Abstract
Background
Mechanisms triggering the pathogenesis of chronic spontaneous urticaria (CSU) have been identified as type I autoallergic (which is associated with IgE antibodies against autoantigens) and type IIb autoimmune (which is driven by autoantibodies to FceR1 and/or IgE).
Objective
Our aim was to define presumptive endotypes in patients with CSU by using tests amenable to use in routine clinical practice.
Methods
A retrospective analysis of the medical records of 394 patients with CSU with or without chronic inducible urticaria or angioedema was performed. Patients were assigned to 1 of 4 groups as follows: (1) type I endotype of CSU, if they presented at least 1 of the following: allergic disease, total IgE level of at least 40UI/mL, and positive result of skin tests to inhalant allergen(s), (2) type IIb endotype of CSU, if they presented at least 1 of following: autoimmune disease, low total IgE level less than 40 IU/mL, positive autologous serum skin test result, positive for antinuclear antibodies in a titer of at least 1:160, and elevated level of anti–thyroid peroxidase, (3) overlap of type I/type IIb endotypes of CSU, if they presented with at least 1 marker of both type I and type IIb, and (4) non–type I/type IIb endotype of CSU, if they presented with none of the markers of type I or type IIb.
Results
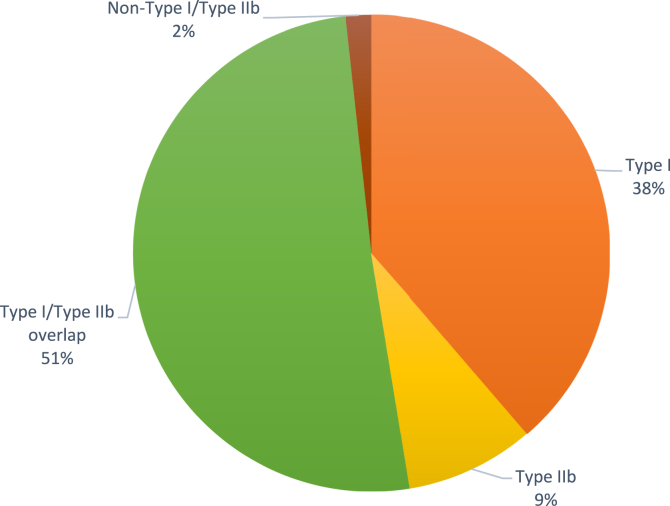
The mean age at onset of symptoms was 34 years; 82.2% of those with CSU were female, and angioedema and chronic inducible urticaria were found in 74.8% and 31.9% of patients, respectively. Of the patients with CSU, 38% presented with the type I endotype and 51% presented with type I/type IIb overlap, whereas 9% presented with the type IIb endotype and 2% presented with the non–type I/type IIb endotype. Eosinopenia was associated with type IIb and type I/type IIb overlap as opposed to the type I and non–type I/type IIb endotypes (P = .02).
Conclusions
Most patients with CSU presented with features of the type 1 (autoallergic) endotype, whether associated with type IIb (autoimmune) endotype or not.
Key words: Urticaria, IgE, omalizumab, autoimmunity, chronic spontaneous urticaria
Introduction
Recent developments in the pathophysiology of chronic spontaneous urticaria (CSU) have highlighted the role of autoimmune mechanisms, and 2 endotypes have emerged: type I autoallergic CSU, which is associated with IgE antibodies against autoantigens, and type IIb aiCSU, which is driven by autoantibodies that activate mast cells, including anti-FcεRI and anti-IgE.1, 2, 3 As proposed by the PURIST study, patients with type IIb autoimmune CSU (aiCSU) should fulfill 3 concomitant criteria: a positive autologous serum skin test (ASST) result, a positive result of an immunoassay for IgG anti-FcεRI or IgG anti-IgE, and a positive basophil reactivity test result (basophil activation test [BAT] result and/or basophil histamine release assay result).4 The results revealed that less than 10% of patients with CSU were triple-positive, and this was associated with high disease activity, low total IgE level, and elevated anti–thyroid peroxidase (anti-TPO) autoantibodies.4 IgE autoantibodies relevant in CSU include those directed to TPO, IL-24, eosinophil peroxidase, eosinophil cationic protein, double-stranded DNA, tissue factor, thyroglobulin, and FcεRI.3 Detection of IgE antibodies to autoallergens is not routinely available; therefore, an increased total IgE level has been considered to be a surrogate marker for autoallergic CSU. To support this assumption, the worldwide experience with anti-IgE therapy with omalizumab since the pivotal study by Maurer et al, which showed high efficacy of omalizumab in antihistamine refractory patients with CSU, has pointed to a major role of IgE and activation of mast cells via FcεRI-linked pathways in the inception of urticaria.5,6 A combination of elevated IgG anti-TPO level and low total IgE level has been shown to be a suitable way to screen patients for type IIb aiCSU and was found to be associated with a positive ASST result, positive BAT result, and other markers of autoimmune pathogenesis.7 These findings have led to the recommendation that patients with CSU be assessed for total IgE level and IgG–anti-TPO level in accordance with the most current revision and update of the international European Academy of Allergy and Clinical Immunology/Global Asthma and Allergy European Network/ EuroGuiDerm/Asia Pacific Association of Asthma, Allergy, and Clinical Immunology urticaria guideline.8 Moreover, the presence of eosinophils in CSU lesions and interactions between mast cells and eosinophils may indicate additional mechanisms leading to clinical symptoms.9
