Abstract
Background
Given the life-threatening nature of food allergy (FA), it is important to assess the level of knowledge among families with food-allergic patients and their ability to cope with anaphylaxis. This study constructed a FA knowledge questionnaire (FAKQ) and confidence in FA management skills (CIFAMS) questionnaire to assess understanding and attitudes toward FA management in food-allergic families.
Methods
Items from literature review and expert panel showing ≥80% content validity index and semantic equivalence were selected for translation into Chinese. These questionnaires underwent feasibility pilot testing followed by cross-sectional validation to assess their psychometric properties of internal consistency, test–retest reliability, and construct validity with a FA quality-of-life questionnaire and discriminant validity. Exploratory factor analysis was performed to confirm their factor structure.
Results
A total of 155 subjects (104 patients and 51 parents) completed a 20-item FAKQ and 10-item CIFAMS. Both tools showed acceptable internal consistency in baseline and retest groups. FAKQ and CIFAMS correlated for all subjects (P = .002) and for adults (P = .002), and similarly between CIFAMS and parent-reported FA independent measure (P = .005). Total score of FAKQ was sensitive to within-group differences of patients hospitalized for FA (P < .001). FAKQ and CIFAMS items were factored into 4 and 2 domains, respectively. Subjects scored the lowest on FAKQ items about signs of allergic reaction and CIFAMS items on epinephrine autoinjector use.
Conclusion
FAKQ and CIFAMS developed by our group are valid and reliable in assessing knowledge and confidence in FA management in patients and parents. These tools are crucial for formulating education programs and advocacy campaigns for FA.
Key words: Food allergy, knowledge, confidence, attitude, emergency management skills, validation, education
Food allergy (FA), predominantly caused by milk, eggs, nuts, fish, crustaceans, shellfish, wheat, soy, and sesame, is a significant global health problem affecting 26 million adults in the United States.1 The prevalence of FA among children in the United States and Australia is 6.7% and 11%, respectively, with eggs and peanuts being the main food allergens.2 In contrast, shellfish was the main food causing adverse reaction in Hong Kong preschool children.3 FA may present with severe anaphylactic reactions. Loke et al reported a 41% increase in the prevalence of pediatric anaphylaxis from 2009 (1.0%) to 2014 (1.4%) in Australia.4 In a retrospective study in Hong Kong, the 10-year incidence of anaphylaxis was 9.8 and 3.6 per 100,000 person-years in children and adults, respectively.5,6 Of these, half of anaphylaxis events were misdiagnosed, while less than one tenth of patients received epinephrine before reaching the hospital.5
Knowing how patients understand their FA is crucial for us to plan for appropriate management program. Earlier studies reported knowledge deficits related to FA management in parents of food-allergic children. For example, parents were reported to be stressed and anxious because they did not know what to do if their child developed anaphylactic shock.7,8 It is equally important to assess how confident FA patients feel in the management of their disease, such as preparation and/or avoidance of foods as well as recognition of allergic symptoms and skill in administering epinephrine autoinjectors. A survey involving 122 children with FA in the United Kingdom revealed that 69% of parents did not know how to use an epinephrine autoinjector on their children during an anaphylactic reaction.9 In this regard, it is important to assess the level of knowledge of people with FA and their confidence to manage anaphylaxis.
Only a few validated instruments assess knowledge on FA (see Table E1 in the Online Repository at www.jaci-global.org), and these are mainly targeted to parents, dietitians, school nurses, and food handlers; we found no instrument specifically designed for adults with FA. In addition to knowledge on FA, practice is directly affected by the confidence level of food-allergic patients and/or their caregivers. Therefore, we sought to fill this knowledge gap by constructing and validating a FA knowledge questionnaire (FAKQ) and a confidence in FA management skills (CIFAMS) questionnaire to assess the level of FA knowledge and confidence in both Chinese pediatric and adult patients with FA, as well as parents of children with FA.
Methods
The study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, and subjects and their parents of those younger than 18 years provided informed verbal assent to participate.
Development of FAKQ and CIFAMS
The FAKQ and CIFAMS assessment tools were constructed and validated after the following 3 phases: phase 1, generation and reduction of items; phase 2, translation; and phase 3, validation. We turn to each of these in turn.
Phase 1
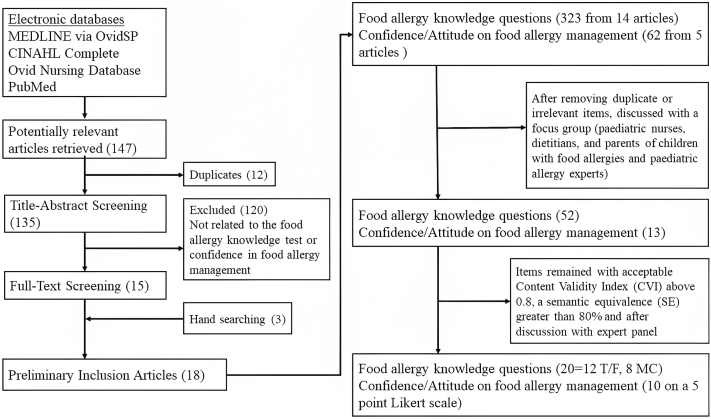
Important items on the knowledge and management skills in FA were generated through focus groups, expert opinion, and literature search for relevant publications. The search was conducted in 4 electronic databases: Medline via OvidSP–In-Process & Other Non-indexed Citations, Ovid Nursing Database, CINAHL Complete, and PubMed (Fig 1). Additional articles were retrieved by manually searching the sites using the following terms about FA: knowledge∗, test∗, question∗ or confidence∗, attitude∗. Search terms were truncated and used in various combinations. We did not restrict the date of publication, and only English-language articles were included.
