Abstract
Background
Anaphylaxis is a life-threatening allergic reaction that poses a considerable burden on populations across all ethnicities and age groups. The Hong Kong Multidisciplinary Anaphylaxis Management Initiative (HK-MAMI) was established to streamline the assessment of patients with anaphylaxis via a multidisciplinary and protocol-driven approach.
Objective
This prospective study aims to define the etiology, clinical manifestations, and treatment of patients with anaphylaxis in Hong Kong.
Methods
Prospective clinical data from allergologic investigations from patients who completed evaluation by the HK-MAMI pathway between January 2017 and August 2022 were analyzed.
Results
Of the 161 patients referred via the HK-MAMI, 131 (81.4%) met the diagnostic criteria for anaphylaxis. The median delay in diagnosis was 2 years (range 0-30 years). The majority of anaphylaxis cases were attributed to food-dependent exercise-induced anaphylaxis (FDEIA), especially wheat-dependent exercise-induced anaphylaxis. In acute management settings, paired tryptase samples were taken in only around one-third of anaphylaxis cases, with 82.5% of the samples demonstrating significant elevation. There was a general underprescription of adrenaline autoinjectors, especially for food-related anaphylaxis. Patients with FDEIA had later ages of onset and diagnosis, and they presented with more cardiovascular manifestations. Skin prick tests and specific IgE level tests were able to diagnose 95% of FDEIA cases.
Conclusion
Our study highlights the significant burden of FDEIA, and especially WDEIA, in Hong Kong, its association with severe presentations, and difficulties encountered in emergency or primary care settings. We advocate appropriate adrenaline use during acute-care management and discharge plans, as well as taking serum mast cell tryptase samples during acute episodes. Interdisciplinary collaboration remains crucial to upholding proper and optimized care for patients with anaphylaxis in Hong Kong.
Key words: Anaphylaxis, epidemiology, exercise-induced, food-dependent, multidisciplinary
Anaphylaxis is an acute, systemic, and life-threatening allergic reaction that poses a considerable burden to populations across all ethnicities and age groups.1 In Hong Kong, the estimated incidence rate of anaphylaxis is 3.57 per 100,000 person years, and similar to the anaphylaxis admissions rate for international cohorts, admissions for anaphylaxis have increased significantly over the past 10 years.2, 3, 4, 5, 6 Patients with anaphylaxis often present in acute care settings; therefore, proper management of these patients requires collaborations between allergists, nurses, dietitians, and primary health care and emergency medicine physicians. Early recognition and rapid intramuscular administration of adrenaline (usually in the form of an adrenaline autoinjector [AAI]) are cornerstones of optimal anaphylaxis management. Timely collection of acute mast cell tryptase levels allows for subsequent paired tryptase analysis that aids in diagnosis. Appropriate postdischarge management, including allergen avoidance and prescription of AAI with allergist referral, have previously been advocated in both local and international recommendations.1,7,8
The underdiagnosis and suboptimal management of anaphylaxis survivors lead to impaired quality of life and increased morbidity and mortality, and they impose a significant economic burden on individuals and health care systems.9, 10, 11, 12, 13 Despite this, a significant proportion of anaphylaxis survivors are unaware of the etiology of their previous life-threatening reactions, and there remains a discrepantly low rate of AAI prescriptions, particularly among adult patients.3,13 Food-dependent exercise-induced anaphylaxis (FDEIA), especially wheat-dependent exercise-induced anaphylaxis (WDEIA), has been identified as one of the major causes of anaphylaxis among Hong Kong Chinese adults but nonetheless remains severely overlooked and underdiagnosed.13 Significant room for improvement in the care of anaphylaxis survivors remains, but improvement is limited by the severe shortage of allergy specialists and services in Hong Kong.14
On the basis of the prior successes of similar multidisciplinary allergy service models in recent years, the Hong Kong Multidisciplinary Anaphylaxis Management Initiative (HK-MAMI) was established in 2018 to streamline the assessment and risk stratification of anaphylaxis survivors in Hong Kong.15, 16, 17, 18 The HK-MAMI aims to utilize a multidisciplinary and protocol-driven approach to patients with suspected anaphylaxis. According to the HK-MAMI pathway, all cases of suspected anaphylaxis are initially managed by emergency medicine physicians and subsequently recruited. Referrals of anaphylaxis cases are reinforced by establishment of local combined emergency medicine–allergy pathways as well as recommendations published by the Hong Kong Anaphylaxis Consortium.7 Thereafter, patients are interviewed and counseled by a trained allergist-nurse, with comprehensive history taking and appropriate discharge advice (including AAI prescription and education). Patients who meet the criteria for anaphylaxis are then prioritized to see an allergist after 2 months and within 6 months of the index event for subsequent workup and management. This longitudinal study was conducted in parallel with establishment of the HK-MAMI to investigate the etiology, clinical characteristics, management, and outcomes of patients recruited in this novel pathway.
