Key Points
Question
For pediatric acute sinusitis, is amoxicillin-clavulanate associated with different rates of treatment failure or adverse events compared with amoxicillin?
Findings
In this cohort study of 320 141 children, treatment failure was rare (3.1% overall) and serious treatment failure was very rare (0.05%). There was a slight increased risk of treatment failure and an increased risk of adverse events, specifically gastrointestinal symptoms and yeast infections, among patients treated with amoxicillin-clavulanate.
Meaning
Among children with acute sinusitis treated in the outpatient setting, amoxicillin-clavulanate was associated with more adverse events and a slightly higher risk of treatment failure risk compared to amoxicillin.
Abstract
Importance
Acute sinusitis is one of the most common indications for antibiotic prescribing in children, with an estimated 4.9 million such prescriptions in the US annually. Consensus does not exist regarding the optimal empirical antibiotic.
Objective
To compare amoxicillin-clavulanate vs amoxicillin for the treatment of acute sinusitis in outpatient children.
Design, Setting, and Participants
Cohort study of children and adolescents aged 17 years or younger with a new outpatient diagnosis of acute sinusitis and a same-day new prescription dispensation of amoxicillin-clavulanate or amoxicillin in a nationwide health care utilization database. Propensity score matching was used to mitigate confounding.
Exposure
A new prescription dispensation of amoxicillin-clavulanate or amoxicillin.
Main Outcomes and Measures
Treatment failure, defined as an aggregate of a new antibiotic dispensation, emergency department or inpatient encounter for acute sinusitis, or inpatient encounter for a sinusitis complication, was assessed 1 to 14 days after cohort enrollment. Adverse events were evaluated, including gastrointestinal symptoms, hypersensitivity and skin reactions, acute kidney injury, and secondary infections.
Results
The cohort included 320 141 patients. After propensity score matching, there were 198 942 patients (99 471 patients per group), including 100 340 (50.4%) who were female, 101 726 (51.1%) adolescents aged 12 to 17 years, 52 149 (26.2%) children aged 6 to 11 years, and 45 067 (22.7%) children aged 0 to 5 years. Treatment failure occurred in 3.1% overall; 0.05% had serious failure (an emergency department or inpatient encounter). The relative risk of treatment failure for the amoxicillin-clavulanate group compared to the amoxicillin group was 1.10 (95% CI, 1.05-1.16). The risk of gastrointestinal symptoms (RR, 1.15 [95% CI, 1.05-1.25]) and yeast infections (RR, 1.33 [95% CI, 1.16-1.54]) was higher with amoxicillin-clavulanate. After patients were stratified by age, the risk of treatment failure after amoxicillin-clavulanate was an RR of 1.21 (95% CI, 1.09-1.33) for ages 0 to 5 years; RR was 1.16 (95% CI, 1.05-1.29) for 6 to 11 years; and RR was 0.95 (95% CI, 0.88-1.02) for 12 to 17 years. The age-stratified risk of adverse events after amoxicillin-clavulanate was an RR of 1.23 (95% CI, 1.10-1.37) for ages 0 to 5 years; RR was 1.19 (95% CI, 1.04-1.35) for 6 to 11 years; and RR was 1.04 (95% CI, 0.95-1.14) for 12 to 17 years.
Conclusions and Relevance
In children with acute sinusitis who were treated as outpatients, amoxicillin-clavulanate compared with amoxicillin was associated with a slightly higher risk of treatment failure, defined as lack of effectiveness or intolerability, and amoxicillin-clavulanate was associated with a higher risk of gastrointestinal symptoms and yeast infections. These findings may help inform decisions for empirical antibiotic selection in acute sinusitis.
This cohort study compares the rate of treatment failure and adverse events associated with amoxicillin-clavulanate vs amoxicillin in children newly diagnosed with acute sinusitis in an outpatient setting.
Introduction
Acute sinusitis, one of the most common indications for antibiotic prescribing in children, leads to an estimated 4.9 million such prescriptions in the US annually.1 While sinus aspirates and nasopharyngeal specimens in children with sinusitis recover a bacterium in only 68% to 72% of cases, antibiotics are prescribed in 85% of outpatient encounters.1,2,3,4 Amoxicillin and amoxicillin-clavulanate are the most frequently prescribed antibiotics, collectively accounting for 65% of antibiotics prescribed for acute sinusitis in children.2
Evidence-based guidelines indicate a lack of consensus on the optimal choice of empirical antibiotic for pediatric acute sinusitis. The Infectious Diseases Society of America recommends amoxicillin-clavulanate,5 while the American Academy of Pediatrics recommends amoxicillin with or without clavulanate6,7 as first-line empirical antibiotic treatment for an initial diagnosis of acute sinusitis. Understanding whether amoxicillin or amoxicillin-clavulanate is preferred as first line is of substantial clinical importance. Clavulanate adds an additional antimicrobial spectrum through inhibition of bacterial β-lactamases,8 but the increased breadth of coverage may come at the cost of increased adverse events and further perpetuation of antimicrobial resistance.9,10
Three organisms, Streptococcus pneumoniae, nontypeable Haemophilus influenzae, and Moraxella catarrhalis, account for the majority of acute bacterial sinusitis cases.3,6,11 Among these, M catarrhalis isolates universally express a β-lactamase, while H influenzae isolates do so in as many as 65% of cases, with substantial geographic variability.12 S pneumoniae resistance to amoxicillin is not mediated via a β-lactamase and thus amoxicillin-clavulanate adds no benefit for these isolates.
To our knowledge, the effectiveness and safety of amoxicillin-clavulanate vs amoxicillin for the treatment of new diagnoses of acute bacterial sinusitis in children has not been compared since the first introduction of conjugate pneumococcal vaccines in 2000.13,14 Since that time, the routine use of this vaccine and increasing antibiotic resistance rates may have shifted the microbiology of acute bacterial sinusitis, with reports of reductions in the contributions of S pneumoniae and increases in the rates of β-lactamase–producing H influenzae.12,15 This study aimed to address the current gap in evidence on this topic by comparing treatment failure and adverse events associated with amoxicillin-clavulanate vs amoxicillin to treat new diagnoses of acute sinusitis in children.
Methods
Data Source
This nationwide cohort study used the MarketScan Commercial Claims and Encounters Database to identify persons aged 17 and younger with new diagnoses of acute sinusitis between January 1, 2017, and December 31, 2021. This longitudinal health care utilization database includes demographic information, medical visits and hospitalizations, diagnoses, procedures, and outpatient prescription medication dispensations.