Fig 1.
Process of item generation and reduction for developing FAKQ and CIFAMS.
A total of 147 articles in English were retrieved from electronic databases. Twelve replicated articles and 120 articles deemed irrelevant to our topics were excluded after initial abstract screening. Together with 3 articles that were retrieved by manual website search, we compiled a total of 18 articles including 7981 subjects that were related to FA knowledge, attitudes, and/or confidence after full-text screening (Table E1). Seven articles involved parents of food-allergic children, whereas the others involved school nurses, nutritionists, doctors, and/or food handlers. Among these retrieved articles, 14 articles included a total of 323 questions on FA knowledge, while 5 articles provided a total of 62 questions on confidence or attitude about FA management. After removal of duplicates or irrelevant items, all remaining questions were discussed among a focus group involving pediatric allergists, pediatric nurses, dietitians, and parents of food-allergic children. The members were either our research staff or collaborators, while FA children were randomly selected from our allergy clinic. At the end, 52 questions were considered relevant to FA knowledge (see Table E2 in the Online Repository at www.jaci-global.org). We categorized them into the following 8 domains: (a) general concepts, (b) clinical manifestations and diagnoses, (c) FA avoidance measures and treatment, (d) life-saving measures during emergencies, (e) food allergen labeling, (f) management of FA at home, (g) management of FA at school, and (h) management of FA in restaurants. In addition, we included 13 questions that measured subjects’ confidence in FA management (see Table E3 in the Online Repository).
These selected questions on FA knowledge and confidence in FA management were then distributed to 12 panel members, including 2 pediatric allergists, 1 general pediatrician, 3 pediatric nurses, 3 dietitians, 1 FA patient, and 2 parents whose children had FA. They were requested to provide independent assessment on the relevance of these items in assessing FA knowledge and confidence in FA management based on a 4-point scale from “not relevant,” “somewhat relevant,” “quite relevant,” to “very relevant.” The panel also scored items for their semantic equivalence to suitability for the Chinese population using a 4-point Likert scale from “not appropriate,” “somewhat appropriate,” “quite appropriate,” to “very appropriate.” Among the 52 items, the content validity index (CVI) and semantic equivalence (SE) for FA knowledge questions ranged from 0.48 to 0.98 and 47.9% to 100%, respectively. Twenty-two questions fulfilled the criteria of ≥0.8 for both CVI and SE.10 Two ambiguous questions were subsequently deleted, with one being unrelated to FA in Hong Kong and the other having no prospective data confirmed by oral food challenge. The CVI and SE for questions on confidence in FA management ranged from 0.79 to 0.94 and 77.1% to 95.8%, respectively, and 10 questions were selected on the basis of their acceptable CVI and SE. These 30 questions were further reviewed and fine-tuned by panel members to make the wording clearer for both children and adults.
Phase 2
Our final set of 20-item FAKQ and 10-item CIFAMS instruments was translated by bilingual experts from English into Chinese according to standard forward and backward translation methods.11 Both English and Chinese versions were reviewed by pediatric allergists and nurses to ensure clarity and comprehension for local Chinese. After minor modifications, the finalized items were pilot tested for feasibility on 13 subjects consisting of the same 9 pediatric allergists, general pediatricians, nurses, and dietitians who assessed the selected questions, as detailed above, as well as 2 research assistants, 1 mother of a food-allergic child, and 1 food-allergic patient. The members were either our research staff or collaborators, while FA children were randomly selected from our allergy clinic. In addition, we distributed the questionnaires to children over 8 years old to check for comprehensibility and feasibility. We found that children younger than 12 could not understand the questions. Finally, all items, as described in Table E4 (available in the Online Repository at www.jaci-global.org), were deemed appropriate and understandable for completion by subjects aged ≥12 years old.
Phase 3
This cross-sectional study targeted enrollment of 150 subjects ≥12 years of age with self-reported adverse food reactions (AFRs) in the recent 2 years and parents of patients with AFR within 2 years who were younger than 12 from our pediatric outpatient clinics. After completing the questionnaires, subjects’ FA diagnosis was ascertained by pediatric allergists’ assessment of their AFRs together with positive results from skin prick testing (ALK Abelló, Hørsholm, Denmark) and/or serum-specific IgE (Thermo Fisher Scientific, Waltham, Mass) for cow’s milk, egg yolk, egg white, wheat, soy, peanut, mixed fish, mixed shellfish, almond, and other foods as clinically indicated according to subjects’ allergic history. For uncertain cases, subjects underwent either open-label or double-blind, placebo-controlled food challenges. In other words, these subjects had either IgE-mediated (immediate) or delayed-onset FA. All subjects could read traditional Chinese. Subjects were excluded if they were unable to give verbal informed assent or had a mental illness that affected their judgment or ability to learn. All subjects completed a questionnaire on demographics, type and number of food restrictions and FA, and details of previous allergic reactions. They also filled out the FAKQ, the CIFAMS questionnaire, and the age-appropriate FA quality-of-life questionnaire (FAQLQ). Forty subjects were randomly selected to repeat these questionnaires 5 days later to evaluate test–retest reliability.