Methods
The HK-MAMI pathway was established in 2017 and has been in effect in the Hong Kong West Cluster (HKWC) under the Hospital Authority since then; it receives referrals following episodes of suspected anaphylaxis from the whole of Hong Kong (both the public and private sector). Only referrals for adult patients (defined as aged 18 years or older) are accepted. Patients who have experienced perioperative anaphylaxis are recruited into another dedicated pathway and excluded from the HK-MAMI. All patients diagnosed with suspected anaphylaxis are first interviewed by a trained nurse based on a protocol-driven questionnaire (see Fig E1 in the Online Repository at www.jaci-global.org) to ensure that they meet the diagnostic criteria and clarify relevant history.8,19 The HK-MAMI adopted the definition and diagnostic criteria for anaphylaxis from the World Allergy Organization anaphylaxis guidelines.20 All nurse-led interviews are subsequently verified with an allergist. Patients meeting the criteria are then referred for evaluation and workup at the anaphylaxis clinic after 2 months and within 6 months of the index event. Evaluation includes a comprehensive history and allergologic investigations (including skin prick tests [SPTs], intradermal tests, and specific IgE [sIgE] tests with or without challenges) at the discretion of the attending allergist. Specifically for FDEIA, all patients are diagnosed based on a compatible history and demonstration of IgE sensitization to the culprit food.21 Patients with suspected FDEIA are first offered an SPT during their first consultation and, if the SPT result is negative, they undergo sIgE tests to the culprit food. For research purposes, we also took blood samples from all patients with WDEIA and performed sIgE tests. Patients with confirmed anaphylaxis were offered regular follow-ups at the anaphylaxis clinic every 4 to 6 months thereafter to review whether there are any recurrent events or use of AAI following diagnosis.
Longitudinal clinical data and results from allergologic investigations of patients who completed evaluation via the HK-MAMI pathway between January 2017 and August 2022 were anonymized and collected. Only patients who completed a workup, met the diagnostic criteria, and were diagnosed by their attending allergists were included.8,19 The data collected include baseline demographics; age at the first episode of allergy or anaphylaxis; smoking status; history of asthma and/or chronic obstructive pulmonary disease, hypertension, or chronic urticaria; manifestations of an index reaction; acute and baseline tryptase results (if available); use of adrenaline during the index episode; prescription of an AAI following the index episode; the allergist’s decision regarding AAI prescription after review; any recurrence or use of an AAI after diagnosis; and duration of follow-up. A World Allergy Organization systemic allergic reaction grade was retrospectively assigned to each patient to denote reaction severity.8 In this classification, reactions were classified as grade 1 to 5, with grades 3 to 5 constituting anaphylaxis. A significant elevation of tryptase level was defined as the acute sample (taken within 30 minutes to 6 hours of symptom onset) reaching a level greater than 1.2 times the baseline tryptase level plus 2 ng/L (with the baseline measurement taken at least 24 hours after the reaction). Substances used in traditional Chinese medicine (TCM) were also categorized as drugs. All patients gave informed consent, and the study was approved by the institutional review board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster.
Unless otherwise specified, categoric variables were expressed as numbers (percentages), and continuous variables were expressed as medians (ranges). Comparisons between clinical characteristics and outcomes were performed between patients experiencing anaphylaxis of different etiologies. Categoric variables were compared by chi-square test or Fisher exact test, as appropriate. For continuous variables, significance of differences was examined by an independent t test. A P value less than .05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics, version 28.0 (IBM, Armonk, NY).
Results
During the study period, a total of 161 patients were referred via the HK-MAMI pathway. Of those 161 patients, 131 (81.4%) met the diagnostic criteria for anaphylaxis. The overall male-to-female ratio was 1:1.3, and the median age was 48 years (range 20-86 years). The median delay in diagnosis (ie, duration from the first episode of symptoms to diagnosis) was 2 years (range 0-30 years). The demographics, clinical characteristics, and outcomes of patients with anaphylaxis according to different etiologies are shown in Table I. Both age at diagnosis (42 years [range 1-84 years] vs 54 years [range 18-82 years] [P < .001]) and age at first episode (35 years [range 1-80 years] vs 51 years [18-81 years] [P < .001]) were significantly lower in patients with food- rather than drug-related anaphylaxis. However, there was no difference between the delay in diagnosis between the 2 groups (3 years [range 0-26 years] in the case of food allergy vs 1 year [range 0-30 years] in the case of drug allergy [P = .107]).
Table I.
