Abstract
Introduction and importance
endovascular repair is an alternative to open repair for abdominal aortic aneurysms (AAA), which lowers morbidity and mortality but may presents infectious complications. Endograft infection is a rare but serious life-threatening condition with a mortality rate up to 50 %. We reported a case of aortic endograft infection by Francisella tularensis, rare and highly virulent gram-negative coccobacillus known for use in bioterrorism.
Case presentation
A 79-year-old man presented with asthenia, weight loss, night sweats and one episode of fever. In 2007, he underwent aorto-bi-iliac endograft repair for AAA without any complication. The diagnostic workup showed some signs of inflammation, but negative blood cultures and no sign of infection on CT scan. The combination of positron emission tomography (PET) and white blood cell (WBC) scintigraphy led to the diagnosis of aortic endograft infection. The management was antimicrobial therapy and surgery. Perioperative analysis shows the presence of Francisella Tularensis.
Discussion and conclusions
Aortic endograft infection is a serious complication with a high mortality rate. Its diagnosis may be difficult, but the combination of WBC scintigraphy and PET scan may improve identification of the infection, even if blood cultures and CT scan are negative. The gold standard treatment is removal of the endograft, debridement, and in situ reconstruction along with antibacterial therapy.
Keywords: EVAR, Late infection, Tularemia, Case report
Highlights
-
•
First case of EVAR infection by Francisella tularensis, a rare and highly virulent coccobacillus known in bioterrorism
-
•
Diagnosis of EVAR infection can be challenging due to nonspecific symptoms and possibly negative CT scan and blood cultures
-
•
Use of 99mTc-HMPAO-labeled WBC scintigraphy and PET scan for identification of EVAR infection is very helpful
-
•
Antimicrobial therapy associated to the removal of the endograft and in situ reconstruction is the first choice treatment
1. Introduction
Endovascular aneurysm repair (EVAR) is an alternative to open repair (OR) for patients with abdominal aortic aneurysm (AAA) [1]. Postoperative complications can be frequent (16 %–30 %), may require reintervention in up to 19 % of cases, and can be life-threatening [2]. Widespread EVAR use has increased infectious complications [3]. Aortic endograft infection (AEGI) occurs in <1 % of EVAR cases with high mortality rates (25 %–50 %) [4]. The diagnosis of AEGI may be challenging because of nonspecific symptomatology, possibly negative computed tomography (CT) scan and negative cultures in up to one-third of cases [3]. The gold standard management is complete removal of the endograft, debridement and reconstruction. However, conservative treatment, such as percutaneous, or surgical drainage or life-long antibiotic therapy, can be acceptable for patients with high surgical risk [3].
We reported a case of AEGI by Francisella tularensis. This work was reported in line with the SCARE criteria [5].
To our best knowledge, this is the first documented case, in patient referred to our University Hospital, of AEGI with F. tularensis which is a highly virulent gram-negative coccobacillus that causes a rare bacterial zoonotic disease and may be transmitted to humans by multiple ways (Contaminated water or food ingestion; arthropod or animals bites; direct contact with infected animals, animal tissues, or fluids; or inhalation of aerosols) [6,7]. The incidence of tularemia varies widely. In Europe, only 800 cases was reported annually [8]. Tularemia manifests as an ulceroglandular, glandular, oculoglandular, oropharyngeal, pneumonic, or typhoidal disease [6]. Reported cases of pericarditis, meningitis, and endocarditis had been rare, with only two cases in Europe and two in the United States, probably because of difficulties in obtaining positive blood cultures [9].
2. case presentation
A 79-year-old man presented with asthenia, weight loss for 1 month, night sweats and only one fever episode. There were systemic symptoms. He had no recent travel. He lived in the countryside. He had no animals or history of hunting, fishing, or contact with animals or their feces. He had contact with a dead fox 1 year ago.
From his medical history, we noted arterial hypertension, sleep apnea syndrome, coronary artery disease treated by coronary stents in 2007 and coronary bypass in 1999. In 2007, he underwent EVAR with aorto-bi-iliac endograft (Gore Excluder) for 56 mm AAA; there was no complication. The patient remained asymptomatic during the follow-up period with progressive decrease in aortic aneurysm size to 39 mm in 2010.
His physical examination was normal. Blood tests showed minor signs of inflammation. The blood and urine cultures and serology tests were negative. Gastroscopy, colonoscopy echocardiography, chest radiograph and thoracoabdominal CT scan were normal.