To investigate endotypes among Brazilian patients with CSU, we performed a retrospective, descriptive, noninterventional study, with analysis of medical records of a group of 394 patients who presented with CSU with or without chronic inducible urticaria or angioedema. We have proposed criteria to define CSU endotypes that could be readily available to use in clinical practice, and we have shown that most Brazilian patients with CSU presented with the autoallergic presumptive endotype, with or without features of autoimmunity. For details on the study methods, please see the Methods section of the Online Repository (at www.jaci-global.org). The study was approved by the ethics committee of Ribeirão Preto Medical School Hospital (protocol no. 82879318.7.0000.5440).
Results and discussion
Demographic data were collected, as were the results of in vivo and in vitro tests performed at our clinic or in central laboratories (see Fig E1 and Table E1 in this article's Online Repository at www.jaci-global.org). An anti-TPO level of 35 IU/mL or higher was considered high, a total IgE level less than 40 IU/mL was considered low, and an antinuclear antibody (ANA) titer of 1:160 titer or higher was considered to be a positive ANA test result.7 ASST was performed as described.10,11 Autoimmune diseases were present in 67 of the patients with CSU (16.9%), with Hashimoto thyroiditis being the most common (Fig E2 in this article's Online Repository at www.jaci-global.org).
On the basis of the data from a preliminary analysis (described in detail in the Online Repository at www.jaci-global.org) and available literature, we categorized patients as having type I CSU if they presented with at least 1 of the following criteria: associated allergic disease, total IgE level of 40 UI/mL or higher, and a positive result of skin tests to inhalant allergen(s). Patients were categorized as having type IIb CSU if they presented with at least 1 of these features: autoimmune disease, low total IgE level (<40 IU/mL), positive ASST result, positive antinuclear antibody test result (titer of 1:160 or higher), and elevated anti-TPO level. Patients were categorized as having type I/type IIb overlap CSU if they presented with at least 1 marker of both type I and type IIb CSU, and they were categorized as having non–type I/type IIb CSU if they presented with none of the markers of either type I or type IIb CSU.
Our results showed that most of our patients presented with type I CSU (38%) or type I/type IIb overlap (51%), which are endotypes presumptive of CSU (Fig 1 and Table I). In keeping with the results of the PURIST study,7 only 9% of the patients presented with the type IIb endotype (Fig 1 and Table I). Overall, peripheral blood eosinopenia was found in 16 of 388 patients (4.1%). Mean eosinophil counts were significantly higher but eosinopenia was more frequent in the type I and type I/type IIb overlap groups than in the type IIb and non–type I/type IIb groups (Table I). No other significant differences were found within the groups (Table I). To allow for relative risk analysis, we conducted further evaluation of eosinophils in peripheral blood by grouping together those patients with type IIb and type I/type IIb overlap (those presenting with any of the type IIb markers) and type I and non–type I/type IIb (those who were negative for all type IIb markers). Eosinopenia was significantly more frequent in combined type IIb and type I/type IIb overlap (in 14 of 232 patients [6.03%]) than in combined type I and non–type I/type IIb (in 2 of 156 patients [1.28%]) (relative risk = 4.70 [95% CI = 1.08-20.42]; Fisher exact test P = .02), suggesting an association of eosinopenia with features of autoimmunity in patients with CSU, as reported by Kolkhir et al.12 In addition, eosinophil counts were lower in combined type IIb and type I/type IIb overlap (mean eosinophil count = 0.187 ± 0.170 eosinophils × 109/L) than in combined type I and non–type I/type IIb (mean eosinophil count = 0.216 ± 0.184 eosinophils × 109/L) (Wilcoxon nonparametric test P = .04 [data not shown]).
