Abstract
Background
Imported fire ant (IFA) venom immunotherapy (VIT) is the only disease-modifying treatment reported to be effective at decreasing the risk of systemic reactions (SRs) to IFA stings.
Objective
Our aims were to determine the baseline rates of IFA sensitization in subjects, describe IFA VIT prescribing patterns across the military health system (MHS), and retrospectively evaluate the safety and efficacy of IFA VIT.
Methods
We prospectively compared IFA sensitization in participants with and without an SR to flying Hymenoptera venom. Separately, IFA VIT prescription records were extracted from a centralized repository, and rates were described across the MHS. Additionally, we retrospectively reviewed the clinical course of patients being treated with IFA VIT at 11 military treatment facilities.
Results
The in vitro IFA sensitization rates in our prospective cohort ranged from 19.1% to 24.1%. Sensitization rates did not differ statistically between the subjects with or without an SR to flying Hymenoptera venom. We found that 60.9% of all MHS IFA VIT prescriptions (491 of 806) were from the 11 facilities in this study. We retrospectively identified 137 subjects actively undergoing IFA VIT. Among the subjects actively undergoing IFA VIT, 28 reported an SR to IFA venom and repeat stings by IFAs after reaching VIT maintenance, and 85.7% (24 of 28) of the subjects noted symptoms no worse than a large swelling reaction after a repeat IFA sting. Notably, only 2.9% of the subjects (4 of 137) had an SR due to VIT.
Conclusion
This study's results align with those of prior IFA sensitization reports. A substantial proportion of patients undergoing IFA VIT experienced protection against anaphylaxis with reexposure, with relatively few adverse events.
Key words: Imported fire ant, Hymenoptera, venom, immunotherapy, anaphylaxis, ImmunoCap, hypersensitivity
Imported fire ants (IFAs) (order Hymenoptera) are invasive and spreading with significant global health implications.1, 2, 3, 4, 5, 6 They are endemic in the United States and many parts of the world.1,2,4 IFAs are a considerable cause of morbidity, yet the allergic reaction to them remains poorly characterized.1,6,7 Venom immunotherapy (VIT) is a well-established modality to treat IgE-mediated reactions to Hymenoptera venom, and it is the only disease-modifying treatment available, changing the immune response so that the allergens that once caused symptoms are then tolerated with significantly less severe symptoms or no symptoms at all.1,8 IFA whole-body extract contains the relevant venom allergens and has been used effectively for VIT.1,9,10 IFA VIT is a disease-modifying treatment reported to be effective at decreasing the risk of systemic reactions (SRs) to subsequent IFA stings and is thus recommended for those with an SR to IFA sting.1,8, 9, 10 Nevertheless, few studies examining IFA VIT safety, efficacy, and characterization exist.11 Successful immunotherapy requires a significant time commitment, ideally 3 to 5 years. Unfortunately, a significant portion of patients fail to complete treatment.12 Poor adherence adds to the challenge of long-term efficacy and safety evaluation of a large pool of patients. Large-scale studies done across multiple facilities are needed to evaluate the safety and efficacy of IFA VIT.
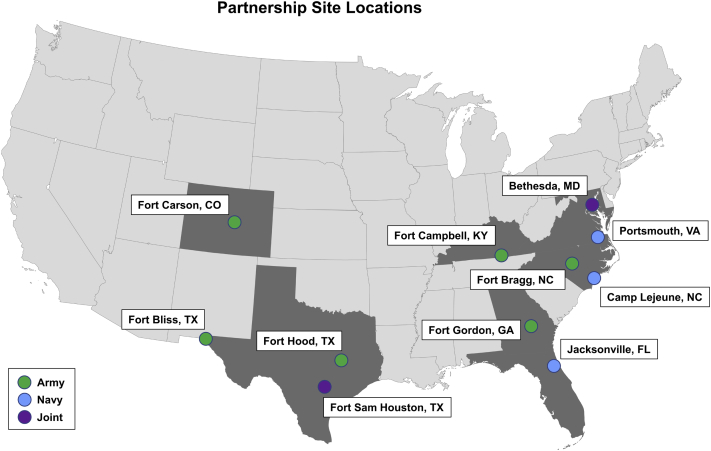
The US Military Health System (MHS) has multiple facilities in IFA-endemic areas (Fig 1).1,13,14 For all branches of the military served by MHS facilities, most allergen extract preparations, including VIT extracts, are generated by the US Army Centralized Allergen Extract Laboratory (USACAEL).15,16 A significant portion of military personnel reside and train in IFA-endemic regions; avoidance of IFAs in endemic areas is extremely difficult; thus, management of patients with IFA hypersensitivity should center on IFA VIT.15 IFA VIT is therefore a significant military readiness subject. Overall, we sought to further provide both military and civilian allergists with additional information regarding IFA sensitization and IFA VIT characterization.
Fig 1.
Multisite partnership sites and location. Location of all facilities in our study. Blue indicates a Navy facility, green indicates an Army facility, and purple indicates a joint facility.
