Abstract
Background
Respiratory syncytial virus (RSV) is the most frequent cause of bronchiolitis. Precise and updated information about demographic characteristics, clinical manifestations, and risk factors for severe disease are needed for optimal implementation of upcoming new therapeutic and preventive interventions.
Objectives
The main goals of this study were to define the epidemiology of acute bronchiolitis in hospitalized young children during 5 calendar years in Spain; evaluate the differences in clinical manifestations between children hospitalized with RSV infection and those hospitalized with non-RSV infection; and identify demographic characteristics, clinical parameters, and risk factors associated with disease severity.
Methods
We performed a retrospective review of the medical records of children younger than 2 years who were hospitalized with bronchiolitis between January 2015 and December 2019. We constructed multivariable models to identify independent predictors of disease severity defined as length of hospital stay (LOS), pediatric intensive care unit (PICU) admission, and need for a high-flow-nasal canula (HFNC).
Results
From January 2015 to December 2019, 1437 children were hospitalized with bronchiolitis and met the inclusion criteria. The proportion of children hospitalized with bronchiolitis caused by RSV increased significantly during the study period, from 60% to 65% (P = .03). The children with RSV bronchiolitis were younger than those with non-RSV bronchiolitis (median age = 3 months [interquartile range = 1.5-6.5 months] vs 4 months [interquartile range = 2-7.5 months], respectively (P < .01). The children younger than 6 months with RSV bronchiolitis had enhanced disease severity compared with those with non-RSV bronchiolitis, as defined by an LOS of more than 4 days, severity scores, need for an HFNC, intravenous fluids, enteral feeding, and PICU admissions (P < .01). Age younger than 6 months and RSV-positive etiology were independently associated with greater odds of PICU admission, need for an HFNC, and longer LOS.
Conclusion
This study identified differences in disease severity between young children with RSV bronchiolitis and those with non-RSV bronchiolitis. These differences are particularly significant in children younger than 6 months, who comprise a group of infants with suboptimal innate immunity to RSV and may benefit from new preventive strategies.
Key words: Bronchiolitis, RSV, epidemiology, age
Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis; it represents about 60% to 80% of all cases, especially during epidemic peaks. However, several other viruses are associated with bronchiolitis; they include rhinovirus, parainfluenza virus, human metapneumovirus, seasonal coronaviruses, and adenovirus among others.1, 2, 3
Previously published studies on the epidemiology and clinical aspects of bronchiolitis in hospitalized patients have reported contradictory results. Different studies conducted in the United States in the 2000s suggested that there was a decrease in the incidence of bronchiolitis hospitalizations.4,5 In England, however, the rates of hospitalization for bronchiolitis in infants younger than 1 year increased an average of 1.8% per year between 2004 and 2011, and in children younger than 2 years, higher rates of hospitalization per 1000 children per year were observed between 2001 and 2016.6
Despite the burden of bronchiolitis on children’s health, specific treatments or vaccines are not yet available. However, it is expected that in the coming years we will have new preventive and therapeutic agents against RSV.7,8 Precise and updated information about the demographic characteristics, clinical manifestations, temporal trends, and risk factors for disease severity in young children of different ages with acute bronchiolitis and with different underlying conditions are relevant for evaluation and clinical implementation of new therapeutic and preventive interventions. This will help define the groups of patients who would benefit the most from these upcoming RSV prevention strategies, such as maternal vaccines and prolonged half-life mAbs.9, 10, 11, 12, 13, 14
The main goals of this study were to define the epidemiology of acute bronchiolitis in hospitalized young children during 5 calendar years (from January 2015 to December 2019) in Spain, a European country with an excellent and easily accessible public health care system, to evaluate the differences in clinical manifestations between children hospitalized with RSV infection and those hospitalized with non-RSV infection, as well as to identify demographic characteristics, clinical parameters, and risk factors associated with disease severity.
Methods
Study population
We conducted a retrospective observational study that included all children younger than 2 years who were hospitalized with a diagnosis of acute bronchiolitis at the Department of Pediatrics of the Hospital Gregorio Marañón over a period of 5 calendar years from January 1, 2015, to December 31, 2019. This pediatric hospital is a university-based tertiary care facility in Madrid, Spain, with more than 1600 children hospitalized in the general pediatric wards every year.
Patients were identified by using the pediatric wards’ registry and discharge diagnosis codes. After review of the medical records, only those children with a first episode of bronchiolitis in the first 2 years of life were selected. Patients with incomplete data were excluded.
Data collection
Data were collected from electronic health care records and included epidemiologic, demographic, clinical, and laboratory data, as well as diagnostic test results, disease severity parameters, and interventions during the hospitalization. In addition, we collected data on exposures and the presence of chronic underlying conditions such as the following: prematurity; chronic lung disease; exposure to secondhand smoke; breast-feeding; food allergies; malnutrition; day care attendance; and family history of atopy, asthma, or allergies.
Also, we collected data on the results of laboratory tests (RSV rapid antigen test and Alere BinaxNOW RSV Card [Abbott, Chicago, Ill]); hemoglobin levels; white blood cell counts with differential; C-reactive protein levels; chest radiograph findings; and use of medical interventions such as bronchodilators, systemic steroids, antibiotics, enteral feedings, and intravenous fluids at any time during the hospitalization.
