Abstract
Background
ABX464 (obefazimod) is a small molecule that upregulates a single microRNA (miR-124) in immune cells and reduces the production of various inflammatory cytokines and chemokines.
Objective
We assessed the efficacy and safety of the standard of care (SoC) plus oral obefazimod (SoC plus ABX464), 50 mg once daily, versus the SoC plus placebo for prevention of severe acute respiratory syndrome in patients with coronavirus disease 2019 (COVID-19) who are at risk for severe disease.
Methods
Eligible patients for this phase 2/3 double-blind, placebo-controlled miR-AGE study were randomized (2:1) into 2 groups: SoC-ABX464 (n = 339) and SoC-placebo (n = 170). The primary end point was the percentage of patients who did not require use of high-flow oxygen or invasive or noninvasive mechanical ventilation within 28 days. The safety analyses included patients who had been randomly assigned and had received at least 1 dose of the study treatment.
Results
At the time of the interim analysis, obefazimod showed no benefit over placebo when added to the SoC; the study enrollment was stopped for futility. The evaluation of the safety of obefazimod in 505 patients showed significantly more treatment-emergent adverse events in the SoC-ABX464 group than in the SoC-placebo group (P = .007). Frequently reported AEs in the SoC-ABX464 group included headache (14.6%), abdominal pain (9.6%), diarrhea (9.0%), back pain (6.9%), and nausea (6.0%). No treatment-related changes in laboratory parameters were reported.
Conclusion
For patients who have severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and are at risk for severe COVID-19, obefazimod, 50 mg, provided no benefit over placebo when added to the SoC, although it did have a good safety profile (comparable to that reported in many therapeutic areas).
Key words: ABX464, obefazimod, miR-124, COVID-19
The novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in 2019 in Wuhan, China, and soon confirmed as the cause of the coronavirus disease 2019 (COVID-19). SARS-CoV-2 infection can lead to a cytokine storm syndrome, acute respiratory distress syndrome, and respiratory and multiple organ failure.1 The hyperinflammatory syndrome associated with COVID-19 includes increased monocyte chemoattractant protein-1 (MCP-1), IL-1β, TNF-α, IL-17, G-CSF, and IL-6 levels. More specifically, elevated proinflammatory cytokines involved in TH17 cell responses in patients infected with COVID-19 may be the cause of vascular permeability and leakage.2
ABX464 (obefazimod), a small molecule that induces the selective upregulation of miR-124 in immune cells,3,4 significantly improved moderate-to-severe active ulcerative colitis (UC)5 and was safe and well tolerated in more than 850 volunteers and patients in clinical trials.6 The upregulation of the single miR-124 has been demonstrated in healthy subjects as well as in patients with HIV infection and patients with UC.3,7 From a mechanistic perspective, obefazimod was shown to bind the cap binding complex (CBC), a protein complex at the 5' ends of RNAs involved in fueling cellular RNA integrity (eg, splicing). As a result, obefazimod enhances the splicing of a long noncoding RNA to upregulate miR-124, which is a modulator of inflammation and innate immunity and has an antiproliferative effect (by affecting some of the important cell cycle transcripts).8 Developed initially as an inhibitor of HIV replication9, 10, 11 and HIV reservoir reduction,12 obefazimod–cap binding complex interactions were shown to strengthen the RNA quality control of HIV-RNA biogenesis preventing the production of unspliced HIV RNA,9 thereby reducing the viral reservoirs of patients infected with HIV.12 In addition, a reduction of SARS-CoV-2 replication in an in vitro reconstituted human airway epithelial model showed comparable efficacy between remdesivir (5 μM) and obefazimod (0.1, 1, and 5 μM) (Julien Santo, Abivax, personal communication, 2020).
Through its effects on miR-124, obefazimod has the potential to provide therapeutic restitution of physiologic pathways impaired by inflammatory diseases. miR-124 is a well-known physiologic inhibitor of systemic and pulmonary inflammation (potent reduction of MCP-1, IL-1β, TNFα, IL-17, G-CSF, and IL-6). miR-124 exerts a direct effect on the translational downregulation of MCP-1, monocyte chemoattractant protein,13 as well as the IL-6 receptor.8 In conjunction with these effects, an increase in miR-124 has been associated with the maintenance of M2 differentiation of the tissue-resident macrophage. Indeed, M2 macrophages control tissue inflammation by producing type 2 cytokines such as IL-4 and IL-10, both of which are mediators of tissue homeostasis.14 Obefazimod affected the immune system in vitro and demonstrated a marked decrease in the inflammatory colitis in murine model of inflammatory bowel disease as well as in patients with UC.7 These effects of obefazimod15,16 were associated with the prevention of proliferation of TH17 cells, which produce IL-17 in both peripheral blood and mesenteric lymph nodes.7
Given the antiviral and anti-inflammatory effects of obefazimod, we compared the efficacy and safety of the standard of care (SoC) plus 50 mg of oral ABX464 (the SoC-ABX464 group) once daily with the efficacy and safety of the SoC plus placebo for prevention of severe acute respiratory syndrome in patients with COVID-19 who are at risk for severe disease.