Methods
Study design and organization
We sought to characterize IFA sensitization patterns, treatment approaches, and clinical outcomes in IFA VIT by using 3 distinct designs. First, a prospective diagnostic study was performed to evaluate IFA sensitization patterns and relevant biomarkers in participating subjects with a history of flying Hymenoptera sting (106 participants in total [Table I]). Second, a retrospective analysis of a centralized immunotherapy repository was performed to quantify the number of IFA VIT prescriptions across the MHS (3613 prescriptions within US Department of Defense [DoD] military facilities and 1904 prescriptions within partnership facilities [for a detailed breakdown, see Table E1 in the Online Repository at www.jaci-global.org]). Lastly, a multisite retrospective chart review was performed to assess IFA VIT efficacy and safety (137 subjects).
Table I.
Fire ant IgE sensitization and baseline characteristics
| Variable | SR-negative in response to flying Hymenoptera | SR-positive in response to flying Hymenoptera |
|---|---|---|
| No. of patients | 47 | 59 |
| IFA IgE (kU/L), median (range)∗ | 0 (0-2.61) | 0 (0-14) |
| Positive test result, no. (%)† | 9 (19.1%) | 15 (24.1%) |
| 95% CI for % | (9.1%-33.3%) | (15.0%-38.4%) |
| Positive test result (plus equivocal result), no. (%)‡ | 12 (25.5%) | 23 (39.0%) |
| 95% CI for % | (13.9%-40.3%) | (26.5%-52.6%) |
| Age (y), average (SD) | 31.4 (12.5) | 39.9 (12.5) |
| Men, no. (%) | 26 (55.3%) | 31 (52.5%) |
| Women, no. (%) | 21 (44.7%) | 28 (47.5%) |
| Race | ||
| Asian, no. (%) | 2 (4.3%) | 2 (3.4%) |
| Black, no. (%) | 4 (8.5%) | 13 (22.0%) |
| White, no. (%) | 29 (61.7%) | 42 (71.2%) |
| Hispanic, no. (%) | 10 (21.3%) | 1 (1.7%) |
| Other, no. (%)§ | 2 (4.3%) | 1 (1.7%) |
| Asthma , no. (%) | 1 (2.1%) | 14 (23.7%) |
| Atopy, no. (%) | 5 (10.6%) | 22 (37.3%) |
| Tryptase level (μg/L), average (SD)‖ | 3.5 (1.7) | 4.5 (2.1) |
| Total IgE level (IU/mL), median (range)¶ | 55 (0-813) | 73 (2-817) |
The Fisher exact P value for comparison of percentage of positivity according to SR status is .49. The Fisher exact P value for comparison of percentage of positive (plus equivocal) reactions by SR status is .15. Boldface indicates test results most allergists would look for as positive, versus the alternative with equivocal results.
FireAnt ImmunCap.
IgE level > 0.31 kU/L.
IgE level ≥ 0.10 kU/L; equivocal result may be considered as positive.
Other indicates unknown or undisclosed.
Tryptase reference interval is from 2.2 to 13.2 μg/L.
IgE reference interval is from 6 to 495 IU/mL.
IFA sensitization assessment
Sera from participating subjects at Walter Reed National Military Medical Center (WRNMMC) were collected as part of a prospective study published previously.17 The subjects were adult MHS beneficiaries who presented to the allergy and immunology clinic for evaluation of suspected hypersensitivity to flying Hymenoptera venom between 2012 and 2020. Subjects were evaluated for SR absence (SR-negative [n = 47 subjects]) or presence (SR-positive [n = 59 subjects]) immediately after a flying Hymenoptera sting (Table I) and grouped as previously described.17 IFA-specific IgE levels were measured by using ImmunoCAP (ICAP) (Thermo Fisher Scientific, Waltham, Mass). From the same sera, tryptase and IgE levels were also collected. For this study, absolute values of the IFA ICAP result were recorded in kU/L and sensitization rates were calculated with cutoff levels for a positive test defined as a value of 0.32 kU/L or higher to avoid equivocal results. All subjects provided written informed consent to participate in the study, and the study was approved and monitored by the institutional review board.
IFA VIT prescription review
A data retrieval from the USACAEL’s Extract Lab Management System was performed to identify all IFA prescriptions written from January 2016 to March 2021 (4012 prescriptions). The USACAEL prepares most allergen extracts for all branches of the military.16 In addition, the USACAEL also supplies extracts to the Veterans Health Administration, as well as to civilian practices.15
IFA VIT efficacy and safety review
A partnership of 11 separate health care facilities consisting of 6 Army, 3 Navy, and 2 joint partners across 8 US states was formed (Fig 1). The Army facilities were Evans Army Community Hospital (Fort Carson, Colo), Dwight D. Eisenhower Army Medical Center (Fort Gordon, Ga), Blanchfield Army Community Hospital (Fort Campbell, Ky), Womack Army Medical Center (Fort Bragg, NC), William Beaumont Army Medical Center (Fort Bliss, Tex), and Carl R. Darnall Army Medical Center (Fort Hood, Tex). The Navy facilities were Naval Hospital Jacksonville (Jacksonville, Fla), Naval Medical Center Camp Lejeune (Camp Lejeune, NC), and Naval Medical Center Portsmouth (Portsmouth, Va). The Joint facilities were Brooke Army Medical Center (Fort Sam Houston, Tex) and WRNMMC (Bethesda, Md). An audit of all patients actively undergoing IFA VIT from January 2021 to March 2021 at all partnership sites was requested. A standardized questionnaire was developed to gather demographics, IFA hypersensitivity history, relevant clinical history, IFA VIT course, and history of IFA sting reexposure. The retrospective chart review was conducted by using this questionnaire for all patients actively undergoing IFA VIT at the time of audit (137 subjects [see Fig 2 and Table E1 for a detailed breakdown]). Clinical history of hypersensitivity symptoms (severity and characteristics as reported by the subject) was classified according to the Mueller grading system.18
Fig 2.