Disease severity was assessed on the basis of a clinical disease severity score (Wood-Downes score modified by Ferré score)15 and standard parameters of severity, including the following: length of hospital stay (LOS), need for and duration of supplemental O2 use such as via a nasal cannula or high-flow nasal cannula (HFNC), need for pediatric intensive care unit (PICU) admission, ventilatory support such as continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), and mechanical ventilation. The disease severity score was assessed at the time of admission. Scoring ranged from 0 (normal) to 14 (most abnormal) and comprised 6 parameters: respiratory rate, heart rate, wheezing, auscultation, cyanosis, and retractions. According to the Wood-Downes score modified by Ferré score, values of 8 or more are considered severe bronchiolitis.15 Malnutrition was defined as a weight-for-age percentile below the 3rd percentile or z score of 2 or lower.16
Finally, we collected data on pneumonia and atelectasis diagnosed on the basis of radiographic changes as defined by pediatric radiologists. Pneumonia was considered when the radiographic reports included words such as infiltrate, consolidation, and pneumonia. Atelectasis was defined by the presence of volume loss.17
Statistical analyses
Descriptive analyses were performed by using frequency distributions and rates. Means (± SDs) or medians (25th-75th percentiles) were used to summarize patients’ demographic and baseline characteristics. The chi-square test for trends was used to determine significant changes in hospitalization rates over time. Comparisons of continuous variables were carried out by using the Student t test, Mann-Whitney U test, 1-way ANOVA, or Kruskal Wallis test according to data distribution and the number of groups. Comparisons of categoric variables were performed by using the chi-square test or Fisher exact test, as appropriate. The Benjamini-Hochberg test was applied to correct for multiple comparisons when applicable. The relationships between quantitative variables were examined by using the Spearman correlation coefficient. Multivariable models were constructed to identify independent predictors of severity defined as LOS, PICU admission, and need for an HFNC by linear or logistic regression as appropriate. Variables that were significant in univariate analyses (P < .2) or were biologically meaningful were introduced in the models. Patients who did not undergo viral testing were excluded from the multivariate models. Associations of risk factors with qualitative variables (HFNC use and PICU admissions) were displayed as odds ratios (ORs) and 95% CIs; for LOS, they were described as risk ratios and 95% CIs, which represent the antilog of the regression parameter estimates and confidence limits. The goodness of fit of the final set of predictors was expressed as the coefficient of determination for multivariate analysis (R2). We used the Akaike information criterion to compare multivariate models. Assumptions of multicollinearity were evaluated in all models. Statistical analysis was performed by using R statistical software, version 3.5.3.18 A 2-tailed P value less than .05 was considered significant.
Ethical issues
The study was approved by the Hospital Gregorio Marañón institutional review board, with waived informed consent (institutional review board no. CEIC 437/15).
Results
Bronchiolitis admissions and seasonality
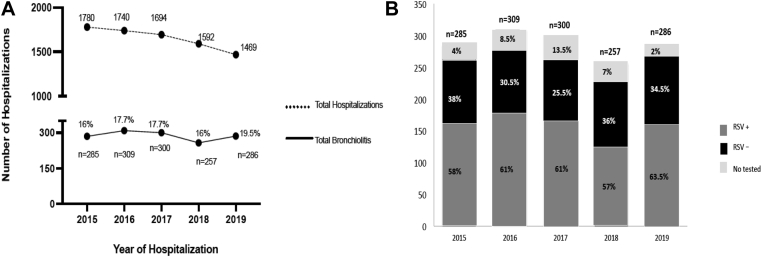
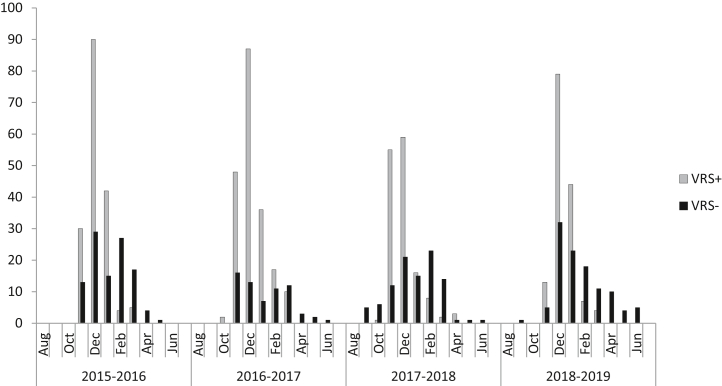
From January 1, 2015, to December, 31, 2019, 1437 children were hospitalized for bronchiolitis and met the inclusion criteria. An RSV diagnostic test was performed on 1315 patients (92%), with 838 of them (63.7%) testing positive for RSV. There was a trend toward an increasing proportion of hospitalizations for bronchiolitis in relation to all-cause hospitalizations during the study period, from 16% in 2015 to 19.5% in 2019 (P = .06). The proportion of children hospitalized with bronchiolitis caused by RSV increased significantly from 60% to 65% (P = .03) (Fig 1, A and B), whereas the percentage of cases of non-RSV bronchiolitis decreased from 38% in 2015 to 34.5% in 2019 (P = .04). The highest proportions of hospitalizations occurred in December (n = 507 [35.5%]), November (242 [16.8%]), and January (241 [16.7%]). The initial cases of RSV were commonly identified in the second half of October, and the last cases were identified most commonly in mid-March (Fig 2).
Fig 1.
A, Bronchiolitis hospitalizations increased significantly from 2015 to 2019. The x-axis represents each study year. The y-axis reflects the total number of admissions to the infant ward and the total number and percentage of bronchiolitis hospitalizations, which increased from 2015 to 2019 (P =.06). B, RSV bronchiolitis and non-RSV bronchiolitis hospitalizations in Gregorio Marañón Hospital per year from 2015 to 2019. Hospitalizations attributed to RSV bronchiolitis increased significantly from 2015 to 2019 (P = .03).
Fig 2.
Monthly distribution of hospitalizations for RSV bronchiolitis and non-RSV bronchiolitis. The horizontal axis (x-axis) represents the 4 seasons analyzed and the months of each season; the vertical axis (y-axis) shows the total number of RSV-positive and RSV-negative bronchiolitis cases identified per month during the study period.
Demographic and clinical characteristics
We included 1437 children younger than 2 years who were hospitalized with bronchiolitis. Overall, the median age of the study patients was 3.0 months (interquartile range [IQR] = 1.5-7 months); 56.6% of the infants were males. The main demographic and clinical characteristics according to their RSV status (RSV-positive vs RSV-negative) are summarized in Table I.
Table I.
