Abstract
Involvement of the abdomen and pelvis is common in lymphoma. Nodal and extra-nodal abdominal and pelvic lymphoma may present with various complications. Complications are most common in high grade lymphomas, especially diffuse large B-cell lymphoma. Complications may occur as the initial manifestation of lymphoma, during treatment course, or late following complete disease remission. Most complications are associated with worse prognosis and increased mortality. Imaging is essential in evaluation of disease extent and diagnosis of complications. Therefore, radiologists should be familiar with the clinical context and imaging features of abdominal and pelvic lymphoma complications. We provide a comprehensive, organ-system based approach, clinical and imaging review of complications of abdominal and pelvic lymphoma along with radiologic images of illustrated cases of the most commonly encountered complications.
Keywords: Lymphoma, Abdominal lymphoma, Gastrointestinal Lymphoma, Complication, Oncologic Emergency, Emergency Radiology
Introduction:
Abdomen and pelvis are common sites of nodal and extra-nodal involvement in Hodgkin (HL) and non-Hodgkin lymphoma (NHL). Spleen and gastrointestinal tract are one of the most common sites of extra-nodal lymphoma involvement, especially the stomach and small bowel [1–3]. Imaging features of lymphoma in the abdomen or pelvis varies with type of lymphoma and site of involvement. The imaging appearances of lymphoma may resemble or differ from infectious, inflammatory and neoplastic conditions. Radiologists should be familiar with those imaging appearances. Furthermore, abdominal or pelvic involvement in lymphoma may lead to various complications. Complications are more frequent in high grade lymphomas, such as diffuse large B-cell lymphoma (DLBCL) or T-cell lymphoma (TCL). Complications are common and can occur as the initial presentation, following treatment, or after complete remission. Most complications are associated with worse prognosis and increased mortality. Imaging is essential in evaluation and diagnosis of complications. It is important for radiologists to be acquainted with the spectrum of presentations, patterns of disease and multimodality imaging of both common and less common complications of lymphoma in the abdomen and pelvis to increase diagnostic accuracy and direct therapy [2]. There is paucity of literature discussing the complications related to abdominal and pelvic lymphoma; however, there are numerous case reports describing various complications. To our knowledge, this is the first article that provides a comprehensive review of the various complications of lymphoma in the abdomen and pelvis. Using an organ system approach, we first describe the clinical presentation, incidence, common types of lymphoma and patterns of involvement. We then provide a comprehensive review of the different complications of lymphoma in the abdomen and pelvis and describe their radiologic findings in different imaging modalities. Radiologic images of illustrated cases of most commonly encountered complications are included in each section. We hope this review paper provides a guide for radiologists to better understand and interpret the complication of lymphoma in the abdomen and pelvis.
1. Gastric complications:
The most common site of extra-nodal NHL involvement is the stomach with an incidence reaching up to 50–70% of all gastrointestinal (GI) lymphomas [2–4]. The primary histopathologic types of gastric lymphomas include DLBCL and mucosa-associated lymphoid tissue (MALT) lymphoma. Mantle cell, follicular lymphoma (FL), and TCL are less common types [1–4]. Differential diagnoses on imaging include gastric adenocarcinoma, metastasis, gastritis, peptic ulcer disease, Menetrier disease, gastrointestinal stromal tumors (GIST) [2]. The imaging appearance of gastric lymphoma includes diffuse submucosal wall thickening, infiltrative, mass-like, polypoidal, or ulcerative lesions [3] . On CT, gastric lymphoma is usually characterized by extensive gastric wall thickening, mild enhancement, and preservation of perigastric fat planes with a relatively homogenous and monotonous soft tissue density pattern. Regional nodal enlargement, when present, can be a helpful sign especially when located below the renal veins [2, 3]. On MR, lesions show homogeneous intermediate signal intensity on T1WI and mild hyperintensity on T2WI [2].
The main complications of gastric lymphoma are gastric bleeding, gastric outlet obstruction (GOO), perforation, and fistula formation [3–6]. These can occur as the initial manifestation, during active disease and treatment, or after disease remission [3, 7]. Bleeding usually occurs during active disease [7] . GOO uncommonly occurs in the active phase of aggressive lymphoma and more commonly stenotic obstruction after disease remission with stricture formation [4, 5]. Perforation, free or contained with or without fistula formation, is uncommon and usually happens after chemotherapy administration.
Gastric bleeding is the most common complication with a reported incidence of 2–3.5% [6] in a few studies and reaching up to 7–19% in other studies [4, 5, 8]. The risk of gastric bleeding after chemotherapy is increased with a prior history of bleeding before treatment. Upper gastrointestinal bleeding can occur as the first presentation of the disease or during chemotherapy treatment [4, 7]. Bleeding may present with overt symptoms such as hematemesis and melena, or may be occult, presenting with hemoglobin drop. The role of imaging, like other causes of upper gastrointestinal bleeding, is to detect the source of bleeding and evaluate for the presence of active bleeding [9]. Multi-phasic computed tomography (CT) angiography is usually performed in patients with significant bleeding and prior to embolization or surgical planning. CT is valuable to identify arterial anatomy, presence of active contrast extravasation, pseudoaneurysm formation, evaluate local disease extent and co-existing complications [9–11]. Gastric bleeding can be managed conservatively, with endoscopic techniques, embolization or gastrectomy depending on the patient’s clinical status, severity of bleeding, local disease extent and other associated complications and comorbidities [12].
Gastric outlet obstruction (GOO) occurs in 3–11% of patients [3–6]. Active gastric lymphoma uncommonly results in GOO due to preserved gastric distensibility [2, 4]. Gastric, duodenal, and pancreatic adenocarcinoma are more commonly known to cause GOO. However, late gastric stricture and obstruction is relatively more common after disease remission and after multiple sessions of chemotherapy [4, 5]. GOO usually presents with repeated non-bilious vomiting, abdominal pain and abdominal distention. CT or MRI show marked gastric distension, obstructive infiltrative tumor, or stenotic stricture (Fig. 1) [9, 10]. GOO can be managed with surgical gastrojejunostomy or endoscopic balloon dilatation [5].
Fig. 1:

Gastric outlet obstruction. Axial (a) and coronal (b) contrast enhanced CT images of a patient with relapsed follicular lymphoma. There is an infiltrative mass in the gastric antrum and duodenum (white arrow) with marked gastric distention (white asterisk) indicating gastric outlet obstruction. There is contiguous retroperitoneal and peri-portal adenopathy (black arrow) encasing the celiac axis. Axial FDG-PET (c) prior to obstruction showing FDG-avid gastric antral mass and adenopathy.
Gastric perforation is an uncommon complication with a reported incidence of 1–2% but is associated with a high mortality rate [4, 6–8]. Perforation commonly occurs after institution of chemotherapy [13]. The risk factors include ulcerative and necrotic tumor, higher grade, large tumor more than 10 cm and disseminated extranodal involvement (Lugano Stage IV) [7]. Pre-phase low dose chemotherapy, in comparison to conventional dose, is associated with lower incidence of perforation and bleeding complications [13]. Perforation clinically presents with abdominal pain, signs of peritonitis and may lead to sepsis and shock. Pneumoperitoneum, ulcerated tumor, peri-gastric fat stranding, and defect in the gastric wall are the imaging findings of perforation on CT (Fig. 2) [9, 10]. Gastric perforation is treated surgically and is associated with worse prognosis [7].
Fig. 2:

Gastric perforation. Axial FDG-PET/CT image (a) of a patient with diffuse large B-cell lymphoma. There are nodular FDG-avid lesions in the greater and lesser gastric curvatures (white arrow) and FDG-avid hepatic masses. Axial contrast enhanced CT (b) following chemotherapy treatment shows a perforation (black arrow) in the greater curvature at the site of prior FDG-avid disease involvement, pneumoperitoneum (white asterisk) and ascites.
Another rare complication is gastrosplenic fistula that typically occurs after chemotherapy in patients with contiguous gastric and splenic involvement. Post chemotherapy necrosis of the infiltrative tumor creates a tract between the stomach and spleen [14]. It can present with massive gastrointestinal bleeding [15] features of sepsis due to associated splenic abscess [14, 16], or asymptomatic and found incidentally on imaging [17]. On cross sectional imaging, a necrotic tract could be seen communicating gastric lumen with a necrotic collection within the spleen containing gas (Fig. 3) [14, 16, 17]. The necrotic fistulous tract and the splenic collection would be non-FDG-avid usually surrounded by FDG-avid tissue. The mainstay of management is surgical resection which includes gastrectomy and splenectomy, however there are reported cases of management with chemotherapy and radiotherapy [14, 15, 17]. The mortality rate is high, and it is associated with a high postoperative complication rate [15, 16]. Formation of fistula with other organs such as gastric-pleural is very rare [18].
Fig. 3:

Gastrosplenic fistula. Axial CT with oral contrast (a), intravenous contrast (b), and FDG-PET/CT (c) of a patient with gastric and splenic involvement by large B-cell lymphoma, on chemotherapy. There is a gas containing collection (white asterisk) in the center of the FDG-avid splenic hilar lesion (white arrow) with a necrotic tract communicating with the stomach (black arrow), indicating a gastro-splenic fistula, with splenic abscess formation.
2. Bowel complications:
The second most common site of involvement of GI lymphoma is the bowel, more frequent in the small bowel than the large bowel [2, 3, 19, 20]. In the small bowel, ileal involvement occurs most frequently, followed by the jejunum and duodenum, and multifocal involvement occurs in 10–25% of cases [2, 20]. Differential diagnosis include adenocarcinoma, carcinoid tumor, GIST, metastasis, familial polyposis syndromes, and inflammatory bowel disease. The colon and less commonly rectum may be involved in lymphoma [2, 3, 20]. The most common symptoms at presentation are abdominal pain, nausea, vomiting and weight loss [21]. There are rare cases of appendiceal involvement by NHL, which may present as acute appendicitis [19].
Types of lymphoma that can involve the bowel are DLBCL, extranodal marginal zone B-cell lymphoma (ENMZL), mantle cell lymphoma, FL, Burkitt lymphoma, TCL and HL [1, 22]. T-cell lymphoma has worse prognosis in comparison to B-cell lymphoma [20, 22]. Risk factors include immunosuppressed states such as HIV or organ transplant, celiac disease and inflammatory bowel disease [3].
The imaging appearance of small bowel lymphoma include wall thickening, nodular, infiltrative or polypoid masses, ulcerated lesions, aneurysmal dilatation or cavitation. [2]. Bowel lymphoma may show subserosal infiltration [23]. Extension of tumor beyond the submucosa is associated with worse prognosis[22]. In the small bowel, lymphoma is characterized by a lack of desmoplastic reaction, therefore intestinal obstruction is less common [1–3]. Bowel lymphoma can cause bowel dilatation. In contrast, primary malignancies such as adenocarcinoma and inflammatory processes such as Crohn’s disease show infiltration with focal (occasionally eccentric) wall thickening, ulceration, luminal narrowing and bowel obstruction [2].
In the large bowel, lymphoma can demonstrate various patterns including polypoid mural lesions, circumferential wall thickening with smooth mucosal surface or ulceration, cavitation, mucosal fold thickening, or focal mucosal nodularity. Diffuse large bowel lymphoma may have a diffuse nodular or ulcerative pattern [1–3, 7, 24].
Complications of lymphoma in the small and large bowel include perforation, obstruction and stricture formation, intussusception, enteric fistula, and bleeding [7, 9, 25, 26]. Similar to gastric complications, bowel complications can occur as the initial manifestation, following treatment, or late after disease remission [7, 26]. Bleeding usually occurs during active disease [7, 26]. Intussusception may be the initial presentation of bowel lymphoma [27, 28]. Bowel obstruction is more commonly seen after treatment secondary to stricture formation [26, 29]. Perforation or fistula formation usually occurs after chemotherapy [25, 26, 30]. Patients may present with multiple bowel complications [7].
Perforation is a significant life-threatening complication of GI lymphoma with an incidence of 2–9% and a high morbidity and mortality rate [20, 22, 25, 30]. Perforation is associated with worse prognosis and leads to significant morbidity from sepsis, multiorgan failure, prolonged hospitalization [7, 22, 23, 26, 30]. Perforation can occur as an initial presentation of the primary disease or following chemotherapy [7, 26, 30]. Perforation is mostly seen with DLBCL, and TCL and its risk increases with more aggressive tumors [7, 20, 25, 26, 30]. Concentric, ulcerative, transmural, and long segment (more than 10 cm) lesions and disseminated extranodal involvement (Lugano Stage IV) are associated with a higher perforation rate (Fig. 4) [7, 30, 31]. The most common site of perforation is the small bowel followed by the large bowel [26, 30]. Lymphoma patients with new onset abdominal pain must be promptly evaluated clinically and radiologically to exclude perforation [26, 30]. CT is the modality of choice in urgent care for identifying perforation, free or contained, and evaluating associated intestinal and extraintestinal findings (Fig. 5) [9, 31].
Fig. 4:

Aneurysmal bowel dilatation with necrotic wall at risk of perforation. Coronal (a) and axial (b) CT images with intravenous and oral contrast of a patient with Burkitt lymphoma. There is marked circumferential wall thickening and aneurysmal dilation of a segment of small bowel (black arrow) with areas of necrosis within the wall of the lesion (white arrow). These features indicate a high risk for bowel perforation. Note peritoneal thickening and ascites due to peritoneal lymphomatosis.
Fig. 5:
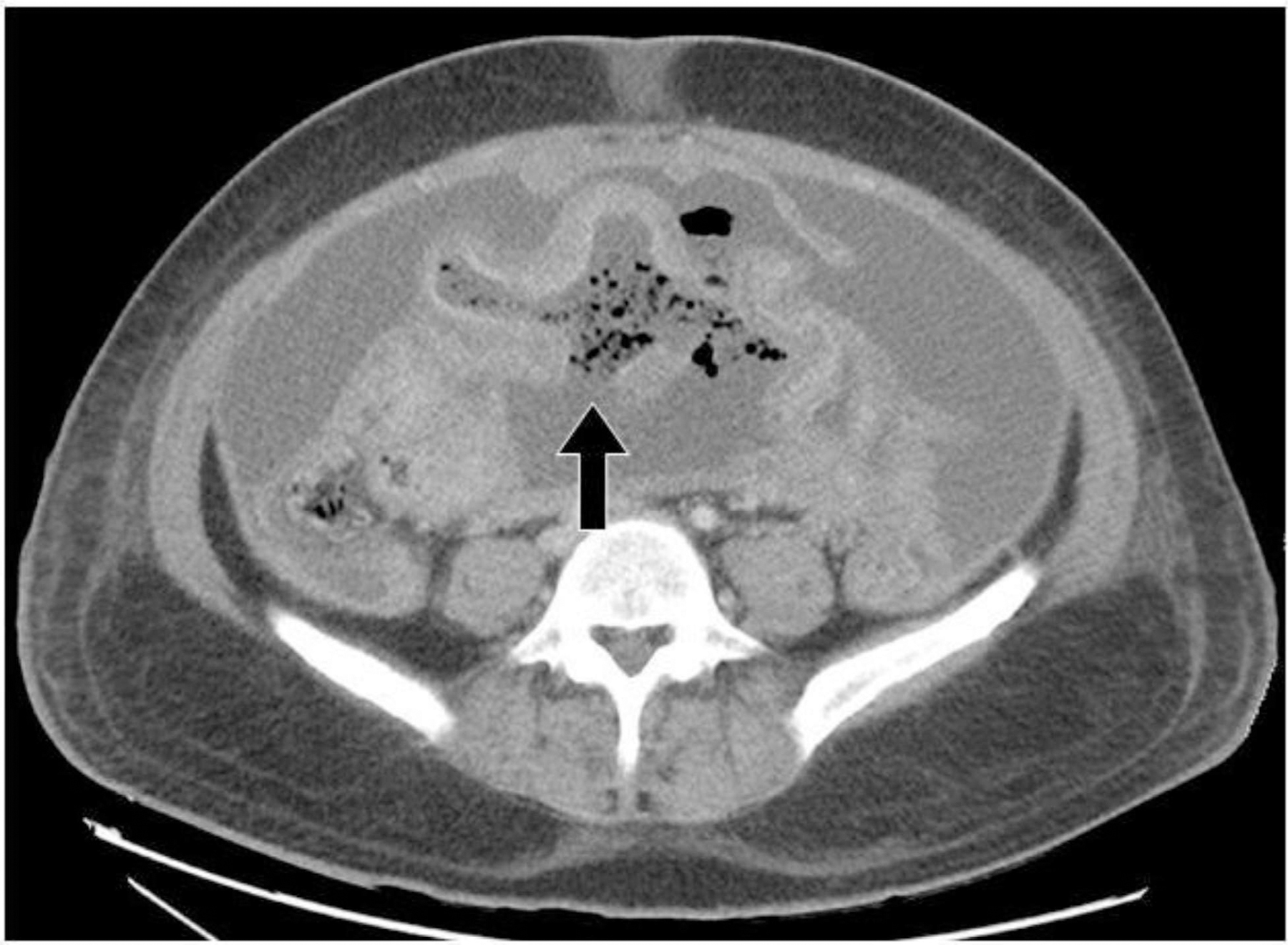
Small bowel perforation. Axial contrast enhanced axial CT image of a patient with B-cell lymphoma on chemotherapy. There is wall thickening and aneurysmal dilation of a segment of small bowel, with a large defect in the posterior wall of the involved small bowel loop (black arrow) indicating perforation with resultant pneumoperitoneum, ascites and peritonitis.
Gastrointestinal bleeding may be the initial manifestation of bowel lymphoma [7, 25, 26]. Bleeding may occur following therapy or secondary to vascular erosion by cavitary aneurysmal bowel lesions. The reported bleeding rate is 2–7% with varying degrees of severity [20, 25, 32]. Gastrointestinal bleeding could lead to worse prognosis in comparison to cases without bleeding [7]. Bleeding may present with melena or fresh blood per rectum or uncommonly as hemoperitoneum. CT angiography is usually performed in patients with significant gastrointestinal bleeding and prior to embolization or surgical planning [9, 11]. CT is valuable to identify arterial anatomy, presence of active contrast extravasation, pseudoaneurysm formation, evaluate local disease extent and co-existing complications (Fig. 6) [9, 11]. Bleeding can be managed conservatively, with endoscopy, embolization or surgically depending on the patient’s condition, severity of bleeding, local disease extent and other associated complications and co-morbidities (Fig. 7) [12, 26].
Fig. 6:
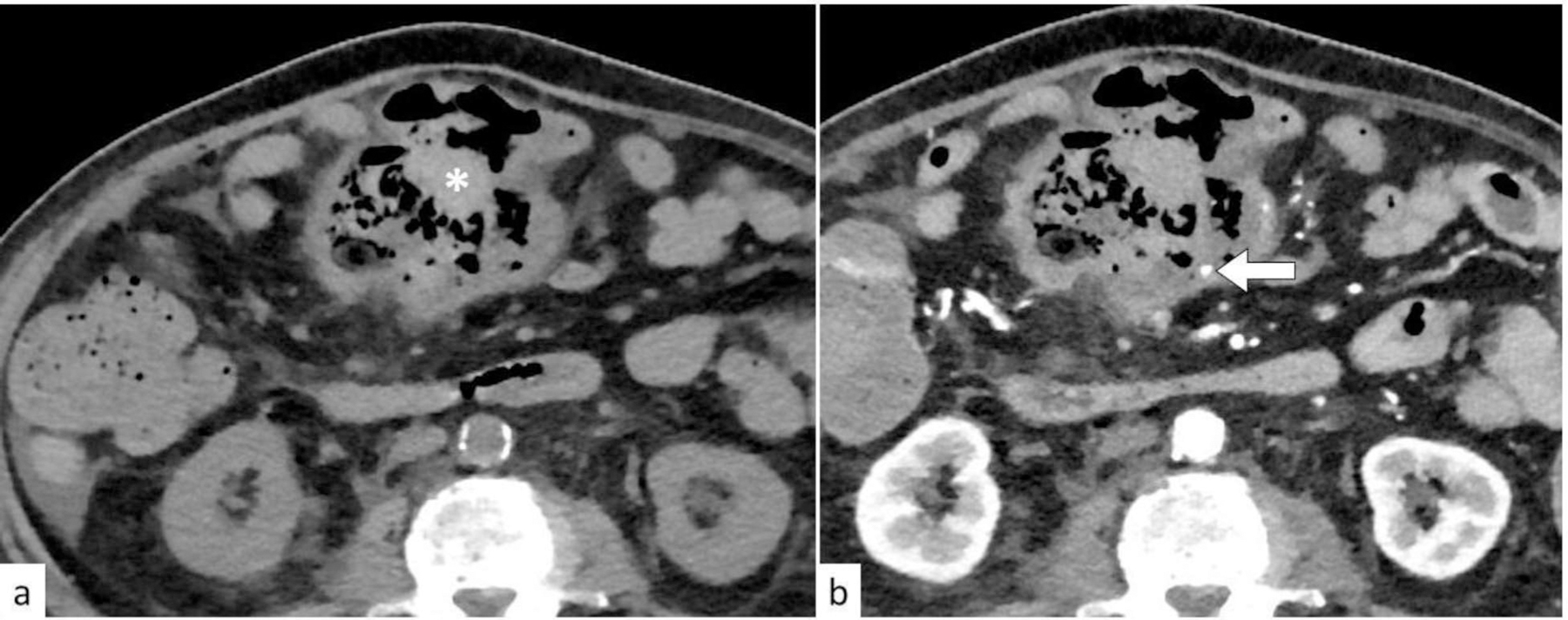
Active gastrointestinal bleeding. Axial unenhanced (a) and arterial-phase enhanced (b) CT images of a patient with T-cell lymphoma on chemotherapy presenting with abdominal pain and fresh bleeding per rectum. There is a cavitary small bowel mass in the mid abdomen with hyperdense luminal contents representing blood clots (white asterisk) and a bleeding mesenteric artery within the cavitary mass (white arrow).
Fig. 7:

Gastrointestinal bleeding treated with endovascular embolization. Axial FDG-PET/CT image (a) of a 46-year-old male with T-cell lymphoma shows intensely FDG avid mesenteric and retroperitoneal lymph nodes with focal FDG-avid wall thickening of a jejunal loop in the left mid abdominal quadrant (white arrow). During chemotherapy treatment the patient developed melena and axial pre-contrast (b) and arterial-phase post-contrast (c) CT images were obtained. There are foci of contrast extravasation (black arrows) at the site of jejunal involvement, indicating active bleeding. Selective digital subtraction angiography of a jejunal branch of superior mesenteric artery shows contrast extravasation and confirms active bleeding. Endovascular embolization was performed to achieve hemostasis.
Lymphoma uncommonly causes direct bowel obstruction (without intussusception) as the initial presentation [7]. Bowel obstruction is more common with adenocarcinoma and carcinoid. Aggressive lymphoma, especially DLBCL may present with a large tumor causing obstruction [7, 25]. Post-treatment bowel stricture may occur at the site of initial disease involvement and present with obstruction after disease remission [29]. The reported incidence of bowel obstruction is 5–16% [20, 23, 25]. Bowel obstruction presents with abdominal pain, distention, vomiting and constipation [26]. Plain radiograph may reveal dilated bowel loops. CT is the modality of choice to detect the site of obstruction and evaluate the presence of underlying mass and possible complications such as ischemia, pneumatosis and perforation (Fig. 8, Fig. 9 and Fig. 10) [9]. Surgery is the treatment of choice [26, 29].
Fig. 8:

Small bowel obstruction. Axial (a) and coronal (b) contrast enhanced CT images of a patient presenting with small bowel obstruction as the initial manifestation of diffuse large B-cell lymphoma. There is a homogeneously enhancing, circumferential tumor in the terminal ileum (black arrow) causing small bowel obstruction with marked dilatation of the proximal loops. Axial (a) and coronal (b) contrast enhanced CT images of the same patient following disease remission after 7 years presenting with features of bowel obstruction. There is a stricture at the site of the treated primary tumor (white arrow) with proximal bowel dilatation and small bowel feces sign.
Fig. 9:

Small bowel obstruction. Coronal contrast enhanced CT image of a patient presenting with small bowel obstruction as initial manifestation of diffuse large B-cell lymphoma. There is a homogeneously enhancing, circumferential tumor in the jejunum (white arrow) causing small bowel obstruction with dilatation of the proximal bowel loops.
Fig. 10:
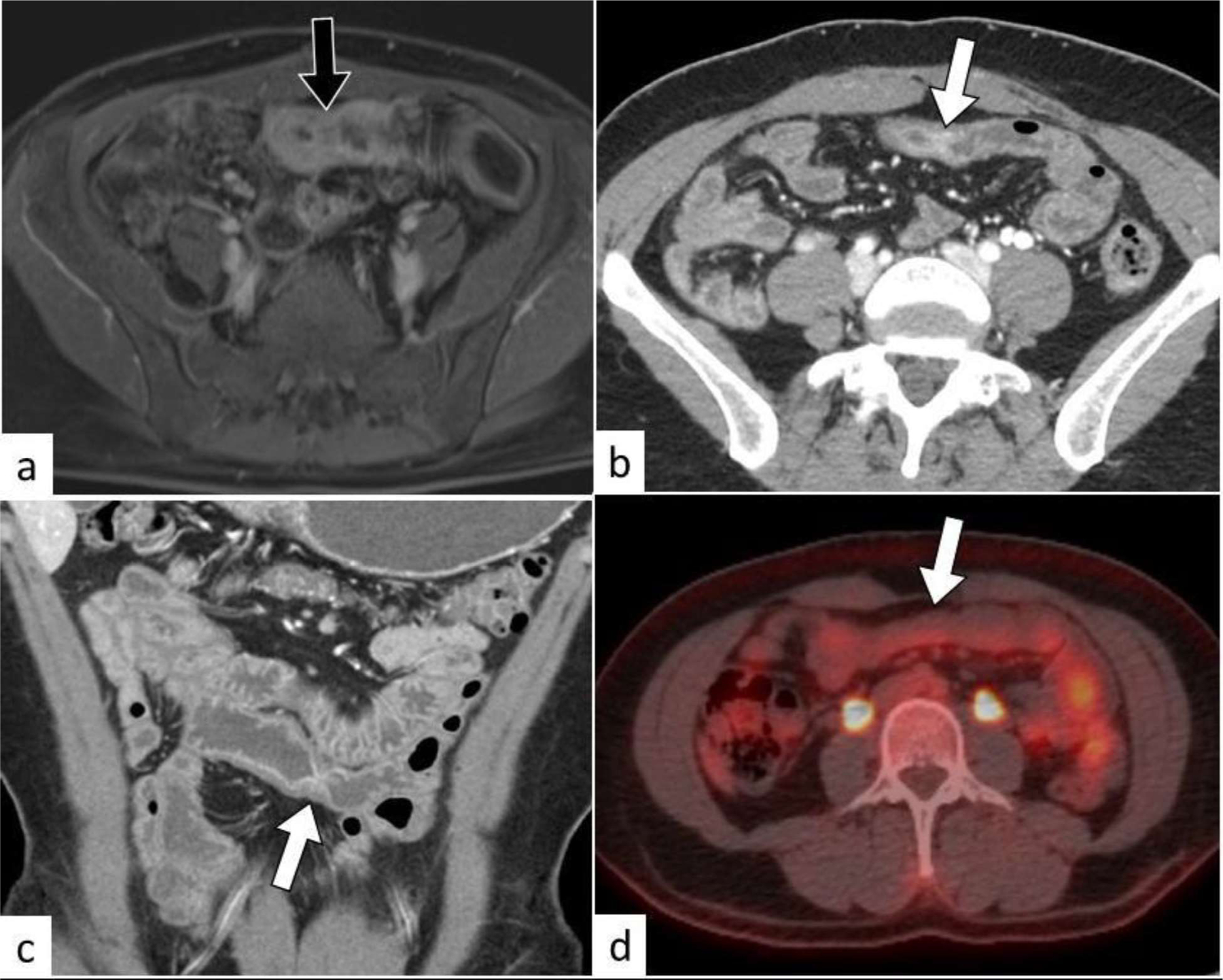
Small bowel stricture. Axial T1W fat sat contrast enhanced MR image (a) of a patient with non-Hodgkin lymphoma of the small bowel showing a circumferential non-obstructing tumor (black arrow). Axial (b) and coronal (c) contrast enhanced CT following chemotherapy and disease remission shows a short segment small bowel stricture (white arrow). Note no increased FDG-uptake on the PET/CT image (d) at the site of the stricture (white arrow).
Small bowel lymphoma can also present as intussusception with or without bowel obstruction [28]. The incidence of intussusception is 7–17% in patients with bowel lymphoma [27, 28]. Clinical symptoms include recurrent, episodic and colicky abdominal pain, vomiting, palpable mass, blood in stool, and anorexia [28]. Lymphoma is one of the pathologic lead points of intussusception in children [28]. The chronicity of symptoms and weight loss are two important factors when considering diagnosis of a malignant cause of intussusception, particularly GI lymphoma. The peak age of GI Burkitt lymphoma is 5–15 years which is above the age of idiopathic intussusception (6 months to 3 years) [27]. Burkitt lymphoma patients presenting with intussusception often have completely respectable disease [27]. Intussusception is rare in adults with the vast majority of cases having an underlying pathological process [33]. MALT lymphoma and DLBCL are the most common subtypes. It most commonly involves the cecum, transverse, and sigmoid colon. Ultrasound is highly specific and sensitive for diagnosis of intussusception showing typical donut sign, the edematous thickened bowel wall (hypoechoic rim) surrounding the intussusceptum and adjacent mesenteric fat (hyperechoic center) [9]. CT also is an accurate modality for diagnosis of intussusception, identifying underlying lead-point and is more informative for extra-intestinal findings (Fig. 11 and Fig. 12) [9]. Longer segment intussusception with larger diameter, obstruction and infiltration are more likely to be associated with a lead-point (Fig. 11) [33].
Fig. 11:

Intussusception. Axial CT (a-c) images with oral contrast of a patient with diffuse large B-cell lymphoma. There is ileo-ileal intussusception (white arrow). Note the prominent lobular wall thickening of the intussuscipiens loop.
Fig. 12:

Intussusception. Axial (a, b) and oblique coronal reformatted (c) CT images with intravenous contrast of an 11-year-old patient with ileo-cecal intussusception (white arrow). There is a nodular enhancing lesion within the intussusception. Distal ileal loops are mildly dilated. Surgical pathology confirmed Burkitt lymphoma with no extra-intestinal involvement.
Another major but rare complication of GI lymphoma is enteral fistula, which most commonly occurs with DLBCL [25, 34]. A fistula can develop as sequela of chemotherapy, radiotherapy or surgery; but can manifest as the first presentation of primary GI lymphoma [25, 34–36]. Symptoms include abdominal pain, diarrhea, malnutrition, and weight loss [34, 37] in cases of enteric fistula, symptoms of sepsis in cases of fistula with spleen and abscess formation [35] or massive GI bleeding in case of fistula formation with arteries and aorta [36]. Fistula formation is a chronic process whereby the tumor invades the serosa and perforates into the adjacent mesentery, forming a contained abscess or fistulous tract with the adjacent bowel loops, spleen, bladder or even aorta [35–37]. It is usually misdiagnosed as Crohn’s disease. Imaging findings include focal wall thickening, fistulous tract communicating with adjacent bowel loops or organs, air fluid levels, pockets of gas within the lesion and enlarged lymph nodes (Fig. 13 and Fig. 14) [34–37].
Fig. 13:
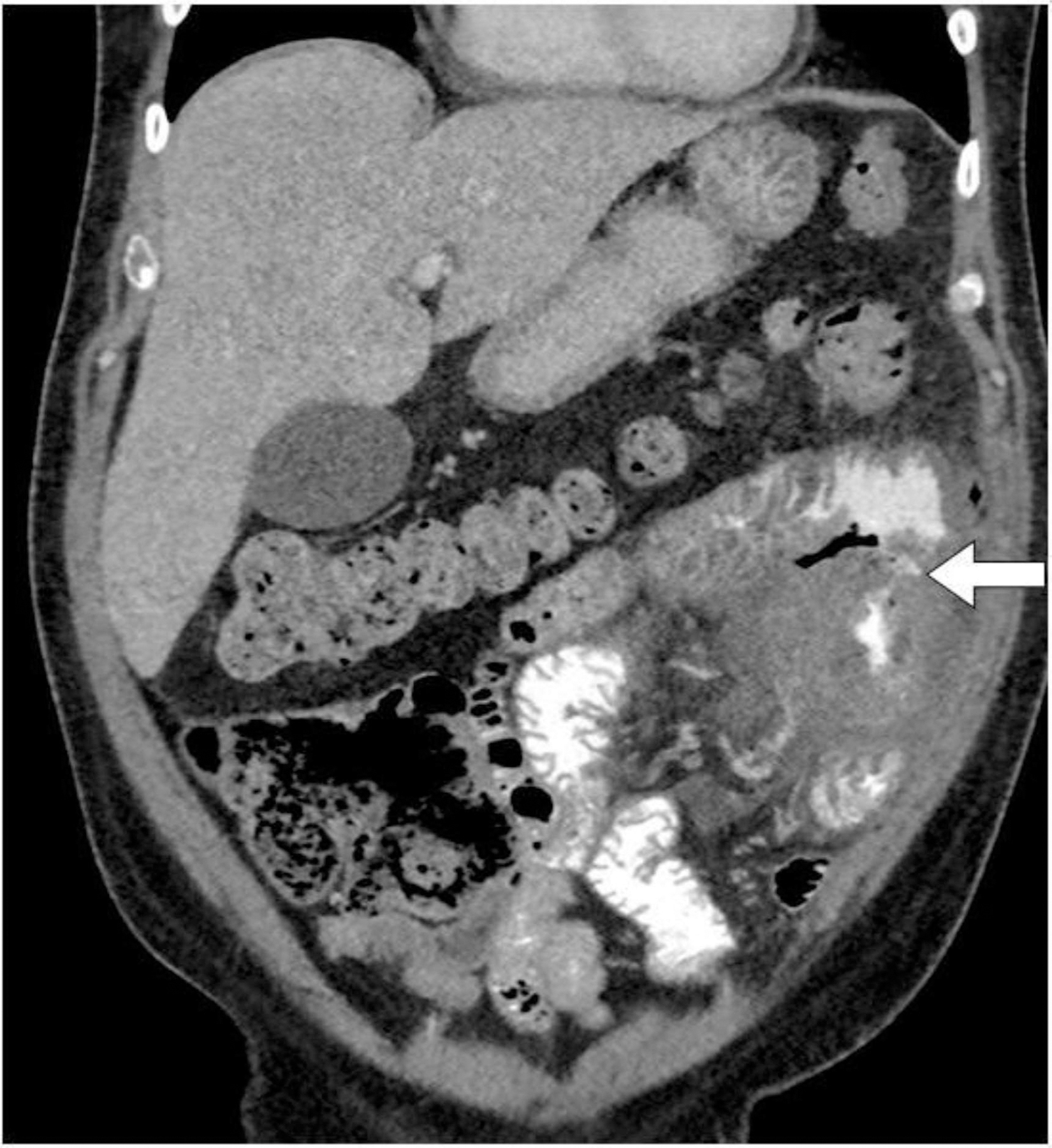
Entero-enteric fistula. Coronal CT image with intravenous and oral contrast of a patient with anaplastic large cell lymphoma of the small bowel on chemotherapy. There is an infiltrative small bowel tumor in the left upper quadrant with a fistula tract between two adjacent small bowel loops (white arrow).
Fig. 14:

Colo-splenic fistula. Coronal contrast enhanced CT (a) and coronal FDG-PET/CT (b) demonstrate enhancing and FDG-avid wall thickening in the splenic flexure and descending colon (white arrow). There is an ill-defined heterogeneously enhancing and FDG-avid soft tissue in the spleen (black arrow) with a central necrotic non-FDG-avid fluid and gas containing collection (asterisk). The necrotic splenic collection is continuous with the colonic splenic flexure consistent with perforation into the spleen (colo-splenic fistula). FDG-PET/CT also demonstrates intense uptake in the mesenteric and left para-aortic lymph nodes and left pleura. The patient was diagnosed with diffuse large B-cell lymphoma.
3. Splenic complications:
The spleen is commonly involved in both NHL and HL. On CT, splenic lymphoma appears as diffuse infiltration with or without focal hypoenhancing lesions. Marked splenomegaly and lymphadenopathy are also characteristic findings. On MR, deposits from lymphoma are hypointense on T1W and T2W images [2]. Differential diagnosis include fungal micro-abscesses, sarcoidosis, hemangioma and splenic infarcts [2].
Spontaneous splenic rupture is a rare complication of lymphoma, more common in NHL and particularly DLBCL, and occurs approximately in 0.2% of cases of lymphoma [38–40]. Symptoms include severe abdominal pain radiating to the shoulder due to phrenic nerve irritation, and signs of hypovolemic shock. Imaging findings include splenic enlargement, parenchymal hematoma, subcapsular hemorrhage, contrast extravasation and splenic infarcts (Fig 15 and Fig 16) [38]. Emergent management by splenectomy or endovascular embolization is required [39]. Neoplastic splenic rupture, including lymphoma, has worse prognosis in comparison to other non-neoplastic etiologies [40].
Fig. 15:

Splenic rupture. Axial (a) and coronal (b) CT of the abdomen and axial CT of the pelvis (c) with oral and intravenous contrast. There is massive splenomegaly with infarcts in the upper pole (white asterisk). There is peri-splenic and dependent hemoperitoneum (white arrows), indicating spontaneous splenic rupture.
Fig. 16:
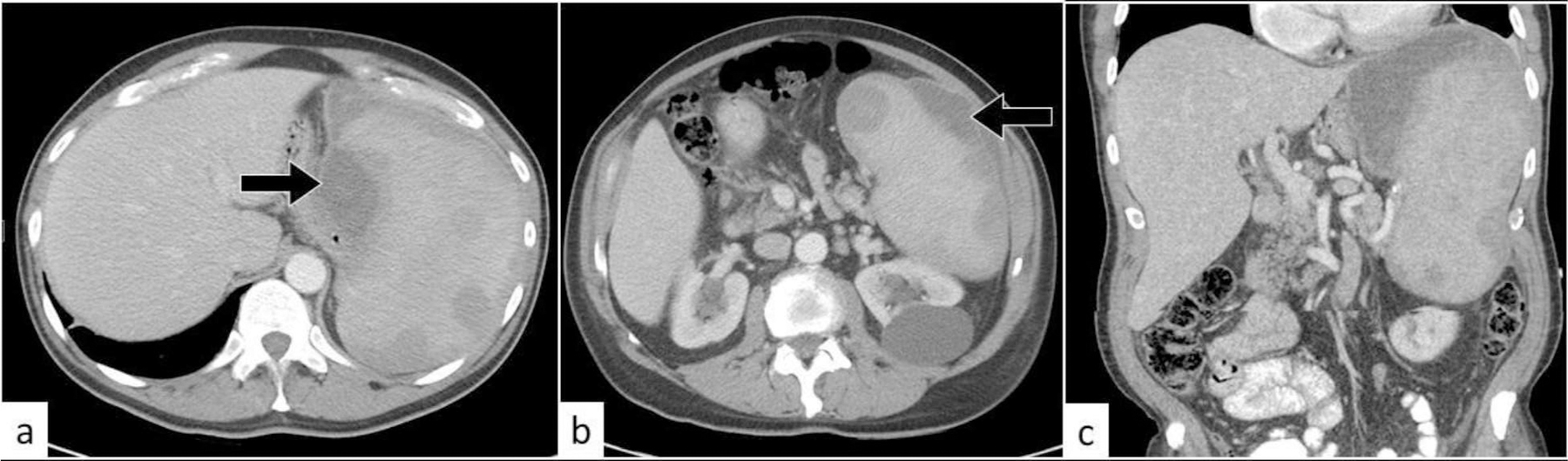
Contained splenic rupture. Axial (a, b) and coronal (c) CT of the abdomen with oral and intravenous contrast. There is splenomegaly with multiple hypodense splenic lesions. There are contained small subcapsular splenic hematomas (black arrows).
4. Hepatobiliary and pancreatic complications:
The liver can be involved in primary and secondary NHL. Primary hepatic lymphoma is rare, representing <1% of all extranodal lymphomas [41, 42]. Primary hepatic disease presents as one of the following: solitary lesion, multiple focal lesions, diffuse hepatic infiltration or periportal infiltration [2, 42]. Differential diagnosis includes hepatocellular carcinoma (HCC), metastasis, abscesses, hepatitis, and focal nodular hyperplasia. On CT, primary hepatic lymphoma typically appears as homogeneous or heterogeneous hypodense lesions, sometimes with central necrosis. Lesions can be homogeneously hypoenhancing or can show patchy or rim enhancement. Primary hepatic lymphoma tends to be more heterogeneous than secondary hepatic lymphoma [42]. Lesions are not hypervascular in the arterial phase but may show washout in the portal and delayed phase. On MRI, lesions have low signal intensity on T1WI and high signal intensity on T2WI. In contrast, HCC is an arterially hyperenhancing mass with washout in the portal and delayed phases in a background of cirrhosis [2].
Complications of lymphoma involving the hepatobiliary system include biliary obstruction and stricture formation [41, 43, 44]. Gallbladder and extrahepatic bile duct lymphomas may present with cholecystitis-like symptoms or complicated cholecystitis [45, 46]. Portal vein occlusion, pseudoaneurysm formation and hemobilia are extremely rare complications [47].
Obstructive jaundice is a rare clinical manifestation of NHL, particularly DLBCL, with an estimated prevalence of less than 1% of all malignant causes of biliary obstruction [43, 44, 48]. It usually suggests a poor prognosis [48]. Biliary duct obstruction is usually secondary to extrinsic compression by hepatic, periportal and peripancreatic lymph nodes and less commonly by primary biliary lymphoma [43, 44, 48]. Presenting symptoms include jaundice associated with dark urine and pale stool, abdominal pain, vomiting, pruritus, fever and weight loss [44]. Differential diagnosis include cholangiocarcinoma, pancreatic carcinoma, and sclerosing cholangitis. Primary biliary lymphoma should be suggested when cholangiography shows mild narrowing of the extrahepatic biliary duct without mucosal irregularity. The degree of luminal narrowing and the structure may be discrepantly milder than expected when compared to severity of stenosis on CT and MRI [49]. This may be due to lack of desmoplastic reaction in contrast to cholangiocarcinoma [49]. Imaging appearances on cholangiography reveal narrowing or complete obliteration of the CBD, presence of multiple focal narrowing and dilatation. CT or MRI shows intrahepatic and extrahepatic biliary ductal dilatation and enlarged portal, paraceliac and peripancreatic lymph nodes (Fig. 17 and Fig. 18) [9, 48].
Fig. 17:

Biliary obstruction. Coronal contrast enhanced CT images (a, b) of a patient with diffuse large B-cell lymphoma. There are multiple hypoenhancing pancreatic masses, a mass in the left hepatic lobe and submucosal hypoenhancing nodules in the gastric wall (white arrows in a). There is obstruction of the common bile duct due to pancreatic head masses with mild extra and intra hepatic biliary ductal dilatation (black arrow in a). Resolution of biliary ductal dilatation following retrievable CBD stent placement (black arrow in b) and post treatment necrosis in the pancreatic and hepatic masses (white arrows in b).
Fig. 18:

Biliary obstruction. Axial contrast enhanced CT image of a patient with B-cell lymphoma. There is an infiltrative mass (white arrow) involving the hepatic hilum, gastrohepatic region and encasing the celiac axis and portal vein confluence. The mass encases the bile duct confluence (black arrow) causing high grade biliary obstruction with severe intrahepatic biliary ductal dilatation. Note a hypodense mass in the splenic hilum.
Complications of treatment include stricture formation involving the common bile duct and portal vein occlusion, with its sequela such as portal hypertension, esophageal varices [44, 48, 50]. Endobiliary or percutaneous stent placement should be considered for biliary decompression in patients presenting with obstructive jaundice [43]. Late obstructive jaundice has worse prognosis than early presentation obstructive jaundice [43].
Pancreatic involvement by lymphoma is another rare manifestation, usually indicating advanced disease. This can be due to direct parenchymal invasion by large peri-pancreatic or retroperitoneal lymph nodes, primary pancreatic mass, or secondary extra nodal deposit. Differential diagnosis includes pancreatic adenocarcinoma, autoimmune pancreatitis, and neuroendocrine tumors. DLBCL and FL are the two most common types of PPL [51]. PPL is frequently misdiagnosed as pancreatic adenocarcinoma [52]. Fine needle aspiration is a valuable method in establishing diagnosis [52]. PPL is predominantly a focal mass or less commonly a diffuse infiltrating lesion [51]. Imaging findings of PPL reveal a focal heterogeneous mass usually at the pancreatic head typically with little or no pancreatic ductal dilatation, in contrast to primary adenocarcinoma [53]. On MR, focal pancreatic lesions show homogeneous relative low signal intensity on T1WI and T2WI and restricted diffusion. Diffuse infiltration and pancreatic enlargement can be seen. Pancreatic lymphoma may lead to obstructive jaundice or duodenal obstruction [51, 52, 54, 55]. Acute pancreatitis is also an unusual presentation of lymphoma [52, 56].
5. Genitourinary complications:
Genitourinary tract NHL lymphomas constitute around 8% of extranodal lymphomas [57]. The most common subtypes are DLBCL, MALT lymphoma and FL [57]. Among these, DLBCL has the worst outcomes [57]. The most common site of involvement is the kidneys [57].
Primary kidney lymphoma is rare (<1%). Most are NHL and particularly DLBCL [58, 59]. Differential diagnosis includes renal cell carcinoma (RCC), transitional cell carcinoma, metastatic lesions, multisystem histiocytosis such as Erdheim-Chester disease and Rosai-Dorfman disease, and retroperitoneal fibrosis [57, 59]. The most common presenting symptoms in kidney lymphoma are hematuria, abdominal or flank pain, fever, and weight loss. Renal function can be preserved, however diffuse renal infiltration by lymphomatous deposits can cause renal impairment and kidney injury secondary to infiltration of the parenchyma (intrinsic) or by obstructive uropathy (post renal) secondary to infiltration of the renal pelvis or ureters and mass effect by enlarged retroperitoneal lymph nodes (Fig. 19 and Fig. 20) [57–62]. Other renal complications include nephrotic syndrome, renal vein thrombosis, hypercalcemia induced nephropathy, amyloidosis and urolithiasis [62, 63].
Fig. 19:

Hydronephrosis. Axial (a) sagittal (b) CT image of a patient with diffuse large B-cell lymphoma. There is a homogeneous retrocaval mass (white arrow) encasing the right pelviureteric junction and causing moderate pelvicalyceal dilatation (black arrow).
Fig. 20:

Hydronephrosis and renal function impairment. Coronal T2W (a), coronal T1W fat sat post contrast (b), axial ADC map (c) MR images of a patient with diffuse large B-cell lymphoma show heterogeneous, T2 hypointense, hypoenhancing renal masses bilaterally with restricted diffusion (black arrow). The right lower pole mass causes obstruction of the calyceal system with moderate dilatation (white arrow). Axial unenhanced CT (d) following disease progression and renal function impairment shows diffuse infiltration and enlargement of the renal parenchyma bilaterally.
Imaging findings of renal involvement include multiple bilateral parenchymal nodules or masses (50–60%) with preserved renal architecture, solitary renal mass (10–25%), diffuse parenchymal infiltration and renal enlargement, renal sinuses involvement without hydronephrosis, peri-renal lesions as well as large retroperitoneal lymph nodes and masses [61]. Lesions can progressively increase in size and coalesce leading to renal enlargement and architectural distortion and deformation of the collecting system. Perirenal lesions can invade the ureter, renal vasculature at the hilum and other retroperitoneal structures [59]. On CT, lymphomatous deposits are hyperdense on precontrast and enhance less than normal renal parenchyma. On MR, they have low to intermediate signal intensity on T1WI and T2WI and high signal on diffusion weighted images. On PET, renal lymphomas are intensely FDG-avid and can be distinguished from RCC. Correlation with contrast enhanced CT is helpful for detection of small focal lesions [57, 59]. CT urography can show delayed excretion secondary to renal impairment and nephrographic irregularities. Other imaging findings are hydronephrosis secondary to sinus infiltration or ureteral infiltration and thickening of Gerota’s fascia.
Urinary tract obstruction occurs in approximately 2% of NHL cases [9, 60]. There are reports in the literature on renal pelvicalyceal rupture secondary to lymphoma. Imaging findings include retroperitoneal free fluid or fluid collection and urine leak on delayed-phase imaging. It is managed conservatively with ureteral stent placement [64]. Fistula formation in the pelvis, such as vesicovaginal fistula, is a rare reported complication of bladder or genital lymphoma [65]. Ureteric involvement with lymphoma is rare. The involvement pattern is either peri-ureteric, transmural or submucosal. Obstruction of the urinary tract if present is usually mild [66].
Involvement of the bladder by lymphoma is rare, and can be primary (0.2%) or secondary due to advanced disease (1.8%) [67]. .The most common subtypes are MALT lymphoma followed by DLBCL [68, 69].TCL is extremely rare [57, 67]. Presenting symptoms are hematuria, dysuria, urinary frequency, nocturia, recurrent urinary tract infections and abdominal and flank pain [57, 67–69]. Bladder lymphoma can rarely present with oliguria, bilateral ureteral obstruction causing bilateral hydronephrosis and post obstructive renal failure [67, 69]. Imaging findings show irregular bladder wall thickening, bladder mass and pelvic lymphadenopathy (Fig. 21) [67]. The appearance may mimic other urothelial cancers [67].
Fig. 21:

Bilateral hydronephrosis and double-J stent placement. Axial contrast enhanced CT images (a, b) of a patient with bladder marginal zone lymphoma. There is a mass in the base and left posterolateral bladder wall (black arrow) infiltrates the vesicoureteric junctions and causes moderate bilateral hydronephrosis, more so on the left (white arrow). Unenhanced axial CT image (c) following bilateral double-J stent placement.
NHL of the genital tract is rare with the most common site involved being the ovaries [70]. Primary ovarian lymphoma constitutes 0.5% of extranodal NHL [71]. The most common subtypes are DLBCL, FL and Burkitt lymphoma [70, 71]. Presenting symptoms can include ascites, abdominal distention, pelvic pain, menstrual irregularities, and B-symptoms. Differential diagnosis includes primary ovarian carcinoma. Imaging findings can show bilateral large adnexal masses, peritoneal deposits, and abdominal and retroperitoneal lymphadenopathy [71, 72]. Complications include ovarian torsion [72] and obstructive hydronephrosis [73]. Mainstay of treatment is chemotherapy, and debulking surgery can be avoided if diagnosis is made preoperatively (Fig. 22) [71].
Fig. 22:

Ovarian lymphoma with ascites diagnosed by percutaneous biopsy. Axial contrast enhanced CT image (a) of a 14-year-old female shows a large solid enhancing right ovarian mass (black arrow) with few foci of necrosis, and ascites (white asterisk). CT-guided percutaneous needle biopsy (b) results revealed diffuse large B-cell lymphoma.
Primary lymphoma of the cervix is rare, occurring in only 0.2% of extranodal lymphomas. Differential diagnosis includes squamous cell carcinomas of the genitourinary tract. Abnormal vaginal bleeding is the most common presenting symptom. It can also lead to ureteral obstruction and can present with bilateral hydronephrosis and kidney impairment. Imaging findings can include distorted and enlarged uterus, irregular bladder wall thickening, and hydronephrosis. Obstructive symptoms are treated with ureteral stenting and insertion of nephrostomy tubes. Prognosis is usually favorable, and treatment options include hysterectomy, radiation, and chemotherapy [74].
Testicular lymphoma may be primary or secondary. Primary testicular lymphoma is uncommon, accounting for <5% of testicular malignancy and 1–2% of NHL [75]. Lymphoma may relapse in the testicles. Testicular lymphoma is the most common malignancy in men older than 60 years [76]. DLBCL is the most common type [75]. They may present with testicular mass, B symptoms, hematospermia and clinical features of extra testicular involvement [75, 77]. Hydrocele occurs in 40% of cases [75]. They appear as hypoechoic testicular masses on ultrasound with increased vascularity (Fig. 23) [76, 78]. Testicular lymphoma infiltrates through the tubules, preserving the normal vascular architecture of the testis [78]. Spread along the spermatic cord and gonadal veins into the retroperitoneum is a feature of testicular lymphoma (Fig. 23) [79].
Fig. 23:

Hematospermia, hydrocele, and retroperitoneal extension. Longitudinal ultrasound (a) and axial FDG-PET/CT (b) of a patient with high-grade B-cell lymphoma showing a hypoechoic testicular mass (black arrow in a) and mild hydrocele. The mass is intensely FDG-avid (black arrow in b). Maximum intensity projection of FDG-PET (c) and coronally reconstructed FDG-PET/CT (d) of a different patient presented with large left scrotal mass and hematospermia. There is a large intensely FDG-avid left scrotal mass (black arrow in c) extending to suprarenal retroperitoneal space (white arrow in d). Note FDG-avid lesions in the right scrotum, kidneys and supra and infra diaphragmatic lymph nodes. Biopsy of the left supraclavicular lymph node confirmed diffuse large B-cell lymphoma.
6. Adrenal complications:
Primary adrenal lymphoma constitutes less than 1% of NHL. DLBCL is the most common subtype in primary adrenal lymphoma [80–82]. Presenting symptoms can be nonspecific, with deteriorating general state, weight loss and abdominal pain [82, 83]. Signs and symptoms of adrenal insufficiency are common and can be seen in 50–70-% of cases [84], especially when the disease involves both adrenal glands [80–82]. Differential diagnosis of bilateral adrenal masses include adrenal metastasis, adrenocortical carcinoma, pheochromocytoma and adrenal hemorrhage [80, 84]. On CT, bilateral adrenal masses are seen in 70% of cases, lesions are bilateral, large and can appear homogenous or heterogenous with variable CT density (Fig. 24)[83]. On MRI, lesions show low signal on T1WI and intermediate to high signal on T2WI [80, 81, 83, 84]. Immediate glucocorticoid therapy is required when adrenal insufficiency is suspected. Chemotherapy is usually needed for definitive treatment [84]. Adrenal lymphoma may lead to spontaneous adrenal hemorrhage [85].
Fig. 24:

Adrenal insufficiency. Coronal contrast enhanced CT image of the abdomen. There are large bilateral homogeneously enhancing adrenal masses (black arrows) due to involvement by large B-cell lymphoma, in a patient presenting with features of adrenal insufficiency.
7. Peritoneal complications:
Peritoneal lymphomatosis (PL) is a rare presentation of extranodal lymphomas and is usually secondary to high grade NHL. Primary peritoneal lymphoma is less frequent [86]. The most common subtype for extranodal involvement of the peritoneal cavity is DLBCL and less commonly Burkitt lymphoma [86, 87]. It is characterized by diffuse peritoneal thickening, extensive infiltration with multiple nodules and mesenteric masses, with or without ascites [87]. Differential diagnosis includes peritoneal carcinomatosis, peritoneal mesotheliomas, tuberculous peritonitis, sarcomatosis, diffuse peritoneal leiomyomatosis and benign splenosis [86]. Presenting symptoms can be nonspecific and include fatigue, fever, chills, night sweats, weight loss, abdominal pain, abdominal distention and ascites [86, 87]. Imaging appearance of PL is characterized by homogeneous enhancing nodular thickening of the peritoneum with ascites (Fig. 25) [87]. Additional signs include extensive abdominal and retroperitoneal lymphadenopathy, large confluent adenopathic masses without significant necrosis with coating of the mesenteric vessels “sandwich sign” and splenomegaly [87]. The mainstay of treatment is systemic chemotherapy without surgery in contrast to peritoneal carcinomatosis [86, 87].
Fig. 25:

Peritoneal lymphomatosis with ascites. Axial contrast enhanced CT image shows lymphomatous peritoneal nodules and masses (black arrow) with moderate volume ascites.
8. Vascular complications:
Lymphoma is a risk factor for hypercoagulable state and increases the risk of bland venous thromboembolism [88]. Tumor thrombus is a rare complication of lymphoma, which results from direct extension of either an adjacent primary or extranodal lymphomatous mass [89]. Tumor thrombus more commonly occurs in other primary solid malignancies such as HCC, RCC and adrenocortical carcinoma. The IVC and portal vein are most commonly involved [89]. The most common subtype is DLBCL, followed by FL), mixed DLBCL/FL and HL. TCL and HIV-associated plasmacytic lymphoma can also be seen [89]. Imaging appearance usually consists of a large infiltrative mass with secondary extension into the adjacent vessel and tumor thrombus. In contrast to bland thrombus, In contrast to bland thrombus, tumor thrombus shows enhancement and FDG-avidity similar to that of primary nodal or extranodal disease (Fig. 26, Fig. 27 and Fig. 28) [9, 89]. It is important to recognize this complication in patients with extranodal lymphomatous involvement and establish the correct diagnosis to direct treatment accordingly. Tumor thrombus from lymphoma is treated with systemic chemotherapy, in contrast to surgery such as nephrectomy in case of RCC [89].
Fig. 26:

Bland venous thrombus. Axial contrast enhanced CT image of the abdomen of a patient with diffuse large B-cell lymphoma. There are hypoenhancing masses in the pancreatic head and both kidneys with a non-occlusive bland thrombus in the inferior vena cava (black arrow).
Fig. 27:
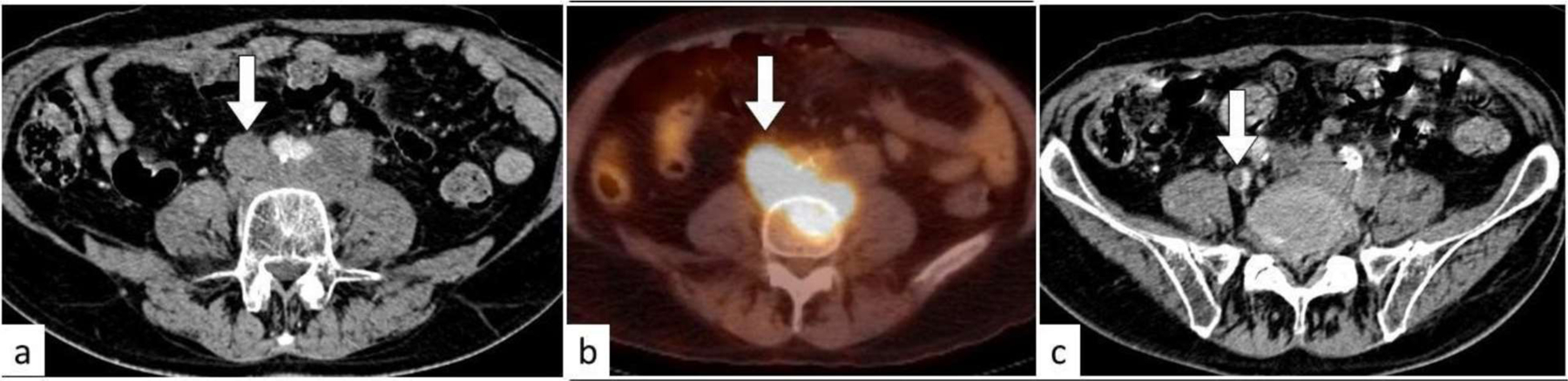
Tumor thrombus. On the axial contrast enhanced CT image of the lower abdomen (a), there are enlarged retroperitoneal lymph nodes and enhancing thrombus (white arrow) expanding the inferior vena cava. The thrombus is FDG-avid on PET/CT (b). On the axial contrast-enhanced CT image of the pelvis (c), the thrombus (white arrow) extends in the right common iliac vein partially occluding the lumen.
Fig 28:

Tumor thrombus. Axial (a) and coronal (b) contrast enhanced CT images of a patient with diffuse large B-cell lymphoma. There is an enhancing tumor thrombus (white arrow) in the left femoral vein extending to the tributaries showing similar enhancing density of the conglomerate left inguinal adenopathy. There is a contiguous bland thrombus more distally (black arrow) with no enhancement. The left lower extremity is edematous and swollen. Note in addition the periaortic adenopathy.
Lymphomatous masses in the abdomen and pelvis may cause external compression on the veins and result in dilated venous collaterals and varices [90, 91]. Gastric varices may be present with upper gastrointestinal bleeding due to occlusion of the splenic vein (Fig. 29) [90, 91]. A rare vascular complication of aggressive lymphomas is arterial invasion and formation of pseudoaneurysm which may present with hemorrhage [90, 92].
Fig. 29:

Gastric varices. Axial contrast enhanced CT (a) and FDG-PET/CT (b) of a patient with diffuse large B-cell lymphoma. There is an FDG-avid tumor in the third part of the duodenum (black asterisk) with retroperitoneal adenopathy causing occlusion of the splenic vein (white arrow). Axial contrast enhanced CT (c, d) after disease remission showing chronic occlusion of the splenic vein (white arrow) with formation of gastric varices (black arrow).
In conclusion, lymphoma in the abdomen and pelvis may lead to various complications. Imaging is essential in evaluation of disease extent and diagnosis of complications. We hope this review paper provides a guide for radiologists to better understand and interpret the complications of lymphoma in the abdomen and pelvis.
Funding:
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Statements and Declarations:
Conflict of interest:
The author declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics committee approval:
Ethics approval was not required for this review paper.
Informed consent:
Informed consent was not applicable since the manuscript does not contain any patient data.
Contributor Information
Mihran Khdhir, Department of diagnostic radiology, American University of Beirut Medical Center, Riad El-Solh 1107 2020, PO Box 11-0236, Beirut, Lebanon.
Tamara El Annan, Department of diagnostic radiology, American University of Beirut Medical Center, Beirut Lebanon..
Mohammad Ali El Amine, Department of radiology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Muhammed Shareef, Department of radiology and biomedical imaging, Yale New Haven Hospital, New Haven, CT, USA.
References:
- 1.Lewis RB, et al. From the radiologic pathology archives: gastrointestinal lymphoma: radiologic and pathologic findings. Radiographics, 2014. 34(7): p. 1934–1953. [DOI] [PubMed] [Google Scholar]
- 2.Hedgire SS, et al. Extranodal lymphomas of abdomen and pelvis: imaging findings and differential diagnosis. Abdominal Radiology, 2017. 42(4): p. 1096–1112. [DOI] [PubMed] [Google Scholar]
- 3.Ghai S, et al. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. Radiographics, 2007. 27(5): p. 1371–1388. [DOI] [PubMed] [Google Scholar]
- 4.Spectre G, et al. Bleeding, obstruction, and perforation in a series of patients with aggressive gastric lymphoma treated with primary chemotherapy. Annals of surgical oncology, 2006. 13(11): p. 1372–1378. [DOI] [PubMed] [Google Scholar]
- 5.Kadota T, et al. Complications and outcomes in diffuse large B-cell lymphoma with gastric lesions treated with R-CHOP. Cancer medicine, 2019. 8(3): p. 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couto ME, et al. Gastric Diffuse Large B-Cell Lymphoma: A Single-Center 9-Year Experience. Indian Journal of Hematology and Blood Transfusion, 2021. 37(3): p. 492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Y, et al. Influence of Severe Gastrointestinal Complications in Primary Gastrointestinal Diffuse Large B-Cell Lymphoma. Cancer Management and Research, 2021. 13: p. 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matysiak-Budnik T, et al. Gastric MALT lymphoma in a population-based study in France: clinical features, treatments and survival. Alimentary pharmacology & therapeutics, 2019. 50(6): p. 654–663. [DOI] [PubMed] [Google Scholar]
- 9.Morani AC, et al. Imaging of acute abdomen in cancer patients. Abdominal Radiology, 2020. 45(8): p. 2287–2304. [DOI] [PubMed] [Google Scholar]
- 10.Guniganti P, et al. CT of gastric emergencies. Radiographics, 2015. 35(7): p. 1909–1921. [DOI] [PubMed] [Google Scholar]
- 11.Guglielmo FF, et al. Gastrointestinal bleeding at CT angiography and CT enterography: imaging atlas and glossary of terms. RadioGraphics, 2021. 41(6): p. 1632–1656. [DOI] [PubMed] [Google Scholar]
- 12.Stanley AJ and Laine L, Management of acute upper gastrointestinal bleeding. Bmj, 2019. 364. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y, et al. Safety and efficacy of low-dose pre-phase before conventional-dose chemotherapy for ulcerative gastric diffuse large B-cell lymphoma. Leukemia & Lymphoma, 2015. 56(9): p. 2613–2618. [DOI] [PubMed] [Google Scholar]
- 14.Palmowski M, et al. Large gastrosplenic fistula after effective treatment of abdominal diffuse large-B-cell lymphoma. Annals of hematology, 2008. 87(4): p. 337–338. [DOI] [PubMed] [Google Scholar]
- 15.Sousa M, et al. Massive gastrointestinal bleeding after chemotherapy for gastric lymphoma. International journal of surgery case reports, 2016. 21: p. 41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerem M, et al. Spontaneous gastrosplenic fistula in primary gastric lymphoma: Surgical management. Asian journal of surgery, 2006. 29(4): p. 287–290. [DOI] [PubMed] [Google Scholar]
- 17.Saito M, et al. Successful Treatment of Gastrosplenic Fistula Arising from Diffuse Large B-Cell Lymphoma with Chemotherapy: Two Case Reports. Case Reports in Oncology, 2019. 12(2): p. 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warburton C and Calverley P, Gastropleural fistula due to gastric lymphoma presenting as tension pneumothorax and empyema. European Respiratory Journal, 1997. 10(7): p. 1678–1679. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez Turizo MJ, et al. Primary diffuse large B-cell lymphoma presenting as acute appendicitis: A report of 2 cases and a literature review. Clinical case reports, 2020. 8(2): p. 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SJ, et al. Multicenter retrospective analysis of 581 patients with primary intestinal non-hodgkin lymphoma from the Consortium for Improving Survival of Lymphoma (CISL). BMC cancer, 2011. 11(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zinzani PL, et al. Primary intestinal lymphoma: clinical and therapeutic features of 32 patients. Haematologica, 1997. 82(3): p. 305–308. [PubMed] [Google Scholar]
- 22.Nakamura S, et al. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer: Interdisciplinary International Journal of the American Cancer Society, 2003. 97(10): p. 2462–2473. [DOI] [PubMed] [Google Scholar]
- 23.Gou H-F, et al. Clinical prognostic analysis of 116 patients with primary intestinal non-Hodgkin lymphoma. Medical Oncology, 2012. 29(1): p. 227–234. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, et al. Primary colorectal lymphoma: spectrum of imaging findings with pathologic correlation. European radiology, 2002. 12(9): p. 2242–2249. [DOI] [PubMed] [Google Scholar]
- 25.Aoki T, et al. Development and internal validation of a risk scoring system for gastrointestinal events requiring surgery in gastrointestinal lymphoma patients. Journal of Gastroenterology and Hepatology, 2019. 34(4): p. 693–699. [DOI] [PubMed] [Google Scholar]
- 26.Abbott S, Nikolousis E, and Badger I, Intestinal lymphoma—a review of the management of emergency presentations to the general surgeon. International journal of colorectal disease, 2015. 30(2): p. 151–157. [DOI] [PubMed] [Google Scholar]
- 27.Gupta H, et al. Clinical implications and surgical management of intussusception in pediatric patients with Burkitt lymphoma. Journal of pediatric surgery, 2007. 42(6): p. 998–1001. [DOI] [PubMed] [Google Scholar]
- 28.England R, et al. Intussusception as a presenting feature of Burkitt lymphoma: implications for management and outcome. Pediatric surgery international, 2012. 28(3): p. 267–270. [DOI] [PubMed] [Google Scholar]
- 29.Kerr JP, et al. Intestinal strictures: a new complication of treatment for primary gastrointestinal diffuse large B-cell lymphoma. British journal of haematology, 2008. 140(6): p. 712–714. [DOI] [PubMed] [Google Scholar]
- 30.Vaidya R, et al. Bowel perforation in intestinal lymphoma: incidence and clinical features. Annals of oncology, 2013. 24(9): p. 2439–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarid N, et al. CT findings are highly predictive for perforation in patients with diffuse large B-cell lymphoma involving the intestines. Leukemia & lymphoma, 2018. 59(8): p. 1878–1883. [DOI] [PubMed] [Google Scholar]
- 32.Hong Y-W, et al. The role of surgical management in primary small bowel lymphoma: A single-center experience. European Journal of Surgical Oncology (EJSO), 2017. 43(10): p. 1886–1893. [DOI] [PubMed] [Google Scholar]
- 33.Tresoldi S, et al. Adult intestinal intussusception: can abdominal MDCT distinguish an intussusception caused by a lead point? Abdominal imaging, 2008. 33(5): p. 582–588. [DOI] [PubMed] [Google Scholar]
- 34.Zhao R-J, et al. Enteral fistula as initial manifestation of primary intestinal lymphoma. Chinese medical journal, 2020. 133(1): p. 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Zahir AA and Meshikhes A-WN, Colonic lymphoma presenting acutely with perforated colo-splenic fistula. International journal of surgery case reports, 2012. 3(8): p. 368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shulik O, Marling K, and Butler J, Primary aorto-enteric fistula–a unique complication of poorly differentiated large B-cell lymphoma. The American Journal of Case Reports, 2013. 14: p. 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuang N, et al. Rare intestinal fistula caused by primary lymphoma of the gastrointestinal tract: two case reports and literature review. Medicine, 2018. 97(27). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaniappan K, Lim CTS, and Chin PW, Non-traumatic splenic rupture-a rare first presentation of diffuse large B-cell lymphoma and a review of the literature. BMC cancer, 2018. 18(1): p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amaki J, et al. Three cases of spontaneous splenic rupture in malignant lymphoma. International journal of hematology, 2018. 108(6): p. 647–651. [DOI] [PubMed] [Google Scholar]
