Abstract
Introduction and importance
Liver abscesses in neonates are rare. Omphalitis is a very rare cause. We report a case of a voluminous neonatal pyogenic liver abscess following omphalitis, successfully managed in our institution.
Case presentation
A 21-day-old full-term female newborn was brought to our institution for progressive febrile swelling of the right hypochondrium. The parents reported umbilical suppuration. Clinical examination confirmed the presence of a mass extending from the epigastric region to the right hypochondrium in a febrile baby with no other abnormalities.
Laboratory investigations revealed an elevated C-reactive protein level (64 mg/dl) and hyperleukocytosis (20,800/mm3) with neutrophil predominance and normochromic microcytic anemia (hemoglobin 8.2 g/dl).
Her first abdominal ultrasound was interpreted as a cyst of the common bile duct.
Triple antibiotic therapy with cephalosporin, ampicillin and gentamycin was started, but unsuccessful. Abdominal ultrasound was repeated, revealing a hepatic abscess in segment 8, with a volume of approximately 17 ml. Percutaneous echo-guided drainage was performed and antibiotic therapy was readjusted after identification of the germ (Staphylococcus aureus) with good outcome. The baby was discharged two weeks later. At one month follow-up, the baby was completely asymptomatic.
Clinical discussion and conclusion
As neonatal liver abscesses are unusual, they can lead to misdiagnosis. Omphalitis is a very rare cause. Treatment by percutaneous echo-guided drainage is simple and effective.
Keywords: Neonatal pyogenic liver abscess, Omphalitis, Ultrasound, Diagnosis, Percutaneous drainage, Case report
Highlights
-
•
Liver abscesses are uncommon in newborns.
-
•
Omphalitis is a very rare cause.
-
•
Abdominal ultrasound is the gold standard for the diagnosis, but it can be challenging.
-
•
Percutaneous ultrasound-guided drainage in neonates, rigorous antibiotherapy can produce satisfactory results.
1. Introduction
Pyogenic liver abscess is an unusual infection in neonates [[1], [2], [3], [4]]. It is a severe and potentially life-threatening infection, and remains a major cause of mortality [[1], [2], [3]]. Indeed, liver abscess in neonates can be difficult to diagnose and manage, partly due to its rarity and difficulties of clinical suspicion, but it can also present as sepsis with several other localizations and can therefore be missed if not actively searched for in neonates [[4], [5], [6]].
Pyogenic liver abscesses are generally caused by bacteria, the origin of which varies worldwide. In Western countries, ascending infection via umbilical vein catheters is the most common, while infections associated with poor hygiene and septicemia are most frequent in developing countries [2,3,6,7].
Neonatal pyogenic liver abscess (NPLA) following umbilical cord infection is very rarely reported [6,7].
We report a case of NPLA following omphalitis in a 21-day-old new-born successfully managed in our institution. Our aim is to present the etiopathogenic approach, diagnostic difficulties and management. This case report was presented using the SCARE 2020 guidelines [8].
2. Case presentation
The female newborn was born from a 28-year-old multiparous mother with no particular pathological history (maternal infection, maternal diabetes). The pregnancy was well monitored. No pathology diagnosed antenatally. No family history of congenital malformations. She was born by vaginal delivery at 39 gestational weeks in a district health center, with good extrauterine adaptation. APGAR 10-10-10. Birth weight 4800 g. Immediate evolution was good and maternal breastfeeding started immediately. She was discharged at day 2 after a traditional ligature and umbilical cord section. Umbilical cord care was then continued at home by the parents.
On day 17, the child was admitted to the hospital with a progressive painful swelling of the right hypochondrium that had been developing for one week. There was no rhinorrhea, cough, diarrhea, or vomiting. The parents reported umbilical suppuration on the fifth day of life at home.