Differences between RSV and non-RSV bronchiolitis
| Characteristic | Total (n = 1,437)∗ | RSV (n = 838) | Non-RSV (n = 477) | P value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (mo) | 3 (1.5-7) | 3 (1.5-6.5) | 4 (2-7.5) | <.01 |
| Sex (male) | 814 (56.64%) | 454 (54.17%) | 281 (58.90%) | .82 |
| Malnutrition | 295 (20.52%) | 153 (18.26%) | 114 (23.89%) | .01 |
| Previously healthy | 1,059 (73.69%) | 650 (77.57%) | 332 (69.61%) | .85 |
| Underlying conditions | 378 (26.31%) | 188 (22.43%) | 145 (30.39%) | <.01 |
| Heart disease | 33 (2.29%) | 16 (1.91%) | 14 (2.93%) | |
| Neurologic disease | 22 (1.53%) | 10 (1.19%) | 11 (2.31%) | |
| Malformations | 17 (1.18%) | 8 (0.95%) | 6 (1.26%) | |
| Congenital infections | 6 (0.42%) | 6 (0.71%) | 0 (0%) | |
| Endocrinologic disease | 6 (0.42%) | 4 (0.48%) | 2 (0.42%) | |
| Hematologic disease | 4 (0.28%) | 3 (0.36%) | 1 (0.21%) | |
| Others | 10 (0.69%) | 6 (0.71%) | 3 (0.63%) | |
| Preterm births | 280 (19.48%) | 135 (16.11%) | 108 (22.64%) | |
| With other comorbidities | 58 (4.10%) | 24 (2.86%) | 25 (5.24%) | |
| Without other comorbidities | 222 (15.44%) | 111 (13.24%) | 83 (17.40%) | |
| Prematurity: gestational age (wk) | 280 (19.48%) | 135 (16.11%) | 108 (22.64%) | <.01 |
| <28 | 30 (2.10%) | 13 (1.55%) | 15 (3.14%) | .07 |
| 28-31 (+ 6) | 41 (2.85%) | 11 (1.31%) | 21 (4.40%) | <.01 |
| 32-36 (+ 6) | 209 (14.54%) | 111 (13.24%) | 72 (15.10%) | .36 |
| Laboratory test results | ||||
| Hemoglobin level (g/dL) | 11.2 (10.2-12.5) | 11.1 (10.2-12.2) | 11.2 (10.4-12) | .91 |
| WBC count | 11,500 (9,000-14,800) | 11,300 (8,750-14,600) | 12,200 (9,000-14,550) | .37 |
| Absolute neutrophil count | 4,900 (3,200-7,200) | 4,800 (3,000-7,000) | 5,000 (3,300-7,975) | .41 |
| Absolute lymphocyte count | 4,600 (3,400-6,200) | 4,650 (3,400-15,300) | 4,450 (3,475-6,200) | .84 |
| CRP level (mg/dL) | 1.6 (0.5-3.7) | 1.65 (0.6-4.15) | 1.5 (0.5-3.45) | .42 |
| Radiologic findings | ||||
| Radiologic evaluation | 579 (40.3%) | 359 (42.8%) | 183 (38.4%) | .11 |
| No pathologic findings | 292 (50.4%) | 173 (48.2%) | 93 (50.8%) | .58 |
| Atelectasis | 46 (7.9%) | 37 (10.3%) | 8(4.3%) | .02 |
| Focal consolidation | 238 (41.1%) | 146 (40.6%) | 82 (44.8%) | .35 |
| Pneumothorax | 3 (0.5%) | 3 (0.8%) | 0 (0%) | .55 |
| Treatments | ||||
| Antibiotics | 386 (26.86%) | 241 (28.75%) | 125 (26.20%) | .33 |
| Bronchodilators | 1,113 (77.45%) | 617 (73.62%) | 392 (82.18%) | <.01 |
| Systemic steroids | 131 (9.12%) | 59 (7.10%) | 60 (12.57%) | <.01 |
| Intravenous fluids | 499 (34.72%) | 348 (41.52%) | 124 (25.99%) | <.01 |
| Enteral feeding | 236 (16.42%) | 177 (21.12%) | 53 (11.11%) | <.01 |
| Parameters of disease severity | ||||
| Severity score ≥8 | 433 (30.13%) | 305 (36.39%) | 102 (21.38%) | <.01 |
| Apnea | 173 (12.03%) | 108 (12.88%) | 60 (12.57%) | .93 |
| LOS (d) | 4 (3-5.7) | 5 (3-7) | 4 (2-6) | <.01 |
| Received O2 | 1,319 (91.78%) | 800 (95.46%) | 415 (87.01%) | <.01 |
| Duration of O2 therapy (d) | 3 (2-6) | 4 (2-6) | 2 (1-5) | <.01 |
| HFNC | 448 (31.17%) | 310 (36.99%) | 113 (23.68%) | <.01 |
| PICU admission | 230 (16.01%) | 174 (20.76%) | 51 (10.69%) | <.01 |
| Time in PICU (d) | 4 (2-7) | 4 (2-6.5) | 3 (2-7) | .09 |
| CPAP/BiPAP | 206 (14.33%) | 159 (18.97%) | 42 (8.81%) | <.01 |
| MV | 28 (1.94%) | 22 (2.62%) | 4 (0.83%) | <.01 |
This table displays total bronchiolitis cases (ie, RSV bronchiolitis and non-RSV bronchiolitis cases) but patients not tested were excluded. Wood-Downes severity scores higher than 8 are considered severe. The treatment section reflects the need for antibiotics, bronchodilators, steroids, intravenous fluids, or enteral nutrition at any time during admission. In 122 cases, no viral testing was performed. Continuous variables were analyzed by using the Mann-Whitney U test, and categoric variables were analyzed by using the Fisher exact or chi-square test. Data are reported as medians and IQRs (25%-75%). Boldface indicates statistical significance.
CRP, C-reactive protein; MV, mechanical ventilation; WBC, white blood cell.
