 condition (long COVID). December 7, 2022. Accessed February 25, 2023. https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition
- 2.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133-146. doi: 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodin P, Casari G, Townsend L, et al. ; COVID Human Genetic Effort . Studying severe long COVID to understand post-infectious disorders beyond COVID-19. Nat Med. 2022;28(5):879-882. doi: 10.1038/s41591-022-01766-7 [DOI] [PubMed] [Google Scholar]
- 4.Quinn KL, Katz GM, Bobos P, et al. Understanding the post COVID-19 condition (long COVID) in adults and the expected burden for Ontario. Ontario COVID-19 Science Advisory Table . September 7, 2022. Accessed March 3, 2023. https://covid19-sciencetable.ca/sciencebrief/understanding-the-post-covid-19-condition-long-covid-in-adults-and-the-expected-burden-for-ontario
- 5.Statistics Canada . Long-term symptoms in Canadian adults who tested positive for COVID-19 or suspected an infection, January 2020 to August 2022. October 17, 2022. Updated October 20, 2022. Accessed March 1, 2023. https://www150.statcan.gc.ca/n1/daily-quotidien/221017/dq221017b-eng.htm
- 6.National Center for Health Statistics; Centers for Disease Control and Prevention . Post-COVID conditions. June 6, 2022. Updated July 20, 2023. Accessed August 14, 2023. https://data.cdc.gov/NCHS/Post-COVID-Conditions/gsea-w83j
- 7.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsampasian V, Elghazaly H, Chattopadhyay R, et al. Risk factors associated with post−covid-19 condition: a systematic review and meta-analysis. JAMA Intern Med. 2023;183(6):566-580. doi: 10.1001/jamainternmed.2023.0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meagher T. Long COVID: an early perspective. J Insur Med. 2021;49(1):19-23. doi: 10.17849/insm-49-1-1-5.1 [DOI] [PubMed] [Google Scholar]
- 10.Buttery S, Philip KEJ, Williams P, et al. Patient symptoms and experience following COVID-19: results from a UK-wide survey. BMJ Open Respir Res. 2021;8(1):e001075. doi: 10.1136/bmjresp-2021-001075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adeloye D, Elneima O, Daines L, et al. ; International COVID-19 Airways Diseases Group . The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir Med. 2021;9(12):1467-1478. doi: 10.1016/S2213-2600(21)00286-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz GM, Bach K, Bobos P, et al. Understanding how post-COVID-19 condition affects adults and health care systems. JAMA Health Forum. 2023;4(7):e231933. doi: 10.1001/jamahealthforum.2023.1933 [DOI] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence; National Library of Medicine . COVID-19 rapid guideline: managing the long-term effects of COVID-19 (NG188). December, 2020. Accessed, July 11, 2022. https://www.ncbi.nlm.nih.gov/books/NBK567264/ [PubMed]
- 14.Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Shi H, Liu X, Sun T, Wu J, Liu Z. Effect of pulmonary rehabilitation for patients with post-COVID-19: a systematic review and meta-analysis. Front Med (Lausanne). 2022;9:837420. doi: 10.3389/fmed.2022.837420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Xia W, Zhan C, et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): a randomised controlled trial. Thorax. 2022;77(7):697-706. doi: 10.1136/thoraxjnl-2021-217382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;39:101166. doi: 10.1016/j.ctcp.2020.101166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teixeira DO Amaral V, Viana AA, Heubel AD, et al. Cardiovascular, respiratory, and functional effects of home-based exercise training after COVID-19 hospitalization. Med Sci Sports Exerc. 2022;54(11):1795-1803. doi: 10.1249/MSS.0000000000002977 [DOI] [PubMed] [Google Scholar]
- 19.De Souza Y, Macedo J, Nascimento R, et al. Low-intensity pulmonary rehabilitation through videoconference for post-acute COVID-19 patients. Am J Respir Crit Care Med. 2021;203:A4124. doi: 10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts [DOI] [Google Scholar]