Breakdown of demographics, clinical characteristics, and outcomes of patients with anaphylaxis according to different etiologies
| Characteristic | All (N = 131) | Food (n = 83) | Drug (n = 28) | Other∗ (n = 20) |
|---|---|---|---|---|
| Male sex, no. (%) | 57 (43.5) | 41 (49.4) | 9 (32.1) | 7 (35.0) |
| Age (y), median (range) | 48 (20-86) | 44 (20-86) | 55 (20-83) | 48 (22-76) |
| Age at first episode (y), median (range) | 42 (1-81) | 35 (1-80) | 51 (18-81) | 45 (12-74) |
| Delay in diagnosis (y), median (range) | 2 (0-30) | 3 (0-26) | 1 (0-30) | 1 (0-16) |
| Smoker, no. (%) | 15 (11.5) | 8 (9.6) | 5 (17.9) | 2 (10.0) |
| History of chronic urticaria, no. (%) | 38 (29.0) | 26 (31.3) | 5 (17.9) | 7 (35.0) |
| Hypertension, no. (%) | 20 (15.3) | 10 (12.0) | 6 (21.4) | 4 (20.0) |
| Asthma and/or COPD, no. (%) | 18 (13.7) | 16 (19.3) | 1 (3.6) | 1 (5.0) |
| Clinical presentation, no. (%) | ||||
| Mucocutaneous | 125 (95.4) | 81 (97.6) | 25 (89.3) | 19 (95.0) |
| Cardiovascular | 101 (77.1) | 63 (75.9) | 22 (78.6) | 16 (80.0) |
| Respiratory | 73 (55.7) | 47 (56.6) | 16 (57.1) | 10 (50.0) |
| Gastrointestinal | 24 (18.3) | 14 (16.9) | 4 (14.3) | 6 (30.0) |
| WAO systemic allergic reaction grade, no. (%)† | ||||
| Grade 3 | 22 (16.8) | 13 (15.7) | 5 (17.9) | 4 (20.0) |
| Grade 4 | 2 (1.5) | 2 (2.4) | 0 (0.0) | 0 (0.0) |
| Grade 5 | 107 (81.7) | 68 (81.9) | 23 (82.1) | 16 (80.0) |
| Acute management, no. (%) | ||||
| Paired tryptase sample taken | 47 (35.9) | 24 (28.9) | 16 (57.1) | 7 (35.0) |
| Significant rise | 37/47 (78.7) | 19/24 (79.2) | 14/16 (87.5) | 4/7 (57.1) |
| Adrenaline administered | 74 (56.5) | 45 (54.2) | 15 (53.6) | 14 (70.0) |
| Discharged with AAI | 62 (47.3) | 47 (56.6) | 2 (7.1) | 13 (65.0) |
| After allergist review | ||||
| AAI prescribed, no. (%) | 102 (77.9) | 83 (100.0) | 1 (3.6) | 18 (90.0) |
| Recurrence, no. (%) | 9 (6.9) | 7 (8.4) | 0 (0.0) | 2 (10.0) |
| Adrenaline administered, no. (%) | 5 (3.8) | 4 (4.8) | 0 (0.0) | 1 (5.0) |
| Follow-up duration (y), median (range) | 1.55 (0.20-5.69) | 1.83 (0.20-5.69) | 1.20 (0.28-3.81) | 1.28 (0.45-4.23) |
COPD, Chronic obstructive pulmonary disease; WAO, World Allergy Organization.
Other: idiopathic (n = 13), venom (n = 4), exercise-induced anaphylaxis (n = 2), and hamster (n = 1).
In this classification, anaphylaxis includes only grade 3 to grade 5 reactions.
The majority of anaphylaxis cases are attributed to FDEIA, especially WDEIA
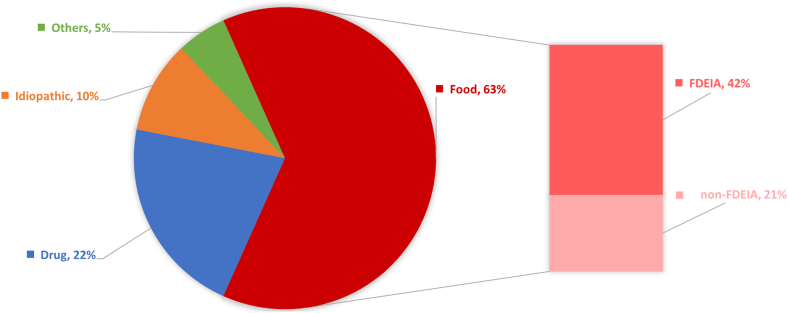
Specific etiologies of anaphylaxis were identified in 90.1% of the patients (118 of 131) after detailed allergologic workup, with 13 patients diagnosed as having “idiopathic anaphylaxis” by exclusion. A breakdown of the confirmed etiologies of anaphylaxis is shown in Fig 1. The majority of anaphylaxis cases were triggered by food (63% [83 of 131]), followed by cases triggered by a drug (22% [28 of 131]). The frequencies of the culprits of drug-related anaphylaxis are shown in Table E1 (in the Online Repository at www.jaci-global.org).