Comparisons between RSV positive and negative children
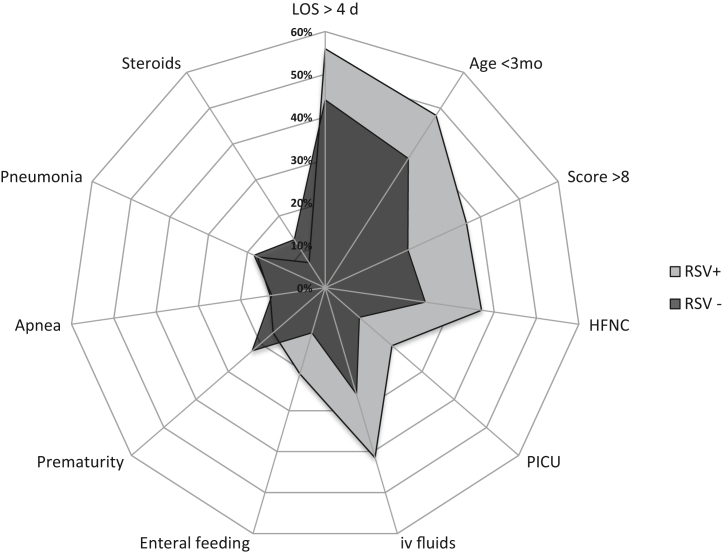
Among the 1437 children hospitalized with acute bronchiolitis, RSV was detected in 838 (58.3%); the detection rate when we included only those patients on whom a diagnostic test was performed was 63.2% (838 of 1326). Fig 3 shows the main differences between children with RSV bronchiolitis and those with non-RSV bronchiolitis. The children with RSV bronchiolitis were younger than those with non-RSV bronchiolitis (3 months [IQR = 1.5-6.5] vs 4 months [IQR = 2-7.5] [P < .01]). The proportion of children with underlying conditions was significantly lower in the RSV group than in the non-RSV group (22.43% vs 30.39%, respectively [P < .01]). No differences were observed in the use of antibiotics between groups; however, among children with documented pneumonia or atelectasis on chest radiograph, the proportion of antibiotic use was lower in children with RSV than in the non-RSV group (77% vs 89% [P = .03]). Total white blood cell counts and neutrophil and lymphocyte percentages did not differ significantly between patients with RSV bronchiolitis and patients with non-RSV bronchiolitis (Table I). Disease severity was worse in children hospitalized with RSV bronchiolitis in all parameters evaluated, including the following: longer LOS (5 days [IQR = 3-7 days] vs 4 days [IQR = 2-6 days] [P < .01]), higher percentage of children with a Wood-Downes severity score of 8 or higher (36.4% vs 21.3% [P < .01]), higher proportion of children requiring an HFNC (37% vs 23.6% [P < .01]), need for CPAP/BiPAP (19% vs 8.8% [P < .01]), need for mechanical ventilation (2.62% vs 0.8% [P < .01]), and higher proportion of PICU admissions (20.7% vs 10.6% [P < .01]) (Table I).
Fig 3.
Differences between RSV positivity and RSV negativity. Results were expressed as percentages
Disease severity in patients with RSV bronchiolitis and non-RSV bronchiolitis according to age
We analyzed whether bronchiolitis severity differed according to age. To this end, we divided the cohort into children younger than 6 months and children older than 6 months. We found that children younger than 6 months with RSV bronchiolitis had enhanced disease severity compared with children with non-RSV bronchiolitis. Disease severity was defined by an LOS exceeding 4 days, severity scores, need for an HFNC, intravenous fluids, enteral feeding, and PICU admissions (P < .01).
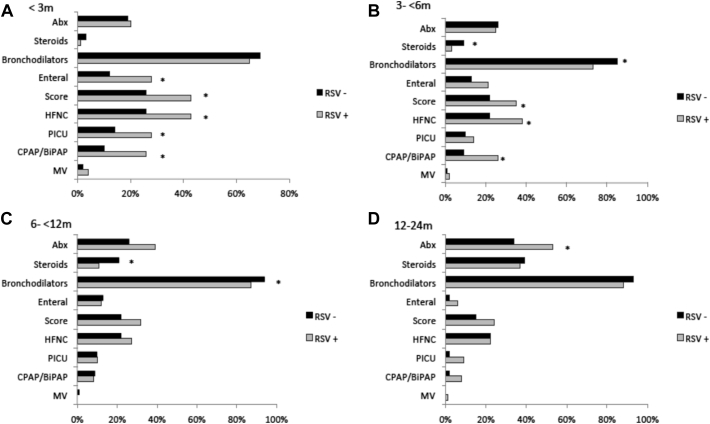
Next, we stratified the RSV bronchiolitis and non-RSV cohorts into 4 different age groups: 0 months to younger than 3 months (n = 577 [43.9%]), 3 months to younger than 6 months (n = 317 [24.1%]), 6 months to 12 months (n = 282 [21.4%]), and older than 12 months to 24 months (n = 139 [10.6%]) (Table II and Fig 4). Using this approach, we observed that disease severity was greater in infants younger than 3 months of age with RSV bronchiolitis than in those with non-RSV bronchiolitis in terms of LOS (6 days [IQR = 4-9 days] vs 4 days [2-6.2 days] [P < .01]), duration of supplemental O2 use [5 days (IQR = 2-7 days) vs 2 days [IQR = 1-5 days] [P < .01]), need for PICU admission (28.6% vs 14.5% [P < .01]), need for an HFNC (43.7% vs 26.7% [P < .01]), and need for CPAP/BiPAP therapy (26.5% vs 10.5% [P < .01]). In addition, the percentage of children with a severity score 8 or higher was greater in the RSV group (43% vs 26% [P < .01]) (Table II and Fig 4).
Table II.