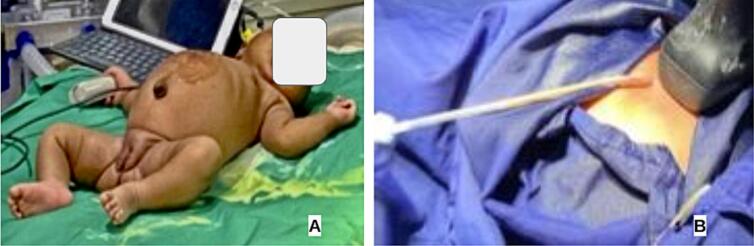
Clinically, the baby was irritable, pale, and febrile with an inflammatory mass extending from the epigastric region to the right hypochondrium (Fig. 1).
Fig. 1.
Clinical images (A) before drainage, (B) percutaneous ultrasound-guided drainage.
The rest of the abdomen was normal. Otolaryngologic, pleuropulmonary, and osteoarticular examinations were normal.
We found a biological infectious syndrome with C-reactive protein of 64 mg/dl and hyperleukocytosis of 20,800/mm, predominantly neutrophilic, and normochromic microcytic anemia (hemoglobin 8.2 g/dl). Blood culture was performed.
The first abdominal ultrasound revealed a choledochal cyst. Due to the infectious syndrome, the pediatrician hospitalized the child and started triple antibiotic therapy (cephalosporin, ampicillin and gentamicin) after sampling blood cultures.
We were then requested for a surgical examination at day 21. Our examination revealed a febrile neonate weighing 4600 g. No cholestasis syndrome. We found an inflammatory mass of the hypochondrium measuring 10 cm in long axis, well limited, renitent, fixed to the deep plane.
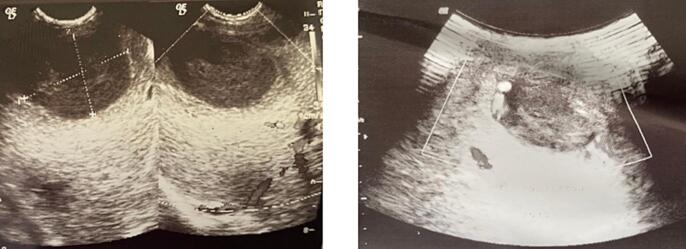
Because of the clinical-radiologic discordance, we repeated the ultrasound, this time with a sonographer accustomed to pediatrics. It showed a hypoechoic formation with fine mobile echoes, oval, well circumscribed, thick-walled and regular, vascularized peripherally on color Doppler, located in segment VIII of the liver, suggesting an abscess. Volume 17 ml (Fig. 2). No intra- or extrahepatic bile duct abnormalities.
Fig. 2.
Pre drainage ultrasound images.
Percutaneous echo-guided drainage was performed (Fig. 2). This evacuated 14 ml of frank pus. The abscess was then irrigated and drained.
Cytobacteriological examination of the pus isolated Staphylococcus aureus and Klebsiella ozaenae, as did blood cultures. Appropriate antibiotic therapy (amikacin and azithromycin) was initiated for a total of four weeks.
The child was discharged from the hospital after 72 h of apyrexia (14 days after the procedure).
At the one-month follow-up, the clinical examination was normal and the ultrasound showed a residual pouch without collection (Fig. 3).
Fig. 3.
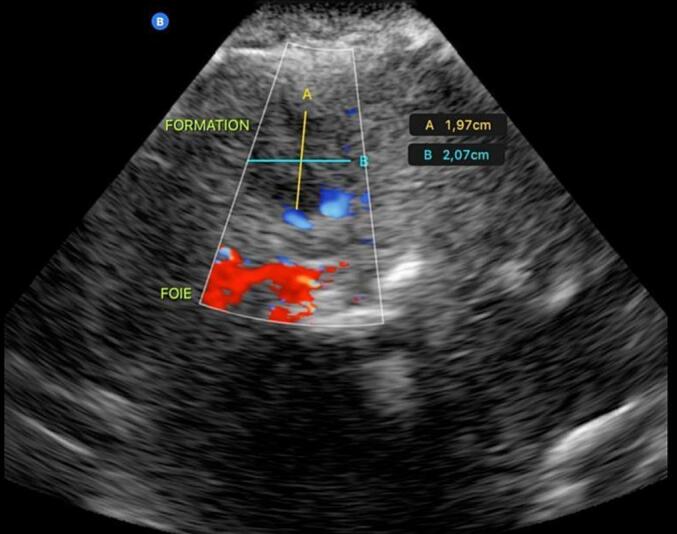
Ultrasound images at one month follow-up.