Stacked Venn diagram comparing source of subjects. Gray indicates all subjects in USACAEL (5 years), green indicates subjects within DoD facilities only (5 years), and blue indicates subjects within partnership facilities (5 years). Yellow indicates those subjects in the ALX group in partnership facilities during the first quarter of 2021, and red indicates those subjects in the ALX group who had an SR before VIT, reached maintenance, and were stung after maintenance (the STX group). Q, Quarter.
Statistical analysis
The demographic, clinical, and laboratory characteristics of the WRNMMC cohort evaluated for Hymenoptera venom hypersensitivity were reported for patient by SR status. Fisher exact tests were used to compare IFA sensitization by SR status. Frequencies and percentages of MHS IFA VIT prescriptions were reported by facility to describe overall prescribing patterns across the MHS. Descriptive statistics were reported for patient demographic and clinical characteristics for the retrospective cohort of patients identified as actively undergoing IFA VIT across the study sites. Proportions of reactogenicity outcomes were reported with their exact CIs.
Results
IFA sensitization
Over 8 years, the WRNMMC allergy and immunology clinic collected serum and relevant patient characteristics from 59 SR-positive and 47 SR-negative participants with flying Hymenoptera sting (Table I). SR-positive participants were more likely to be atopic (37.3% vs 10.6%), asthmatic (23.7% vs 2.1%), and older on average (39.9 vs 31.4 years) than the SR- participants.
Serum was tested for IFA IgE via ICAP as well as by measuring tryptase and total IgE levels (Table I). The average tryptase levels for SR-positive and SR-negative subjects were 3.5 and 4.5 ng/mL, respectively (<11.4 μg/L).19 Only 1 subject in the SR-positive group had tryptase levels considered increased (12 μg/L). The median and average IgE levels for both groups were within normal reference values, although they were slightly elevated in the SR-positive group (median level 55 IU/mL; average level 91.2 IU/mL) versus in the SR-negative group (median level 73 IU/mL; average level 135.1 IU/mL). Thus, it was unlikely that the baseline IgE levels had an undue influence on ICAP positivity.
Overall, baseline IFA sensitization rates were determined to be 19.1% (95% CI = 9.1%-33.3%) for SR-negative subjects versus 24.1% (95% CI = 15.0%-38.4%) for SR-positive subjects (P = .49). With lower sensitization thresholds of 0.10 kU/L instead of 0.31 kU/L, which may add equivocal results, the sensitization rates were 25.5% (95% CI = 13.9%-40.3%) versus 39.0% (95% CI = 26.5%-52.6%) for SR-negative versus SR-positive participants, respectively (P = .15).
IFA VIT prescription review in MHS
Data from USACAEL revealed 4012 IFA prescriptions for Solenopsis invicta (2076) or Solenopsis richteri (1936) from January 2016 to March 2021. Of these prescriptions, 3613 (90.1%) were from DoD military facilities and 1904 were from our 11 partnership facilities (or 47.5% of all USACAEL prescriptions or 52.7% of all DoD facilities). The frequencies of prescriptions by ordering facility within our partnership are shown in Table E1.
All these IFA VIT prescriptions noted previously from USACAEL were derived from 876 unique patients. Of these, 809 unique patients (92.3%) were from DoD facilities (Fig 2). Of these patients, 491 unique patients had IFA VIT prescriptions from our 11 partnership facilities, representing 56.1% of all USACAEL or 60.7% of all DoD facility patients in the period previously specified (Fig 2). Of note, some patients had received prescriptions at multiple facilities, likely owing to permanent change of station by military personnel.
Patients actively undergoing IFA VIT
We tallied all patients actively undergoing IFA VIT at the time of chart review (January to March 2021) and identified 137 patients within our partnership facilities (see Table E1). The proportion of all potential subjects within USACAEL as well as DoD is represented in Fig 2. Frequencies of subjects by study site as well as relevant demographics and clinical history are shown in Table II. Table E1 compares the proportions of the active subjects who underwent further review here with the USACAEL prescription proportions for our facilities. These 137 subjects are also labeled as all subjects actively undergoing IFA VIT in the partnership facilities (the ALX group) in this article (Table III).
Table II.