Instruments
FAKQ
The 20-item FAKQ included 12 true–false questions and 8 multiple-choice questions. One point was awarded for each correct answer in these questions, while no mark was given to incorrect or unknown answers. Patients must select all the correct items for each question because we want them to be able to recognize all the signs of allergic reactions.
CIFAMS
Each question of the CIFAMS 10-item tool was scored on a 5-point Likert scale, ranging from 0 “not confident” to 4 “very confident.”
FAQLQ series
The FAQLQ series consisted of 4 separate questionnaires. The FAQLQ’s parental form was used for parents of children aged ≤12 years,12 which consisted of 14 items for parents of children aged 0-3 years, 26 items for parents of children aged 4-6 years, and 30 items for parents of children aged 7-12 years. The FAQLQ–children’s form with 24 items was applicable to patients aged 8-12 years,13 the FAQLQ–teenager form with 23 items was applicable to patients aged 13-17 years,14 and the adult form (FAQLQ-AF) with 29 items was applicable for patients aged ≥18 years.15 These questionnaires adopted a 7-point numerical scale from 0 “not at all” to 6 “very much.” Higher scores indicated greater clinical impact on quality of life (QoL). FA-independent measure (FAIM) was included in the last section of FAQLQ that contained questions on outcome expectation and independent measurement on a 7-point Likert scale, with higher scores indicating higher perceived severity.16
Statistical analyses
Internal consistency was evaluated by Cronbach alpha, with ≥0.70 indicating good internal consistency.17 The test–retest reliability of intraclass correlation coefficient was assessed in 40 subjects, with 0.4 to 0.7 considered acceptable.18 Pearson coefficient and Spearman rho were used to assess convergent validity for parametric and nonparametric variables, respectively. The construct validity of FAKQ and CIFAMS was assessed by their correlation with FAQLQ series, with coefficients 0.4 to 0.7 indicating moderate correlation.19 Discriminant validity referred to the sensitivity of potentially relevant covariates such as age, sex, type and number of food restrictions and FA, and symptoms of previous allergic reactions. Finally, exploratory factor analysis was performed to determine the underlying relationship among measured questionnaire items. Principal component analysis was used to identify potential relationship between the measured variables of FAKQ and CIFAMS. Factor loading of ≥0.30 was considered to be acceptable.20 All 2-sided statistical analyses were performed by SPSS v23 software (IBM, Armonk, NY, USA), with P < .05 being considered statistically significant.
Results
Study population
A convenience sample of 174 subjects with AFRs and parents of children with AFR were approached, of whom 155 (89%) agreed to participate. Table E5 (available in the Online Repository at www.jaci-global.org) summarizes their demographic and clinical features. Fifty-one parents consented to join the study and completed both questionnaires, including 17 parents with children in the 0-3-year-old group, 8 in the 4-6-year-old group, and 26 in the 7-12-year-old group. The remaining 104 food-allergic patients consisted of 2 patients in the 8-12-year-age range, 26 in the 13-17-year-age range, and 76 adults ≥18 years old. After consulting with allergists, 137 subjects (88%) were confirmed to have FA. About 70% of subjects were female, and two fifths had family history of allergic diseases. Most subjects (88%) reported FA to 1-6 kinds of food, with 62% being allergic to shellfish. Data for 2 patients in 8-12-year-old group were not analyzed because they could not understand the questions in our tools, as we mentioned above.
Table E6 (available in the Online Repository at www.jaci-global.org) illustrates the scores of different tools at baseline. Parents of children aged 0-12 years had the highest mean scale scores on FA knowledge (mean 10.1), while children aged 13-17 years had the highest confidence score in FA management (mean 22.2). However, this group of children had the lowest FAKQ score (mean 6.5). Mean scores for FAQLQ were the highest in parents of children aged 0-3 years (mean 2.7) and lowest in children aged 13-17 years (mean 1.9). For FAIM, adult patients reported the highest scores (mean 10.5).
Performance of FAKQ and CIFAMS
Internal consistency and test–retest reliability
Cronbach alpha for FAKQ was 0.69, suggesting acceptable but not very good internal consistency. Cronbach alpha remained at 0.65 to 0.70 if any single FAKQ item was deleted. In contrast, CIFAMS had good internal consistency, with Cronbach α ≥ 0.80, ranging between 0.83 and 0.86 if any single item was deleted. In the test–retest group, correlation between baseline and repeat FAKQ was very good, with the intraclass correlation coefficient being 0.74, which remained at 0.71 to 0.75 if any single item was deleted. The baseline and repeat FAKQ showed significant correlation, with P < .001. The correlation between baseline and second CIFAMS was also very good, with an intraclass correlation coefficient of 0.89, which was 0.87 to 0.89 if any single item was deleted. The baseline CIFAMS also strongly correlated with that repeated 5 days later (P < .001).