Fig 1.
Breakdown of confirmed etiologies of anaphylaxis (n = 131). Idiopathic anaphylaxis (n = 13), venom- (n = 4), exercise- (n = 2), and hamster-induced anaphylaxis (n = 1).
Of those cases with food-related anaphylaxis, two-thirds were attributable to FDEIA (42% overall [55 of 131]), and the remaining one-third (21% overall [28 of 131]) were non-FDEIA primary food allergies. Among food allergens, wheat was the most commonly implicated food, accounting for the majority of food allergy cases (57.8% [48 of 83]). In particular, WDEIA was the most common cause of FDEIA (81.8% of cases [45 of 55]) and food-related anaphylaxis overall (54.2% of cases). Shellfish was the second most common allergen implicated in FDEIA (in 16.7% of cases [9 of 55]) and food-related anaphylaxis overall (in 13.3% of cases).
Low rate of paired tryptase samples taken and adrenaline use during acute management
Paired tryptase samples were taken in only around one-third (35.9%) of anaphylaxis cases. Significantly more paired tryptase samples were available for patients with drug- versus food-related anaphylaxis (57.1% vs 28.9% [P = .007]). Of the 40 patients with paired tryptase level results available, 82.5% demonstrated significant elevation. The rate of significant tryptase level elevation did not differ statistically between drug- and food-related anaphylaxis (87.5% vs 79.2% [P = .681]). Only around half of the patients were administered adrenaline intramuscularly, subcutaneously, or intravenously during their index event (drug- vs food-related anaphylaxis [53.6% vs 54.2%] [P = .953]). Specifically, only 56 patients received adrenaline via the intramuscular route. In addition, only 44.1% were prescribed an AAI at discharge (drug- vs food-related anaphylaxis [7.1% vs 56.6%] [P < .001]).
Underprescription of AAI before allergist review
The prescription rate and concordance of AAI before and after allergist review are shown in Fig 2. Most decisions to prescribe AAI were deemed appropriate after allergist review (60 of 62 of cases [96.8%]), whereas 39.1% of the decisions to not prescribe AAI (27 of 69) were subsequently revised. All patients with food-related anaphylaxis were prescribed AAI because of a history of severe reactions and high risk of allergen reexposure. One patient with a drug allergy to TCM was also prescribed an AAI because of a history of severe reaction and unidentifiable ingredient in his herbal TCM.
Fig 2.
Flow diagram of prescription and concordance of AAI before and after allergist review.
Patients with FDEIA had later diagnosis and onset, with more cardiovascular manifestations
The clinical characteristics and outcomes of patients diagnosed with FDEIA and those of patients with non-FDEIA food anaphylaxis were compared and are shown in Table II. Both age at diagnosis (45 years [range 19-84 years] vs 35 years [range 1-66 years] [P < .033]) and age at first episode (38 years [range 15-80 years] vs 31 years [range 1-62 years] [P < .046]) were significantly greater in patients with FDEIA versus in those in the non-FDEIA group. Significantly fewer patients with FDEIA had concomitant asthma and/or chronic obstructive pulmonary disease (10.9% vs 35.7% [P = .007]). Patients with FDEIA were more likely to experience cardiovascular manifestations (90.9% vs. 46.4% [P < .001]) but less likely to experience respiratory manifestations (47.3% vs 75.0% [P = .016]) than were patients in the non-FDEIA group. In our cohort of 55 patients with FDEIA, all (100%) of them reported exercise as a cofactor, with concurrent cofactors of nonsteroidal anti-inflammatory drug use (3 of 55 [5.5%]) and alcohol use (2 of 55 [3.6%]) present in a few patients (see Fig E2 in the Online Repository at www.jaci-global.org).
Table II.