Retrospective review of subject demographics at the 11 study sites
| Variable | Value | ||
|---|---|---|---|
| Site subjects, no. | SR only, no.∗ | SR and Mtce, no.† | |
| Site location | |||
| Fort Carson, Colo | 5 | 5 | 3 |
| Jacksonville, Fla | 5 | 5 | 5 |
| Fort Gordon, Ga | 17‡ | 16 | 11 |
| Fort Campbell, Ky | 4 | 4 | 3 |
| Bethesda, Md | 7 | 7 | 5 |
| Camp Lejeune, NC | 27 | 27 | 18 |
| Fort Bragg, NC | 21‡ | 20 | 20 |
| Fort Bliss, Tex | 11 | 11 | 7 |
| Fort Hood, Tex | 15 | 15 | 4 |
| Fort Sam Houston, Tex | 18‡ | 17 | 17 |
| Portsmouth, Va | 7 | 7 | 6 |
| Total | 137 | 134 | 99 |
| Demographic variables | |||
| Asian, no. (%) | 7 (5.1%) | ||
| Black/African American, no. (%) | 13 (9.5%) | ||
| Hawaiian/Pacific Islander, no. (%) | 3 (2.2%) | ||
| White, no. (%) | 74 (54.0%) | ||
| Other, no. (%) | 20 (14.6%) | ||
| Unknown, no. (%) | 20 (14.6%) | ||
| Subject age (y), average (range) | 32.4 (4-64) | ||
| Female, no. (%) | 56 (40.9%) | ||
| Male, no. (%) | 81 (59.1%) | ||
| Concurrent medical conditions, no. (%) | |||
| Allergic rhinitis | 47 (34.3%) | ||
| Asthma | 13 (9.5%) | ||
| Atopic dermatitis | 9 (6.6%) | ||
| Chronic urticaria | 8 (5.8%) | ||
| Eosinophilic esophagitis | 1 (0.7%) | ||
Mtce, Maintenance.
Number of subjects who had an SR to IFA.
Number of subjects who had an SR to IFA and reached Mtce with IFA VIT.
Includes patients undergoing IFA VIT who did not have SR to the initial Hymenoptera sting.
Table III.
IFA VIT characteristics for all review subjects
| Variable | Count |
|---|---|
| Total ALX study subjects, no. | 137 |
| Subjects with SR to IFA sting before VIT, no. (%) | 134 of 137 (97.8%) |
| Subjects who reached Mtce, no. (%) | 101 of 137 (73.7%) |
| Subjects who reached Mtce and SR to IFA before VIT, no. (%)∗ | 99 of 137 (72.2%) |
| Subjects with LLR to IFA sting before VIT, no. (%)†,‡ | 64 of 137 (46.7%) |
| Subjects who had an SR to IFA VIT, no. (%)§ | 4 of 137 (2.9%) |
| Subjects with epinephrine use for SR to VIT after Mtce, no. (%)‖ | 2 of 101 (2.0%) |
| Subjects stung after VIT start, no. (%) | 33 of 137 (24.1%) |
| Subjects stung after VIT Mtce and before VIT SR (STX group), no. (%)¶ | 28 of 137 (20.4%) |
| Subjects stung after VIT Mtce and before VIT LLR (LRX group), no. (%)‡,# | 16 of 137 (11.7%) |
| IFA VIT aspects for ALX study subjects | |
| Duration of Mtce IFA VIT (mo), median (range)∗∗ | 13 (0-312) |
| Concentration of Mtce VIT dose, no. (%) | |
| 1:100 wt/vol | 112 of 137 (81.8%) |
| 1:66.5 wt/vol | 12 of 137 (8.8%) |
| 1:200 wt/vol | 7 of 137 (5.1%) |
| Other (1:40, 1:50, 1:125, 1:142.5 wt/vol) | 6 of 137 (4.4%) |
| IFA VIT aspects for STX group | |
| Duration of Mtce IFA VIT (mo), mean (SD)∗∗ | 65.3 (69.2) |
| SR to IFA VIT in STX group subjects, no. (%)§ | 2 of 28 (7.1%) |
Mtce, Maintenance IFA VIT. Boldface indicates populations that are referenced throughout the article.
Subjects who had an SR to IFA sting initially, who reached IFA VIT Mtce.
Subjects who had an LLR to IFA sting initially before to IFA VIT initiation.
LLR does not exclude an SR.
SR induced by IFA VIT treatment.
SR induced by IFA VIT treatment but after IFA VIT Mtce was reached.
Subjects who had an SR to IFA sting initially and stung to IFA after IFA VIT Mtce.
Subjects who had an LLR to IFA sting initially and stung to IFA after IFA VIT Mtce.
At time of retrospective chart review.
Notably, we evaluated 137 patients actively undergoing IFA VIT, of whom 134 had an SR to IFAs before VIT. Of these patients, 99 had reached maintenance at the time of our review (Table III). Of the 137 potential subjects, 28 had an SR to IFAs before IFA VIT, had reached maintenance to IFA VIT, and had subsequently been stung by IFAs after maintenance (subjects labeled as belonging to the group of IFA sting study subjects with a prior SR [STX]). Similarly, 16 subjects were identified as having had a large local reaction (LLR) to IFAs before IFA VIT, having reached maintenance to IFA VIT, and having subsequently been stung by IFAs after maintenance (subjects labeled as IFA sting study subjects with a prior LLR [LRX]). The LRX subjects’ LLRs and SRs were not mutually exclusive.
