Abstract
Introduction and importance
Our study aims to expose the experience of our department in dealing with bladder leiomyosarcomas and illustrate the management tools of this rare pathology.
Case presentation
We present a retrospective study of 4 cases of bladder leiomyosarcoma, gathered in the Department of Urology during the period of 1996–2022. All our patients were exclusively male and aged between 35 and 73 years. No history of pelvic irradiation nor chemotherapy was found in our patients. Three patients had high-grade leiomyosarcoma and pT2 stage whereas only one had a low-grade tumour. Three patients underwent radical treatment by cystoprostatectomy with extensive bilateral pelvic lymph node dissection and one patient was treated by endoscopic re-resection and endoscopic monitoring. We noticed 2 lymph nodes and liver metastasis recurrences in 2 patients treated by radical surgery while 2 patients didn't present recurrences at two years of follow-up.
Clinical discussion
To date, there is no clear and precise therapeutic approach for the treatment of bladder leiomyosarcoma. Little is known about the long term survival associated with these tumours. All studies agree that the prognosis for bladder leiomyosarcoma is poor, if not diagnosed early, especially those presenting with an undifferentiated tumour grade, distant metastatis and treated without surgical therapy.
Conclusion
Bladder leiomyosarcoma is a rare and highly aggressive tumour. The anatomopathological examination provides diagnosis and prognosis assessment. Radical surgery remains the most suitable therapeutic approach.
Keywords: Leiomyosarcoma, Bladder cancer, Cystectomy, Chemotherapy
Highlights
-
•
Bladder leiomyosarcoma is rare and highly malignant. Diagnosis and prognosis are based on pathological examination.
-
•
The FNCLCC system seems to have the best performance in terms of objectivity and assessment of differentiation, mitotic activity and necrosis.
-
•
There is no consensus on management.
-
•
Radical surgery is the most suitable treatment option.
-
•
Chemotherapy and radiotherapy are promoted by some teams.
1. Introduction
Leiomyosarcoma of the bladder is a rare and potentially mesenchymal aggressive tumour. It represents about 0.1 % of bladder cancers [1]. Only small series and case reports are described in the literature because of the rarity of this pathology. Clinical and radiological presentations are comparable to urothelial cancers with more locally advanced and metastatic spreading forms. The risk factors are not yet well identified [2,3]. Commonly, leiomyosarcomas of the bladder have a poor prognosis. [1] The natural history and management are still nonconsensual.
Through a few cases, our study aims to expose the experience of our department in dealing with this pathology and illustrate the management tools.
2. Presentation of cases
We report a retrospective study of four cases of bladder leiomyosarcoma. We used the files of patients who had surgery of bladder tumours in our urology department from 1996 to 2022.
This work has been reported in line with the SCARE criteria.
All our patients were exclusively male with ages ranging from 35 to 73 years. No history of pelvic irradiation nor chemotherapy was observed. The clinical presentation was predominantly hematuria. Only one patient out of four didn't suffer from hematuria and the disease was revealed by combined lower urinary tract symptoms. Physical Examination showed in one case a hard, mobile hypogastric mass of 7 cm better felt by intra-rectal palpation. Ultrasonography showed the tumour in all four cases. All patients underwent transurethral resection of the bladder (TURB). Clinical, radiological and endoscopic characteristics are summarized in Table 1.
Table 1.
Clinical and endoscopic characteristics of patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age (years) | 35 | 46 | 73 | 50 |
| Medical hystory | Bladder surgery for lithiasis in childhood | – | Diabetes Hyepertension Coronary artery disease |
– |
| Tobacco (package years) | No | Yes (30 PY) | No | Yes (30 PY) |
| Hematuria | Final | No | Total | Total |
| Endoscopy |
|
|
|
|
Pathological examination and immunohistochemical analysis confirmed the presence of bladder leiomyosarcoma (shown in Fig. 1, Fig. 2). The different findings are shown in Table 2.