Methods
Study design and participants
The miR-AGE study is a phase 2/3 randomized, double blind, placebo-controlled study to evaluate the efficacy and the safety of the SoC plus ABX464 in treating inflammation and preventing COVID-19–associated acute respiratory failure in patients aged 65 years or older and patients aged 18 years or older who have at least 1 additional risk factor for severe COVID-19 and are infected with SARS-CoV-2.
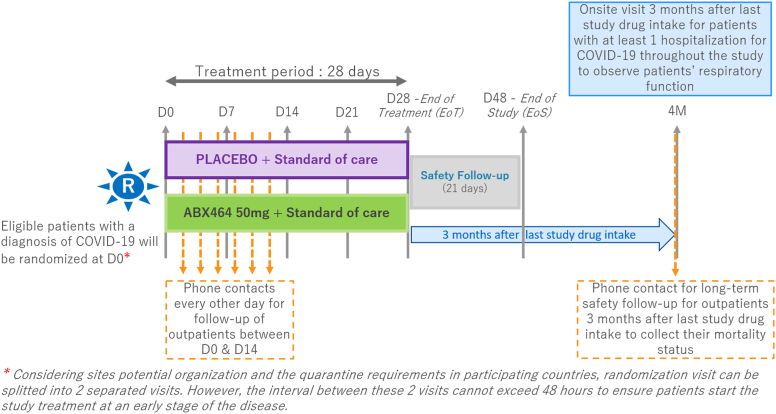
The study consisted of 3 periods: period 1, a treatment phase during which randomized patients were treated for 28 days; period 2, a 21-day safety follow-up phase; and period 3, a phase comprising exploratory follow-up of pulmonary function 3 months after the last intake of the study drug (Fig 1).
Fig 1.
Study design. The study consisted of 3 periods: a treatment phase (randomized patients were treated for 28 days), a 21-day safety follow-up phase, and an exploratory phase consisting of follow-up of the patients' pulmonary function 3 months after the last study drug intake. From day 0 onward, randomized patients were followed by the investigational site ondays 7, 14, 21, and 28 (±2 days) after randomization.
Patients were recruited from 56 centers in 9 countries (Belgium, Brazil, France, Germany, Italy, Mexico, Peru, Spain, and the United Kingdom). Eligible patients were (1) adult men or women (aged ≥18 years), whether hospitalized or not hospitalized, who had been diagnosed with SARS-CoV-2 infection by PCR or a rapid antigen test (approximately 48 hours before randomization) and had at least 1 associated risk factor (obesity defined as BMI ≥ 30, a recent history of uncontrolled high blood pressure, treated diabetes [type I or II], and a history of ischemic cardiovascular disease) for severe COVID-19 and (2) older adults (aged ≥65 years). At least 1 of the following symptoms was present at enrollment: fever or perceived fever for more than 24 hours, headache, sore throat, dry cough, fatigue, chest pain or choking sensation (with no associated respiratory distress), myalgia, anosmia, ageusia, and gastrointestinal symptoms. Other inclusion criteria were a specific oxygen saturation as measured by pulse oximetry (Spo2) of 92% or higher, hemoglobin level higher than 9.0 g/dL, absolute neutrophil count of 1000/mm, platelet count of 100,000/mm3 or more, creatinine clearance of at least 50 mL per minute as determined by the Cockcroft-Gault formula, total serum bilirubin level less than twice the upper limit of normal (ULN), alkaline phosphatase level less than twice the ULN, and aspartate transaminase (serum glutamate oxaloacetate transaminase) and alanine transaminase (serum glutamate pyruvate transaminase) levels less than 3 times the ULN.
Randomization
Eligible patients were randomized (2:1) into 2 groups: SoC plus obefazimod, 50 mg daily (the SoC-ABX464 group), and SoC plus placebo (the SoC-placebo group). After randomization, patients were stratified according to age (aged ≥65 years or <65 years with at least 1 risk factor) and inpatient or outpatient status at the time of enrollment.