IFA VIT safety analysis
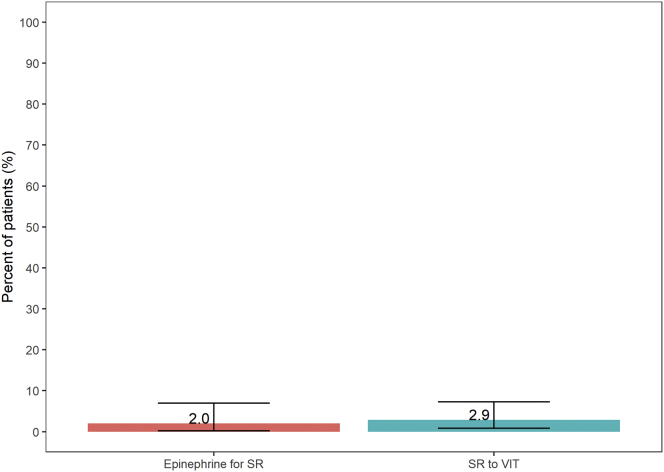
Adverse reactions among ALX subjects are shown in Table III. We found that 4 of 137 patients (2.9%) had an SR specifically to IFA VIT at all stages of immunotherapy (Fig 3). Separately, 2 of 101 subjects (2.0%) who reached maintenance had an SR to IFA VIT that required epinephrine use. Of the 137 subjects, 36 did not reach maintenance (101 of the 137 did reach maintenance), and there was 1 subject with more than 26 years of IFA VIT.
Fig 3.
Subject adverse events. Comparison of notable subject subsets as defined in Table III. Pink indicates subjects who used epinephrine for an SR to IFA VIT treatment specifically after maintenance (2 of 101 [2%]), and cyan indicates subjects who had SR to IFA VIT treatment specifically (4 of 137 [2.9%]).
The recommended maintenance IFA VIT concentration is 1:100 wt/vol.1 Our subjects’ most commonly prescribed concentration was 1:100 wt/vol (112 of the 137 subjects in the ALX group [81.8%] and 21 of 28 subjects in the STX group [75.0%]). Of note, all subjects in the STX group or ALX group who had an SR to IFA VIT were receiving maintenance concentrations of 1:100 wt/vol. However, no subjects in this study who reached maintenance with IFA VIT concentrations higher than 1:100 wt/vol (n = 9) had an SR to IFA VIT. Also, 4 of these 9 subjects had an SR to IFA before VIT but following a repeat sting after VIT maintenance, and none had an SR (1 had no reaction, 1 had local irritation, and 2 had large swelling).
IFA VIT efficacy analysis
We assessed the efficacy of IFA VIT in all subjects who subsequently had an IFA sting after VIT maintenance (Table IV). We found 28 subjects who met the criteria of having had an SR to IFAs before VIT, having reached maintenance, and having been subsequently stung (the STX group). Separately, we found 16 subjects who met the criteria of having had an LLR to IFAs before VIT, having reached maintenance, and having been subsequently stung (the LRX group). Of note, an LLR in the LRX group does not exclude an SR; in fact, 15 of 16 had an SR with an LLR, and 1 subject had an LLR without an SR before VIT initiation.
Table IV.
Characteristics of challenged IFA VIT subjects
| Variable | Count (%) | 95% CI for percentage |
|---|---|---|
| IFA sting subjects with VIT Mtce and pre-VIT SR (STX group) | ||
| STX group subjects, no. (%)∗ | 28 of 137 (20.4%) | — |
| STX group subject age at review (y), average (SD)† | 37.2 (16.0) | |
| IFA sting reaction for STX group, no (%) | ||
| None | 11 of 28 (39.3%) | (21.5%-59.4%) |
| Local irritation | 8 of 28 (28.6%) | (13.2%-48.7%) |
| Large swelling | 5 of 28 (17.8%) | (6.1%-36.9%) |
| Systemic cutaneous | 2 of 28 (7.1%) | (0.9%-23.5%) |
| Anaphylaxis | 2 of 28 (7.1%) | (0.9%-23.5%) |
| Pre-IFA VIT aspects for STX group | ||
| Age at initial IFA SR (y), average (SD) | 28.0 (12.4) | |
| No. of stings at first IFA SR, median (range) | 2-5 (1 to ≥10)‡ | |
| Subjects with epinephrine use for IFA SR before VIT, no. (%) | 12 of 28 (42.9%) | (24.5%-62.8%) |
| No. of IFA sting episodes before VIT, median (range) | 2 (1-15)‡ | |
| Mueller SR grade for initial IFA SR for STX group, no (%)§ | ||
| Mueller grade 1: slight general reaction | 3 of 28 (10.7%) | (2.3%-28.2%) |
| Mueller grade 2: general reaction | 13 of 28 (46.4%) | (27.5%-66.1%) |
| Mueller grade 3: severe general reaction | 8 of 28 (28.6%) | (13.2%-48.7%) |
| Mueller grade 4: shock reaction | 4 of 28 (14.2%) | (4.0%-32.7%) |
| IFA sting subjects with VIT Mtce and Pre-VIT LLR (LRX group) | ||
| No. of LRX group subjects‖,¶ | 16 of 137 | — |
| LRX group subjects with less than an LLR after sting, no. (%) | 12 of 16 (75%) | (47.6%-92.7%) |
| LRX group subjects noting decreased reaction severity after sting, no. (%) | 15 of 16 (93.8%) | (69.8%-99.8%) |
Mtce, Maintenance IFA VIT.
Subjects who had an SR to an IFA sting initially and stung to IFA after IFA VIT Mtce.
At time of retrospective chart review.
Based on known reported count, which was unknown for some subjects.
According to Mueller.18
Subjects who had an LLR to an IFA sting initially and stung to IFA after IFA VIT Mtce.
LLR does not exclude SR.