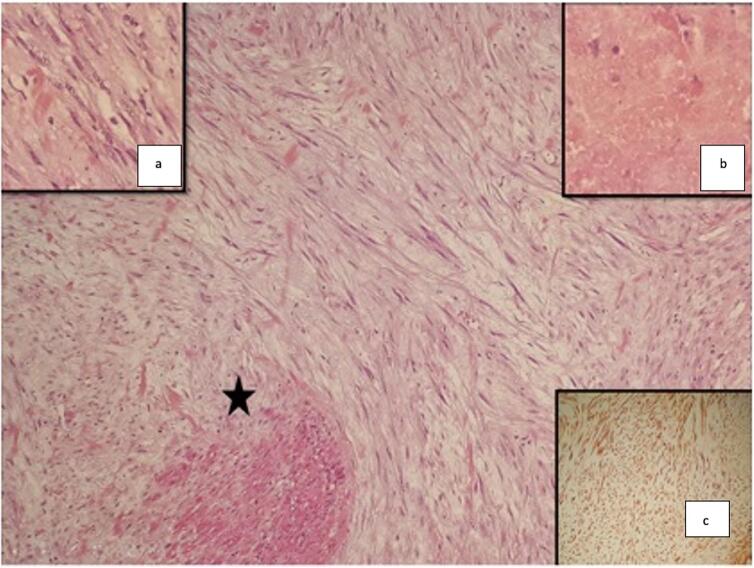
Fig. 1.
Bladder leiomyosarcoma on endoscopic resection samples. (HE × 100): *Fusiform tumour proliferation splitting the musculosa (HE × 250) a: Fusiform cells with enlarged nuclei showing no marked atypia, b: Focus of the tumour necrosis, c: Immunostaining with anti-H caldesmon.
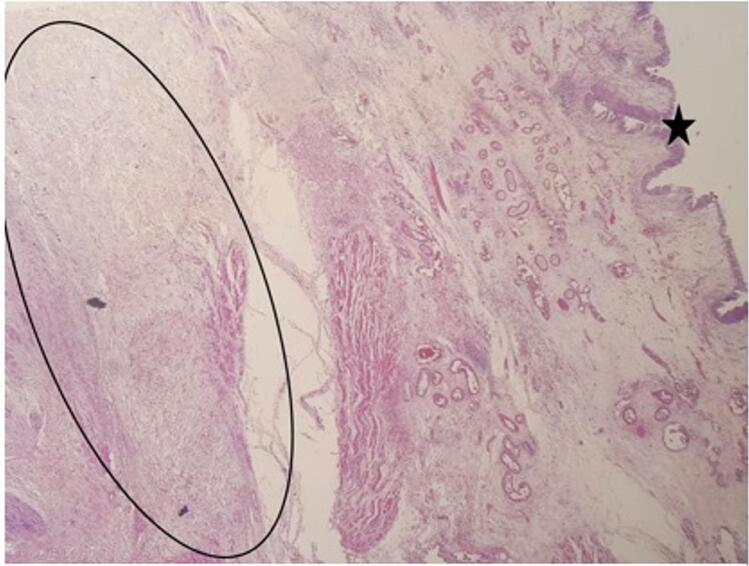
Fig. 2.
Bladder leiomyosarcoma on cystoprostatectomy specimen (HE × 40): * regular bladder mucosa, oval form: mesenchymal proliferation dissecting the musculosa.
Table 2.
Pathology data.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Differenciation | Well differentiated | Well differentiated | Unspecified | Well differentiated |
| Mitotic figures/10 high-powered fields | 20 | 12 – |
34 | 3 – |
| Grade | High grade | High grade | High grade | Low grade |
| Necrosis | + | + (>50 %) | + | + (<50 %) |
| FNCLCC | Grade 3 | Grade 2 | Grade 3 | Grade 1 |
| IHC | Actin smooth muscle + Anti-cytokeratin - Anti-desmin - |
Actin smooth muscle + Desmin + h-Caldesmon + |
Actin smooth muscle + Anti- CK + |
Actin smooth muscle + h-Caldesmon + |
| Pathology on cystectomy specimen | pT3 Negative surgical margins 2 N- Left 4 N – Right |
pT2 Negative surgical margins 3 N- Right 2 N- Left |
– |
pT2 Negative surgical margins 6 N- Left 6 N- Right |
Staging showed in two patients a locally advanced tumour cT3 with bilateral pelvic nodes and bilateral hydronephrosis. The 3rd patient had a negative staging with a normal bladder appearance. The latter patient was lost to follow-up for 20 months after resection and the extension findings on re-examination showed a bilateral N+ cT4 with no distant staging.