Differences between RSV and non-RSV bronchiolitis at different ages
| Characteristic | < 3 mo |
3 to <6 mo |
6 to <12 mo |
12-24 mo |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RSV (405) | No RSV (172) | P value | RSV (184) | No RSV (133) | P value | RSV (163) | No RSV (119) | P value | RSV (86) | No RSV (53) | P value | |
| Demographic characteristics | ||||||||||||
| Sex ( male) | 48% | 38% | .02 | 46.2% | 41.4% | .42 | 43% | 40% | .62 | 39% | 51% | .16 |
| Preterm | 12% | 17% | .10 | 20.1% | 32.3% | .01 | 16.6% | 25.2% | .09 | 27.9% | 11.3% | .03 |
| Malnutrition | 18.6% | 24% | .17 | 15.8% | 27.3% | .01 | 14.7% | 23.5% | .06 | 29% | 17.6% | .15 |
| Laboratory test results | ||||||||||||
| Hemoglobin level | 11 (10-12) | 11 (10-13) | .44 | 11 (10-12) | 11 (10-11.5) | .10 | 11 (10-11.5) | 11 (10-12) | .56 | 11(10-12) | 12 (11-13) | .68 |
| WBC count (× 103) | 10.3 (8-13) | 10 (8-13) | .84 | 12 (9-16) | 13 (9-15) | .76 | 12 (9-15) | 13 (10-14) | .52 | 12 (9-15) | 11 (8-16) | .73 |
| Neuthrophil count (× 103) | 4.1 (2.5-6) | 3.7 (2-5) | .74 | 6 (3-7.6) | 5 (4-8.5) | .62 | 6 (4-8) | 6 (4-8.7) | .55 | 6 (5-9) | 6 (4-11) | .91 |
| Lymphocyte count (× 103) | 4.7 (3.2-6) | 5 (3.5-7) | .33 | 5 (4-7.1) | 4 (3-5.9) | .13 | 4.6 (3-5) | 4.4 (3-6) | .89 | 4 (3-5) | 3 (2 -4) | .60 |
| CRP level | 1.5 (0.4-3) | 1 (0.2-2) | .52 | 1.5 (0.6-3) | 1 (0.5-2) | .56 | 2 (0.8-5) | 1.9 (0.8-4) | .62 | 2 (0.7-4) | 1.6 (0.7-4) | .73 |
| Radiologic findings | ||||||||||||
| Radiologic evaluation | 150 (37%) | 52 (30.2%) | .12 | 73 (39.7%) | 55 (41.4%) | .80 | 85 (52.1%) | 56 (47%) | .46 | 51 (59.3%) | 20 (37.7%) | .01 |
| No findings | 89 (59.3%) | 29 (55.8%) | .74 | 24 (32.8%) | 29 (52.7%) | .04 | 43 (50.6%) | 30 (53.6%) | .73 | 17 (33.3%) | 5 (25%) | .58 |
| Atelectasis | 19 (12.7%) | 4 (7.7%) | .45 | 14 (19.2%) | 4 (7.3%) | .07 | 4 (4.7%) | 0 (0%) | .15 | 0 (0%) | 0 (0%) | .99 |
| Focal consolidation | 40 (26.7%) | 19 (36.5%) | .21 | 35 (47.9%) | 22 (40%) | .47 | 37 (43.5%) | 26 (46.4%) | .86 | 34 (66.7%) | 15 (75%) | .57 |
| Pneumothorax | 2 (1.3%) | 0 (0%) | .99 | 0 (0%) | 0 (0%) | .99 | 1 (1.2%) | 0 (0%) | .99 | 0 (0%) | 0 (0%) | .99 |
| Treatments | ||||||||||||
| Antibiotics (%) | 20% | 19% | .73 | 25% | 26% | .89 | 39% | 32% | .31 | 53% | 34% | .03 |
| Bronchodilators | 65% | 69% | .47 | 73% | 85% | .01 | 87% | 94% | .02 | 88% | 93% | .72 |
| Systemic steroids | 0.5% | 2.5% | .06 | 2.5% | 8.5% | .03 | 11% | 21% | .02 | 37% | 39% | .85 |
| Intravenous fluids | 43.5% | 28.5% | <.01 | 44.6% | 21.8% | <.01 | 35% | 28.6% | .30 | 38.5% | 22.7% | .07 |
| Enteral feeding | 28% | 12% | <.01 | 21.2% | 13.5% | .10 | 12.3% | 10% | .70 | 6% | 2% | .40 |
| Parameters of disease severity | ||||||||||||
| Severity score ≥ 8 (%) | 43% | 26% | <.01 | 36.4% | 22% | .01 | 32% | 22% | .14 | 24% | 15% | .32 |
| Severity score | 5 (4-9) | 5 (4-8) | <.01 | 5 (4-8) | 5 (4-6) | <.01 | 5 (4-8) | 5 (4-6) | .27 | 5 (4-7) | 5 (4-6) | .84 |
| Apnea | 22% | 25% | .84 | 8% | 9% | .81 | 1.5% | 3.5% | .24 | 2.3% | 0% | .52 |
| LOS | 6 (4-9) | 4 (2-6.2) | <.01 | 5 (3-7) | 5 (3-8) | .35 | 4 (3-7) | 4 (2-6) | .08 | 4 (2-6) | 3 (2-5) | .14 |
| Duration of O2 (d) | 5 (2-7) | 2 (1-5) | <.01 | 4 (2-6) | 3 (2-5) | .04 | 3 (2-5) | 2 (1-4) | .02 | 3 (1-5) | 2 (1-3) | .10 |
| HFNC | 43.7% | 26.7% | <.01 | 38% | 22% | <.01 | 27% | 21% | .26 | 22.1% | 22.6% | .90 |
| PICU admission | 28.6% | 14.5% | <.01 | 18.5% | 10.5% | .05 | 9.8% | 9.2% | .90 | 9.3% | 1.9% | .15 |
| Time in PICU (d) | 4 (2-7) | 3 (2-7) | .29 | 4 (2-5) | 3 (2-5) | .44 | 3 (1-4) | 2.5 (2-4) | .76 | 5 (2-9) | 3 (3-3) | .80 |
| CPAPP/BiPAP | 26.5% | 10.5% | <.01 | 17.4% | 9% | .04 | 8% | 9.25% | .82 | 8% | 1.9% | .15 |
| MV | 4.2% | 1.7% | .21 | 2.2% | 0% | .14 | 0% | 0.8% | .42 | 1.2% | 0% | .90 |
This table displays total bronchiolitis cases (ie, RSV bronchiolitis and non-RSV bronchiolitis cases). The treatment section reflects the need for antibiotics, bronchodilators, steroids, intravenous fluids, or enteral nutrition at any time during admission. Continuous variables were analyzed by using the Mann-Whitney U test, and categoric variables were analyzed by using the Fisher exact or chi-square test. Data are reported as medians and IQRs (25%-75%). Boldface indicates statistical significance.
CRP, C-reactive protein; MV, mechanical ventilation; WBC, white blood cell.
Fig 4.
Treatments and parameters of disease severity according RSV status and stratified age. All items were stratified according RSV (blue) versus no RSV (orange) and by age younger than 3 months (A), between 3 and 6 months (B), older than 6 months to12 months (C), and older than 12 months to 24 months (D). The y-axis represents the treatments and parameters of disease severity in the 2 groups, and the x-axis represents the frequency of each item (%). Comparisons were done by using the Fisher exact test. Asterisk indicates significant 2-sided P values. This figure reflects only the treatments administered during the time of admission. Bronchodilators were always used via inhalation. Abx, Need for antibiotics at any time during admission; MV, mechanical ventilation.
Greater disease severity in children with RSV bronchiolitis was also documented in children between 3 and 6 months of age, who also had a longer duration of supplemental O2 use (4 days [IQR = 2-6 days] vs 3 days [IQR = 2-5 days], respectively [P = .04]) but not in terms of LOS (5 days [IQR = 4-8 days] vs 5 days [4-6 days] [P = .35]). The proportion of children requiring an HFNC was higher in the RSV group (38% vs 22% [P < .01]) as well as in terms of PICU admissions (18.5% vs 10.5% [P = .05]) and the need for CPAP/BiPAP (17.4% vs 9% [P = .04]) (Fig 4 and Table II).