3. Discussion
We report an exceptional case of NPLA following omphalitis successfully treated in a developing country. The ultrasound findings, which were confused with a choledochal cyst, and the etiopathogenic mechanism are specific to this case.
The majority of cases published in the literature are in children, with an annual prevalence of 1 to 100 cases and a mean age of approximately 7 years [2,3,5]. Neonatal cases have been rarely reported and have involved preterm or very low birth weight infants [1,6,7].
The most common cause of liver abscess in newborns is reported to be due to malposition of the umbilical catheter in Western countries [7]. In our conditions, infection related to poor hygiene including umbilical cord infection seems to be the most risk factors.
Omphalitis and umbilical sepsis are relatively rare in the developed world (1/200 newborns), but are endemic in less developed regions [9,10]. In a recent study in Zambia, 6.2 % of newborns presented with omphalitis [11].
Following omphalitis, liver infection may occur by contiguous or hematogenous route [9,12]. Other portal entries are rarely reported, such as skin infection, sepsis, gingival in Papillon-Lefevre syndrome [12].
In our case, omphalitis seems to be the most likely favoring factor. In fact, the newborn has no perinatal pathologic history, but after ligation (suture) and umbilical cord section, the care at home was done with poor hygiene, leading to omphalitis and purulent discharge. Otherwise, the clinical examination was normal.
Abdominal ultrasound is the first choice because it is noninvasive, nonirradiating and reproducible, allowing to follow the evolution of the disease. However, its operator-dependent nature may lead to diagnostic errors, especially since the pathology is not so common and may mimic certain congenital disorders of the biliary tree. In our patient, there was a discrepancy between the ultrasound, which showed a choledochal cyst, and his clinical condition. There are other differential diagnoses of hepatic cystic lesions in children, including neoplastic (hepatoblastoma, infantile hemangioendothelioma, and mesenchymal hamartoma), posttraumatic (hepatic hematoma, biloma), or iatrogenic (umbilical vein catheter). Familiarity with imaging findings and clinical features is essential for accurate diagnosis of pediatric hepatic cystic lesions, which in turn can guide appropriate clinical management [13]. Liver abscesses tend to be hypoechoic and are usually solitary. In contrast, tumors typically have a solid appearance and may be calcified. Serum alpha-fetoprotein levels may be helpful in differentiating malignant and benign hepatic masses from liver abscesses [13,14].
Ultrasound also specifies the location, number, and size of the abscess, reveals associated biliary anomalies, and allows for percutaneous drainage [2,[12], [13], [14]]. The sensitivity of ultrasound, CT and MRI is approximately the same [12,14], but the latter two are not operator dependent and therefore provide a more accurate diagnosis. In our context, these examinations were financially inaccessible.
However, ultrasound should be repeated in case of doubt. Thus, because of the clinical and imaging discordance, a second ultrasound allowed us to confirm the diagnosis.
Percutaneous echo-guided drainage is currently the treatment of choice, with good results in very specific indications (abscesses larger than 4 cm, increase in abscess size under treatment or lack of clinical response after 72 h, solitary abscess) [4,6,7,11,12,[14], [15], [16]].
In contrast to adults, in whom amoebae are the main cause of liver abscesses, pyogenic abscesses predominate in children [3]. The most common bacteria are Staphylococcus aureus, followed by Enterobacteriaceae (Escherichia coli, Klebsiella, Enterobacter, Pseudomonas) [4,6,9]. Enterobacteriaceae sometimes surinfect an amoebic abscess, especially in neonates [12]. Abscesses caused by Haemophilus parainfluenzae, Yersinia enterocolitica, and Clostridium perfringens have also been described [12,[15], [16], [17]]. In 17 to 33 % of cases, the pathogen is not identified [4,6,9]. Medical management with antibiotics is then sufficient to treat pyogenic abscesses in 36–55 % of cases, especially in multiple diffuse abscesses and in unstable patients with shock or coagulopathy [7,12].