Construct validity
FAKQ and CIFAMS correlated significantly in the overall group (P = .002), among parents of children aged 4-12 years (P = .005), and in adult patients (P = .002) (see Table E7 in the Online Repository at www.jaci-global.org). There was no correlation between FAKQ and FAQLQ, and we found significant correlation between CIFAMS and parent-report FAIM (P = .005); between CIFAMS and total FAQLQ score (P = .015); and between CIFAMS and FAIM (P = .005).
Discriminant validity
The ability of FAKQ and CIFAMS to discriminate between different subgroups of potentially relevant covariates was studied (Table I). The total score on FAKQ differed significantly among subgroups in the following ways: by subject age (P = .002), with respect to food restriction (P = .027), with peanut allergy (P = .009), and whether patients were hospitalized for FA (P < .001). For the CIFAMS total score, they showed significant associations for sex (P = .026), egg allergy (P = .032), and milk allergy (P = .019). There were also significant correlations between children with chronic urticaria and their parents’ total FAKQ score (P = .002), and between parental and patients’ FAKQ scores and hospitalization due to FA (P = .022 and .029, respectively).
Table I.
Sensitivity by significant within-group differences among potentially relevant variables for FAKQ and CIFAMS
| Variable | FAKQ |
CIFAMS |
||||
|---|---|---|---|---|---|---|
| Overall | Parents | Patients | Overall | Parents | Patients | |
| Age (who answers) | 0.248§ | 0.024 | 0.140 | −0.050 | −0.015 | 0.030 |
| Age (patient) | −0.122 | 0.104 | 0.140 | 0.099 | 0.040 | 0.030 |
| Relationship | 0.279§ | — | — | −0.109 | — | — |
| Sex | 0.045 | −0.202 | −0.031 | −0.179∗ | −0.118 | −0.178 |
| With food restriction | 0.177∗ | 0.198 | 0.110 | 0.010 | 0.063 | 0.026 |
| With FA | 0.032 | 0.043 | −0.008 | −0.114 | −0.037 | −0.131 |
| Egg allergy | 0.125 | 0.019 | −0.041 | −0.172∗ | −0.221 | −0.078 |
| Cow’s milk allergy | 0.065 | −0.068 | 0.036 | −0.189∗ | −0.170 | −0.179 |
| Peanut allergy | 0.210† | 0.151 | 0.089 | −0.079 | −0.093 | −0.009 |
| Fish allergy | 0.105 | 0.098 | −0.048 | −0.141 | −0.077 | −0.132 |
| Shellfish allergy | −0.030 | 0.111 | −0.010 | −0.032 | −0.044 | −0.070 |
| Asthma | −0.007 | −0.007 | 0.031 | 0.017 | −0.231 | 0.103 |
| Allergic rhinitis | 0.096 | 0.190 | 0.085 | 0.006 | −0.084 | 0.034 |
| Chronic urticaria | 0.201∗ | 0.429§ | −0.033 | −0.006 | 0.176 | −0.094 |
| Eczema (atopic dermatitis) | 0.040 | 0.034 | −0.096 | 0.029 | 0.216 | 0.012 |
| Require hospitalization for FA | 0.276§ | 0.320∗ | 0.214∗ | 0.050 | 0.050 | 0.070 |
Analyzed by logistic regression for continuous independent variable (age) and Pearson correlation coefficients for binary independent variables.
Correlation significant at .05 level (2-tailed).
Correlation significant at .01 level (2-tailed).
Correlation significant at .005 level (2-tailed).
Factor analysis
When we analyzed data by varimax rotation with Kaiser normalization on the 4 factors with eigenvalues greater than 1, all 20 items of FAKQ had strong factor loading of ≥0.3 and could be categorized into 4 domains, as follows: administration of autoinjector (6 items), avoidance of exposure (4 items), anaphylaxis and symptoms (6 items), and basic FA knowledge (4 items) (Table II). For CIFAMS, all items also exhibited strong loads of ≥0.3. These items were grouped into 2 domains: general concepts about FAs (7 items) and administration of autoinjectors (3 items) (Table III).
Table II.
Exploratory factor analysis for 20-item FAKQ
| Item | Factor |
Domain | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Q2 | 0.722 | Administration of autoinjectors | |||
| Q3 | 0.612 | 0.313 | Administration of autoinjectors | ||
| Q7 | 0.797 | Administration of autoinjectors | |||
| Q16 | 0.750 | Administration of autoinjectors | |||
| Q17 | 0.660 | Administration of autoinjectors | |||
| Q19 | 0.355 | −0.314 | Administration of autoinjectors | ||
| Q5 | 0.636 | Avoiding exposure | |||
| Q11 | 0.481 | Avoiding exposure | |||
| Q13 | 0.452 | −0.378 | Avoiding exposure | ||
| Q15 | 0.635 | Avoiding exposure | |||
| Q4 | 0.369 | −0.497 | Anaphylaxis and symptoms | ||
| Q6 | 0.417 | Anaphylaxis and symptoms | |||
| Q9 | 0.603 | Anaphylaxis and symptoms | |||
| Q10 | 0.506 | Anaphylaxis and symptoms | |||
| Q12 | 0.588 | Anaphylaxis and symptoms | |||
| Q14 | 0.303 | 0.502 | Anaphylaxis and symptoms | ||
| Q1 | 0.510 | Basic FA knowledge | |||
| Q8 | 0.594 | Basic FA knowledge | |||
| Q18 | 0.305 | Basic FA knowledge | |||
| Q20 | 0.328 | Basic FA knowledge | |||
Table III.