Study Design
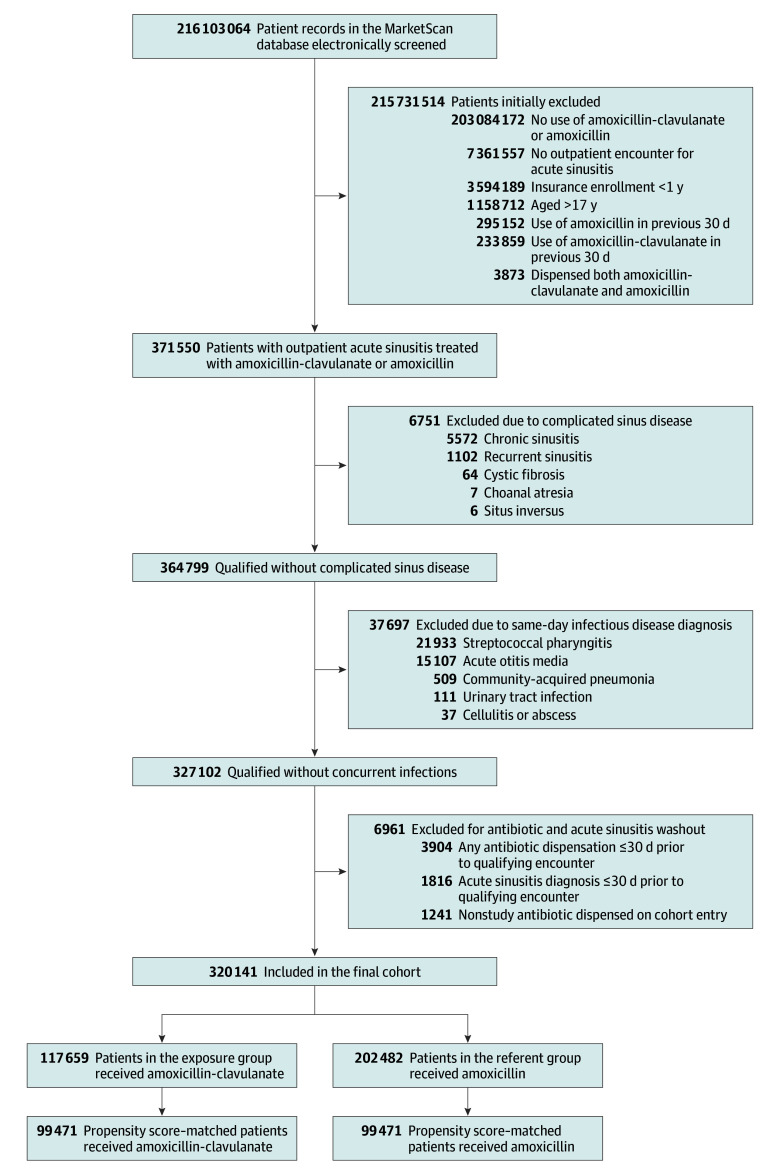
This study used a new-user, active comparator design (eFigure 1 in Supplement 1).16,17 Cohort entry was defined as an outpatient encounter with an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code for acute sinusitis (J01.×0) and a same-day prescription dispensation for either amoxicillin or amoxicillin-clavulanate, a definition with a positive predictive value of 92% (95% CI, 87%-97%) for new diagnoses of acute bacterial sinusitis.18 Patients were excluded if they were 18 years or older at cohort entry, lacked at least 365 days of continuous enrollment in their insurance plan prior to their qualifying encounter; or had chronic sinus disease, a same-day additional infectious disease diagnosis, or an acute sinusitis diagnosis or oral antibiotic dispensation in the 30 days prior to their qualifying encounter (see eTable 1 in Supplement 1 for definition of exclusion criteria). Patients who were dispensed both amoxicillin-clavulanate and amoxicillin or who were dispensed any antibiotic in addition to amoxicillin-clavulanate or amoxicillin at the index encounter were excluded. Each patient contributed only 1 treatment episode to the analyses.
Exposures
Patients dispensed amoxicillin-clavulanate were considered exposed; patients dispensed amoxicillin comprised the referent group.
Outcomes
The primary outcome was treatment failure, an aggregate outcome assessed 1 to 14 days after cohort entry. The outcome window was chosen to capture treatment failure while on and shortly after completion of treatment, as recent data suggest that 80% of pediatric patients receive 10-day courses of antibiotics for acute sinusitis.2 Treatment failure was defined as the first occurrence of any of the following individual outcomes: (1) a new antibiotic dispensation, different from the index antibiotic, in the absence of an outpatient encounter (eg, a prescription called in after an unbilled phone encounter); (2) a new antibiotic dispensation, different from the index antibiotic, with a same-day outpatient encounter for acute sinusitis; (3) an emergency department encounter for acute sinusitis; (4) an inpatient encounter for acute sinusitis; or (5) an inpatient encounter for a complication of sinusitis (see eTable 2 in Supplement 1 for outcome definitions). Secondary analyses included the 5 component treatment failure outcomes individually, and an aggregate of the emergency department and inpatient encounters, termed serious treatment failure.
We assessed adverse events, including gastrointestinal symptoms, hypersensitivity and skin reactions, acute kidney injury, yeast infections, and Clostridioides difficile infections (see eTable 3 in Supplement 1 for adverse outcome definitions and ascertainment windows).19
Covariates
We considered several prespecified potential confounders and proxies for confounders previously shown to be associated with the receipt of broader antibiotics in the outpatient setting.20,21,22,23,24,25 These included patient factors (older age, chronic conditions, rural residence, residence in a southern state), prescriber factors (clinician specialty), and care setting (emergency department vs urgent care vs outpatient clinic, defined by Place of Service coding).20,22,23,24,25 To mitigate confounding by indication, we included covariates potentially associated with disease severity at the time of evaluation, including days’ supply of the index antibiotic dispensation, comorbidities (via the pediatric comorbidity index26 and individually), and corticosteroid prescription. We captured prior use of amoxicillin or amoxicillin-clavulanate (before the 30-day washout window) in the event this was associated with the study antibiotic selection. Because health care access and health-seeking behavior could affect the likelihood of the primary outcome, we captured several measures of health care utilization including number of previous health care encounters, number of distinct medications, and insurance plan type. In general, covariates were assessed during the 365 days prior to cohort entry, with several covariates assessed only in closer proximity to cohort entry (eg, corticosteroids in the 30 days prior to cohort entry) (detailed in eFigure 1 in Supplement 1 and see eTable 4 in Supplement 1 for a comprehensive list of covariates).