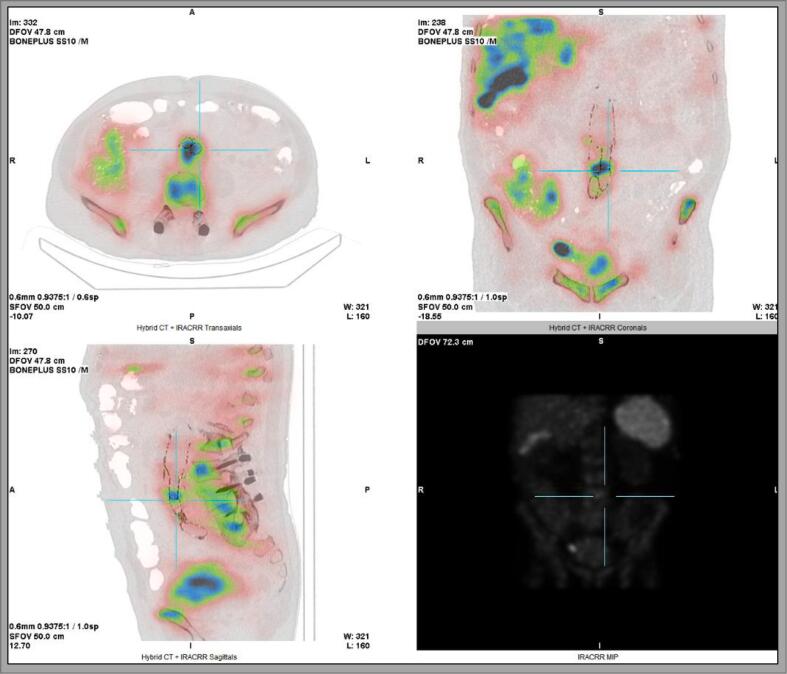
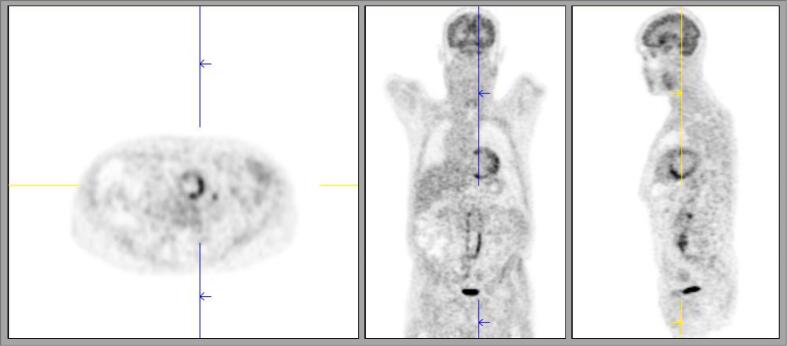
99mTc-Hexamethylpropyleneamine oxime-labeled WBC scintigraphy (Fig. 1) showed accumulation of labeled WBCs at three spots. One of them, localized at the aortoiliac bifurcation, was suspected to be a zone of quiet infection. Positron emission tomography (PET) (Fig. 2) favored inflammation or AEGI. Based on those combined findings we concluded to aortic endograft infection and decided to proceed with surgery.
Fig. 1.
99mTc-HMPAO-labeled WBC scintigraphy.
There is accumulation of labeled WBCs at the aortoiliac bifurcation.
WBC, white blood cell.
Fig. 2.
Positron emission tomography.
The findings favor infectious and/or inflammatory processes in the aortic graft, in relation to the L4–L5 junction.
A midline laparotomy was performed. We exposed the abdominal aorta and iliac arteries. The tissues were inflamed with numerous lymph nodes. After heparin administration, the aorta was clamped below the renal arteries, and the iliac arteries were clamped distally. Upon aneurysmal sac opening, we found around 10 mL of cloudy fluid surrounding the endograft. The aortic sac was freed from the surrounding structures and removed. The two distal endograft limbs were removed from the common iliac arteries and clamped, and the aortic clamp was moved above the renal arteries to explant the endograft. Once the endograft was extracted, we clamped the aorta below the renal arteries (clamping time < one minute). The common iliac arteries and aorta were cleaned and silver-coated polyesters aortic graft was anastomosed end-to-end to the infrarenal aorta and to the aorta above its bifurcation. Empiric tazobactam and vancomycin were started.
Cultures of the periaortic and aortic tissues showed F. tularensis. We shifted the antibiotics to ciprofloxacin and intravenous gentamicin for 2 weeks, followed by ciprofloxacin alone for 10 weeks [10,11]. The inflammatory markers decreased progressively.