When we performed a similar analysis in children between 6 and 12 months of age according RSV status, we did not find significant differences, except for a longer duration of supplemental O2 use in the RSV group (3 days [IQR = 2-5 days] vs 2 days [IQR = 1-4 days] [P = .02]). Differences in disease severity between children with RSV bronchiolitis and those with non-RSV bronchiolitis were no longer identified in children between 12 and 24 months of age. Taken together, these results indicate that bronchiolitis caused by RSV is more severe than that caused by other viruses, especially in infants younger than 6 months.
Independent predictors of disease severity
Lastly, we analyzed which factors were independently associated with disease severity defined by PICU admission, need for an HFNC, and longer LOS as dependent variables. Age younger than 6 months (OR = 3.03 [95% CI = 1.72-5.26]), RSV positivity (OR = 1.71 [95% CI = 1.01-2.96]), apnea (OR = 3.52 [95% CI = 1.95-6.46]), and lower absolute lymphocyte counts (OR = 1.03 [95% CI = 1.01-1.06]) were independently associated with greater odds of PICU admission (Table III). Likewise, age younger than 6 months (OR = 2.17 [95% CI = 1.58-3.03]), RSV positivity (OR = 1.91 [95% CI = 1.46-2.52]), apnea (OR = 2.25 [95% CI = 1.57-3.24]), malnutrition (OR = 1.71 [95% CI = 1.26-2.32]), and atelectasis (OR = 3.71 [95% CI = 1.59-14.0]) were independently associated with greater odds of HFNC use (Table III). Lastly, we found that age younger than 6 months, malnutrition, apnea, RSV positivity, and documented pneumonia and/or atelectasis were also independent risk factors for longer LOS (Table IV).
Table III.
Risk factors for PICU admission and need for HFNC determined by multivariate logistic regression analysis
| Characteristic | Need for PICU admission/HFNC |
||
|---|---|---|---|
| PICU admission | |||
| OR | 95% CI | P value | |
| Age < 6 mo | 3.03 | (1.72-5.26) | <.01 |
| Sex (male) | 1.15 | (0.73-1.81) | .53 |
| Malnutrition | 1.69 | (0.98-2.90) | .05 |
| Apnea | 3.52 | (1.95-6.46) | <.01 |
| RSV | 1.71 | (1.01-2.96) | .04 |
| Low absolute lymphocyte count | 1.03 | (1.01-1.06) | <.01 |
| Need for HFNC | |||
| Age < 6 mo | 2.17 | (1.58-3.03) | .01 |
| Malnutrition | 1.71 | (1.26-2.32) | .01 |
| Apnea | 2.25 | (1.57-3.24) | <.01 |
| RSV | 1.91 | (1.46-2.52) | <.01 |
| Atelectasis | 3.71 | (1.59-14.0) | <.01 |
For the first model (with PICU admission as the dependent variable), R2 = 0.30 and Akaike information criterion = 472.13; for the second model, (with need for HFNC as the dependent variable), R2 = 0.40 and Akaike information criterion = 1228.1. Both models were made by excluding those patients not tested for RSV (122 patients). Boldface indicates statistical significance.
Table IV.
Risk factors for LOS determined by multivariate lineal regression analysis
| Characteristic | Risk ratio | 95% CI | P value |
|---|---|---|---|
| Age < 6 mo | 5.26 | 2.95-9.10 | <.01 |
| Malnutrition | 3.98 | 2.84-5.59 | <.01 |
| Apnea | 6.76 | 4.44-10.2 | <.01 |
| RSV | 3.04 | 2.27-4.05 | <.01 |
| Pneumonia/atelectasis | 7.46 | 3.96-10.81 | <.01 |
R2 = 0.28; F statistic (5) = 44.97.
Age, RSV, apnea, malnutrition and pneumonia/atelectasis were analyzed as categoric variables in relation with LOS. Associations of risk factors in lineal regression were displayed as ratios and 95% CIs, which represent the antilog of the regression parameter estimates and confidence limits. Boldface indicates statistical significance.
Discussion
The present study shows that RSV remains the main etiologic agent of severe bronchiolitis requiring hospitalization during the respiratory season in Spain (November-March) and that the percentage of bronchiolitis caused by RSV increased throughout the study period. We confirmed that RSV bronchiolitis was more severe in terms of LOS, duration of supplemental O2 use, severity score, PICU admission, and need for an HFNC and ventilatory support, especially in infants younger than 6 months of age. Even in a cohort of hospitalized children only, age had a significant impact in disease severity in children with RSV infection. It is well known that there are currently no effective therapies for the treatment of bronchiolitis, except for the administration of O2 and respiratory support in the most severe cases (see Table E1 in the Online Repository at www.jaci-global.org).
In agreement with the findings of previous studies, we found that the proportion of hospitalizations for bronchiolitis increased significantly during the study period, from 16% in 2015 to 19.5% in 2019. In addition, the percentage of cases caused by RSV increased from 58% in 2015 to 63.5% in 2019. Other investigators previously found an increase in rates of hospitalizations for bronchiolitis during the periods 2002-2007 and 2001-2016,6,19,20 although yet other authors have reported reductions in hospitalization rates.4,5,11
We found that 73.69% of children hospitalized with bronchiolitis had no underlying medical conditions. In addition, RSV-positive infants had a lower percentage of underlying conditions than did those with non-RSV bronchiolitis (22.4% vs 30.3%). These results are in agreement with those of Garcia et al,19 who also found a major proportion of underlying conditions in the non-RSV group, which likely reflects the implementation of prophylaxis with anti-RSV mAbs in the high-risk groups. In addition, the detection of RSV as a cause of bronchiolitis was associated with less frequent antibiotic administration. Perhaps, the lack of knowledge of the etiologic agent in cases of respiratory infection led physicians to prescribe antibiotics more frequently in those cases. This observation provides another important argument to encourage viral testing in patients hospitalized for bronchiolitis.