Analyses
The balance of covariates between patients treated with amoxicillin-clavulanate and those treated with amoxicillin was assessed using absolute standardized differences, with differences greater than 0.1 interpreted as indicating substantial imbalance.27 To achieve balance in measured covariates, propensity scores for the likelihood to be dispensed amoxicillin-clavulanate were estimated using logistic regression, accounting for all prespecified covariates (eTable 4 in Supplement 1).28,29 Propensity score nearest neighbor 1:1 matching with a maximum caliper of 1% was performed, and treatment effects were evaluated without further adjustment since all covariates were balanced in the matched cohort. Odds ratios (ORs) were estimated using logistic regression, which can be interpreted as relative risk (RR) in cohort studies with infrequent end points. Subgroup analyses were conducted for 3 age strata: 0 to 5 years, 6 to 11 years, and 12 to 17 years. These assessed whether contributions from possible differential antimicrobial resistance patterns (eg, antimicrobial resistance associated with daycare exposure), age-based differences in symptom reporting, or age-related health seeking behavior (eg, parents more likely to seek care for young children than older children) would affect the exposure-outcome relationship.6,15,30
Sensitivity analyses were conducted to evaluate the robustness of the primary results. First, to account for potential residual confounding, we estimated high-dimensional propensity scores that included 200 empirically identified covariates with the strongest potential for confounding in addition to the prespecified covariates.31 Second, as the risk of infection with antibiotic resistant organisms is related to the recent receipt of antibiotics, we extended the period of exclusion of prior antibiotic use and prior acute sinusitis diagnosis from 30 days to 180 days and then to 365 days.30 Third, to address potential outcome misclassification, we included ICD-10 codes for recurrent sinusitis (J01.×1) in addition to those for acute sinusitis when defining treatment failure. Fourth, we extended the outcome assessment window to 30 days to evaluate for the risk of both treatment failure and relapse of infection. Fifth, we conducted a post-hoc analysis to restrict the cohort to only patients dispensed 10 days of antibiotics, hypothesizing that these patients were more ill than patients who were dispensed shorter courses. Lastly, we evaluated 3 negative control outcomes, which we anticipated would not differ between treatment groups: wart removal, ingrown nail, and tendinitis/tendinopathy (eTable 3 in Supplement 1). For most instances of these diagnoses, an individual could very reasonably care for the problem at home or seek care through an outpatient evaluation. Therefore, any difference in risk of these outcomes between groups would suggest residual confounding due to differences in health-seeking behavior.
All analyses were prespecified (unless otherwise noted) and conducted using the Aetion Evidence Generation Platform, R version 3.4.2, and SAS version 9.4.32,33,34 The Brigham and Women’s Hospital institutional review board waived the need for informed consent. This study followed the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) guidelines.35,36
Results
Characteristics of the Study Cohort
The cohort consisted of 320 141 patients who met the inclusion criteria (Figure 1). More than 90% of patients were dispensed 10 or more days of antibiotics and nearly 90% of qualifying encounters occurred in the office setting (Table). Utilization of urgent care at the initial visit was more common among patients dispensed amoxicillin-clavulanate (9.6%) than amoxicillin (5.9%). Among those dispensed amoxicillin-clavulanate, 56.4% were adolescents (aged 12-17 years) compared with 32.7% of those dispensed amoxicillin. Patients were included from all 50 US states (eFigure 2 in Supplement 1). After propensity score matching, all covariates were balanced (Table; eFigure 3 and eTable 4 in Supplement 1).
Figure 1. Screening and Patient Selection for Study of Amoxicillin-Clavulanate and Amoxicillin in Pediatric Sinusitis.
Table. Baseline Demographic and Clinical Characteristics of the Cohort by Sinusitis Treatment.
| Covariate | Unadjusted | Propensity score–matched | ||||
|---|---|---|---|---|---|---|
| Amoxicillin-clavulanate (n = 117 659)a | Amoxicillin (n = 202 482)a | Absolute standardized difference | Amoxicillin-clavulanate (n = 99 471)a | Amoxicillin (n = 99 471)a | Absolute standardized difference | |
| Demographics | ||||||
| Age | ||||||
| 0-5 y | 23 812 (20.2) | 74 677 (36.9) | 0.38 | 22 656 (22.8) | 22 411 (22.5) | 0.01 |
| 6-11 y | 27 480 (23.4) | 61 676 (30.5) | 0.16 | 26 046 (26.2) | 26 103 (26.2) | 0.00 |
| 12-17 y | 66 367 (56.4) | 66 129 (32.7) | 0.49 | 50 769 (51.0) | 50 957 (51.2) | 0.00 |
| Female | 59 087 (50.2) | 103 135 (50.9) | 0.01 | 50 094 (50.4) | 50 246 (50.5) | 0.00 |
| Male | 58 572 (49.8) | 99 347 (49.1) | 0.01 | 49 377 (49.6) | 49 225 (49.5) | 0.00 |
| Medical history | ||||||
| Allergic rhinitis | 21 016 (17.9) | 31 651 (15.6) | 0.06 | 16 964 (17.1) | 17 066 (17.2) | 0.00 |
| Anxiety | 11 091 (9.4) | 12 792 (6.3) | 0.13 | 8527 (8.6) | 8524 (8.6) | 0.00 |
| Asthma | 6268 (5.3) | 9190 (4.5) | 0.04 | 5037 (5.1) | 5046 (5.1) | 0.00 |
| Chronic rhinitis | 2909 (2.5) | 4916 (2.4) | 0.00 | 2345 (2.4) | 2342 (2.4) | 0.00 |
| Type 1 diabetes | 549 (0.5) | 613 (0.3) | 0.03 | 411 (0.4) | 414 (0.4) | 0.00 |
| Type 2 diabetes | 431 (0.4) | 438 (0.2) | 0.03 | 325 (0.3) | 313 (0.3) | 0.00 |
| Any malignancy | 245 (0.2) | 315 (0.2) | 0.01 | 196 (0.2) | 186 (0.2) | 0.00 |
| Pediatric comorbidity index, mean (SD)b | 1.34 (2.09) | 1.03 (1.76) | 0.16 | 1.24 (1.97) | 1.24 (1.99) | 0.00 |
| Prior sinusitis diagnosis, mean (SD)c | 0.43 (1.38) | 0.30 (1.07) | 0.03 | 0.36 (1.22) | 0.35 (1.22) | 0.00 |
| Medications | ||||||
| Influenza vaccined | 44 219 (37.6) | 88 773 (43.8) | 0.13 | 38 559 (38.8) | 38 575 (38.8) | 0.00 |
| Nasal corticosteroidse | 14 310 (12.2) | 16 666 (8.2) | 0.13 | 10 877 (10.9) | 10 904 (11.0) | 0.00 |
| Oral corticosteroidse | 12 082 (10.3) | 13 226 (6.5) | 0.14 | 8655 (8.7) | 8657 (8.7) | 0.00 |
| No. of distinct prescriptions, median (IQR)d | 2 (1-5) | 2 (0-4) | 0.19 | 2 (1-4) | 2 (1-4) | 0.00 |
| No. prior amoxicillin uses, mean (SD)c | 0.36 (0.70) | 0.51 (0.84) | 0.19 | 0.38 (0.73) | 0.39 (0.72) | 0.01 |
| No. prior amoxicillin-clavulanate uses, mean (SD)c | 0.26 (0.66) | 0.13 (0.43) | 0.23 | 0.19 (0.52) | 0.19 (0.53) | 0.00 |
| Supply of initial antibiotic dispensation, d | ||||||
| ≤4 | 210 (0.2) | 449 (0.2) | 0.00 | 190 (0.2) | 207 (0.2) | 0.00 |
| 5-7 | 13 335 (11.3) | 9781 (4.8) | 0.24 | 8112 (8.2) | 8042 (8.1) | 0.00 |
| 8-9 | 390 (0.3) | 714 (0.4) | 0.02 | 348 (0.3) | 350 (0.4) | 0.02 |
| 10 | 91 977 (78.2) | 173 585 (85.7) | 0.20 | 81 148 (81.6) | 81 288 (81.7) | 0.00 |
| 11-14 | 8838 (7.5) | 14 800 (7.3) | 0.01 | 7431 (7.5) | 7374 (7.4) | 0.00 |
| ≥15 | 2909 (2.5) | 3153 (1.6) | 0.06 | 2242 (2.3) | 2210 (2.2) | 0.01 |
| Care setting | ||||||
| Office | 101 895 (86.6) | 184 682 (91.2) | 0.15 | 87 700 (88.2) | 87 673 (88.1) | 0.00 |
| Urgent care | 11 316 (9.6) | 12 005 (5.9) | 0.14 | 8329 (8.4) | 8367 (8.4) | 0.00 |
| Emergency department | 901 (0.8) | 707 (0.3) | 0.07 | 601 (0.6) | 568 (0.6) | 0.00 |
| Retail health clinic | 718 (0.6) | 579 (0.3) | 0.04 | 454 (0.5) | 453 (0.5) | 0.00 |
| Other/missing/unknown | 2829 (2.4) | 4509 (2.2) | 0.01 | 2387 (2.4) | 2410 (2.4) | 0.00 |
All values are reported as number (%) unless otherwise indicated.