Comparison between characteristics and outcomes of patients diagnosed with FDEIA and non-FDEIA food anaphylaxis
| Characteristic | Food (n = 83) | FDEIA (n = 55) | Non-FDEIA (n = 28) | P value |
|---|---|---|---|---|
| Male sex, no. (%) | 41 (49.4) | 28 (50.9) | 13 (46.4) | .699 |
| Age (y), median (range) | 44 (20-86) | 47 (23-86) | 38 (20-67) | .096 |
| Age at first episode (y) | 35 (1-80) | 38 (15-80) | 31 (1-62) | .046 |
| Delay in diagnosis (y) | 3 (0-26) | 3 (0-26) | 3 (0-17) | .383 |
| Smoker, no. (%) | 8 (9.6) | 4 (7.3) | 4 (14.3) | .433 |
| Asthma and/or COPD, no. (%) | 26 (31.3) | 6 (10.9) | 10 (35.7) | .007 |
| Hypertension, no. (%) | 10 (12.0) | 9 (16.4) | 1 (3.6) | .153 |
| History of chronic urticaria, no. (%) | 16 (19.3) | 20 (36.4) | 6 (21.4) | .165 |
| Clinical presentation, no. (%) | ||||
| Mucocutaneous | 81 (97.6) | 54 (98.2) | 27 (96.4) | 1.000 |
| Cardiovascular | 63 (75.9) | 50 (90.9) | 13 (46.4) | <.001 |
| Respiratory | 47 (56.6) | 26 (47.3) | 21 (75.0) | .016 |
| Gastrointestinal | 14 (16.9) | 9 (16.4) | 5 (17.9) | 1.000 |
| WAO systemic allergic reaction grade, no. (%)∗ | <.001 | |||
| Grade 3 | 13 (15.7) | 1 (1.8) | 12 (42.9) | |
| Grade 4 | 2 (2.4) | 1 (1.8) | 1 (3.6) | |
| Grade 5 | 68 (81.9) | 53 (96.4) | 15 (53.6) | |
| Acute management, no. (%) | ||||
| Paired tryptase sample taken | 24 (28.9) | 16 (29.1) | 8 (28.6) | .961 |
| Significant elevation | 19/24 (79.2) | 13/16 (81.3) | 6/8 (75.0) | 1.000 |
| Adrenaline administered | 45 (54.2) | 30 (54.5) | 15 (53.6) | .933 |
| Discharged with AAI | 47 (56.6) | 30 (54.5) | 17 (60.7) | .592 |
| After allergist review | ||||
| AAI prescribed, no. (%) | 83 (100.0) | 55 (100.0) | 28 (100.0) | N/A |
| Recurrence, no. (%) | 7 (8.4) | 4 (7.3) | 3 (10.7) | .683 |
| Adrenaline administered, no. (%) | 4 (4.8) | 1 (1.8) | 3 (10.7) | .109 |
| Follow-up duration (y), median (range) | 1.83 (0.20-5.69) | 1.76 (0.20-5.69) | 2.03 (0.20-3.97) | .677 |
Boldface indicates statistical significance (P < .05).
COPD, Chronic obstructive pulmonary disease; NA, not available; WAO, World Allergy Organization.
In this classification, anaphylaxis includes only grade 3 to grade 5 reactions.
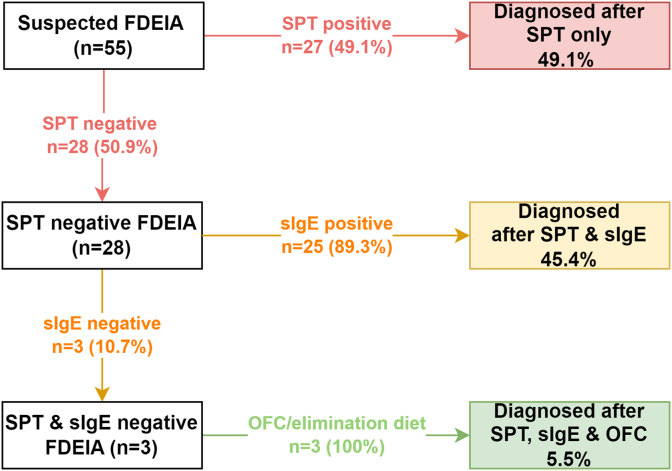
Half of FDEIA cases could be diagnosed with SPTs alone, and 95% were diagnosed with a combination of SPTs and sIgE tests
According to departmental protocol, patients with a compatible history of FDEIA would first be offered an SPT during their first consultation and, if the result is negative, sIgE tests to culprit food are offered. The diagnostic approach and outcomes for all patients with FDEIA are shown in Fig 3. Half of the cases (27 of 55 [49.1%]) were confirmed by an SPT, and almost all of the remaining cases (25 of 28 [89.3%]) were then diagnosed by sIgE tests. Multiplex sIgE test panels (ImmunoCAP ISAC microarray, Thermofisher Phadia) were used in 3 cases with both negative SPT and sIgE test results; the test panels did not yield additional diagnostic information. The remaining 3 cases were clinically diagnosed by either an oral food challenge or an oral elimination diet (ie, resolution of FDEIA following avoidance of culprit foods). For WDEIA, 22 patients had a positive SPT result, with all of the cases involving an SPT with a positive result also demonstrating positive sIgE test results.
Fig 3.
Flowchart of the diagnostic approach and outcomes of 55 patients with FDEIA. OFC, Oral food challenge.