On six months follow-up, the patient was doing well without signs of new or recurrent infection.
3. Discussion
F. tularensis is a highly infectious gram-negative coccobacillus. The most recent epidemiological report on tularemia in 2019 showed 1463 cases, with a notification rate of 0.3/100,000 [12]. Only four cases of endocarditis, and one prosthetic valve endocarditis were reported. To our best knowledge, we report the first documented case of EVAR infection with F. tularensis.
EVAR has specific complications, such endoleak, iliac branch occlusion and spinal cord ischemia. The high postprocedural complications rate need lifelong imaging surveillance [4].
The risk factors for AEGI are emergency setting of the procedure, the need for surgical revision, use of the radiology suite instead of the operating room, the presence of fever before the procedure, and too short prophylactic antibiotic therapy [3]. AEGI had been associated with endoleaks, percutaneous coiling, and placement of sealant or glue in the aneurysmal sac [3,13]. The mean interval between endograft placement and infection symptoms is 28.3 months [3]. In our case, this interval is much longer (15 years).
Patients usually present fever (71.4 %), back pain (57.1 %), and sometimes weight loss associated with inflammation and leukocytosis in approximately two-thirds of cases [4,14,15].
The most commonly isolated microorganisms in blood cultures are gram-positive cocci (66 %). Only 26.7 % of blood cultures could be positive [3]. CT scan could show mesenteric fat stranding adjacent to the stent graft, perigraft fluid collections, abnormal enhancement, air bubbles and/or adjacent structures erosion [14,16]. Technetium-labeled WBC scanning is useful for aortic graft infection diagnosis, particularly in the low-grade stages [17]. PET can be useful in confirming the diagnosis in highly suspicious clinical situations with negative blood tests and inconclusive CTA [3]. Our patient presents high suspicion of endograft infection with negative blood cultures. PET and labeled WBC scintigraphy were compatible with infection, which was later confirmed by perioperative tissues culture.
The patient's underlying condition, virulence of the infectious organism, the presence of prosthetic–duodenal fistula (PDF) and the AEGI treatment modalities influenced the patient's prognosis. In case of surgical treatment, suprarenal aortic clamping and endograft fixation increased the mortality rate [3]. The different surgical treatment modalities include in situ reconstruction (ISR) or extra-anatomic reconstruction (EAR) [18]. During the 1980s the most common treatment was graft excision with EAR, but this technique has low patency, a high amputation rate and a significant risk of rupture along the aortic suture line [12,19]. To minimize these problems, ISR using different conduits [i.e., autogenous veins, cryopreserved allografts (CAG), synthetic grafts made from polyester/polytetrafluoroethylene (PTFE), and either rifampicin-bonded or silver-coated prosthesis] was introduced [19]. ISR showed lower complication and mortality rates than after EAR [20].
The choice of graft type depends on local protocols and the patient's underlying condition [20]. Although no ideal graft material exists, biological material conduit is preferred because its lowest reinfection rate (<10 %) and higher survival rates [13,15]. An autologous vein might be preferred for young patients [20]. Some teams prefer a cryopreserved arterial homograft ISR because of its capacity to mimic the structural and hemodynamic characteristics of autologous vessels while avoiding the harvesting [3].
Silver/rifampicin polyesters and cryopreserved allograft may be more suitable if PDF is identified. For a low virulence infection, rifampicin-soaked or silver grafts were reported to be effective. For large perigraft abscesses and methicillin-resistant Staphylococcus aureus infections, EAR should be considered [20].
Standard expanded PTFE and polyester grafts had significantly worse results for graft occlusion and amputation [10,20]. The reinfection rates did not significantly differ among the types of conduits except for standard polyester/PTFE [20].
In some cases, the surgical treatment may not be the most suitable option for the patient, but the conservative treatment (intravenous antibiotics, anti-inflammatory therapy, drainage or local irrigation) presents high-rate mortality (58 %–100 %) [13,21].
Considering the patient's age, comorbidities and severity of the infection, our strategy was to combine antimicrobial therapy with ISR using silver-coated polyesters graft (Intergard Synergy).