Pediatric comorbidity index predicts the 1-year risk of hospitalization in children based on 24 conditions assessed via International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes and was developed and validated using the MarketScan database (score range, 0-29; each point increase is associated with 1.39 increased odds of hospitalization).26
Assessed 365 to 30 days prior to cohort entry.
Assessed in 365 days prior to cohort entry.
Assessed in 30 days prior to and including the day of cohort entry.
Risk of Treatment Failure
In the propensity score–matched analysis, treatment failure occurred in 3209 (3.2%) patients dispensed amoxicillin-clavulanate and 2928 (2.9%) patients dispensed amoxicillin (RR, 1.10 [95% CI, 1.05-1.16]; Figure 2 and eTable 6 in Supplement 1). The pattern of utilization seen in treatment failure episodes differed between the amoxicillin-clavulanate and the amoxicillin groups. The most frequent indication of a treatment failure was an antibiotic dispensation without a same-day outpatient encounter. This occurred in 2179 (2.2%) patients dispensed amoxicillin-clavulanate and 1550 (1.6%) patients dispensed amoxicillin (RR 1.42 [95% CI, 1.33-1.51]). Cephalosporins were the most frequent newly dispensed antibiotics without a same-day encounter in both the amoxicillin-clavulanate (873 prescriptions [40.1%]) and the amoxicillin (477 prescriptions [30.8%]) groups (eFigure 4 in Supplement 1). The next most frequently dispensed antibiotics were macrolides (623 patients [28.6%]) and amoxicillin (408 patients [18.7%]) among the amoxicillin-clavulanate-treated group, and macrolides (456 patients [29.4%]) and amoxicillin-clavulanate (428 patients [27.6%]) among the amoxicillin-treated group. A new antibiotic was dispensed with a same-day acute sinusitis outpatient encounter in 1010 (1.0%) patients dispensed amoxicillin-clavulanate and 1405 (1.4%) patients dispensed amoxicillin (RR, 0.72 [95% CI, 0.66-0.78]). In these episodes, cephalosporins were the most frequently dispensed antibiotics in each group: 474 patients (46.2%) in the amoxicillin-clavulanate group and 560 patients (39.2%) in the amoxicillin group (eFigure 5 in Supplement 1).
Figure 2. Treatment Failure After Propensity Score Matching.
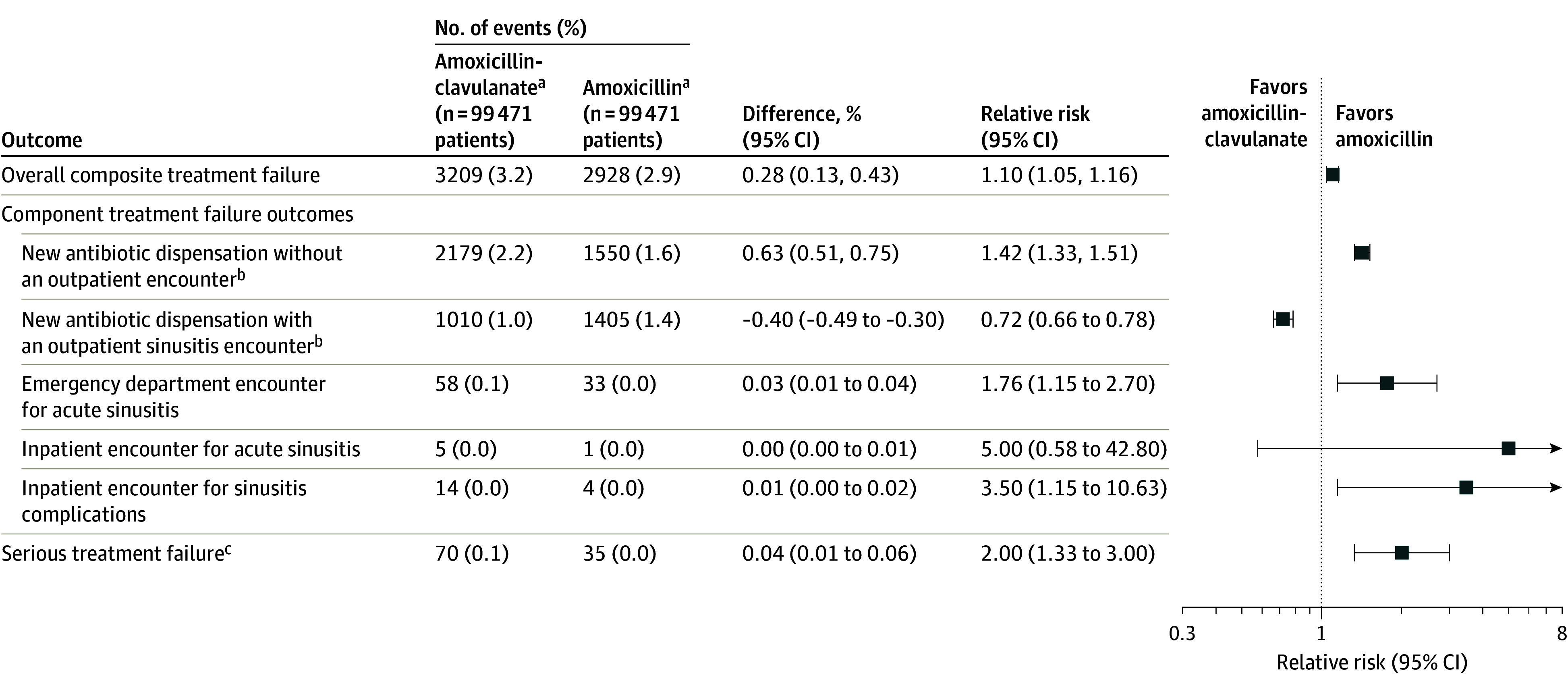
aAmoxicillin-clavulanate indicates the exposure group, and amoxicillin indicates the referral group.
bA new antibiotic could be any oral antibiotic except the index antibiotic dispensed on the cohort entry date.
cSerious treatment failure indicates the first occurrence of (1) emergency department encounter for acute sinusitis; (2) inpatient encounter for acute sinusitis; and (3) inpatient encounter for sinusitis complications.