Discussion
This longitudinal study reports the etiology, clinical characteristics, management, and outcome of patients recruited in the HK-MAMI. As our data were obtained via the sole adult referral center for anaphylaxis in the public health care system, they are representative of the majority of anaphylaxis cases in the entire territory over the past 5 years. Our findings outline the distinctive etiologies of anaphylaxis among Chinese adults with a disproportionately high rate of FDEIA or WDEIA, as well as its association with severe clinical manifestations.
Akin to previous reports, our study demonstrates that food allergy remains the leading cause of anaphylaxis among adults in Hong Kong.22 Patients with food-related anaphylaxis presented and were also diagnosed at a younger age than patients with drug-associated anaphylaxis. However, there was no difference between the delays in diagnosis between the 2 groups. This likely reflects the later sensitization and exposure to causative drugs than to food. Unlike patients with drug-associated anaphylaxis, patients with severe food allergies often require more detailed counseling (including dietary avoidance and dietitian referral), regular follow-up, and prescriptions for AAI. It is therefore imperative that future strategies further emphasize the role of allied health professionals, especially allergy nurses, dietitians, and pharmacists, in achieving comprehensive and continuous care without overloading the very limited allergist labor force in Hong Kong.
Despite adrenaline being universally advocated as the first-line treatment for anaphylaxis, we identified a significantly low rate of adrenaline administration in the acute care setting. It was observed that certain patients in our cohort received subcutaneous injections of adrenaline, which may be less effective than intramuscular injections. This emphasizes the importance of continued education for the latest evidence, especially for health care professionals at the first point of contact, such as paramedics and emergency medicine physicians. There was also suboptimal documentation for acute mast cell tryptase levels, with around only one-third of patients with anaphylaxis having paired tryptase samples available for analysis. Among those patients for whom paired tryptase results were available, more than 80% demonstrated significant elevation (ie, sensitivity) regardless of etiology. Interestingly, physicians were significantly more likely to collect tryptase from patients with drug- rather than food-related anaphylaxis. This may reflect the relative ease of physicians recognizing early signs of anaphylaxis following drug exposure (as opposed to food exposure, especially in cases of FDEIA) and therefore missing the time window for blood sampling among patients with delayed diagnoses. Given their utility and importance in anaphylaxis management, we propose that rates of adrenaline administration and tryptase sampling be included in future quality control studies and service audits.
FDEIA (especially WDEIA) remains the most common cause of anaphylaxis among adults in Hong Kong Chinese individuals, accounting for more than 40% of cases. FDEIA as a disease entity was likely underreported and undertreated before establishment of the HK-MAMI. In our cohort, two-thirds of cases of food-related anaphylaxis were cases of FDEIA. Wheat was the top food allergen, accounting for the majority of food allergy cases and more than 80% of FDEIA cases. This contrasts with the findings of previous studies, which reported a low prevalence of wheat anaphylaxis in Hong Kong.22 This discrepancy may be attributed to a lack of disease awareness and vigilance, especially in nonspecialist settings. FDEIA is a relatively less emphasized condition and was not systematically taught in either of the medical schools in Hong Kong before 2017. The presentation of FDEIA can also be very heterogeneous, with irregular temporal relationships and variable presence of cofactors leading to inconsistent symptom presentations. Furthermore, we discovered that Chinese patients with FDEIA among Chinese present differently than White patients do—with more cardiovascular but fewer cutaneous manifestations, which may further compound diagnostic difficulty.13 Compared with other cohorts, our cohort in Hong Kong was characterized by a significantly larger burden of FDEIA. For instance, FDEIA was responsible for only 13.2% and 38.3% of total and food-related anaphylaxis cases in Korea, respectively.23 Another recent Korean study even reported that less than 20% of food anaphylaxis was caused by FDEIA.24 This disparity may be attributed either to genuine biologic differences among different ethnicities or to dietary habits/exposures across localities that result in distinct sensitisation profiles. Furthermore, it is possible that there was a selection bias, with diagnostically more challenging patients (such as those with FDEIA) more likely to reach our service than those with obvious culprits, who might decline referral for further workup. The fact that we manage only adult patients may also have led to an etiologic distribution different from that in prior studies. Further interethnic and interregional studies will be of great interest. Given its underrecognition and underdiagnosis despite an overwhelmingly high prevalence and importance as a cause of anaphylaxis among Chinese individuals, FDEIA should be deemed a priority in anaphylaxis evaluation protocols and promotion of disease awareness among the public and health care professionals.
We have demonstrated that a protocol-driven, multidisciplinary (nurse-led history taking with allergist confirmation and evaluation) pathway is effective in screening and evaluating patients with FDEIA. Via the HK-MAMI pathway, almost 90% of patients with FDEIA in our cohort were diagnosed on the basis of clinical history and demonstratable IgE-sensitization to the culprit food (by either SPTs or sIgE assays). Diagnosed patients are counseled and educated by nurses and dietitians on a personalized emergency plan, including AAI technique, and they are also given allergen and cofactor avoidance advice. Having a well-established diagnostic pipeline reinforces confidence in collaborating personnel and allows more patients with FDEIA to be safely screened, identified, and treated in our locality.