Exploratory factor analysis for 10-item CIFAMS
| Item | Factor |
Domain | |
|---|---|---|---|
| 1 | 2 | ||
| Q7 | 0.849 | FA: General concepts | |
| Q8 | 0.817 | FA: General concepts | |
| Q9 | 0.744 | FA: General concepts | |
| Q1 | 0.731 | FA: General concepts | |
| Q6 | 0.688 | FA: General concepts | |
| Q2 | 0.660 | FA: General concepts | |
| Q10 | 0.591 | FA: General concepts | |
| Q5 | 0.881 | Administration of autoinjectors | |
| Q4 | 0.876 | Administration of autoinjectors | |
| Q3 | 0.696 | Administration of autoinjectors | |
Subjects’ responses in FAKQ and CIFAMS
Less than two fifths of subjects provided accurate answers to 11 items in FAKQ. To identify the questions that subjects had when they answered FAKQ and CIFAMS, we found that they had the lowest scores for items 11, 13, and 14 of FAKQ (Table IV) and items 3 to 5 of CIFAMS (Table V). Question 13 asked how to introduce new foods to a baby with FA, and question 14 was a multiple choice on signs of an allergic reaction. For CIFAMS, items 3 to 5 enquired when and how to confidently use an epinephrine autoinjector.
Table IV.
Summary of correct responses at baseline and retest for different FAKQ items
| Item | Baseline |
Retest |
||||
|---|---|---|---|---|---|---|
| Overall (n = 155) | Patients (n = 104) | Parents (n = 51) | Overall (n = 41) | Patients (n = 30) | Parents (n = 11) | |
| Q1 | 130 (83.9) | 83 (79.8) | 47 (92.2) | 38 (92.7) | 28 (93.3) | 10 (90.9) |
| Q2 | 31 (20.0) | 13 (12.5) | 18 (35.3) | 12 (29.3) | 6 (20.0) | 6 (54.5) |
| Q3 | 38 (24.5) | 22 (21.2) | 16 (31.4) | 14 (34.1) | 9 (30.0) | 5 (45.5) |
| Q4 | 27 (17.4) | 17 (16.3) | 10 (19.6) | 7 (17.1) | 3 (10.0) | 4 (36.4) |
| Q5 | 99 (63.9) | 65 (62.5) | 34 (66.7) | 27 (65.9) | 21 (70.0) | 6 (54.5) |
| Q6 | 61 (39.4) | 38 (36.5) | 23 (45.1) | 21 (51.2) | 15 (50.0) | 6 (54.5) |
| Q7 | 45 (29.0) | 16 (15.4) | 29 (56.9) | 12 (29.3) | 6 (20.0) | 6 (54.5) |
| Q8 | 113 (72.9) | 76 (73.1) | 37 (72.5) | 31 (75.6) | 22 (73.3) | 9 (81.8) |
| Q9 | 52 (33.5) | 37 (35.6) | 15 (29.4) | 19 (46.3) | 14 (46.7) | 5 (45.5) |
| Q10 | 73 (47.1) | 46 (44.2) | 27 (52.9) | 26 (63.4) | 19 (63.3) | 7 (63.6) |
| Q11 | 123 (79.4) | 81 (77.9) | 42 (82.4) | 33 (80.5) | 23 (76.3) | 10 (90.9) |
| Q12 | 88 (56.8) | 56 (53.8) | 32 (62.7) | 29 (70.7) | 20 (66.7) | 9 (81.8) |
| Q13 | 18 (11.5) | 9 (8.7) | 9 (17.6) | 4 (9.8)∗ | 0 | 4 (36.4) |
| Q14 | 11 (7.1)∗ | 7 (6.7) | 4 (7.8) | 4 (9.8)∗ | 3 (10.0) | 1 (9.1) |
| Q15 | 106 (68.4) | 72 (89.2) | 34 (66.7) | 34 (82.9) | 24 (80.0) | 10 (90.9) |
| Q16 | 54 (34.8) | 26 (25.0) | 28 (54.9) | 17 (41.5) | 9 (30.0) | 8 (72.7) |
| Q17 | 63 (40.6) | 37 (35.6) | 26 (51.0) | 20 (48.8) | 15 (50.0) | 5 (45.5) |
| Q18 | 108 (69.7) | 69 (66.3) | 39 (76.5) | 30 (73.2) | 23 (76.7) | 7 (63.6) |
| Q19 | 94 (60.6) | 59 (56.7) | 35 (68.6) | 21 (51.2) | 15 (50.0) | 6 (54.5) |
| Q20 | 20 (12.9) | 12 (11.5) | 8 (15.7) | 9 (22.0) | 6 (20.0) | 8 (72.7) |
Data are presented as nos. (%).
Questions with lowest numbers of correct responses.
Table V.