Among the STX group, the majority of subjects had Mueller grade 2 reactions (Table IV) and 12 of 18 (42.9%) reported the use of epinephrine for their SR before initiation of VIT.18 The subjects reported a median of 2 to 5 stings that triggered their first SR to IFA and noted a median of 2 IFA sting episodes before their initiation of VIT. Distinctly, no symptoms were reported in 11 of 28 subjects (39.3% [95% CI = 21.5%-59.4%]), and local irritation was reported in 8 of 28 subjects (28.6% [95% CI = 13.2%-48.7%]) for subsequent stings after VIT maintenance, despite all having had a prior SR to IFAs (Fig 4 and Table IV). Large swelling was reported in 5 of 28 patients (17.8% [95% CI = 6.1%-36.9%). There were 2 subjects (2 of 28 [7.14%] [95% CI = 0.9%-23.5%]) with anaphylaxis and 2 subjects (2 of 28 [7.14%] [95% CI = 0.9%-23.5%]) with systemic cutaneous reaction within STX (Fig 4 and Table IV).
Fig 4.
Efficacy and sting challenge reaction distribution. IFA sting reaction distribution for subjects who reached maintenance and had an SR to IFA before VIT as defined in Table IV. Yellow indicates a reaction with no more than local irritation (19 of 28 [67.9%]), orange indicates large swelling (5 of 28 [17.8%]), salmon indicates a systemic cutaneous reaction (2 of 28 [7.1%]), and red indicates anaphylaxis (2 of 28 [7.1%]).
Of the 4 patients with the most severe reactions, 2 subjects (patients 1 and 2) had anaphylaxis in response to an IFA sting after VIT maintenance, and both of these patients noted epinephrine use for these reactions. Patients 1 and 2 also reported epinephrine use for their SR to an IFA sting before VIT initiation. Patients 1 and 2 reported Mueller grade 2 and 4 SRs, respectively, before VIT. The other 2 patients (patients 3 and 4) had reported a systemic cutaneous reaction to an IFA sting after VIT maintenance; however, patient 3 noted epinephrine use for the systemic cutaneous reaction whereas patient 4 did not. Patients 3 and 4 did not report using epinephrine for their SR before VIT and reported Mueller grade 3 and 4 SRs, respectively, before VIT.
Within the STX group, there were a total of 4 patients who used epinephrine following their sting after VIT (2 of 4 for anaphylaxis, 1 of 4 for systemic cutaneous reaction, and 1 of 4 who noted no symptoms). The patient who noted no symptoms but used epinephrine after being stung after VIT had previously had a Mueller grade 4 reaction with no epinephrine use before VIT. Lastly, within the LRX group, 12 of 16 patients (75% [95% CI = 47.6%-92.7%]) had symptoms less severe than an LLR (local irritation or none) with IFA sting after maintenance VIT (Table IV). Of the 16 patients, 15 (93.8% [95% CI = 69.8%-99.8%]) reported decreased severity of their LLR or no LLR to IFA sting after maintenance VIT (Table IV). The 1 patient who did not notice decreased severity to LLR had a pre-VIT Mueller grade 3 reaction for which epinephrine was used. This patient reported large swelling in response to IFA sting after maintenance VIT and had been undergoing maintenance for 60 months at the time of chart review. Of note, none of the patients who had anaphylaxis or a systemic cutaneous reaction to sting on in the subset STX had an LLR and would not have met the criteria to be in the subset LRX.
Discussion
Sensitization
In the current WRNMMC cohort we found baseline IFA IgE ICAP sensitization rates between 19.1% and 24.1% in subjects with exposure to flying Hymenoptera. Sensitization rates for other Hymenoptera have been published previously.17 There were no statistically significant differences in IFA sensitization rates between the subjects with and those without an SR to flying Hymenoptera sting. Suspicion for IFAs as the cause of SR was low, as this study was performed in a nonendemic area. However, the study did include service members who move frequently and may have been stationed in endemic areas at 1 point. One study noted sensitization rates of 17% in subjects living in an IFA-endemic area and 2% in those living in a nonendemic area.20 However, in another study, the rate of IFA IgE presence among patients living in endemic areas ranged from 35.7% to 57.5% when there was a cutoff at less than 0.35 kUa/L.21 Overall, the rate of venom sensitization in the general population with no previous case history is estimated to be between 9.3% and 28.7%.1,22 The current cohort of MHS beneficiaries receiving care at WRNMMC (a nonendemic area) had IFA IgE sensitization rates within the ranges expected from prior publications.