- 40.Renzulli P, et al. Systematic review of atraumatic splenic rupture. Journal of British Surgery, 2009. 96(10): p. 1114–1121. [DOI] [PubMed] [Google Scholar]
- 41.Ugurluer G, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors, 2016. 8(3): p. 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajesh S, et al. The imaging conundrum of hepatic lymphoma revisited. Insights into imaging, 2015. 6(6): p. 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross WA, et al. Outcomes in lymphoma patients with obstructive jaundice: a cancer center experience. Digestive diseases and sciences, 2010. 55(11): p. 3271–3277. [DOI] [PubMed] [Google Scholar]
- 44.Ravindra K, et al. Non-Hodgkin lymphoma presenting with obstructive jaundice. Journal of British Surgery, 2003. 90(7): p. 845–849. [DOI] [PubMed] [Google Scholar]
- 45.Mani H, et al. Gall bladder and extrahepatic bile duct lymphomas: clinicopathological observations and biological implications. The American journal of surgical pathology, 2010. 34(9): p. 1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah K, et al. Primary gallbladder lymphoma presenting with perforated cholecystitis and hyperamylasaemia. The Annals of The Royal College of Surgeons of England, 2016. 98(2): p. e13–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walton H, et al. Portal vein pseudoaneurysm secondary to pancreatic lymphoma and biliary stent insertion: a rare cause of haemobilia. CVIR endovascular, 2018. 1(1): p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ödemiş B, et al. Biliary tract obstruction secondary to malignant lymphoma: experience at a referral center. Digestive diseases and sciences, 2007. 52(9): p. 2323–2332. [DOI] [PubMed] [Google Scholar]
- 49.Yoon MA, et al. Primary biliary lymphoma mimicking cholangiocarcinoma: a characteristic feature of discrepant CT and direct cholangiography findings. Journal of Korean medical science, 2009. 24(5): p. 956–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts K, et al. Biliary and portal vein strictures following treatment of Hodgkin’s lymphoma. The Annals of The Royal College of Surgeons of England, 2012. 94(7): p. e18–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadot E, et al. Clinical features and outcome of primary pancreatic lymphoma. Annals of surgical oncology, 2015. 22(4): p. 1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du X, et al. Primary pancreatic lymphoma: a clinical quandary of diagnosis and treatment. Pancreas, 2011. 40(1): p. 30–36. [DOI] [PubMed] [Google Scholar]
- 53.Ramesh J, et al. Frequency of occurrence and characteristics of primary pancreatic lymphoma during endoscopic ultrasound guided fine needle aspiration: a retrospective study. Digestive and Liver Disease, 2014. 46(5): p. 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boninsegna E, et al. CT imaging of primary pancreatic lymphoma: experience from three referral centres for pancreatic diseases. Insights into imaging, 2018. 9(1): p. 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Facchinelli D, et al. Primary pancreatic lymphoma: clinical presentation, diagnosis, treatment, and outcome. European journal of haematology, 2020. 105(4): p. 468–475. [DOI] [PubMed] [Google Scholar]
- 56.Amodio J and Brodsky JE, Pediatric Burkitt lymphoma presenting as acute pancreatitis: MRI characteristics. Pediatric radiology, 2010. 40(5): p. 770–772. [DOI] [PubMed] [Google Scholar]
- 57.Lontos K, et al. Primary urinary tract lymphoma: rare but aggressive. Anticancer research, 2017. 37(12): p. 6989–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, et al. Primary renal lymphoma: a population-based study in the United States, 1980–2013. Scientific reports, 2019. 9(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Purysko AS, et al. Imaging manifestations of hematologic diseases with renal and perinephric involvement. Radiographics, 2016. 36(4): p. 1038–1054. [DOI] [PubMed] [Google Scholar]
- 60.Da’as N, et al. Kidney involvement and renal manifestations in non-Hodgkin’s lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. European journal of haematology, 2001. 67(3): p. 158–164. [DOI] [PubMed] [Google Scholar]
- 61.Sheth S, Ali S, and Fishman E, Imaging of renal lymphoma: patterns of disease with pathologic correlation. Radiographics, 2006. 26(4): p. 1151–1168. [DOI] [PubMed] [Google Scholar]
- 62.Corlu L, et al. Renal dysfunction in patients with direct infiltration by B-cell lymphoma. Kidney International Reports, 2019. 4(5): p. 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SW, et al. Urolithiasis in patients suffering from malignant hematologic diseases. Yonsei Medical Journal, 2010. 51(2): p. 244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrari D, et al. Recurrent MALT lymphoma presenting as renal calyceal-pelvic rupture. What is the cause of this break? Radiology case reports, 2019. 14(8): p. 1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans DA and Moore AT, The first case of vesico-vaginal fistula in a patient with primary lymphoma of the bladder–a case report. Journal of Medical Case Reports, 2007. 1(1): p. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghersin E, et al. Multimodality imaging of direct ureteric involvement in non-Hodgkin’s lymphoma using PET/CT, CT urography and antegrade CT pyelography. The British journal of radiology, 2007. 80(959): p. e283–e286. [DOI] [PubMed] [Google Scholar]
- 67.Kawaguchi Y, et al. Malignant lymphoma of the bladder with bilateral hydronephrosis. Rare tumors, 2019. 11: p. 2036361318825165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hughes M, Morrison A, and Jackson R, Primary bladder lymphoma: management and outcome of 12 patients with a review of the literature. Leukemia & lymphoma, 2005. 46(6): p. 873–877. [DOI] [PubMed] [Google Scholar]
- 69.Bates A, Norton A, and Baithun S, Malignant lymphoma of the urinary bladder: a clinicopathological study of 11 cases. Journal of clinical pathology, 2000. 53(6): p. 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nasioudis D, et al. Primary lymphoma of the female genital tract: an analysis of 697 cases. Gynecologic Oncology, 2017. 145(2): p. 305–309. [DOI] [PubMed] [Google Scholar]
- 71.Shokralla HA, Fathalla AE, and Sidhom NF, Primary Ovarian Non-Hodgkin’s Lymphoma: Retrospective Study of 16 Patients. Journal of Cancer Therapy, 2016. 7(01): p. 55. [Google Scholar]
- 72.Taylor JS, et al. Burkitt’s lymphoma presenting as ovarian torsion. American journal of obstetrics and gynecology, 2012. 207(2): p. e4–e6. [DOI] [PubMed] [Google Scholar]
- 73.Volpi E, et al. Ovarian lymphoma and hydronephrosis. JSLS: Journal of the Society of Laparoendoscopic Surgeons, 2013. 17(4): p. 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Novotny S, Ellis T, and Stephens J, Primary B-cell lymphoma of the cervix presenting with bilateral hydronephrosis. Obstetrics & Gynecology, 2011. 117(2): p. 444–446. [DOI] [PubMed] [Google Scholar]
- 75.Cheah CY, Wirth A, and Seymour JF, Primary testicular lymphoma. Blood, The Journal of the American Society of Hematology, 2014. 123(4): p. 486–493. [DOI] [PubMed] [Google Scholar]
- 76.Coursey Moreno C, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. Radiographics, 2015. 35(2): p. 400–415. [DOI] [PubMed] [Google Scholar]
- 77.Mittal PK, et al. Hematospermia evaluation at MR imaging. Radiographics, 2016. 36(5): p. 1373–1389. [DOI] [PubMed] [Google Scholar]
- 78.Bertolotto M, et al. Grayscale and color Doppler features of testicular lymphoma. Journal of Ultrasound in Medicine, 2015. 34(6): p. 1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sabarwal KS and Ismail EHM, A case of primary testicular lymphoma with continuous spread along the gonadal vein and spermatic cord. BJR| case reports, 2019. 5(1): p. 20180063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laurent C, et al. Adrenal lymphoma: presentation, management and prognosis. QJM: An International Journal of Medicine, 2017. 110(2): p. 103–109. [DOI] [PubMed] [Google Scholar]
- 81.Rashidi A and Fisher SI, Primary adrenal lymphoma: a systematic review. Annals of hematology, 2013. 92(12): p. 1583–1593. [DOI] [PubMed] [Google Scholar]
- 82.Majidi F, et al. Clinical spectrum of primary adrenal lymphoma: results of a multicenter cohort study. European journal of endocrinology, 2020. 183(4): p. 453–462. [DOI] [PubMed] [Google Scholar]
- 83.Zhou L, et al. Primary adrenal lymphoma: radiological; pathological, clinical correlation. European journal of radiology, 2012. 81(3): p. 401–405. [DOI] [PubMed] [Google Scholar]
- 84.Karimi F, Primary adrenal lymphoma presenting with adrenal failure: a case report and review of the literature. International journal of endocrinology and metabolism, 2017. 15(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arslan S, et al. Imaging findings of spontaneous intraabdominal hemorrhage: neoplastic and non-neoplastic causes. Abdominal Radiology, 2022: p. 1–30. [DOI] [PubMed]
- 86.Flores E, et al. A case series of diffuse large B-cell lymphoma and burkitt lymphoma presenting with peritoneal lymphomatosis. International journal of surgery case reports, 2016. 28: p. 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karaosmanoglu D, et al. CT findings of lymphoma with peritoneal, omental and mesenteric involvement: peritoneal lymphomatosis. European journal of radiology, 2009. 71(2): p. 313–317. [DOI] [PubMed] [Google Scholar]
- 88.Mahajan A, et al. Lymphoma and venous thromboembolism: influence on mortality. Thrombosis research, 2014. 133: p. S23–S28. [DOI] [PubMed] [Google Scholar]
- 89.Chauhan A, et al. Tumor thrombus as a rare presentation of lymphoma: a case series of 14 patients. American Journal of Roentgenology, 2015. 204(4): p. W398–W404. [DOI] [PubMed] [Google Scholar]
- 90.Shih I-L, et al. Retroperitoneal follicular lymphoma presenting with gastric varices and splenic artery pseudoaneurysm. Annals of hematology, 2013. 92(9): p. 1283. [DOI] [PubMed] [Google Scholar]
- 91.Chen B-C, et al. Isolated gastric variceal bleeding caused by splenic lymphoma-associated splenic vein occlusion. World Journal of Gastroenterology: WJG, 2013. 19(40): p. 6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Masamoto Y, et al. A case report of non-traumatic renal artery pseudoaneurysm due to chemotherapy for diffuse large B-cell lymphoma. Annals of hematology, 2010. 89(1): p. 107–108. [DOI] [PubMed] [Google Scholar]