Outcomes
The primary end point was the percentage of patients who did not require use of high-flow oxygen (use of high-flow oxygen being defined as a setting of ≥3 L per minute with ≥1 Spo2 measurement of <92%, with or without O2 supplementation) or invasive or noninvasive mechanical ventilation within 28 days and remained alive at the end of the 28-day period.
The secondary end points during the study were as follows: proportion of patients requiring hospitalization; proportion of patients reporting each severity rating on a 7-point ordinal scale; improvement in score on the 7-point ordinal scale and time to an improvement in 1 category of the 7-point on an ordinal scale from baseline; effect of the SoC plus ABX464 on oxygen saturation before hospitalization and at the end of the study treatment; effect of the SoC plus ABX464 on immunophenotyping and cytokines levels; proportion of patients requiring oxygen supplementation (>3 L per minute) during the study; time to hospitalization (for all causes and for COVID-19–related hospitalization); time to application and duration of invasive or noninvasive mechanical ventilation or high-flow oxygen therapy or oxygen supplementation; duration of hospital stay for patients hospitalized because of consequences of COVID-19 infection; level of miR-124 expression in the blood; change over time in C-reactive protein, troponin I, and T and D-dimer levels; prevention of COVID-19–related deaths; SARS-CoV-2 infection in patients; and SpO2 evolution from day 0 to day 14.
The safety end points comprised the number and percentages of patients with treatment-emergent adverse events (TEAEs); cumulative incidence of serious adverse events (SAEs), including serious adverse drug reactions and suspected unexpected serious adverse reactions; cumulative incidence of grade 3 and 4 adverse events (AEs); number of patients with a discontinuation or temporary suspension of study drugs (for any reason); long-term effect of ABX464 on all-cause mortality from the first study drug intake to the 3-month follow-up visit; clinical laboratory parameters; vital signs; and electrocardiogram findings.
Interim analysis
An interim analysis was performed on the first 305 patients who completed the day 28 assessment or reached the end of the study to assess the safety of the study treatment, potentially stop the study early for futility, and reassess the total sample size. The results of the interim analysis were assessed by the study data and safety monitoring board (DSMB).
Statistical analysis
Two analysis sets were used: a safety analysis set (SAF) and a full analysis set (FAS). The SAF included all randomized patients who received at least 1 dose of study treatment, and the FAS included all randomized patients.
The primary efficacy end point was analyzed by using the Mantel-Haenszel test stratified for factors used in the randomization.
Descriptive statistics were summarized for all secondary efficacy end points and safety end points, as well as for clinical laboratory parameters, vital signs, and ECG findings.
Additional post hoc safety analyses were performed to compare event rates between treatment groups by using the chi-square test. Statistical analyses were carried out using SAS, version 9.4 or higher (SAS Institute, Inc, Cary, NC).
Results
The study plan called for participation of approximately 800 patients, with the first patient enrolled on July 1, 2020. Following an interim analysis, the study was terminated early. Of the 509 randomized patients included in the FAS, 339 were assigned to the SoC-ABX464 group and 170 were assigned to the SoC-placebo group. In all, 224 patients in the SoC-ABX464 group (66.9%) and 120 (70.6%) in the SoC-placebo group completed the study treatment (see Fig E1 in the Online Repository at www.jaci-global.org).
The demographic and baseline characteristics were similar among the groups. The baseline disease characteristics were generally similar among the treatment groups, with minor exception for the mean time since onset of symptoms (6.6 vs 8.1 days in the SoC-ABX464 vs SoC-placebo groups, respectively) (Table I).
Table I.
Baseline disease characteristics
| Disease characteristic | SoC-ABX464 (n = 339) | SoC-placebo (n = 170) |
|---|---|---|
| SARS-CoV-2 status, no. | 338 | 170 |
| Positive, no. (%) | 338 (100) | 170 (100) |
| Time since onset of symptoms (d) | ||
| no. | 336 | 170 |
| Mean (SD) | 8.1 (20.2) | 6.6 (3.1) |
| Median | 7.0 | 6.0 |
| Risk factors, no. (%) | ||
| Age | 339 | 170 |
| ≥65 y | 98 (28.9) | 50 (29.4) |
| BMI, no. | 334 | 168 |
| ≥30 (kg/m2) | 187 (56.0) | 96 (57.1) |
| Recent history of uncontrolled high blood pressure, no. (%) | 213 (62.8) | 88 (51.8) |
| Treated diabetes (type I or II), no. (%) | 115 (33.9) | 46 (27.1) |
| History of ischemic cardiovascular disease, no. (%) | 0 | 0 |
| No. of concurrent risk factors | ||
| no. | 338 | 169 |
| Mean (SD) | 2.2 (0.9) | 2.1 (0.9) |
| Median | 2.0 | 2.0 |
| SpO2 (visit 1) | ||
| no. | 338 | 170 |
| Mean (SD) | 96.7 (2.0) | 96.9 (2.1) |
| Median | 97.0 | 97.0 |
| WHO ordinal scale (visit 1) | 331 | 168 |
| Not hospitalized, no limitations on activities | 218 (65.9) | 112 (66.7) |
| Not hospitalized, limitation on activities | 46 (13.9) | 22 (13.1) |
| Hospitalized, not requiring supplemental oxygen | 54 (16.3) | 28 (16.7) |
| Hospitalized, requiring supplemental oxygen | 13 (3.9) | 6 (3.6) |
Efficacy
After 305 randomized patients had completed the day 28 assessment or reached the end of this study, the estimated conditional power was 0% and the study met the predefined stopping criterion for futility (conditional power ≤ 10%).