Multisite partnership and centralized repository
Because of military personnel’s frequent moves and change of duty stations, there is a clear need for a centralized allergen extract repository such as the USACAEL. As a result, a centralized repository of all VIT prescriptions as well as standardized VIT vials are available for MHS beneficiaries at a wide variety of locations. We aimed to form a multisite partnership to capture as many patients actively undergoing IFA VIT for our study as possible. Ultimately, we formed a collaboration of 11 partners (Fig 1) while ensuring that we captured facilities located in IFA-endemic locations as well as some facilities in nonendemic locations. On the basis of data from the USACAEL, we estimated that approximately 809 subjects within the DoD in the past 5 years received IFA VIT prescriptions (Fig 2). We noted that 60.6% of patients (n = 491) had received prescriptions from our partnership facilities within the past 5 years (Fig 2). However, at the significantly shorter time of analysis, there were 137 subjects who were actively undergoing IFA VIT within our partnership facilities. This may be partly explained by deployments or duty station reassignments, as well as by separation from military service, but also possibly by poor adherence, with 1 author noting that only 35% of patients remained adherent at that specific study site after 1 year with a similar military population.12
Adherence
Lack of adherence is of significant concern for readiness and successful clinical outcomes. The authors of 1 study did note that they achieved high adherence to VIT with counseling thoroughness.23 We speculate that our population’s high relocation rate and barriers associated with transition of care play a factor in VIT adherence. However, we also want to highlight that this study was not designed to track adherence; the discrepancy between the 491 unique subjects within the prescription database review and the 137 unique active charts within our partnership facilities (Fig 2) is due to multiple factors aside from speculated poor adherence. These factors include service members continuing their VIT in facilities outside of the MHS. Thus, a separate study tracing each subject’s VIT course individually would answer the question of whether the discrepancy is truly due to poor adherence.
Safety and efficacy
Our descriptive findings support the safety and efficacy of IFA VIT, consistent with previous publications. Among our retrospective cohort, we found that 2.9% of subjects had an SR to IFA VIT. Furthermore, in subjects with a history of SR to index sting, we found that 85.7% of subjects (24 of 28) who reached VIT maintenance had reactions no more severe than an LLR after subsequent IFA. Of those patients, 92.86% (26 of 28) did not have anaphylaxis in response to a repeat sting. Moreover, 93.8% of subjects (15 of 16) who reached VIT maintenance noted decreased severity of LLR after a repeat sting compared with their initial LLR.
In this cohort, 81.8% of all study subjects were receiving an IFA VIT maintenance concentration of 1:100 wt/vol, which aligns with the most commonly prescribed maintenance dose noted in 1 study.15 Other authors have recommended a maintenance concentration of 1:100 wt/vol, and based on sting challenges, their efficacy ranged between 97.9% and 98.2%.9,10 In our study, there were 15 subjects who were given doses at concentrations higher than 1:100 wt/vol, and 4 of these subjects were subsequently stung after IFA VIT. None of these subjects had an SR to the IFA VIT or an SR to IFA sting after maintenance. This sample is small, but it supports efficacy and safety at concentrations higher than 1:100 wt/vol.
In the current cohort, 33 of 137 subjects (24.1%) experienced a repeat sting after the initiation of IFA VIT. Prior studies have shown that IFA stings are difficult to avoid, with 1 author proposing that 30% to 60% of the population in urban areas infested by IFAs are stung annually.24,25 Thus, because of this risk of a repeat sting, management should center on IFA VIT, and IFA VIT efficacy should be a subject of readiness discussions.
Future studies and outlook
The evaluations of the prescriptions, safety, and efficacy were all retrospective studies. With a similar multisite partnership, a prospective study of similar setup but with an additional sting challenge would provide stronger evidence regarding the questions asked in our study. IFA dosage schedule is also poorly defined, and a future prospective study could stratify and compare different protocols.1 We are also designing a prospective study that traces the adherence of this study’s subject to IFA VIT to clarify the issue of low adherence to IFA VIT, as well as its potential causes.12 Lastly, although our study involved 11 partners, additional partnerships would strengthen the results by increasing the relatively small cohort size.
Clinical implications.
An 11-site study has reconfirmed the efficacy and safety of IFA VIT in decreasing the risk of SRs to IFA stings.
Acknowledgments
We thank James A. Hagerty and Susan E. Kosisky from the US Army Centralized Allergen Extract Lab for their technical support of this project.
Footnotes
Supported by the Department of Research Programs at Walter Reed National Military Medical Center.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest. The identification of specific products or scientific instrumentation is considered an integral part of the scientific endeavor and does not constitute endorsement or implied endorsement on the part of the author, US Department of Defense, or any component agency. The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the US Department of Defense or the US Government.