- 20.Capin JJ, Jolley SE, Morrow M, et al. Safety, feasibility and initial efficacy of an app-facilitated telerehabilitation (AFTER) programme for COVID-19 survivors: a pilot randomised study. BMJ Open. 2022;12(7):e061285. doi: 10.1136/bmjopen-2022-061285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Corral T, Fabero-Garrido R, Plaza-Manzano G, Fernández-de-Las-Peñas C, Navarro-Santana M, López-de-Uralde-Villanueva I. Home-based respiratory muscle training on quality of life and exercise tolerance in long-term post-COVID-19: randomized controlled trial. Ann Phys Rehabil Med. 2023;66(1):101709. doi: 10.1016/j.rehab.2022.101709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimeno-Almazán A, Buendía-Romero Á, Martínez-Cava A, et al. Effects of a concurrent training, respiratory muscle exercise, and self-management recommendations on recovery from post-COVID-19 conditions: the RECOVE trial. J Appl Physiol (1985). 2023;134(1):95-104. doi: 10.1152/japplphysiol.00489.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimeno-Almazán A, Franco-López F, Buendía-Romero Á, et al. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: a randomized controlled trial. Scand J Med Sci Sports. 2022;32(12):1791-1801. doi: 10.1111/sms.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNarry MA, Berg RMG, Shelley J, et al. Inspiratory muscle training enhances recovery post-COVID-19: a randomised controlled trial. Eur Respir J. 2022;60(4):2103101. doi: 10.1183/13993003.03101-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okan F, Okan S, Duran Yücesoy F. Evaluating the efficiency of breathing exercises via telemedicine in post-Covid-19 patients: randomized controlled study. Clin Nurs Res. 2022;31(5):771-781. doi: 10.1177/10547738221097241 [DOI] [PubMed] [Google Scholar]
- 26.Philip KEJ, Owles H, McVey S, et al. An online breathing and wellbeing programme (ENO Breathe) for people with persistent symptoms following COVID-19: a parallel-group, single-blind, randomised controlled trial. Lancet Respir Med. 2022;10(9):851-862. doi: 10.1016/S2213-2600(22)00125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romanet C, Wormser J, Fels A, et al. Effectiveness of endurance training rehabilitation after hospitalisation in intensive care for COVID-19-related acute respiratory distress syndrome on dyspnoea (RECOVER): a randomised controlled, open-label multicentre trial. medRxiv.Preprint posted online August 20, 2022. doi: 10.1101/2022.08.29.22279327 [DOI]
- 28.Sari F, Bayram S, Pala GG, Çömçe F, Küçük H, Oskay D. Effects of inspiratory muscle training in patients with post-COVID-19. Harran Üniversitesi Tıp Fakültesi Dergisi. 2022;19(3):581-588. doi: 10.35440/hutfd.1136549 [DOI] [Google Scholar]
- 29.Şahın H, Naz İ, Karadeniz G, Süneçlı O, Polat G, Ediboğlu O. Effects of a home-based pulmonary rehabilitation program with and without telecoaching on health-related outcomes in COVID-19 survivors: a randomized controlled clinical study. J Bras Pneumol. 2023;49(1):e20220107. doi: 10.36416/1806-3756/e20220107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Blanco C, Bernal-Utrera C, Anarte-Lazo E, Gonzalez-Gerez JJ, Saavedra-Hernandez M. A 14-day therapeutic exercise telerehabilitation protocol of physiotherapy is effective in non-hospitalized post-COVID-19 conditions: a randomized controlled trial. J Clin Med. 2023;12(3):776. doi: 10.3390/jcm12030776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 34.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 35.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407-415. doi: 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 36.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol. 2011;64(12):1277-1282. doi: 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 37.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64(12):1283-1293. doi: 10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 38.Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group . GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64(12):1294-1302. doi: 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 39.Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group . GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011;64(12):1303-1310. doi: 10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 40.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97-111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 41.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 42.University of Cambridge School of Clinical Medicine MRC Biostatistics Unit . The BUGS Project. March 2014. Accessed February 25, 2023. https://www.mrc-bsu.cam.ac.uk/software/bugs/