A recent study9 reported that age differences had a significant impact on clinical manifestations and severity in children with RSV bronchiolitis. The study highlighted the importance of age stratification to better understanding of this disease and its pathogenesis. In the present study, when we stratified the patient cohorts by age groups and according to RSV status, we found significant differences in most of the parameters of disease severity analyzed (LOS, duration of O2 therapy, PICU admission, need for an HFNC, and need for mechanical ventilation). Young infants (ie, those younger than 3 months) who were RSV positive had a more severe disease, and the differences in disease severity parameters were documented until 6 months of age. This is consistent with the findings of other studies in which younger infants were at higher risk of need for supplemental O2 administration and PICU admission.11,21, 22, 23, 24, 25, 26 These clinical data are correlated quite precisely with improved understanding of the infant's immune system, in which the innate immune response against RSV, especially the interferon response, does not appear to be protective until after 6 months of age.27,28
These results demonstrate the importance of thoroughly analyzing the impact of age on this disease, as new preventive interventions such as vaccines and prolonged half-life mAbs are being evaluated in clinical trials.9 In this new era, it is essential to define the burden of disease in each subgroup of patients rather than only in those at high-risk, because the new prolonged half-life mAbs and maternal vaccines are directed to all at-term previously healthy infants, who represent approximately 70% to 80% of hospitalized children, as shown in the present study. Having precise and updated clinical information in terms of disease severity of the different age groups will be important to guide decisions for the implementation of these new prevention strategies.7,8,29,30 An additional challenge is to also obtain similar accurate data for outpatients with RSV infection so as to better define the burden of disease in this important subgroup.
Adjusted analyses confirmed that younger age, RSV etiology, apnea, and lymphopenia were independently associated with disease severity. Other authors31,32 have reported an association between lower lymphocyte counts and worse disease severity in previously healthy infants younger than 12 months with RSV bronchiolitis. Similarly, lymphopenia has been identified as a risk factor in other viral infections such as COVID-19 or influenza.33,34 Nevertheless, information regarding the role of lymphopenia in bronchiolitis disease severity is still limited. In our data set, lymphopenia was barely significant as an independent risk factor for PICU admission, suggesting that low lymphocyte counts are indeed associated with worse clinical disease severity.
Our study has several limitations. The definition of bronchiolitis is controversial; in this study we have included infants as old as 24 months, even though other definitions suggest including only infants no older than 12 months. Our study’s retrospective design may have introduced some bias due to missing data. In addition, no viral diagnostic studies were performed outside the respiratory season, and even during the epidemic period we only performed an RSV-specific test. Thus, the RSV-negative group is not well characterized, and we were unable to analyze the role of viral coinfections. Nevertheless, the number of cases analyzed during the 5-year period allowed us to identify important and clinically relevant differences between children with RSV bronchiolitis and those with non-RSV bronchiolitis.
In conclusion, this study identified differences in disease severity between young children with RSV infections and those with non-RSV infections, and it showed that these differences are especially significant in children younger than 6 months. The majority of children hospitalized with RSV bronchiolitis during the 5 study seasons were previously healthy and without any apparent risk factors. Infants younger than 6 months of age with or without risk factors for severe RSV infection constitute an important group that may benefit from novel preventive strategies.
Acknowledgments
We want to thank the nurses of the Hospital General Universitario Gregorio Marañón infant ward for their help with the study, and especially the patients and families that participated in this study.
Footnotes
Supported in part by the Fondo de Investigaciones Sanitarias (grants PI16/00822 and grant PI 21/00840 [to R.R.-F.]).
Disclosure of potential conflict of interest: R. Rodriguez-Fernandez has received fees for lectures from Abbvie, and Sanofi; has received fees for participation in Advisory Boards from Sanofi, Astra Zeneca, and Merck; and has received research grants from FIS (Fondo de Investigaciones Sanitarias). A. Mejias has received research grants from Janssen, Merck, the NIH, and the Bill & Melinda Gates foundation, and fees for participation in Advisory Boards from Janssen, Merck, Sanofi, and Reviral, and for lectures from Sanofi. O. Ramilo has received research grants from Janssen, Merck, the NIH, and the Bill & Melinda Gates foundation, and fees for participation in Advisory Boards from Sanofi, Merck, Pfizer, and Adagio, and for lectures from Pfizer, Sanofi, and AstraZeneca. The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Florin T.A., Plint A.C., Zorc J.J. Viral bronchiolitis. Lancet. 2017;389:211–224. doi: 10.1016/S0140-6736(16)30951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall C.B., Simőes E.A., Anderson L.J. Clinical and epidemiologic features of respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:39–57. doi: 10.1007/978-3-642-38919-1_2. [DOI] [PubMed] [Google Scholar]
- 3.Bont L., Checchia P.A., Fauroux B., Figueras-Aloy J., Manzoni P., Paes B., et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in Western countries. Infect Dis Ther. 2016;5:271–298. doi: 10.1007/s40121-016-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasegawa K., Tsugawa Y., Brown D.F., Mansbach J.M., Camargo C.A., Jr. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132:28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujiogi M., Goto T., Yasunaga H., Fujishiro J., Mansbach J.M., Camargo C.A., Jr., et al. Trends in bronchiolitis hospitalizations in the United States: 2000-2016. Pediatrics. 2019;144 doi: 10.1542/peds.2019-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green C.A., Yeates D., Goldacre A., Sande C., Parslow R.C., McShane P., et al. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch Dis Child. 2016;101:140–146. doi: 10.1136/archdischild-2015-308723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin M.P., Yuan Y., Takas T., Domachowske J.B., Madhi S.A., Manzoni P., et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383:415–425. doi: 10.1056/NEJMoa1913556. [DOI] [PubMed] [Google Scholar]