Summary of subjects with “very confident” score in different CIFAMS items
| Item | Baseline |
Retest |
||||
|---|---|---|---|---|---|---|
| Overall (n = 155) | Patients (n = 104) | Parents (n = 51) | Overall (n = 41) | Patients (n = 30) | Parents (n = 11) | |
| Q1 | 27 (17.4) | 24 (23.1) | 3 (5.9) | 11 (7.1) | 11 (10.6) | 0 |
| Q2 | 14 (9.0) | 12 (11.5) | 2 (3.9) | 7 (4.5) | 6 (5.8) | 1 (2.0) |
| Q3 | 4 (2.6)∗ | 4 (3.8) | 0 | 4 (2.6) | 4 (3.8) | 0 |
| Q4 | 2 (1.3)∗ | 2 (1.9) | 0 | 1 (0.6)∗ | 1 (1.0) | 0 |
| Q5 | 3 (1.9)∗ | 3 (2.9) | 0 | 2 (1.3)∗ | 2 (1.9) | 0 |
| Q6 | 29 (18.7) | 26 (25.0) | 3 (5.9) | 11 (7.1) | 11 (10.6) | 0 |
| Q7 | 28 (18.1) | 25 (24.0) | 3 (5.9) | 8 (5.2) | 8 (7.7) | 0 |
| Q8 | 13 (8.4) | 13 (12.5) | 0 | 6 (3.9) | 6 (5.8) | 0 |
| Q9 | 15 (9.7) | 14 (13.5) | 1 (2.0) | 6 (3.9) | 5 (4.8) | 1 (2.0) |
| Q10 | 14 (9.0) | 12 (11.5) | 2 (3.9) | 5 (3.2) | 5 (4.8) | 0 |
Data are presented as nos. (%).
Questions with lowest score for “very confident.”
Discussion
This study constructed and validated a 20-item FAKQ and a 10-item CIFAMS (Table E4) as 2 new tools for assessing FA knowledge and confidence in FA management in 155 Chinese subjects. Both tools significantly correlated with each other and with FAIM, and showed acceptable internal consistency in baseline and retest groups. Total score of FAKQ also discriminated patients with prior hospitalization for FA. FAKQ items were grouped into 4 domains and CIFAMS items into 2 domains. On the bases of these 2 questionnaires, subjects were most worried about their lack of knowledge regarding signs of allergic reaction and had poor confidence using an epinephrine autoinjector.
Most subjects who completed the questionnaires were female because most children were accompanied to our clinics by their mothers as primary caregivers. Therefore, high mean scores for FAKQ were understandable because mothers are often the ones who encounter food choices and avoidance decisions for FA children.21 In addition, this group scored highest in disrupted QoL, a result also reported by Polloni et al.22 Interestingly, children aged 8-12 reported the highest confidence in FA management and the least impact of FA on their QoL, yet they had the lowest FAKQ scores of all groups. They either overestimated their ability to deal with FAs or knew that they were protected by their families23 such that they did not need to worry about their FAs.
This study found Cronbach alpha for FAKQ at baseline and on repeat to be acceptable but not very good (<0.8). This might be due to the nature of these questionnaires, where questions received lower scores if they involved situations that subjects have not encountered before. Because these allergic events could be unique to anyone, our tool might not yield a high degree of internal consistency. Tavakol and Dennick described that heterogeneous test items could lead to lower Cronbach alpha values because they were not intercorrelated.24 However, we thoroughly discussed and revised all retrieved items with high CVI and SE instead of removing them, even though some items might marginally affect the overall Cronbach alpha. Our construct validity results showed significant correlation between FAKQ and CIFAMS. Regarding subjects’ QoL, we could not identify any literature that reported a relationship between FA knowledge and QoL. While FAQLQ was not significantly associated with FAKQ in this study, the former was associated with CIFAMS, possibly because well-being, as reflected by QoL, is often influenced by patients’ and parents’ perceptions of FA, as indicated by their attitudes and confidence in FA management. A study found that greater parental confidence in managing FA predicted a better QoL.25
Among patients hospitalized for FA, FAKQ showed significant results for the entire group or in parent and patient subgroups (Table I). Thus, people with a history of more severe AFR were more aware of the importance of FA knowledge and management skills. This study also found significant association between total FAKQ score and parents of children with chronic urticaria. It is understandable that these parents would search for more information about the management of chronic urticaria and related allergies.26 Our results revealed significant associations between CIFAMS and egg and milk allergies that might be explained by the higher confidence of parents in managing these allergies when their children have outgrown such problems.27,28 Nonetheless, this finding contradicts those of a previous local study in which impaired QoL in parents was significantly associated with cow’s milk and egg allergies in their children.29 These discrepant observations suggest that assessing FA-specific QoL and management confidence are separate and complementary dimensions when managing families with children with FA.