Emergency department visits were infrequent, occurring in 58 (0.1%) patients treated with amoxicillin-clavulanate and 33 (0.0%) patients treated with amoxicillin (RR, 1.76 [95% CI, 1.15-2.70]). Inpatient encounters for acute sinusitis or sinusitis complications (including orbital cellulitis, intracranial abscess, and meningitis) were rare, occurring in 19 patients treated with amoxicillin-clavulanate and 5 patients treated with amoxicillin (Figure 2; eTable 5 in Supplement 1). Among patients with treatment failure, a hypersensitivity reaction during the follow-up period was documented in 39 of 3209 (1.2%) patients treated with amoxicillin-clavulanate and 37 of 2928 (1.3%) patients treated with amoxicillin.
Risk of Adverse Events
Overall, adverse events were more common among patients dispensed amoxicillin-clavulanate (2249 [2.3%]) than among patients dispensed amoxicillin (1970 [2.0%]) (RR, 1.15 [95% CI, 1.08-1.22]; Figure 3) after propensity score matching. The risk of gastrointestinal symptoms (RR, 1.15 [95% CI, 1.05-1.25]) and yeast infections (RR, 1.33 [95% CI, 1.16-1.54]) were higher with amoxicillin-clavulanate. The risk of C difficile infection was also higher with amoxicillin-clavulanate, but the association was estimated imprecisely as reflected in the width of the CI (RR, 1.83 [95% CI, 0.91-3.71]) and the risk difference was small (0.01 [95% CI, 0.00-0.02]).
Figure 3. Adverse Outcomes After Propensity Score Matching.
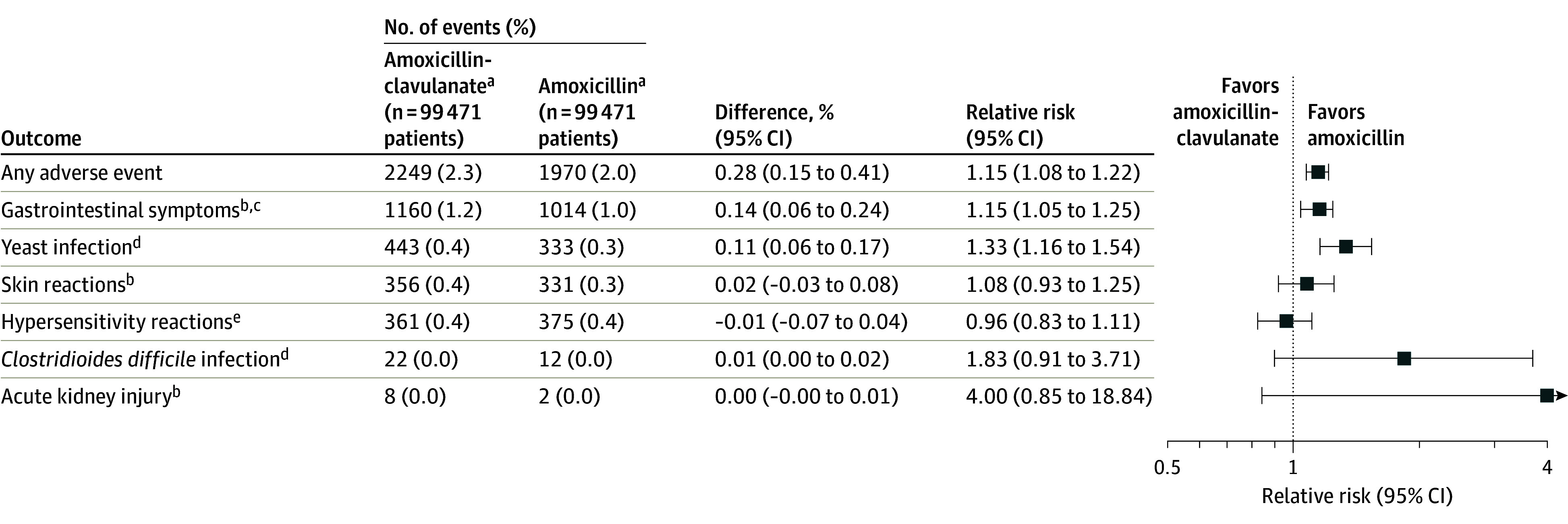
aAmoxicillin-clavulanate indicates the exposure group, and amoxicillin indicates the referral group.
bMeasured from day 1 to day 14 after treatment initiation.
cThe first occurrence of nausea/vomiting, abdominal pain, non–Clostridioides difficile diarrhea.
dMeasured from day 1 to day 90 after treatment initiation.
eIndicates the first occurrence of anaphylaxis, laryngeal edema, angioedema, unspecified allergy, and urticaria. Anaphylaxis, laryngeal edema, and angioedema were measured from day 0 to day 2 after treatment initiation, while unspecified allergy and urticaria were measured from day 1 to day 14 after treatment initiation.
Subgroup Analysis by Age
In propensity score–matched analyses, there were 22 573 patients per exposure group aged 0 to 5 years, 25 975 patients per group aged 6 to 11 years, and 49 582 patients per group aged 12 to 17 years (Figure 4; eTable 8 in Supplement 1). For patients aged 0 to 5 years, there was an increased risk of treatment failure among patients dispensed amoxicillin-clavulanate (RR, 1.21 [95% CI, 1.09-1.33]). Adverse events were more frequent among those dispensed amoxicillin-clavulanate (RR, 1.23 [95% CI, 1.10-1.37]). Among patients aged 6 to 11 years, the RR of treatment failure was 1.16 (95% CI, 1.05-1.29) while the RR of adverse events was 1.19 (95% CI, 1.04-1.35). For patients aged 12 to 17 years, there was no difference in treatment failure risk (RR, 0.95 [95% CI, 0.88-1.02]) or adverse events between patients dispensed amoxicillin-clavulanate and those dispensed amoxicillin (RR, 1.04 [95% CI, 0.95-1.14]).
Figure 4. Treatment Failure and Adverse Outcomes Stratified by Age After Propensity Score Matching.