Supplementary data
References
- 1.Golden D.B., Demain J., Freeman T., Graft D., Tankersley M., Tracy J., et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28–54. doi: 10.1016/j.anai.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 2.Ascunce M.S., Yang C.C., Oakey J., Calcaterra L., Wu W.J., Shih C.J., et al. Global invasion history of the fire ant Solenopsis invicta. Science. 2011;331:1066–1068. doi: 10.1126/science.1198734. [DOI] [PubMed] [Google Scholar]
- 3.Bertelsmeier C. Globalization and the anthropogenic spread of invasive social insects. Curr Opin Insect Sci. 2021;46:16–23. doi: 10.1016/j.cois.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Kemp S.F., deShazo R.D., Moffitt J.E., Williams D.F., Buhner W.A., 2nd Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683–691. doi: 10.1067/mai.2000.105707. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y.S., Huang S.A., Lin I.L., Lin C.C., Lai H.K., Yang C.H., et al. Establishment and social impacts of the red imported fire ant, Solenopsis invicta, (Hymenoptera: Formicidae) in Taiwan. Int J Environ Res Public Health. 2021;18:5055. doi: 10.3390/ijerph18105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamith-Miranda D., Fox E.G.P., Monteiro A.P., Gama D., Poublan L.E., de Araujo A.F., et al. The allergic response mediated by fire ant venom proteins. Sci Rep. 2018;8:14427. doi: 10.1038/s41598-018-32327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracy J.M., Demain J.G., Quinn J.M., Hoffman D.R., Goetz D.W., Freeman T.M. The natural history of exposure to the imported fire ant (Solenopsis invicta) J Allergy Clin Immunol. 1995;95:824–828. doi: 10.1016/s0091-6749(95)70125-7. [DOI] [PubMed] [Google Scholar]
- 8.Jutel M., Agache I., Bonini S., Burks A.W., Calderon M., Canonica W., et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 9.Freeman T.M., Hylander R., Ortiz A., Martin M.E. Imported fire ant immunotherapy: effectiveness of whole body extracts. J Allergy Clin Immunol. 1992;90:210–215. doi: 10.1016/0091-6749(92)90073-b. [DOI] [PubMed] [Google Scholar]
- 10.Tankersley M.S., Walker R.L., Butler W.K., Hagan L.L., Napoli D.C., Freeman T.M. Safety and efficacy of an imported fire ant rush immunotherapy protocol with and without prophylactic treatment. J Allergy Clin Immunol. 2002;109:556–562. doi: 10.1067/mai.2002.121956. [DOI] [PubMed] [Google Scholar]
- 11.La Shell M.S., Calabria C.W., Quinn J.M. Imported fire ant field reaction and immunotherapy safety characteristics: the IFACS study. J Allergy Clin Immunol. 2010;125:1294–1299. doi: 10.1016/j.jaci.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 12.Stokes S.C., Quinn J.M., Sacha J.J., White K.M. Adherence to imported fire ant subcutaneous immunotherapy. Ann Allergy Asthma Immunol. 2013;110:165–167. doi: 10.1016/j.anai.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Steigelman D.A., Freeman T.M. Imported fire ant allergy: case presentation and review of incidence, prevalence, diagnosis, and current treatment. Ann Allergy Asthma Immunol. 2013;111:242–245. doi: 10.1016/j.anai.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 14.United States Department of Agriculture, Animal and Plant Health Inspection Service. Imported Fire Ant Quarantine Map. Available at: https://www.aphis.usda.gov/plant_health/plant_pest_info/fireants/downloads/federal-imported-fire-ant-quarantine.pdf. Accessed October 14, 2021.
- 15.Wauters R.H., Brooks D.I., Schwartz D.J. Imported fire ant immunotherapy prescribing patterns in a large health care system during an 11-year period. Ann Allergy Asthma Immunol. 2020;125:577–580. doi: 10.1016/j.anai.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez C.L., Waibel K.H., Kosisky S.E., Nelson M.R., Banks T.A. 15 years of allergen immunotherapy vial sterility testing. Ann Allergy Asthma Immunol. 2017;118:374–375. doi: 10.1016/j.anai.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Park H.J., Brooks D.I., Chavarria C.S., Wu R.L., Mikita C.P., Beakes D.E. Combining discordant serum IgE and skin testing improves diagnostic and therapeutic accuracy for Hymenoptera venom hypersensitivity immunotherapy. J Allergy Clin Immunol Pract. 2021;10:837–843.e3. doi: 10.1016/j.jaip.2021.08.037. [DOI] [PubMed] [Google Scholar]
- 18.Mueller H.L. Diagnosis and treatment of insect sensitivity. J Asthma Res. 1966;3:331–333. doi: 10.3109/02770906609106941. [DOI] [PubMed] [Google Scholar]
- 19.Akin C. Mast cell activation syndromes. J Allergy Clin Immunol. 2017;140:349–355. doi: 10.1016/j.jaci.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Caplan E.L., Ford J.L., Young P.F., Ownby D.R. Fire ants represent an important risk for anaphylaxis among residents of an endemic region. J Allergy Clin Immunol. 2003;111:1274–1277. doi: 10.1067/mai.2003.1453. [DOI] [PubMed] [Google Scholar]
- 21.Partridge M.E., Blackwood W., Hamilton R.G., Ford J., Young P., Ownby D.R. Prevalence of allergic sensitization to imported fire ants in children living in an endemic region of the southeastern United States. Ann Allergy Asthma Immunol. 2008;100:54–58. doi: 10.1016/S1081-1206(10)60405-X. [DOI] [PubMed] [Google Scholar]
- 22.Bilo M.B., Bonifazi F. The natural history and epidemiology of insect venom allergy: clinical implications. Clin Exp Allergy. 2009;39:1467–1476. doi: 10.1111/j.1365-2222.2009.03324.x. [DOI] [PubMed] [Google Scholar]
- 23.Bilo M.B., Kamberi E., Tontini C., Marinangeli L., Cognigni M., Brianzoni M.F., et al. High adherence to Hymenoptera venom subcutaneous immunotherapy over a 5-year follow-up: a real-life experience. J Allergy Clin Immunol Pract. 2016;4 doi: 10.1016/j.jaip.2015.09.014. 327-9 e1. [DOI] [PubMed] [Google Scholar]
- 24.Letz A.G., Quinn J.M. Frequency of imported fire ant stings in patients receiving immunotherapy. Ann Allergy Asthma Immunol. 2009;102:303–307. doi: 10.1016/S1081-1206(10)60335-3. [DOI] [PubMed] [Google Scholar]
- 25.deShazo R.D., Butcher B.T., Banks W.A. Reactions to the stings of the imported fire ant. N Engl J Med. 1990;323:462–466. doi: 10.1056/NEJM199008163230707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.