Fig 1.
Endotypes of patients with CSU in the present study.
Table I.
Clinical, immunologic,∗ and treatment features of patients in the present study with distinct CSU endotypes
| Parameter | Patients with CSU (n = 394) | Type I n = 151 (38%) | Type IIb n = 35 (9%) | Type I/type IIb overlap n = 201 (51%) | Non–type I/type IIb n = 7 (2%) | P value |
|---|---|---|---|---|---|---|
| Age (y), mean (± SD) | 43 (± 16) | 42 (± 16) | 46 (± 17) | 43 (± 15) | 42 (± 22) | .67† |
| Sex | ||||||
| Female, no. (%) | 324 (82.2%) | 116 (76.8%) | 27 (77.1%) | 175 (87%) | 6 (85.7%) | .06‡ |
| Male, no. (%) | 70 (17.7%) | 35 (23.1%) | 8 (22.8%) | 26 (12.9%) | 1 (14.2%) | |
| Age at onset of symptoms (y), mean (± SD) | 34 (± 17) | 34 (± 15) | 39 (± 21) | 33 (± 16) | 44 (± 19) | .24† |
| Age at diagnosis (y), mean (± SD) | 40 (± 16) | 39 (± 14) | 45 (± 21) | 39 (± 15) | 51 (± 15) | .1‡ |
| Presence of angioedema, no. (%) | 295 (74.8%) | 122 (80.7%) | 23 (65.7%) | 144 (71.6%) | 6 (85.7%) | .11‡ |
| Presence of CIndU, no. (%) | 126 (31.9%) | 49 (32.4%) | 9 (25.7%) | 66 (32.8%) | 2 (28.5%) | .9‡ |
| d-Dimer | ||||||
| High (≥500 ng/mL), no. (%) | 40 of 99 (40.4%) | 11 of 34 (32.3%) | 6 of 10 (60%) | 42.5 of 54 (57.4%) | 0 of 1 (0%) | .35‡ |
| Mean ng/mL, (± SD) | 605 (±614) | 583 (± 484) | 622 (± 389) | 623 (± 722) | 190 | .37† |
| Eosinophils | ||||||
| Eosinopenia (<0.05 × 109/L), no. (%) | 16 of 388 (4.1%) | 2 of 149 (1.3%) | 0 of 35 (0%) | 14 of 197 (7.1%) | 0 of 7 (0%) | .04‡ |
| Eosinophil count × 109/L (± SD) | 0.199 (± 0.176) | 0.218 (± 0.186) | 0.142 (± 0.148) | 0.195 (± 0.173) | 0.171 (± 0.111) | .04§ |
| Worsening with NSAIDs, no. (%) | 86 of 357 (24%) | 35 of 136 (26.4%) | 6 of 30 (20%) | 42 of 185 (22.7%) | 3 of 6 (50%) | .4‡ |
| Updosing second-generation H1 antihistamines (2×, 3×, or 4×) | 268 of 389 (68.8%) | 100 of 150 (66.6%) | 24 of 35 (68.5%) | 140 of 197 (71%) | 4 of 7 (57.1%) | .69‡ |
| Use of omalizumab, no. (%) | 39 (9.8%) | 18 (11.9%) | 5 (14.5%) | 16 (7.9%) | 0 (0%) | .41‡ |
| Fast responders to omalizumab, no. (%) | 29 of 39 (74.3%) | 14 of 18 (77.7%) | 5 of 5 (100%) | 10 of 16 (62.5%) | 0 (0%) | .46‡ |
Boldface indicates statistical significance.
CIndU, Chronic inducible urticaria; NSAID, nonsteroidal anti-inflammatory drug.
ASST performed in 61.7% of the patients (243 of 394).
Kruskal-Wallis non-parametric test.
Fisher exact test.
After the Dunn test, type I and type I/type IIb overlap were shown to be different from type IIb and non–type I/type IIb.
Skin biopsies were performed in 44 patients for diagnostic purposes. Among the 34 nonvasculitis biopsy samples, histopathologic analysis revealed many eosinophils in 15 (44.1%); few eosinophils in 10 (29.4%); and lymphocytic, neutrophilic, lymphohistiocytic and lymphoplasmocytic infiltrates in variable frequencies, with more than 1 pattern possible.