Briefly, at time of the interim analysis, the ratio of responders in the SoC-ABX464 group (83.3%) was less than in the SoC-placebo group (85.3%) (Table II). There was no difference between the arms hospitalized at randomization, hospitalized after randomization, and not hospitalized. The rate of oxygen supplementation was numerically higher in the SoC-ABX464 group (23.0%) than in the SoC-placebo group (17.6%). The results of the interim analysis were assessed by the study DSMB, which deemed the study futile and recommended its termination. On the basis of the DSMB's recommendation, no further efficacy analysis was performed.
Table II.
Response probabilities by strata overall (FAS for primary efficacy end point analysis)
| Condition (up to day 28) | Outcome | SoC-ABX464 (n = 203), No. (%) |
SoC-placebo (n = 102), No. (%) |
|---|---|---|---|
| (a1) AND (a2) | 19 (9.4) | 4 (3.9) | |
| (a1) Requires oxygen flow of ≥3 L per minute | 37 (18.2) | 9 (8.8) | |
| (a2) Have ≥1 SpO2 measurement of <92% | 25 (12.3) | 8 (7.8) | |
| b) Requires mechanical ventilation (invasive, noninvasive, or extracorporeal membrane oxygenation) |
15 (7.4) | 6 (5.9) | |
| c) Death | 4 (2.0) | 1 (1.0) | |
| Primary end point (combination of (a), (b), and (c))∗ | Response | 169 (83.3) | 87 (85.3) |
| Nonresponse | 24 (11.8) | 7 (6.9) | |
| Missing | 10 (4.9) | 8 (7.8) |
Percent sign indicates the percentage of patients within a criterion relative to the total number of patients in the FAS.
Response indicates that outcome has been achieved before day 28; nonresponse indicates that outcome has not been achieved before day 28; and missing means that the patient would have been a responder but discontinued the study before day 28.
Safety
As there was no safety signal detected at the time of the interim analysis, the safety evaluation was performed at the end of the study. Overall, 505 patients were included in the SAF. A total of 757 TEAEs were reported in 289 patients (57.2%) during period 1 (Table III). TEAEs were reported significantly more often for patients in the SoC-ABX464 group (for 206 of 335 patients [61.5%]) than for patients in the SoC-placebo group (for 83 of 170 patients [48.8%]) (P = .007). The rates of SAEs were similar among both groups. TEAEs were considered related to the study treatment for 124 patients (91 in the SoC-ABX464 group [27.2%] and 33 in the SoC-placebo group [19.4%]). In all, 25 patients (21 in the SoC-ABX464 group [6.3%] and 4 in the SoC-placebo group [2.4%]) were withdrawn from treatment on account of TEAEs (the rates between treatment groups were not significantly different [P = .055 according to the chi-square test]). During this period, 202 TEAEs occurring in at least 5% of patients were reported in 142 patients (28.1% in total [115 in the SoC-ABX464 group (34.3%) and 27 in the in SoC-placebo group (15.9%)]) (Table IV). The most frequently reported AEs in the SoC-ABX464 group were headache (14.6%), abdominal pain (9.6%), diarrhea (9.0%), back pain (6.9%), and nausea (6.0%).
Table III.