While awaiting the results of blood culture and antibiotic susceptibility testing, authors use to initiate antibiotic therapy active against pyogenic, anaerobic and amoebic bacteria [15].
Four to six weeks of antibiotic therapy is recommended after percutaneous drainage [2,6,11]. Abscesses resolve in six weeks to six months, although cavitary lesions may persist [12].
In our neonate, it was a combination of Staphylococcus aureus and Enterobacteria, which would be a factor of severity and resistance to first-line antibiotic therapy.
In developing countries, liver abscesses, especially in preterm infants, are still associated with high mortality rates of up to 50 %. Survival rates are significantly improved by prompt diagnosis and therapeutic intervention. Late diagnosis and treatment of liver abscesses, which are often multiple and difficult to drain, can lead to septicemia and death [6,7,9,[15], [16], [17], [18]]. Shah et al. [19] reported portal hypertension due to portal vein thrombosis as a serious complication of liver abscess in a 20-day-old neonate.
The favorable outcome in our case could be explained by prompt and appropriate treatment (drainage and antibiotics) after better imaging and microbiologic diagnosis.
4. Conclusion
NPLA is exceptional. Because of its specific etiopathogenesis in our patient, we recommend that asepsis be emphasized in newborns, as even a trivial infection such as omphalitis can cause a liver abscess. Abdominal ultrasound is the gold standard for the diagnosis of liver abscess, but it can be challenging in our context. Percutaneous ultrasound-guided drainage in neonates can give very good results, especially in solitary liver abscess. Follow-up must be rigorous.
Consent
Written informed consent was obtained from the patient's parents for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Ethical approval (N°004/22/CE/SSE/CAJ/DG) for this study was provided by the Ethical Committee of Gynaeco-Obstetric and Paediatric Hospital, Douala, on 12 March 2022.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Guarantor
Dr. Hakima CHAFAAOUI is the guarantor for the work.
Research registration number
Nil.
CRediT authorship contribution statement
Drs. Octave Excupère Désiré Dongmo Miaffo, Hakima Chafaaoui and Beaudelaire Romulus Assan contributed to the concept and design of study, data collection, data interpretation and analysis, approval of final manuscript.
Drs Pauline Mantho and Prince Parfait Ntankeu were involved in data collection, revision and approval of final manuscript.
Conflicts of interest
Authors have no conflicts of interest to declare.
References
- 1.Khabbache K., Alaoui K., Akoudad Z., Oulmaati A., Bouharrou A. Abcès hépatique chez un nouveau-né secondaire à l’emplacement d’un cathéter ombilical. Arch. Pediatr. 2010;17:1–178. [Google Scholar]
- 2.Ba I.D., Sagna A., Thiongane A., Demely I., Ba A., Faye P.M., coll. Abcès du foie chez l’enfant au Sénégal. Rev. Cames Sante. 2015;3(2):17–21. [Google Scholar]
- 3.Mishra K., Basu S., Roychoudhury S., Kumar P. Liver abscess in children: an overview. World J. Pediatr. 2010;6(3):210–216. doi: 10.1007/s12519-010-0220-1. [DOI] [PubMed] [Google Scholar]
- 4.Simeunovic E., Arnold M., Sidler D., Moore S.W. Liver abscess in neonates. Pediatr. Surg. Int. 2009;25:153–156. doi: 10.1007/s00383-008-2307-5. [DOI] [PubMed] [Google Scholar]