Our study showed that Brazilian patients with CSU presented characteristics similar to those from other areas of the world, including female predominance, age at onset of symptoms between the third and fourth decades of life, and associated angioedema and chronic inducible urticaria in two-thirds and one-third of the cases, respectively. Updosing of second-generation H1 antihistamines was required for treatment of approximately 70% of our patients, possibly owing to the tertiary nature of care of our clinic. Most patients presented with clinical features consistent with a presumptive type 1 endotype, associated or not with type IIb endotype. Several biomarkers have been proposed to characterize severity, duration, and response to treatment,13 allowing for endotyping of patients with CSU; however, some issues remain unresolved, including the threshold levels for low total IgE level, which vary in different studies. We chose to use the cutoff of 40 UI/mL based on previous studies, including the PURIST study by Schoepke et al.3,4,7,13 However, other studies showed that in patients with CSU IgE levels are low when they are less than 20 UI/mL.14, 15, 16, 17 Interestingly, our frequency of type I/type IIb CSU overlap was similar to that in previous studies, in particular in the study by Asero et al,18 which is remarkable for testing for the co-occurrence of IgE and IgG autoantibodies to high- and low-affinity IgE receptors (FcεRI and FcεRII), tissue factor, and thyroglobulin in the same patients. The data revealed that more than 50% of patients had IgE and IgG to 1 or more of these autoantigens.18 Moreover, the finding of a low frequency of patients with type IIb only (9%) was consistent with the findings of other studies, even when additional parameters, such as BAT result and IgG anti-FcεRI level and/or IgG anti-IgE level determined by ELISA, were used to characterize the autoimmune endotype.13
Another important issue that remains unresolved is which single marker or combination of markers would be more useful to endotype patients with CSU in a manner allowing for better clinical management, particularly considering the upcoming novel treatments beyond anti-IgE therapy.2 Up to now there have been no defined criteria for CSU endotyping that could be applied worldwide owing to a lack of availability of methods for measuring CSU-relevant antibodies and basophil activation tests in many areas of the world. In the present study, our informed decision to consider the presence of at least 1 marker sufficient for assigning the patients to each of the presumed endotypes was based on the fact that our criteria had differences from those proposed in the PURIST study and in other studies. Response to anti-IgE treatment could be considered an additional criterion for endotyping. In the present study, 29 of 39 of the patients (74.3%) were fast responders to omalizumab within 4 weeks of treatment. However, access to omalizumab in Brazil is limited, and throughout the study period only 39 of 394 patients were taking omalizumab (9.8%), representing those who were granted access to the medication and not necessarily all of those within the group who would have had an indication for anti-IgE therapy. Therefore, response to anti-IgE therapy would be subject to selection bias.
Eosinopenia was less common than previously reported,9,12 perhaps because of higher lifetime exposure to intestinal parasites in our area. However, skin biopsy samples from selected patients often revealed eosinophil-rich inflammatory infiltrates.9 The mechanisms of eosinopenia in patients with CSU are still unknown. It is possible that low blood eosinophil counts may reflect eosinophils' recruitment to the skin during active disease, as suggested by Kolkhir et al.12
Our results suggest an important role for type 2 immune responses in patients with CSU. Further studies addressing natural history and response to the various urticaria treatments in these well-characterized patients will shed light on the usefulness of endotyping patients with CSU in clinical practice. Additional research is needed to identify more specific and consistent markers in patients with CSU from different populations to allow for better understanding of the underlying mechanisms triggering the onset and determining duration of CSU.
Disclosure statement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. Disclosure of potential conflict of interest: L. K. Arruda is a recipient of a Brazilian National Council for Scientific and Technological Development Research Productivity Grant. The rest of the authors declare that they have no relevant conflicts of interest.
Clinical implications.
Most Brazilian patients with CSU presented with a presumptive autoallergic endotype, with or without features of autoimmunity. Endotyping CSU by using routine clinical tests may lead to better prognostication and optimal treatment choices for individual patients.