FAKQ questions were categorized into 4 domains by exploratory factor analysis, while those of CIFAMS were grouped into 2 domains only (Tables II and III). On the one hand, this may be explained by the fact that some FAKQ questions were too unique to be grouped together.30 On the other hand, CIFAMS only enquired about subjects’ responses to certain FA scenarios and the use of epinephrine autoinjectors. Regarding the accuracy of subjects’ responses to FAKQ, we found that they were weak in selecting the signs of anaphylaxis by a multiple-choice question. Many participants found it difficult to indicate all signs of anaphylaxis. Additionally, many participants had the lowest scores on items that assessed when and how to use an epinephrine autoinjector, which remains the single most important step in treating anaphylaxis. A survey revealed that education about emergency treatment should be provided to parents of food-allergic children; any delay might endanger patients’ safety.31
There are several limitations in this study. Given that subjects were recruited from a public hospital, our findings might not be generalizable to those patients who consulted private practitioners for their FA, who might have different health beliefs and practices as well as different socioeconomic status. It is also not possible for us to compare responses between children’s mothers and fathers, as only 6 fathers were recruited (Table E5), and those with lower socioeconomic status. The 2016 Hong Kong census revealed that 80% of people aged ≥15 years received secondary and higher education. Consistent with this finding, close to two thirds of our participants had a tertiary-level education (Table E5). Further, we targeted this study to recruit 150 subjects and children’s parents at baseline and 40 subjects for test–retest reliability. The lack of prescription data on epinephrine autoinjectors did not allow us to analyze subjects’ FA knowledge and management confidence with respect to this variable. In addition to completing FAKQ and CIFAMS, subjects filled out age-appropriate FAQLQ and FAIM tools. As a result of the nature of our convenience sampling, these subjects were not evenly distributed among different subgroups in relation to the 4 FAQLQ questionnaires. There were only 2 subjects for the FAQLQ–children’s form (8-12 years old), while 26 completed the FAQLQ–teenager form (13-17 years old). Despite these limitations, this study found our developed FAKQs and CIFAMS to be valid and appropriately reliable for assessing FA knowledge and confidence in the management of FA. If we were able to recruit a larger sample, we might find cutoff values of the tools that reflected knowledge deficit and inability to manage FA. Educational programs should be developed for parents of children with food allergens to provide consistent and accurate information as well as to address parents’ and food-allergic patients’ confidence in adverse reaction management.
In conclusion, we developed and validated 2 questionnaires, FAKQ and CIFAMS, for assessing knowledge of and confidence in FA management in food-allergic subjects and parents of food-allergic children. These assessment tools can provide important information for understanding deficient dimensions of FA care and formulating appropriate education programs and healthy advocacy for those with FA.
Key message.
-
•
Validated questionnaires can be a useful assessment tool in identifying gaps in FA knowledge and confidence in FA management.
Acknowledgments
We thank the members of Pediatric Allergy team (www.allergycuhk.org/aboutus) at the Chinese University of Hong Kong and the study subjects for helping us develop and validate the questionnaires.
Footnotes
This project was supported by the Health and Medical Research Fund (reference 04180047), Food and Health Bureau of Hong Kong SAR Government; a Hong Kong Institute of Allergy research grant; a Lee Hysan Foundation research grant, United College of the Chinese University of Hong Kong (to A.S.Y.L.); and the Research Impact Fund (reference R4035-19) of the Research Grants Council, Hong Kong SAR Government (to T.F.L.).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Gupta R.S., Warren C.M., Smith B.M., Jiang J., Blumenstock J.A., Davis M.M., et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2018.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellinger S.V., Stateman A., Srour H., Baguley J., Rivera-Mariani F.E. Evaluating differences in prevalence of food allergies between two geographic regions: Australia and US. J Allergy Clin Immunol. 2019;143:AB268. [Google Scholar]
- 3.Leung T.F., Yung E., Wong Y.S., Lam C.W., Wong G.W. Parent-reported adverse food reactions in Hong Kong Chinese pre-schoolers: epidemiology, clinical spectrum and risk factors. Pediatr Allergy Immunol. 2009;20:339–346. doi: 10.1111/j.1399-3038.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 4.Loke P., Koplin J., Beck C., Field M., Dharmage S.C., Tang M.L. Statewide prevalence of school children at risk of anaphylaxis and rate of adrenaline autoinjector activation in Victorian government schools, Australia. J Allergy Clin Immunol. 2016;138:529–535. doi: 10.1016/j.jaci.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Leung A.S., Li R.M., Au A.W., Rosa Duque J.S., Ho P.K., Chua G.T., et al. Changing pattern of pediatric anaphylaxis in Hong Kong, 2010-2019. Pediatr Allergy Immunol. 2022;33 doi: 10.1111/pai.13685. [DOI] [PubMed] [Google Scholar]
- 6.Li P.H., Leung A.S., Li R.M., Leung T.F., Lau C.S., Wong G.W. Increasing incidence of anaphylaxis in Hong Kong from 2009 to 2019—discrepancies of anaphylaxis care between adult and paediatric patients. Clin Transl Allergy. 2020;10:51. doi: 10.1186/s13601-020-00355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knibb R.C., Barnes C., Stalker C. Parental self-efficacy in managing food allergy and mental health predicts food allergy–related quality of life. Pediatr Allergy Immunol. 2016;27:459–464. doi: 10.1111/pai.12569. [DOI] [PubMed] [Google Scholar]