There had been no consensus on the duration and type of antimicrobial therapy. A minimum of 2 weeks of intravenous therapy followed by an oral regimen is needed. The total duration of antimicrobial treatment can be from 3 months to 1 year, and even lifelong if surgery is not possible [10]. For tularemia cases, streptomycin, gentamicin, doxycycline, and ciprofloxacin are the first choice [11]. Our treatment was ciprofloxacin, and intravenous gentamicin for 2 weeks, followed by ciprofloxacin alone for 10 weeks. This management was successful and led to rapid recovery and no signs of recurrence so far.
4. Conclusions
We reported the first documented case of EVAR infection by F. tularensis. The diagnosis may be challenging because of nonspecific symptomatology and possibly negative CT or cultures. 99mTc-HMPAO-labeled WBC scintigraphy and PET scan were very helpful in identifying AEGI. The treatment of choice for AEGI is endograft removal and ISR, but conservative treatment may be acceptable for high-risk patients.
Ethics approval
All authors adhered to the guidelines for authorship mentioned in the “Guide for Authors” of the International Journal of Surgery Case Reports.
Ethics clearance was not necessary as it is not a study but a case report with a discussion according to a literature review.
Consent of patient
Written informed consent was obtained from the patient for publication of this case report and accompanying images in an anonymous way. A copy of the written consent is available for review by the Editor-in-Chief ofthis journal on request.
Consent for publication
All authors agreed with the content and gave explicit consent to submit this work.
CRediT authorship contribution statement
Manuscript writing: MK, AB, and BR; critical revision, manuscript approval, and agreement to be accountable: all authors; obtaining funding: BR and AB.
Declaration of competing interest
None.
References
- 1.Wanhainen A., Verzini F., Van Herzeele I., Allaire E., Bown M., Cohnert T., Dick F., van Herwaarden J., Karkos C., Koelemay M., Kölbel T., Loftus I., Mani K., Melissano G., Powell J., Szeberin Z., Committee Esvs Guidelines, de Borst G.J., Chakfe N., Debus S., Hinchliffe R., Kakkos S., Koncar I., Kolh P., Lindholt J.S., de Vega M., Vermassen F., Reviewers Document, Björck M., Cheng S., Dalman R., Davidovic L., Donas K., Earnshaw J., Eckstein H.H., Golledge J., Haulon S., Mastracci T., Naylor R., Ricco J.B., Verhagen H. Editor’s choice – European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. European Journal of Vascular and Endovascular Surgery: the Official Journal of the European Society for Vascular Surgery. European Society for Vascular Surgery (ESVS) 2019;57(1):8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Geraedts A.C.M., Mulay S., Vahl A.C., Verhagen H.J.M., Wisselink W., de Mik S.M.L., van Dieren S., Koelemay M.J.W., Balm R. ODYSSEUS study group Editor’s choice – post-operative surveillance and long term outcome after endovascular aortic aneurysm repair in patients with an initial post-operative computed tomography angiogram without abnormalities: the multicentre retrospective ODYSSEUS study. European Journal of Vascular and Endovascular Surgery. 2022;63(3):390–399. doi: 10.1016/j.ejvs.2021.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez Prendes C., Riedemann Wistuba M., Zanabili Al-Sibbai A.A., Del Castro Madrazo J.A., Santervas L.A.C., Perez M.A. Infrarenal aortic endograft infection: a single-center experience. Vasc. Endovasc. Surg. 2019;53(2):132–138. doi: 10.1177/1538574418813606. [DOI] [PubMed] [Google Scholar]
- 4.Daye D., Walker T.G. Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management. Cardiovascular Diagnosis and Therapy. 2018;8(Suppl. 1):S138–S156. doi: 10.21037/cdt.2017.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agha R.A., Franchi T., Sohrab C., Mathew G., Kirwan A., Thomas A., et al. The SCARE 2020 guideline: updating consensus surgical case report (SCARE) guidelines. Int. J. Surg. 2020;84(1):226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Rijks J.M., Tulen A.D., Notermans D.W., Reubsaet F.A.G., de Vries M.C., Koene M.G.J., Swaan C.M., Maas M. Tularemia transmission to humans, the Netherlands, 2011–2021. Emerg. Infect. Dis. 2022;28(4):883–885. doi: 10.3201/eid2804.211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurin M., Gyuranecz M. Tularaemia: clinical aspects in Europe. Lancet Infect. Dis. 2016;16(1):113–124. doi: 10.1016/S1473-3099(15)00355-2. [DOI] [PubMed] [Google Scholar]
- 8.Official EU website. European Centre for Disease Prevention and Control. Factsheet on Tularaemia.