Supplementary data
References
- 1.Kolkhir P., Giménez-Arnau A.M., Kulthanan K., Peter J., Metz M., Maurer M. Urticaria. Nat Rev Dis Primers. 2022;8:61. doi: 10.1038/s41572-022-00389-z. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan A., Lebwohl M., Giménez-Arnau A.M., Hide M., Armstrong A.W., Maurer M. Chronic spontaneous urticaria: focus on pathophysiology to unlock treatment advances. Allergy. 2023;78:389–401. doi: 10.1111/all.15603. [DOI] [PubMed] [Google Scholar]
- 3.Kolkhir P., Muñoz M., Asero R., Ferrer M., Kocatürk E., Metz M., et al. Autoimmune chronic spontaneous urticaria. J Allergy Clin Immunol. 2022;149:1819–1831. doi: 10.1016/j.jaci.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Schoepke N., Asero R., Ellrich A., Ferrer M., Gimenez-Arnau A., E H Grattan C., et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: results of the PURIST Study. Allergy. 2019;74:2427–2436. doi: 10.1111/all.13949. [DOI] [PubMed] [Google Scholar]
- 5.Maurer M., Rosén K., Hsieh H.-J., Saini S., Grattan C., Gimenéz-Arnau A., et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 6.Zuberbier T., Bernstein J.A., Maurer M. Chronic spontaneous urticaria guidelines: what is new? J Allergy Clin Immunol. 2022;150:1249–1255. doi: 10.1016/j.jaci.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Kolkhir P., Kovalkova E., Chernov A., Danilycheva I., Krause K., Sauer M., et al. Autoimmune chronic spontaneous urticaria detection with IgG anti-TPO and total IgE. J Allergy Clin Immunol Pract. 2021;9:4138–4146. doi: 10.1016/j.jaip.2021.07.043. [DOI] [PubMed] [Google Scholar]
- 8.Zuberbier T., Abdul Latiff A.H., Abuzakouk M., Aquilina S., Asero R., Baker D., et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734–766. doi: 10.1111/all.15090. [DOI] [PubMed] [Google Scholar]
- 9.Altrichter S., Frischbutter S., Fok J.S., Kolkhir P., Jiao Q., Skov P.S., et al. The role of eosinophils in chronic spontaneous urticaria. J Allergy Clin Immunol. 2020;145:1510–1515. doi: 10.1016/j.jaci.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Konstantinou G.N., Asero R., Maurer R., Sabroe R.A., Schmid-Grendelmeier P., Grattan C.E.H. EAACI/GA(2)LEN task force consensus report: the autologous serum skin test in urticaria. Allergy. 2009;64:1256–1268. doi: 10.1111/j.1398-9995.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 11.Asero R, Ferrer M, Kocaturk E, Maurer M. Chronic spontaneous urticaria: the role and relevance of autoreactivity, autoimmunity, and autoallergy [e-pub ahead of print]. J Allergy Clin Immunol Pract https://doi.org/10.1016/j.jaip.2023.02.022. Accessed August 5, 2023. [DOI] [PubMed]
- 12.Kolkhir P., Church M.K., Altrichter S., Skov P.S., Hawro T., Frischbutter S., et al. Eosinopenia, in chronic spontaneous urticaria, is associated with high disease activity, autoimmunity, and poor response to treatment. J Allergy Clin Immunol Pract. 2020;8:318–325. doi: 10.1016/j.jaip.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Xiang YK, Kolkhir P, Scheffel J, Sauer M, Vera C, Frischbutter S, et al. Most patients with autoimmune chronic spontaneous urticaria also have autoallergic urticaria, but not vice versa [e-pub ahead of print]. J Allergy Clin Immunol Pract https://doi.org/10.1016/j.jaip.2023.02.006, Accessed August 5, 2023. [DOI] [PubMed]
- 14.Straesser M.D., Oliver E., Palacios T., Kyin T., Patrie J., Borish L., et al. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J Allergy Clin Immunol Pract. 2018;6:1386–1388. doi: 10.1016/j.jaip.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weller K., Ohanyan T., Hawro T., Ellrich A., Sussman G., Koplowitz J., et al. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy. 2018;73:2406–2408. doi: 10.1111/all.13586. [DOI] [PubMed] [Google Scholar]
- 16.Ertas R., Ozyurt K., Atasoy M., Hawro T., Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy. 2018;73:705–712. doi: 10.1111/all.13345. [DOI] [PubMed] [Google Scholar]
- 17.Asero R. Clinical variables of severe chronic spontaneous urticaria from total IgE standpoint: a retrospective study. Eur Ann Allergy Clin Immunol. 2022;54:30–33. doi: 10.23822/EurAnnACI.1764-1489.191. [DOI] [PubMed] [Google Scholar]
- 18.Asero R., Marzano A.V., Ferrucci S., Lorini M., Carbonelli V., Cugno M. Co-occurrence of IgE and IgG autoantibodies in patients with chronic spontaneous urticaria. Clin Exp Immunol. 2020;200:242–249. doi: 10.1111/cei.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.