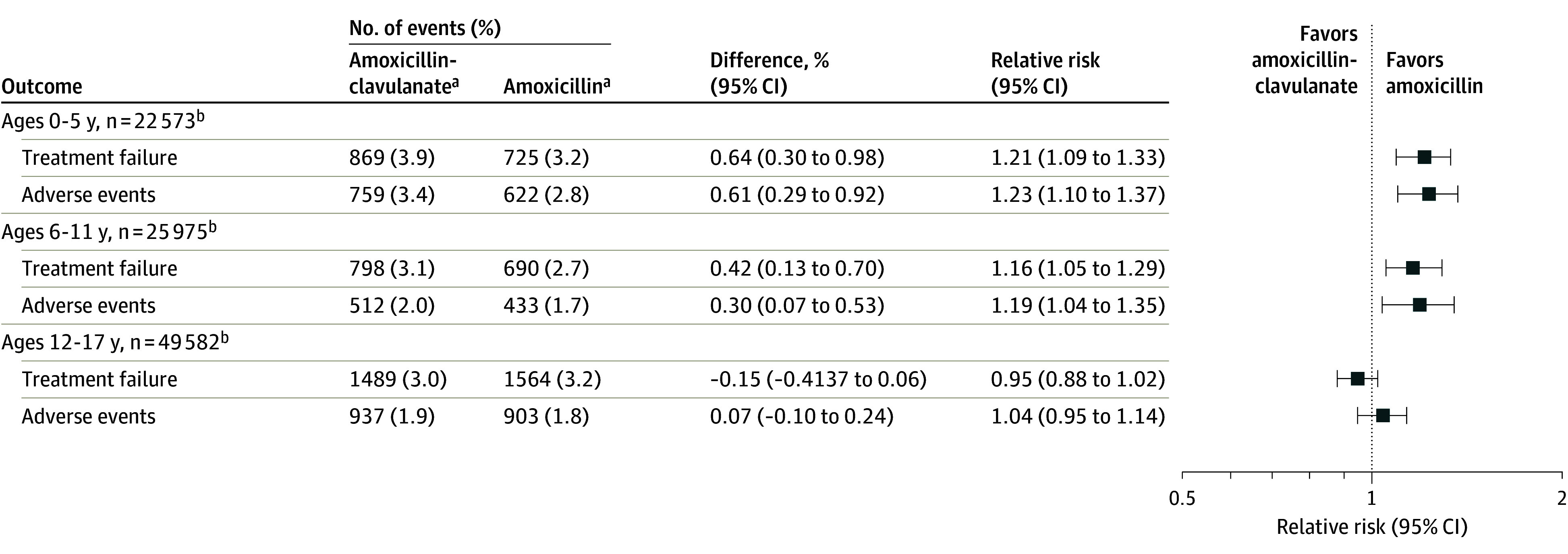
aAmoxicillin-clavulanate indicates the exposure group, and amoxicillin indicates the referral group.
bThe sum of patients across strata does not equal the total number of patients in the primary analyses due to propensity score estimation and matching occurring after patients were stratified by age.
Sensitivity Analyses and Control Outcomes
Results for treatment failure and risk of adverse events were consistent after matching on high-dimensional propensity scores (eTable 6 and eTable 7 in Supplement 1). There were also no changes in the results for individual treatment failure outcomes, although the association weakened somewhat for the risk of an emergency department encounter for acute sinusitis relative to the main analysis (RR, 1.30 [95% CI, 0.88-1.94]) (eTable 6 in Supplement 1). The main analyses were not sensitive to varying the washout period or the outcome definition (eTable 9 in Supplement 1). There was no difference in the risk of treatment failure and relapse evaluated over a 30-day window (RR, 1.03 [95% CI, 0.99-1.08]) (eTable 9 in Supplement 1). The main analyses were also not sensitive to restricting the cohort to only patients treated for 10 days (eTable 9 in Supplement 1). The control outcomes of outpatient encounters for ingrown nail, tendinitis/tendinopathy, and wart removal were not different between patients dispensed amoxicillin-clavulanate vs patients dispensed amoxicillin in the matched population, suggesting no differences in health-seeking behavior (eTable 10 in Supplement 1).
Discussion
In this nationwide cohort of commercially insured children and adolescents treated for acute sinusitis, treatment failure was rare, and there was a slightly higher risk of overall treatment failure for patients dispensed amoxicillin-clavulanate compared to patients dispensed amoxicillin. The risk of adverse events, in particular gastrointestinal symptoms and yeast infections, was higher among those dispensed amoxicillin-clavulanate. When treatment failure did occur, it almost always resulted in a management change in the outpatient setting; emergency department and inpatient encounters were rare.
The increased risk of treatment failure with amoxicillin-clavulanate is likely of little meaningful clinical consequence as the infrequent occurrence of the outcome means an additional 357 patients would need to be treated with amoxicillin to prevent 1 additional treatment failure with amoxicillin-clavulanate. Additionally, in a sensitivity analysis with 30 days of follow up after treatment initiation, there was no difference in the risk of treatment failure or relapse with amoxicillin-clavulanate compared to amoxicillin. In subgroup analyses there was an increased risk of treatment failure with amoxicillin-clavulanate among children aged 11 years and younger, while for adolescents aged 12 to 17 years there was no increased risk. As the most frequent treatment failure component was a new antibiotic dispensation without an encounter, this may indicate more intolerance to amoxicillin-clavulanate among younger children. These findings should be interpreted in the context of other studies demonstrating associations between broad-spectrum antibiotic exposure, including amoxicillin-clavulanate, and the subsequent increased risk of antimicrobial resistant infections.37,38
This study is the first to our knowledge to compare amoxicillin-clavulanate and amoxicillin for the treatment of acute sinusitis since the introduction of the pneumococcal conjugate vaccines more than 20 years ago. Randomized clinical trials published in 1986 and 2001 compared the effectiveness and safety of amoxicillin and amoxicillin-clavulanate to treat acute sinusitis prior to this.13,14 The first study included 93 pediatric patients and found no difference in cure rates between amoxicillin (67%) and amoxicillin-clavulanate (64%), although both were more effective than placebo (43%).13 The second study of 161 pediatric patients found no difference in cure rates between amoxicillin (79%), amoxicillin-clavulanate (81%), and placebo (79%).14 The current study is substantially larger (320 141 patients) and assessed treatment failure requiring a change in management—a new antibiotic dispensation and/or a new health care encounter—rather than evaluating a change in symptoms, as with the first and second studies. In this context, we observed a lower treatment failure risk, and there were no differences in the risk of failure between the 2 groups. The lower treatment failure risk in the current study is likely due to the use of recorded diagnostic codes rather than parent-reported symptoms to define the outcome, but there may also be contributions from differences in the microbiology of the infecting organisms, due to the introduction of the pneumococcal conjugate vaccines. The current findings align with a recent observational study comparing amoxicillin and amoxicillin-clavulanate among a population from the Veterans Health Administration that found no difference in outcomes between adult patients treated with amoxicillin and amoxicillin-clavulanate.39
This study has several strengths. The large cohort permits estimation of the associations with a high level of precision and detection of rare events, such as serious treatment failure. The inclusion of patients from all 50 US states incorporates areas with varying degrees of antibacterial resistance. Lastly, this study is the first to directly compare these treatments for acute sinusitis in children since the introduction of the pneumococcal conjugate vaccines, and thus, it represents current practice patterns and outcomes.