Concerning management (shown in Table 3), we decided to perform cystoprostatectomy with extensive bilateral pelvic lymph node dissection without neoadjuvant therapy for three patients after a multidisciplinary consultation meeting. No particular technical difficulties were noted. Two patients underwent an external Bricker diversion and one a Coffey ureterosigmoidostomy. Postoperative follow-up was normal in two cases while a single patient developed a urinary fistula after 20 days.
Table 3.
Management and follow up.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Neo-adjuvant traitement | No | No | No | No |
| Procedure | Cystectomy + Pelvic lymph node dissection + Coffey diversion | Cystectomy + Pelvic lymph node dissection + Bricker diversion | Re-resection | Cystectomy + Pelvic lymph node dissection + Bricker diversion |
| Adjuvant traitement | No | No | No | Refused by the patient |
| Evolution | Lymph node metastasis and local recurrence after 4 months Death at 10 months |
13 cm liver metastasis after 32 months treated by surgery | Remission at 2 years of follow-up | Remission at 1 year of follow-up |
No adjuvant treatment was indicated for these patients. We indicated adjuvant chemotherapy after multidisciplinary consultation in one case but it was refused by the patient.
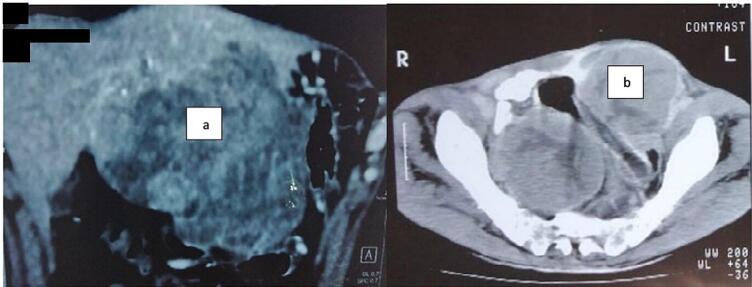
One patient had a fulminant local and lymph node recurrence four months after surgery, for whom we decided an abstention (shown in Fig. 3). The patient survived for five more months.
Fig. 3.
a Liver recurrence after radical surgery for bladder leiomyosarcoma.
b Inguinal lymph node recurrence after radical surgery for bladder leiomyosarcoma.
Another patient was lost to follow-up after cystectomy and reported after 32 months a hard, painless, mobile epigastric mass measuring 10 cm on physical examination. Imaging and percutaneous biopsy of this mass revealed that it was a 13 cm single liver metastasis (shown in Fig. 3). A difficult surgical metastasectomy with segment III and IVb liver resection was then performed. Whereas the 3rd patient who underwent radical treatment had no recurrence within a follow-up of one year.
As for the fourth patient, we decided to perform a re-resection of the tumour base considering his severe condition of advanced coronary artery disease treated by clopidogrel, the complete resection and the normal state of the bladder on postoperative CT scan. This re-resection after 6 weeks did not show any residual tumour. Thus, the patient received endoscopic and radiological monitoring twice a year, confirming remission at two years follow up.
3. Discussion
Sarcoma is the most common mesenchymal tumour affecting the bladder, headed by leiomyosarcoma [4]. It represents 0.1 % of all bladder tumours [2,4,5].
Incidence is higher among males and patients older than 60 years old [[4], [5], [6]]. Our results showed that all our patients were exclusively male, ranging from 35 to 73 years of age.
Risk factors are not clearly defined [4]. Retinoblastoma gene mutation, Li-Fraumeni syndrom, NF-1 gene mutation, history of pelvic irradiation, cyclophosphamide use, chronic bladder inflammation, and the combination of bladder leiomyosarcoma with urothelial carcinoma have been reported in literature. Conversion of leiomyoma to leiomyosarcoma is exceptional [[2], [3], [4],7,8]. None of these risk factors were found in our study.
The clinical, radiological and endoscopic presentation is closely related to urothelial cancers and dominated by hematuria [2,3,6] although in advanced cases there may be a hypogastric mass as in our report [2] or there may be a mass pushing back the bladder wall with normal cystoscopy. Rodriguez et al. [4] reports a case in which there was a posterolateral mass on imaging but cystoscopy showed an intact urothelium. The diagnosis was done by excision surgery of this mass removing a bladder flange and the distal ureter. Some teams report the assessment of disease extension by 18 F-FDG PET as used to predict treatment outcome in leiomyosarcoma [9].