This study is subject to several limitations, such as the sampling method. Our center receives referrals for adult patients only; those who died of anaphylaxis or declined referrals and pediatric patients would not and could not be recruited. There is also potential for selection bias, as we did not have available data on patients who were not referred or were misdiagnosed. Some parameters collected (eg, recurrence, cofactor involvement) rely on patients’ self-reporting only. Furthermore, food cofactor challenge, which is sometimes deemed the criterion standard for FDEIA diagnosis, was not routinely offered at our center owing to facility constraints and operating risks. The optimal diagnostic approach for FDEIA diagnosis remains unknown, and all test results should be interpreted with relevance to individual patient histories and clinical context. For example, in rare cases, SPT might yield false-positive results because of cross-reactivity to grass allergens, and even the result of food cofactor challenge could be falsely negative despite being the most definitive modality.25,26
In conclusion, our study highlights the significant burden of FDEIA, and especially WDEIA, in Hong Kong; its association with severe presentations; and diagnostic difficulties encountered in emergency or primary care settings. It is therefore imperative to continue to promote awareness of FDEIA and WDEIA among nonspecialists. We advocate appropriate adrenaline use during acute care management and discharge plans, as well as collection of serum mast cell tryptase samples during acute episodes. Our HK-MAMI pathway, which incorporates a protocol-driven, multidisciplinary approach to anaphylaxis evaluation, shows promises for increased capacity for timely reviews of suspected anaphylaxis cases. Given a significant deficit of provisional allergy services, collaboration among disciplines remains crucial to upholding proper and optimized care for patients with anaphylaxis in Hong Kong.
Disclosure statement
Supported by funding from the Department of Medicine, The University of Hong Kong.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Supplementary data
Supplementary Figure E1.
Supplementary Figure E2.

References
- 1.Shaker M.S., Wallace D.V., Golden D.B.K., Oppenheimer J., Bernstein J.A., Campbell R.L., et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145:1082–1123. doi: 10.1016/j.jaci.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Koplin J.J., Ho M.H.K., Wong W.H.S., Allen K.J. Increasing hospital presentations for anaphylaxis in the pediatric population in Hong Kong. J Allergy Clin Immunol Pract. 2018;6:1050–1052. doi: 10.1016/j.jaip.2017.09.018. e2. [DOI] [PubMed] [Google Scholar]
- 3.Li P.H., Leung A.S.Y., Li R.M.Y., Leung T.F., Lau C.S., Wong G.W.K. Increasing incidence of anaphylaxis in Hong Kong from 2009 to 2019-discrepancies of anaphylaxis care between adult and paediatric patients. Clin Transl Allergy. 2020;10:51. doi: 10.1186/s13601-020-00355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner P.J., Gowland M.H., Sharma V., Ierodiakonou D., Harper N., Garcez T., et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992-2012. J Allergy Clin Immunol. 2015;135:956–963. doi: 10.1016/j.jaci.2014.10.021. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tejedor-Alonso M.A., Moro-Moro M., Mosquera Gonzalez M., Rodriguez-Alvarez M., Perez Fernandez E., Latasa Zamalloa P., et al. Increased incidence of admissions for anaphylaxis in Spain 1998-2011. Allergy. 2015;70:880–883. doi: 10.1111/all.12613. [DOI] [PubMed] [Google Scholar]
- 6.Turner P.J., Campbell D.E., Motosue M.S., Campbell R.L. Global trends in anaphylaxis epidemiology and clinical implications. J Allergy Clin Immunol Pract. 2020;8:1169–1176. doi: 10.1016/j.jaip.2019.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P.H., Chua G.T., Leung A.S.Y., Chan Y.C., Chan K.K.L., Cheung K.H., et al. Hong Kong Anaphylaxis Consortium consensus statements on prescription of adrenaline autoinjectors in the acute care setting. Asia Pac Allergy. 2021;11:e1. doi: 10.5415/apallergy.2021.11.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardona V., Ansotegui I.J., Ebisawa M., El-Gamal Y., Fernandez Rivas M., Fineman S., et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13 doi: 10.1016/j.waojou.2020.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange L. Quality of life in the setting of anaphylaxis and food allergy. Allergo J Int. 2014;23:252–260. doi: 10.1007/s40629-014-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren C.M., Jiang J., Gupta R.S. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep. 2020;20:6. doi: 10.1007/s11882-020-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flabbee J., Petit N., Jay N., Guenard L., Codreanu F., Mazeyrat R., et al. The economic costs of severe anaphylaxis in France: an inquiry carried out by the Allergy Vigilance Network. Allergy. 2008;63:360–365. doi: 10.1111/j.1398-9995.2007.01513.x. [DOI] [PubMed] [Google Scholar]