- 9.Salit I.E., Liles W.C., Smith Ch. Tularemia endocarditis from domestic pet exposure. Am. J. Med. 2013;126(10) doi: 10.1016/j.amjmed.2013.04.011. e1. [DOI] [PubMed] [Google Scholar]
- 10.Chakfé N., Diener H., Lejay A., Assadian O., Berard X., Caillon J., Fourneau I., Glaudemans A.W.J.M., Koncar I., Lindholt J., Melissano G., Saleem B.R., Senneville E., Slart R.H.J.A., Szeberin Z., Venermo M., Vermassen F., Wyss T.R., Committee E.S.V.S. Guidelines, de Borst G.J., Bastos Gonçalves F., Kakkos S.K., Kolh P., Tulamo R., Vega de Ceniga M., Von Allmen R.S., Van den Berg J.C., Debus E.S., Koelemay M.J.W., Linares-Palomino J.P., Moneta G.L., Ricco J.B., Wanhainen A editor’s choice – European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of vascular graft and Endograft infections. European Journal of Vascular and Endovascular Surgery: the Official Journal of the European Society for Vascular Surgery. 2020;59(3):339–384. doi: 10.1016/j.ejvs.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention: Tularemia. https://www.cdc.gov/tularemia/diagnosistreatment/index.html.
- 12.Official EU website: European Centre for Disease Prevention and Control. Tularaemia – Annual Epidemiological Report for 2019.
- 13.Argyriou C., Georgiadis G.S., Lazarides M.K., Georgakarakos E., Antoniou G.A. Endograft infection after endovascular abdominal aortic aneurysm repair: a systematic review and meta-analysis. Journal of Endovascular Therapy. 2017;24(5):688–697. doi: 10.1177/1526602817722018. [DOI] [PubMed] [Google Scholar]
- 14.Gozzo C., Caruana G., Cannella R., Farina A., Giambelluca D., Dinoto E., Vernuccio F., Basile A., Midiri M. CT angiography for the assessment of EVAR complications: a pictorial review. Insights Into Imaging. 2022;15(1):5. doi: 10.1186/s13244-021-01112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smeds M.R., Duncan A.A., Harlander-Locke M.P., Lawrence P.F., Lyden S., Fatima J., Eskandari M.K. Vascular low-frequency disease consortium treatment and outcomes of aortic endograft infection. J. Vasc. Surg. 2016;63(2):332–340. doi: 10.1016/j.jvs.2015.08.113. [DOI] [PubMed] [Google Scholar]
- 16.Pandey N., Litt H.I. Surveillance imaging following endovascular aneurysm repair. Semin. Interv. Radiol. 2015;32(3):239–248. doi: 10.1055/s-0035-1556878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mark A.S., McCarthy S.M., Moss A.A., Price D. Detection of abdominal aortic graft infection: comparison of CT and in-labeled white blood cell scans. A.J.R. Am. J. Roentgenol. 1985;144(2):315–318. doi: 10.2214/ajr.144.2.315. [DOI] [PubMed] [Google Scholar]
- 18.Batt M., Feugier P., Camou F., Coffy A., Senneville E., Caillon J., Calvet B., Chidiac C., Laurent F., Revest M., Daures J.P. Research group for vascular graft infection a meta-analysis of outcomes after in situ reconstructions for aortic graft infection. Angiology. 2018;69(5):370–379. doi: 10.1177/0003319717710114. [DOI] [PubMed] [Google Scholar]
- 19.Fiorani, P, Speziale, F, Rizzo, L, De Santis, F, Massimi, GJ, Taurino, M, Faraglia, V, Fiorani, L, Baiocchi, P, Santini, C Detection of aortic graft infection with leukocytes labeled with technetium 99m-hexametazime. J. Vasc. Surg., 1993 17 (1), 87–95; discussion 95–96. [PubMed]
- 20.Oderich G.S., Bower T.C., Cherry K.J., Jr., Panneton J.M., Sullivan T.M., Noel A.A., Carmo M., Cha S., Kalra M., Gloviczki P. Evolution from Axillofemoral to in situ prosthetic reconstruction for the treatment of aortic graft infections at a single center. J. Vasc. Surg. 2006;43(6):1166–1174. doi: 10.1016/j.jvs.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 21.Zhu F., Ge X., Ci H., Guan S. Antibiotics and percutaneous drainage for treating stent-graft infection after EVAR. Ann. Vasc. Surg. 2020;65(289):e1–289.e6. doi: 10.1016/j.avsg.2019.12.009. [DOI] [PubMed] [Google Scholar]