Limitations
Despite these strengths, this study has several limitations. First, this study cohort was commercially insured. Insurance type or status would not be expected to affect the biologic relations studied, although rates of treatment failure based on utilization data might vary among commercially insured, uninsured, and Medicaid-insured patients due to differences in health care access and utilization.40,41
Second, this study used medication dispensation but could not evaluate medication adherence. However, treatment failure due to nonadherence (from medication intolerance or for other reasons) is an important factor in clinical practice and is captured in this study.
Third, weight-based dosing was not captured as patient weight was not available in the data, however a recent randomized clinical trial found no difference in outcomes between standard and high-dose amoxicillin and amoxicillin-clavulanate treatment in adults, suggesting that uncaptured differences in dosing strategy are unlikely to change the outcome.42
Fourth, there are several prescriber factors (age and sex of prescriber, practice size, type of clinician) that are associated with prescribing broader antibiotics, which were not available in our data.22,24 While these variables could affect prescribing, they would only introduce bias if they were independently associated with the treatment failure outcome as well.
Fifth, we did not have microbiologic data and could not discern whether acute sinusitis diagnoses were due to viral or bacterial etiologies. The current study’s design reflects current routine clinical practice in which clinicians must make prescribing decisions without knowing the underlying microbiologic etiology of the sinus infection.
Sixth, as with all nonrandomized studies, there is the potential for residual confounding. High-dimensional propensity score analyses attempted to reduce residual confounding through adjustment for proxies of unmeasured variables and found no difference from the primary analysis.
Seventh, residual differences in health-seeking behavior between the groups could lead to more frequent outcome ascertainment in one group. However, sensitivity analyses using negative control outcomes suggested no difference in health-seeking behavior between the study groups.
Eighth, data on race, ethnicity, and socioeconomic status were not available. It was not possible to evaluate how results may have varied in groups defined by these characteristics; this may be a topic for future study. For example, other studies have observed that Black race is associated with receipt of more narrow-spectrum antibiotics and with reduced access to care, which could affect both the treatment and the ascertainment of treatment failure in Black patients.43,44
Conclusions
Among children and adolescents treated with amoxicillin-clavulanate or amoxicillin for a new diagnosis of acute sinusitis, overall treatment failure was rare, and there was a small increased risk of treatment failure with amoxicillin-clavulanate. Gastrointestinal symptoms and yeast infections were more frequent among patients treated with amoxicillin-clavulanate. These findings may inform risk-benefit decisions for empirical antibiotic selection in children and adolescents with acute sinusitis.
eFigure 1. Graphical Depiction of Study Design
eFigure 2. Geographic Distribution of Patients
eFigure 3. Propensity Score Distribution and Overlap
eFigure 4. Antibiotic Switches – No Outpatient Encounter
eFigure 5. Antibiotic Switches – Acute Sinusitis Outpatient Encounter
eTable 1. Exclusion Criteria
eTable 2. Outcome Definitions
eTable 3. Adverse and Control Outcome Definitions
eTable 4. Comprehensive Patient Characteristics
eTable 5. Counts of Complications of Sinusitis Among Patients Who Experienced Treatment Failure
eTable 6. Treatment Failure
eTable 7. Adverse Outcomes
eTable 8. Treatment Failure and Adverse Events by Age
eTable 9. Sensitivity Analyses
eTable 10. Control Outcomes
Data Sharing Statement
References
- 1.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi: 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 2.Savage TJ, Kronman MP, Sreedhara SK, et al. Trends in the antibiotic treatment of acute sinusitis: 2003-2020. Pediatrics. 2023;151(4):e2022060685. doi: 10.1542/peds.2022-060685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaikh N, Hoberman A, Shope TR, et al. Identifying children likely to benefit from antibiotics for acute sinusitis: a randomized clinical trial. JAMA. 2023;330(4):349-358. doi: 10.1001/jama.2023.10854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawada S, Matsubara S. Microbiology of acute maxillary sinusitis in children. Laryngoscope. 2021;131(10):E2705-E2711. doi: 10.1002/lary.29564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow AW, Benninger MS, Brook I, et al. ; Infectious Diseases Society of America . IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72-e112. doi: 10.1093/cid/cis370 [DOI] [PubMed] [Google Scholar]
- 6.Wald ER, Applegate KE, Bordley C, et al. ; American Academy of Pediatrics . Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132(1):e262-e280. doi: 10.1542/peds.2013-1071 [DOI] [PubMed] [Google Scholar]
- 7.Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH; Committee on Infectious Diseases, American Academy of Pediatrics . Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd ed. American Academy of Pediatrics; 2021. Accessed September 5, 2022. https://publications.aap.org/redbook
- 8.Weber DJ, Tolkoff-Rubin NE, Rubin RH. Amoxicillin and potassium clavulanate: an antibiotic combination: mechanism of action, pharmacokinetics, antimicrobial spectrum, clinical efficacy and adverse effects. Pharmacotherapy. 1984;4(3):122-136. doi: 10.1002/j.1875-9114.1984.tb03333.x [DOI] [PubMed] [Google Scholar]
- 9.Gerber JS, Ross RK, Bryan M, et al. Association of broad- vs narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA. 2017;318(23):2325-2336. doi: 10.1001/jama.2017.18715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States, 2019. US Dept of Health and Human Services, Centers for Disease Control and Prevention; 2019. Accessed July 10, 2023. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
- 11.Wald ER, Reilly JS, Casselbrant M, et al. Treatment of acute maxillary sinusitis in childhood: a comparative study of amoxicillin and cefaclor. J Pediatr. 1984;104(2):297-302. doi: 10.1016/S0022-3476(84)81018-5 [DOI] [PubMed] [Google Scholar]
- 12.Kaur R, Pichichero M. Colonization, density, and antibiotic resistance of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis among PCV13 vaccinated infants in the first six months of life in Rochester, New York: a cohort study. J Pediatric Infect Dis Soc. 2023;12(3):135-142. doi: 10.1093/jpids/piad004 [DOI] [PubMed] [Google Scholar]
- 13.Wald ER, Chiponis D, Ledesma-Medina J. Comparative effectiveness of amoxicillin and amoxicillin-clavulanate potassium in acute paranasal sinus infections in children: a double-blind, placebo-controlled trial. Pediatrics. 1986;77(6):795-800. doi: 10.1542/peds.77.6.795 [DOI] [PubMed] [Google Scholar]