- 12.Cardoso B., Moscoso T., Morais-Almeida M., Demoly P., Tanno L.K. Economic burden of drug-induced anaphylaxis: what can we do better? Curr Opin Allergy Clin Immunol. 2022;22:234–241. doi: 10.1097/ACI.0000000000000836. [DOI] [PubMed] [Google Scholar]
- 13.Li P.H., Thomas I., Wong J.C., Rutkowski K., Lau C.S. Differences in omega-5-gliadin allergy: east versus west. Asia Pac Allergy. 2020;10:e5. doi: 10.5415/apallergy.2020.10.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee T.H., Leung T.F., Wong G., Ho M., Duque J.R., Li P.H., et al. The unmet provision of allergy services in Hong Kong impairs capability for allergy prevention-implications for the Asia Pacific region. Asian Pac J Allergy Immunol. 2019;37:1–8. doi: 10.12932/AP-250817-0150. [DOI] [PubMed] [Google Scholar]
- 15.Li P.H., Wong J.C.Y., Chan J.M.C., Chik T.S.H., Chu M.Y., Ho G.C.H., et al. Hong Kong Drug Allergy Delabelling Initiative (HK-DADI) consensus statements for penicillin allergy testing by nonallergists. Front Allergy. 2022;3 doi: 10.3389/falgy.2022.974138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kan A.K., Hui H.K., Li T.S., Chiang V., Wong J.C., Chan T.S., et al. Comparative effectiveness, safety and real-world outcomes of a nurse-led, protocol-driven penicillin allergy evaluation from the Hong Kong Drug Allergy Delabelling Initiative (HK-DADI) J Allergy Clin Immunol Pract. 2023;11:474–480.e2. doi: 10.1016/j.jaip.2022.08.052. [DOI] [PubMed] [Google Scholar]
- 17.Chiang V., Saha C., Yim J., Au E.Y.L., Kan A.K.C., Hui K.S.H., et al. The role of the allergist in coronavirus disease 2019 vaccine allergy safety: a pilot study on a "hub-and-spoke" model for population-wide allergy service. Ann Allergy Asthma Immunol. 2022;129:308–312. doi: 10.1016/j.anai.2022.05.011. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui H.K.S., Li T.S., Lo W.L.W., Kan A.K.C., Ho S.Y., Yeung W.Y.W., et al. Sensitisation profile of Chinese allergic rhinitis patients and effectiveness of a joint allergy-ENT clinic. Allergo J Int. 2022:1–9. doi: 10.1007/s40629-022-00218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson H.A., Muñoz-Furlong A., Campbell R.L., Adkinson N.F., Jr., Bock S.A., Branum A., et al. Second symposium on the definition and management of anaphylaxis: summary report--second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373–380. doi: 10.1016/j.annemergmed.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Simons F.E.R., Ardusso L.R.F., Bilò M.B., El-Gamal Y.M., Ledford D.K., Ring J., et al. World Allergy Organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4:13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang N.N., Wen L.P., Li H., Yin J. A new diagnostic criteria of wheat-dependent, exercise-induced anaphylaxis in China. Chin Med J (Engl) 2018;131:2049–2054. doi: 10.4103/0366-6999.239304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smit D.V., Cameron P.A., Rainer T.H. Anaphylaxis presentations to an emergency department in Hong Kong: incidence and predictors of biphasic reactions. J Emerg Med. 2005;28:381–388. doi: 10.1016/j.jemermed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Yang M.-S., Lee S.-H., Kim T.-W., Kwon J.-W., Lee S.-M., Kim S.-H., et al. Epidemiologic and clinical features of anaphylaxis in Korea. Ann Allergy Asthma Immunol. 2008;100:31–36. doi: 10.1016/S1081-1206(10)60401-2. [DOI] [PubMed] [Google Scholar]
- 24.Lee S.C., Kim S.-R., Park K.H., Lee J.-H., Park J.-W. Clinical features and culprit food allergens of Korean adult food allergy patients: a cross-sectional single-institute study. Allergy Asthma Immunol Res. 2019;11:723. doi: 10.4168/aair.2019.11.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones S., Magnolfi C., Cooke S., Sampson H. Immunologic cross-reactivity among cereal grains and grasses in children with food hypersensitivity. J Allergy Clin Immunol. 1995;96:341–351. doi: 10.1016/s0091-6749(95)70053-6. [DOI] [PubMed] [Google Scholar]
- 26.Brockow K., Kneissl D., Valentini L., Zelger O., Grosber M., Kugler C., et al. Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 2015;135:977–984.e4. doi: 10.1016/j.jaci.2014.08.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.