- 8.Madhi S.A., Polack F.P., Piedra P.A., Munoz F.M., Trenholme A.A., Simões E.A.F., et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426–439. doi: 10.1056/NEJMoa1908380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenes-Chacon H., Garcia-Mauriño C., Moore-Clingenpeel M., Mertz S., Ye F., Cohen D.M., et al. Age-dependent interactions among clinical characteristics, viral loads and disease severity in young children with respiratory syncytial virus infection. Pediatr Infect Dis J. 2021;40:116–122. doi: 10.1097/INF.0000000000002914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muñoz-Quiles C., López-Lacort M., Úbeda-Sansano I., Alemán-Sánchez S., Pérez-Vilar S., Puig-Barberà J., et al. Population-based analysis of bronchiolitis epidemiology in Valencia, Spain. Pediatr Infect Dis J. 2016;35:275–280. doi: 10.1097/INF.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 11.Hervás D., Reina J., Yañez A., del Valle J.M., Figuerola J., Hervás J.A. Epidemiology of hospitalization for acute bronchiolitis in children: differences between RSV and non-RSV bronchiolitis. Eur J Clin Microbiol Infect Dis. 2012;31:1975–1981. doi: 10.1007/s10096-011-1529-y. [DOI] [PubMed] [Google Scholar]
- 12.Cangiano G., Nenna R., Frassanito A., Evangelisti M., Nicolai A., Scagnolari C., et al. Bronchiolitis: analysis of 10 consecutive epidemic seasons. Pediatr Pulmonol. 2016;51:1330–1335. doi: 10.1002/ppul.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbati F., Moriondo M., Pisano L., Calistri E., Lodi L., Ricci S., et al. Epidemiology of respiratory syncytial virus-related hospitalization over a 5-year period in Italy: evaluation of seasonality and age distribution before vaccine introduction. Vaccines. 2020;8:15. doi: 10.3390/vaccines8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui D., Feng L., Chen Y., Lai S., Zhang Z., Yu F., et al. Clinical and epidemiologic characteristics of hospitalized patients with laboratory-confirmed respiratory syncytial virus infection in eastern China between 2009 and 2013: a retrospective study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferres Mataro J., Mangues Bafalluy M.A., Farre Riba R., Julia Brugues A., Bonal de Falgas J. [Subcutaneous adrenaline versus inhaled salbutamol in the treatment of childhood asthmatic crisis] An Esp Pediatr. 1987;27:37–40. [PubMed] [Google Scholar]
- 16.De Cosmi V., Mehta N.M., Boccazzi A., Milani G.P., Esposito S., Bedogni G., et al. Nutritional status, metabolic state and nutrient intake in children with bronchiolitis. Int J Food Sci Nutr. 2017;68:378–383. doi: 10.1080/09637486.2016.1245714. [DOI] [PubMed] [Google Scholar]
- 17.Hilmes M.A., Daniel Dunnavant F., Singh S.P., Ellis W.D., Payne D.C., Zhu Y., et al. Chest radiographic features of human metapneumovirus infection in pediatric patients. Pediatr Radiol. 2017;47:1745–1750. doi: 10.1007/s00247-017-3943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ Available at: Accessed November 19, 2021.
- 19.García C.G., Bhore R., Soriano-Fallas A., Trost M., Chason R., Ramilo O., et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126:e1453–e1460. doi: 10.1542/peds.2010-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho N.T., Thompson C., Nhan L.N.T., Van H.M.T., Dung N.T., Tran My P., et al. Retrospective analysis assessing the spatial and temporal distribution of paediatric acute respiratory tract infections in Ho Chi Minh City, Vietnam. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-016349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan P.W., Lok F.Y., Khatijah S.B. Risk factors for hypoxemia and respiratory failure in respiratory syncytial virus bronchiolitis. Southeast Asian J Trop Med Public Health. 2002;33:806–810. [PubMed] [Google Scholar]
- 22.Boyce T.G., Mellen B.G., Mitchel E.F., Jr., Wright P.F., Griffin M.R. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137:865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez-Auad J.P., Nava-Frías M., Casasola-Flores J., Johnson K.M., Nava-Ruiz A., Pérez-Robles V., et al. The epidemiology and clinical characteristics of respiratory syncytial virus infection in children at a public pediatric referral hospital in Mexico. Int J Infect. 2012;16:e508–e513. doi: 10.1016/j.ijid.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Zurita-Cruz J.N., Gutierrez-Gonzalez A., Manuel-Apolinar L., Fernández-Gárate J.E., Arellano-Flores M.L., Correa Gonzalez R.A., et al. Hospitalizations for viral respiratory infections in children under 2 years of age: epidemiology and in-hospital complications. BMC Pediatr. 2020;20:285. doi: 10.1186/s12887-020-02186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddadin Z., Beveridge S., Fernandez K., Rankin D.A., Probst V., Spieker A.J., et al. Respiratory syncytial virus disease severity in young children. Clin Infect Dis. 2021;73:e4384–e4391. doi: 10.1093/cid/ciaa1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mejias A., Brenes-Chacon H., Garcia-Mauriño C., Moore-Clingenpeel M., Ramilo O. Clinical disease severity scores and viral loads in children with RSV infection. Clin Infect Dis. 2021;72:e1160–e1162. doi: 10.1093/cid/ciaa1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinonen S., Velazquez V.M., Ye F., Mertz S., Acero-Bedoya S., Smith B., et al. Immune profiles provide insights into respiratory syncytial virus disease severity in young children. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aaw0268. [DOI] [PubMed] [Google Scholar]
- 28.Mejias A., Dimo B., Suarez N.M., Garcia C., Suarez-Arrabal M.C., Jartti T., et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mejias A., Rodríguez-Fernández R., Oliva S., Peeples M.E., Ramilo O. The journey to a respiratory syncytial virus vaccine. Ann Allergy, Asthma Immunol. 2020;125:36–46. doi: 10.1016/j.anai.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mejias A., Garcia-Maurino C., Rodriguez-Fernandez R., Peeples M.E., Ramilo O. Development and clinical applications of novel antibodies for prevention and treatment of respiratory syncytial virus infection. Vaccine. 2017;35:496–502. doi: 10.1016/j.vaccine.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aberle J.H., Aberle S.W., Dworzak M.N., Mandl C.W., Rebhandl W., Vollnhofer G., et al. Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med. 1999;160:1263–1268. doi: 10.1164/ajrccm.160.4.9812025. [DOI] [PubMed] [Google Scholar]
- 32.Ramos-Fernández J.M., Moreno-Pérez D., Antúnez-Fernández C., Milano-Manso G., Cordón-Martínez A.M., Urda-Cardona A. [Lower lymphocyte response in severe cases of acute bronchiolitis due to respiratory syncytial virus] An Pediatr. 2018;88:315–321. doi: 10.1016/j.anpedi.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Bermejo-Martin J.F., González-Rivera M., Almansa R., Micheloud D., Tedim A.P., Domínguez-Gil M., et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit Care. 2020;24:691. doi: 10.1186/s13054-020-03398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eşki A., Öztürk G.K., Gülen F., Çiçek C., Demir E. Risk Factors for Influenza Virus related severe lower respiratory tract infection in children. Pediatr Infect Dis J. 2019;38:1090–1095. doi: 10.1097/INF.0000000000002447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.