- 14.Garbutt JM, Goldstein M, Gellman E, Shannon W, Littenberg B. A randomized, placebo-controlled trial of antimicrobial treatment for children with clinically diagnosed acute sinusitis. Pediatrics. 2001;107(4):619-625. doi: 10.1542/peds.107.4.619 [DOI] [PubMed] [Google Scholar]
- 15.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304-309. doi: 10.1097/INF.0b013e3181c1bc48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 17.Sendor R, Stürmer T. Core concepts in pharmacoepidemiology: confounding by indication and the role of active comparators. Pharmacoepidemiol Drug Saf. 2022;31(3):261-269. doi: 10.1002/pds.5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savage TJ, Wardell H, Huybrechts KF. Accuracy of identifying pediatric acute bacterial sinusitis diagnoses in outpatient claims data. Pharmacoepidemiol Drug Saf. 2023;32(8):918-923. doi: 10.1002/pds.5617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler AM, Brown DS, Durkin MJ, et al. Association of inappropriate outpatient pediatric antibiotic prescriptions with adverse drug events and health care expenditures. JAMA Netw Open. 2022;5(5):e2214153. doi: 10.1001/jamanetworkopen.2022.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wattles BA, Jawad KS, Feygin Y, et al. Inappropriate outpatient antibiotic use in children insured by Kentucky Medicaid. Infect Control Hosp Epidemiol. 2022;43(5):582-588. doi: 10.1017/ice.2021.177 [DOI] [PubMed] [Google Scholar]
- 21.Singer A, Fanella S, Kosowan L, et al. Informing antimicrobial stewardship: factors associated with inappropriate antimicrobial prescribing in primary care. Fam Pract. 2018;35(4):455-460. doi: 10.1093/fampra/cmx118 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt ML, Spencer MD, Davidson LE. Patient, provider, and practice characteristics associated with inappropriate antimicrobial prescribing in ambulatory practices. Infect Control Hosp Epidemiol. 2018;39(3):307-315. doi: 10.1017/ice.2017.263 [DOI] [PubMed] [Google Scholar]
- 23.King LM, Kusnetsov M, Filippoupolitis A, et al. Using machine learning to examine drivers of inappropriate outpatient antibiotic prescribing in acute respiratory illnesses. Infect Control Hosp Epidemiol. 2023;44(5):786-790. doi: 10.1017/ice.2021.476 [DOI] [PubMed] [Google Scholar]
- 24.Fleming-Dutra KE, Bartoces M, Roberts RM, Hicks LA. Characteristics of primary care physicians associated with high outpatient antibiotic prescribing volume. Open Forum Infect Dis. 2018;5(1):ofx279. doi: 10.1093/ofid/ofx279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dantuluri KL, Bruce J, Edwards KM, et al. Rurality of residence and inappropriate antibiotic use for acute respiratory infections among young Tennessee children. Open Forum Infect Dis. 2020;8(1):ofaa587. doi: 10.1093/ofid/ofaa587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun JW, Bourgeois FT, Haneuse S, et al. Development and validation of a pediatric comorbidity index. Am J Epidemiol. 2021;190(5):918-927. doi: 10.1093/aje/kwaa244 [DOI] [PubMed] [Google Scholar]
- 27.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 28.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156. doi: 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum PRRD. The central role of the propensity score in observational studies for causal effects. Biometrika. 1984;70(1):41-55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 30.Levine OS, Farley M, Harrison LH, Lefkowitz L, McGeer A, Schwartz B. Risk factors for invasive pneumococcal disease in children: a population-based case-control study in North America. Pediatrics. 1999;103(3):E28. doi: 10.1542/peds.103.3.e28 [DOI] [PubMed] [Google Scholar]
- 31.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20(4):512-522. doi: 10.1097/EDE.0b013e3181a663cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Foundation . R: A language and environment for statistical computing. 2013. Accessed July 20, 2023. http://www.r-project.org
- 33.Aetion Evidence Platform . The system of record for an unruly real world. 2023. Accessed July 20, 2023. https://www.aetion.com
- 34.SAS Institute Inc . Version 9.4. 2023. Accessed July 20, 2023. https://support.sas.com/software/94/
- 35.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aldeyab MA, Harbarth S, Vernaz N, et al. The impact of antibiotic use on the incidence and resistance pattern of extended-spectrum beta-lactamase–producing bacteria in primary and secondary healthcare settings. Br J Clin Pharmacol. 2012;74(1):171-179. doi: 10.1111/j.1365-2125.2011.04161.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben-Ami R, Rodríguez-Baño J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49(5):682-690. doi: 10.1086/604713 [DOI] [PubMed] [Google Scholar]
- 39.Rovelsky SA, Remington RE, Nevers M, et al. Comparative effectiveness of amoxicillin versus amoxicillin-clavulanate among adults with acute sinusitis in emergency department and urgent care settings. J Am Coll Emerg Physicians Open. 2021;2(3):e12465. doi: 10.1002/emp2.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lash TL, VanderWeele TJ, Haneuse S, Rothman KJ. Modern Epidemiology. 4th ed. Wolters Kluwer; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergmark RW, Ishman SL, Phillips KM, Cunningham MJ, Sedaghat AR. Emergency department use for acute rhinosinusitis: insurance dependent for children and adults. Laryngoscope. 2018;128(2):299-303. doi: 10.1002/lary.26671 [DOI] [PubMed] [Google Scholar]
- 42.Gregory J, Huynh B, Tayler B, et al. High-dose vs standard-dose amoxicillin plus clavulanate for adults with acute sinusitis: a randomized clinical trial. JAMA Netw Open. 2021;4(3):e212713. doi: 10.1001/jamanetworkopen.2021.2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleming-Dutra KE, Shapiro DJ, Hicks LA, Gerber JS, Hersh AL. Race, otitis media, and antibiotic selection. Pediatrics. 2014;134(6):1059-1066. doi: 10.1542/peds.2014-1781 [DOI] [PubMed] [Google Scholar]
- 44.Flores G; Committee On Pediatric Research . Technical report–racial and ethnic disparities in the health and health care of children. Pediatrics. 2010;125(4):e979-e1020. doi: 10.1542/peds.2010-0188 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Graphical Depiction of Study Design
eFigure 2. Geographic Distribution of Patients
eFigure 3. Propensity Score Distribution and Overlap
eFigure 4. Antibiotic Switches – No Outpatient Encounter
eFigure 5. Antibiotic Switches – Acute Sinusitis Outpatient Encounter
eTable 1. Exclusion Criteria
eTable 2. Outcome Definitions
eTable 3. Adverse and Control Outcome Definitions
eTable 4. Comprehensive Patient Characteristics
eTable 5. Counts of Complications of Sinusitis Among Patients Who Experienced Treatment Failure
eTable 6. Treatment Failure
eTable 7. Adverse Outcomes
eTable 8. Treatment Failure and Adverse Events by Age
eTable 9. Sensitivity Analyses
eTable 10. Control Outcomes
Data Sharing Statement