- 5.Salahi R., Dehghani S.M., Salahi H., Bahador A., Abbasy H.R., Salahi F. Liver abscess in children: a 10-year single centre experience. Saudi J. Gastroenterol. 2011;17(3):199–202. doi: 10.4103/1319-3767.80384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutsunai T. Abscess of the liver of umbilical origin in infants. Am. J. Dis. Child. 1936;51:1385–1396. [Google Scholar]
- 7.Mannan K., Tadros S., Patel K., Aladangady N. Liver abscess within the first week of life in a very low birthweight infant. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.05.2009.1874. (bcr05.2009.1874) [DOI] [PMC free article] [PubMed] [Google Scholar]; Porras-Ramirez G., Hernandez-Herrera M.H., Porras-Hernandez J.D. Amebic hepatic abscess in children. J. Pediatr. Surg. 1995;30:662–664. doi: 10.1016/0022-3468(95)90684-3. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., SCARE Group, The SCARE guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84(2020):226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Fraser N., Davies B.W., Cusack J. Neonatal omphalitis: a review of its serious complications. Acta Paediatr. May 2006;95(5):519–522. doi: 10.1080/08035250600640422. (PMID: 16825129) [DOI] [PubMed] [Google Scholar]
- 10.Kaplan R.L. Omphalitis: clinical presentation and approach to evaluation and management. Pediatr. Emerg. Care. Mar 1 2023;39(3):188–189. doi: 10.1097/PEC.0000000000002918. [DOI] [PubMed] [Google Scholar]
- 11.Herlihy J.M., Gille S., Grogan C., Bobay L., Simpamba K., Akonkwa B., Chisenga T., Hamer D.H., Semrau K. Can community health workers identify omphalitis? A validation study from Southern Province, Zambia. Tropical Med. Int. Health. Jul 2018;23(7):806–813. doi: 10.1111/tmi.13074. (Epub 2018 Jun 19. PMID: 29752848) [DOI] [PubMed] [Google Scholar]
- 12.Guittet V., Ménager C., Misotte I., Duparc B., Verhaegen Duhamel J.F. Les abcès hépatiques de l’enfant : étude rétrospective de 33 cas observés en Nouvelle-Calédonie de 1985 à 2003. Arch. Pédiatr. 2004;11(9):1046–1053. doi: 10.1016/j.arcped.2004.03.101. [DOI] [PubMed] [Google Scholar]
- 13.Riedesel E.L., Richer E.J., Taylor S.D., Tao T., Gagnon M.H., Braithwaite K.A., Alazraki A.L., Khanna G. Pediatric hepatic cystic lesions: differential diagnosis and multimodality imaging approach. Radiographics. Sep-Oct 2022;42(5):1514–1531. doi: 10.1148/rg.220006. [DOI] [PubMed] [Google Scholar]
- 14.Jha P., Chawla S.C., Tavri S., Patel C., Gooding C., Daldrup-Link H. Pediatric liver tumors: a pictorial review. Eur. Radiol. 2009;19(1):209–219. doi: 10.1007/s00330-008-1106-7. [DOI] [PubMed] [Google Scholar]
- 15.Bosnali O., Moralioglu S., Celayir A.C., Pektas O. Liver abscess: increasing occurrence in premature newborns. J. Neonatal Surg. 2013;2(2):23. [PMC free article] [PubMed] [Google Scholar]
- 16.Rajak C.L., Gupta S., Jain S., Chawla Y., Gulati M., Suri S. Percutaneous treatment of liver abscesses: needle aspiration vs catheter drainage. AJR Am. J. Roentgenol. 1998;170:1035–1039. doi: 10.2214/ajr.170.4.9530055. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A., Srinivasan S., Sharma A.K. Pyogenic liver abscess in children-South Indian experiences. J. Pediatr. Surg. 1998;33:417–421. doi: 10.1016/s0022-3468(98)90081-1. [DOI] [PubMed] [Google Scholar]
- 18.Brook I., Fraizer E.H. Role of anaerobic bacteria in liver abscesses in children. Pediatr. Infect. Dis. J. 1993;12:743–747. doi: 10.1097/00006454-199309000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Shah I., Bhatnagar. Liver abscess in a newborn leading to portal vein thrombosis. Indian J. Pediatr. 2009;76:1268–1269. doi: 10.1007/s12098-009-0244-5. [DOI] [PubMed] [Google Scholar]