- 43.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 44.Valverde-Martínez MÁ, López-Liria R, Martínez-Cal J, Benzo-Iglesias MJ, Torres-Álamo L, Rocamora-Pérez P. Telerehabilitation, a viable option in patients with persistent post-COVID syndrome: a systematic review. Healthcare (Basel). 2023;11(2):187. doi: 10.3390/healthcare11020187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailly M, Pélissier L, Coudeyre E, et al. Systematic review of COVID-19-related physical activity-based rehabilitations: benefits to be confirmed by more robust methodological approaches. Int J Environ Res Public Health. 2022;19(15):9025. doi: 10.3390/ijerph19159025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernal-Utrera C, Montero-Almagro G, Anarte-Lazo E, Gonzalez-Gerez JJ, Rodriguez-Blanco C, Saavedra-Hernandez M. Therapeutic exercise interventions through telerehabilitation in patients with post COVID-19 symptoms: a systematic review. J Clin Med. 2022;11(24):7521. doi: 10.3390/jcm11247521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centeno-Cortez AK, Díaz-Chávez B, Santoyo-Saavedra DR, Álvarez-Méndez PA, Pereda-Sámano R, Acosta-Torres LS. Fisioterapia respiratoria en pacientes adultos post-COVID-19: revisión sistemática de la literatura. Rev Med Inst Mex Seguro Soc. 2022;60(1):59-66. [PMC free article] [PubMed] [Google Scholar]
- 48.Veronese N, Bonica R, Cotugno S, et al. Interventions for improving long COVID-19 symptomatology: a systematic review. Viruses. 2022;14(9):1863. doi: 10.3390/v14091863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmadi Hekmatikar AH, Ferreira Júnior JB, Shahrbanian S, Suzuki K. Functional and psychological changes after exercise training in post-COVID-19 patients discharged from the hospital: a prisma-compliant systematic review. Int J Environ Res Public Health. 2022;19(4):2290. doi: 10.3390/ijerph19042290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fugazzaro S, Contri A, Esseroukh O, et al. ; Reggio Emilia COVID-19 Working Group . Rehabilitation interventions for post-acute COVID-19 syndrome: a systematic review. Int J Environ Res Public Health. 2022;19(9):5185. doi: 10.3390/ijerph19095185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng B, Daines L, Han Q, et al. Prevalence, risk factors and treatments for post-COVID-19 breathlessness: a systematic review and meta-analysis. Eur Respir Rev. 2022;31(166):220071. doi: 10.1183/16000617.0071-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yelin D, Moschopoulos CD, Margalit I, et al. ESCMID rapid guidelines for assessment and management of long COVID. Clin Microbiol Infect. 2022;28(7):955-972. doi: 10.1016/j.cmi.2022.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halabchi F, Selk-Ghaffari M, Tazesh B, Mahdaviani B. The effect of exercise rehabilitation on COVID-19 outcomes: a systematic review of observational and intervention studies. Sport Sci Health. 2022;18(4):1201-1219. doi: 10.1007/s11332-022-00966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vieira AGDS, Pinto ACPN, Garcia BMSP, Eid RAC, Mól CG, Nawa RK. Telerehabilitation improves physical function and reduces dyspnoea in people with COVID-19 and post-COVID-19 conditions: a systematic review. J Physiother. 2022;68(2):90-98. doi: 10.1016/j.jphys.2022.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long COVID Physio . World Health Organization long COVID rehabilitation guidelines. September 15, 2022. Accessed March 23, 2023. https://longcovid.physio/our-work/who-long-covid-rehab-guidelines
- 56.Gore S, Keysor J. COVID-19 postacute sequela rehabilitation: a look to the future through the lens of chronic obstructive pulmonary disease and pulmonary rehabilitation. Arch Rehabil Res Clin Transl. 2022;4(2):100185. doi: 10.1016/j.arrct.2022.100185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sørensen D, Christensen ME. Behavioural modes of adherence to inspiratory muscle training in people with chronic obstructive pulmonary disease: a grounded theory study. Disabil Rehabil. 2019;41(9):1071-1078. doi: 10.1080/09638288.2017.1422032 [DOI] [PubMed] [Google Scholar]
- 58.de Sire A, Moggio L, Marotta N, et al. Impact of rehabilitation on fatigue in post-COVID-19 patients: a systematic review and meta-analysis. Appl Sci (Basel). 2022;12(17):8593. doi: 10.3390/app12178593 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Study Protocol
eTable 1. Research Strategy
eMethods. Models
eTable 2. Excluded Studies–Reasons for Exclusion
eTable 3. Tidier
eFigure 1. Caterpillar Plot for Exercise Capacity
eFigure 2. Caterpillar Plot for Lower Limb Muscle Function
eFigure 3. Caterpillar Plot for Dyspnea
eFigure 4. Sensitivity Analysis for Dyspnea (Allocation Concealment)
eFigure 5. Caterpillar Plot for Respiratory Function (FEV1)
eFigure 6. Caterpillar Plot for Respiratory Function (FVC)
eFigure 7. Caterpillar Plot for Quality of Life Outcomes
eFigure 8. Caterpillar Plot of Physical Health Outcomes
eFigure 9. Caterpillar Plot for Mental Health Outcomes
eTable 4. Adverse Events Results
eTable 5. Model Fit and Model Comparison for Adverse Events
eTable 6. Publication Bias
eFigure 10. Autocorrelation
eFigure 11. Trace Plot
eTable 7. Sensitivity Analysis
eFigure 12. Sensitivity Analysis–Autocorrelation Plot
eFigure 13. Sensitivity Analysis–Trace Plot
Data Sharing Statement