- 8.Abrams E.M., Simons E., Roos L., Hurst K., Protudjer J.L. Qualitative analysis of perceived impacts on childhood food allergy on caregiver mental health and lifestyle. Ann Allergy Asthma Immunol. 2020;124:594–599. doi: 10.1016/j.anai.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Arkwright P.D., Farragher A.J. Factors determining the ability of parents to effectively administer intramuscular adrenaline to food allergic children. Pediatr Allergy Immunol. 2006;17:227–229. doi: 10.1111/j.1399-3038.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 10.Norwood S.L. Prentice-Hall; Upper Saddle River (NJ): 2000. Research strategies for advanced practice nurses. [Google Scholar]
- 11.Brislin R.W. In: Field methods in cross-cultural research. Lonner W.J., Berry J.W., editors. Sage; Beverly Hills (Calif): 1986. The wording and translation of research instrument; pp. 137–164. [Google Scholar]
- 12.DunnGalvin A., de Blok Flokstra B.M., Burks A.W., Dubois A.E., Hourihane J.O. Food allergy QoL questionnaire for children aged 0-12 years: content, construct, and cross-cultural validity. Clin Exp Allergy. 2008;38:977–986. doi: 10.1111/j.1365-2222.2008.02978.x. [DOI] [PubMed] [Google Scholar]
- 13.Flokstra-de Blok B.M., DunnGalvin A., Vlieg-Boerstra B.J., Oude Elberink J.N., Duiverman E.J., Hourihane J.O., et al. Development and validation of a self-administered Food Allergy Quality of Life Questionnaire for children. Clin Exp Allergy. 2009;39:127–137. doi: 10.1111/j.1365-2222.2008.03120.x. [DOI] [PubMed] [Google Scholar]
- 14.Flokstra-de Blok B.M., DunnGalvin A., Vlieg-Boerstra B.J., Oude Elberink J.N., Duiverman E.J., Hourihane J.O., et al. Development and validation of the self-administered Food Allergy Quality of Life Questionnaire for adolescents. J Allergy Clin Immunol. 2008;122 doi: 10.1016/j.jaci.2008.05.008. 139-44.e1442. [DOI] [PubMed] [Google Scholar]
- 15.Flokstra-de Blok B.M., van der Meulen G.N., DunnGalvin A., Vlieg-Boerstra B.J., Oude Elberink J.N., Duiverman E.J., et al. Development and validation of the Food Allergy Quality of Life Questionnaire–Adult Form. Allergy. 2009;64:1209–1217. doi: 10.1111/j.1398-9995.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Velde J.L., Flokstra-de Blok B.M., Vlieg-Boerstra B.J., Oude Elberink J.N., DunnGalvin A., Hourihane J.O., et al. Development, validity and reliability of the food allergy independent measure (FAIM) Allergy. 2010;65:630–635. doi: 10.1111/j.1398-9995.2009.02216.x. [DOI] [PubMed] [Google Scholar]
- 17.Taber K.S. The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res Sci Educ. 2018;48:1273–1296. [Google Scholar]
- 18.Rosner B. 8th ed. Thomson Brooks/Cole; Duxbury (Mass): 2015. Fundamentals of biostatistics. [Google Scholar]
- 19.Schober P., Boer C., Schwarte L.A. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126:1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 20.Ab Hamid M.R., Mustafa Z., Idris F., Abdullah M., Suradi N.R. Measuring value-based productivity: a confirmatory factor analytic (CFA) approach. Int J Business Soc Sci. 2011;2:85–93. [Google Scholar]
- 21.Moen Ø.L., Opheim E., Trollvik A. Parents experiences raising a child with food allergy; a qualitative review. J Pediatr Nurs. 2019;46:e52–e63. doi: 10.1016/j.pedn.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Polloni L., Toniolo A., Lazzarotto F., Baldi I., Foltran F., Gregori D., et al. Nutritional behavior and attitudes in food allergic children and their mothers. Clin Transl Allergy. 2013;3:41. doi: 10.1186/2045-7022-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner E.M., Dahlquist L. Intolerance of uncertainty and protective parenting: the mediating role of maternal appraisals and the moderating role of child health status. Child Health Care. 2022;51:263–284. [Google Scholar]
- 24.Tavakol M., Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–55. doi: 10.5116/ijme.4dfb.8dfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knibb R., Barnes C., Stalker C. Greater confidence in management of food allergy by parents predicts better quality of life. Clin Transl Allergy. 2015;5:O7. [Google Scholar]
- 26.Jaros J., Shi V.Y., Katta R. Diet and chronic urticaria: dietary modification as a treatment strategy. Dermatol Pract Concept. 2019;10 doi: 10.5826/dpc.1001a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sicherer S.H., Wood R.A., Vickery B.P., Jones S.M., Liu A.H., Fleischer D.M., et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol. 2014;133:492–499. doi: 10.1016/j.jaci.2013.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood R.A., Sicherer S.H., Vickery B.P., Jones S.M., Liu A.H., Fleischer D.M., et al. The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol. 2013;131:805–812. doi: 10.1016/j.jaci.2012.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung T.F., Yung E., Wong Y.S., Li C.Y., Wong G.W. Quality-of-life assessment in Chinese families with food-allergic children. Clin Exp Allergy. 2009;39:890–896. doi: 10.1111/j.1365-2222.2008.03192.x. [DOI] [PubMed] [Google Scholar]
- 30.Ferrando P.J., Lorenzo-Seva U. Assessing the quality and appropriateness of factor solutions and factor score estimates in exploratory item factor analysis. Educ Psychol Meas. 2018;78:762–780. doi: 10.1177/0013164417719308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glassberg B., Nowak-Wegrzyn A., Wang J. Factors contributing to underuse of epinephrine autoinjectors in pediatric patients with food allergy. Ann Allergy Asthma Immunol. 2021;126:175–179.e3. doi: 10.1016/j.anai.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.