Late diagnosis may be possible with the discovery of locally advanced forms [4]. Localized forms seem to be more frequent [6]. The rate of metastatic disease at diagnosis is 2 % [3]. In our results, only one patient presented a localized form on staging.
From a pathological point of view, the different publications suggest the use of three main histological and prognostic classifications: the NCI, FNCLCC and Mayo LMS-UB grading system which take into consideration the degree of differentiation, mitosis number (per 10 CFG), necrosis and nuclear atypia [10,11].
The FNCLCC system seems to have the best performance in terms of objectivity and assessment of differentiation, mitotic activity and necrosis [10]. In the present study, we used the FNCLCC system. Immunohistochemistry provides a pathological diagnosis. In fact, the markers vimentin, muscle-specific actin, H-Caldesmon and desmin may be positive, while Epithelial markers and ALK-1 are usually negative [12].
Due to the rarity of this disease and the lack of literature data, prognosis is still uncertain [4]. Histological staging remains the key prognostic indicator [10]. Mattew R et al. reported in a study on 34 bladder leiomyosarcomas that metastatic event and death rates were respectively 53 % and 65 %. Grade 2 and 3 FLCLCC forms were linked to metastatic events and disease-specific death without statistical significance (0–2 %) [10].
The management remains nonconsensual and should be considered within a multidisciplinary strategy [2,4,5]. Neo-adjuvant or adjuvant treatment with radio-chemotherapy may be suggested [3]. In our study, adjuvant chemotherapy was indicated in one case but refused by the patient. Radical cystectomy surgery is most commonly recommended, while partial cystectomy is used for small tumours and patients refusing radical treatment [3,11].
Dayron R et al. reported the largest series in the literature studying 183 cases of bladder leiomyosarcomas. It was reported that 92.9 % of patients had surgical treatment: 41.2 % by trans-urethral bladder resection, 37.1 % by partial or total cystectomy. Fourteen patients (7.7 %) received radiotherapy [6]. All our patients had surgical treatment, 3 out of 4 patients had radical surgery by cystoprostatectomy with pelvic lymph node dissection. None of the patients received chemotherapy nor radiotherapy. Among the patients who underwent radical surgery, we chose an external urinary shunt type Bricker for 2 patients and a ureterosigmoidostomy type Coffey for the other patient. Despite the known poor prognosis of bladder leiomyosarcoma, D. Anakievski et al. reported a single case treated by cystectomy and enterocystoplasty with a follow-up of 12 months free of recurrence [5].
Partial cystectomy has been suggested even for high-grade, small (<4 cm) T1–2 tumours distant from the bladder neck and the trigone [1,4,12].
Minimally invasive treatment methods such as TURB and laser fulguration combined with radio-chemotherapy have been recommended by some authors, especially for small tumours [4]. Ryuta S et al. described a case of bladder leiomyosarcoma treated by TURB followed by re-resection after 70 days. Follow-up was 18 months with no tumour recurrence [11]. In our study, this approach was used for a patient with a two-year recovery. Although these data are promising, endoscopic techniques should be considered as a palliative strategy instead of a curative approach [11].
Margin status remains a key prognostic factor [[2], [3], [4],11].
The overall recurrence rate is about 16 % with a predilection to local recurrences, justifying close clinical and radiological surveillance. The distant metastatic recurrence rate is about 50 % [[3], [4], [5]]. Some series report higher recurrence rates (34 %) mainly involving the retroperitoneum [6]. Two of our patients developed a metastatic recurrence one in 4 months and the second in 32 months after surgery. The recurrence was nodal, locoregional and in the liver for both cases. Dayron R et al. reported an overall median survival of 46 months with a 5-year overall survival of 47 % and 10-year survival of 35 %. It's worth mentioning that patients who underwent cystectomy have a better median survival estimated at 60 months [6].
The principal limits of our study were the retrospective character, the small number of cases and the lack of long-term follow-up.
4. Conclusion
Bladder leiomyosarcoma is rare and highly malignant. Diagnosis and prognosis are based on pathological examination. There is no consensus on management. Radical surgery is the most suitable treatment option. Chemotherapy and radiotherapy are promoted by some teams.
Consent
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Ethical approval is not required for this study in accordance with local or national guidelines.
Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Conception and Design: Bilel Saidani, Ahmed Saadi and Mohamed Ali Bedoui.
Administrative support: Marouene Chakroun and Riadh Ben Slama.
Data acquisition: Bilel Saidani, Ahmed Saadi, Mohamed Ali Bedoui and Selim Zaghbib.
Data analysis: Bilel Saidani and Mohamed Ali Bedoui.
Manuscript writing: Bilel Saidani, Ahmed Saadi, Mohamed Ali Bedoui and Selim Zaghbib.
Final approval of manuscript: All authors.
Critical revision of the manuscript: Ahmed Saadi and Bilel Saidani.
Guarantor
The guarantor for this study Is the corresponding author: Mohamed Ali Bedoui.
Research registration number
It's not a “First in Man” study.
Conflict of interest statement
The authors state no conflitct of interest.
Acknowledgments
Acknowledgement
We are grateful to all the staff of the Urology and Pathology Department of our hospital who contributed to this project.
Methods
The work has been reported in line with the SCARE criteria.
Agha RA, Franchi T, Sohrab C, Mathew G, Kirwan A, Thomas A, et al. The SCARE 2020 guideline: updating consensus Surgical Case Report (SCARE) guidelines. International Journal of Surgery. 2020; 84(1):226–30.
Data availability
The data that support the findings of this study are available from the corresponding author
upon reasonable request.
References
- 1.Hamadalla N.Y., Rifat U.N., Safi K.C., et al. Leiomyosarcoma of the urinary bladder: a review and a report of two further cases. Arab. J. Urol. 2013;11:159–164. doi: 10.1016/j.aju.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youssef K., Dembele O.N., Idriss Z., et al. Le léiomyosarcome de la vessie: à propos de 3 cas. Pan Afr. Med. J. 2018;30:19–21. doi: 10.11604/pamj.2018.30.19.10160. (French) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindberg M.R., Fisher C., Thway K., et al. Leiomyosarcoma of the urinary bladder: a clinicopathological study of 34 cases. J. Clin. Pathol. 2010;63:708–713. doi: 10.1136/jcp.2010.077883. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez D., Preston M.A., Barrisford G.W., et al. Clinical features of leiomyosarcoma of the urinary bladder: analysis of 183 cases. Urol. Oncol. 2014;32:958–965. doi: 10.1016/j.urolonc.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Zieschang H., Koch R., Wirth M.P., et al. Leiomyosarcoma of the urinary bladder in adult patients: a systematic review of the literature and meta-analysis. Urol. Int. 2019;102:96–101. doi: 10.1159/000494357. [DOI] [PubMed] [Google Scholar]
- 6.Ohan H., Minassian G., Minassian H., el al. Retinoblastoma in infancy with subsequent bladder Leiomyosarcoma in adulthood: genomic considerations. Urology. 2020;140:38–40. doi: 10.1016/j.urology.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Anakievski D., Kalchev K. Bladder leiomyosarcoma with total laparoscopic intracoropreal orthotopic managment- case report. Urol. Case Rep. 2020;28 doi: 10.1016/j.eucr.2019.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato R., Watanabe D., Yonemasu H., el al. Total endoscopic management of a patient with urinary bladder leiomyosarcoma presenting with dysuria: a case report. Urol. Case Rep. 2018;20:45–47. doi: 10.1016/j.eucr.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makis W., Rakheja R., Nahal A., et al. Urinary bladder leiomyosarcoma: staging with 18F-FDG PET/CT. Clin. Nucl. Med. 2013;38:218–222. doi: 10.1097/RLU.0b013e3182523e33. [DOI] [PubMed] [Google Scholar]
- 10.Jayarajah U., Fernando M.H., Herath K.B., et al. Partial cystectomy for a primary locally advanced leiomyosarcoma of the bladder: a case report and review of the literature. Clin. Case Rep. 2018;6:883–886. doi: 10.1002/ccr3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon A., Puthalath R., Suresh N., et al. Organ preservation in leiomyosarcoma bladder: case report and review of literature. Urol. Ann. 2018;10:233. doi: 10.4103/UA.UA_109_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saadi A., Bouzouita A., Cherif M., et al. Le léiomyome vésical: À propos de 5 cas. Can. Urol. Assoc. J. 2015;9:471. doi: 10.5489/cuaj.2837. (French) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author
upon reasonable request.