Overall summary of AEs (SAF)
| AE | Group |
||
|---|---|---|---|
| SoC-ABX464 (n = 335) no. patients (%) no. events | SoC-placebo (n = 170) no. patients (%) no. events | Total (N = 505) no. patients (%) no. events | |
| Period 1: AE set on or worsens before or on day 28 | |||
| Any TEAE | 206 (61.5) 549 | 83 (48.8) 208 | 289 (57.2) 757 |
| With grade of ≥3 | 28 (8.4) 31 | 17 (10.0) 21 | 45 (8.9) 52 |
| Classified as serious | 34 (10.1) 38 | 14 (8.2) 16 | 48 (9.5) 54 |
| Leading to treatment withdrawal | 21 (6.3) 21 | 4 (2.4) 4 | 25 (5.0) 25 |
| Leading to study discontinuation | 3 (0.9) 8 | 0 | 3 (0.6) 8 |
| Leading to death | 2 (0.6) 2 | 1 (0.6) 1 | 3 (0.6) 3 |
| Any serious adverse drug reaction | 4 (1.2) 4 | 1 (0.6) 1 | 5 (1.0) 5 |
| Any (SUSARs) | 4 (1.2) 4 | 1 (0.6) 1 | 5 (1.0) 5 |
| Period 2: AE set on or worsens after day 28 | |||
| Any TEAE | 22 (6.6) 38 | 20 (11.8) 35 | 42 (8.3) 73 |
| With grade ≥ 3 | 1 (0.3) 1 | 7 (4.1) 10 | 8 (1.6) 11 |
| Classified as serious | 2 (0.6) 2 | 6 (3.5) 9 | 8 (1.6) 11 |
| Leading to treatment withdrawal | 0 | 1 (0.6) 1 | 1 (0.2) 1 |
| Leading to study discontinuation | 1 (0.3) 1 | 0 | 1 (0.2) 1 |
| Leading to death | 0 | 3 (1.8) 3 | 3 (0.6) 3 |
| Any serious adverse drug reaction | 0 | 1 (0.6) 1 | 1 (0.2) 1 |
| Any (SUSARs) | 0 | 1 (0.6) 1 | 1 (0.2) 1 |
A TEAE is an AE with a start date on or after the date of dispensation of the study drug, or with a start date before the date of dispensation the study drug, the severity of which worsens on or after the date of dispensation of the study drug. A patient is presented once for each system organ class and preferred term according to their worst toxicology grade. AEs are sorted in descending frequency by system organ class, then preferred term within system organ class in the ABX464 group, then in the placebo group, and then alphabetically.
SUSAR, Suspected unexpected serious adverse reaction.
Table IV.
TEAEs occurring in a least 5% of patients in any treatment group during period 1
| TEAE | SoC-ABX464m (n = 335) no. patients (%) no. events | SoC-placebo (N = 170) no. patients (%) no. events | Total (N = 505) no. patients (%) no. events |
|---|---|---|---|
| No. of patients with ≥1 TEAE | 115 (34.3) 161 | 27 (15.9) 41 | 142 (28.1) 202 |
| Gastrointestinal disorders | 73 (21.8) 86 | 14 (8.2) 18 | 87 (17.2) 104 |
| Abdominal pain upper | 32 (9.6) 34 | 5 (2.9) 5 | 37 (7.3) 39 |
| Diarrhea | 30 (9.0) 31 | 6 (3.5) 6 | 36 (7.1) 37 |
| Nausea | 20 (6.0) 21 | 6 (3.5) 7 | 26 (5.1) 28 |
| Nervous system disorders | 49 (14.6) 51 | 15 (8.8) 19 | 64 (12.7) 70 |
| Headache | 49 (14.6) 51 | 15 (8.8) 19 | 64 (12.7) 70 |
| Musculoskeletal and connective tissue disorders | 23 (6.9) 24 | 4 (2.4) 4 | 27 (5.3) 28 |
| Back pain | 23 (6.9) 24 | 4 (2.4) 4 | 27 (5.3) 28 |
Three patients died during period 1 (2 patients in the SoC-ABX464 group and 1 patient in the SoC-placebo group died of COVID-19). Several patients had laboratory values above or below the predefined normal ranges for this study, but none demonstrated clinically significantly abnormal values.
During period 2 (ie, after the end of medication treatment), a total of 73 TEAEs were reported in 42 patients (8.3%) (Table III). No TEAEs occurring in at least 5% of the patients were reported during period 2, and the occurrence of AEs, including SAEs, was higher in the SoC-placebo group than in the SoC-ABX464 group (P < .05). Three patients in the SoC-placebo group died during this period (1 died of COVID-19, 1 died of septic shock, and 1 died of shock).
Discussion
The miR-AGE study was a randomized double-blind, placebo-controlled study that evaluated the efficacy and safety of the SoC plus once-daily oral obefazimod, 50 mg (SoC plus ABX464), in treating inflammation and preventing COVID-19–associated acute respiratory failure in patients aged 65 years or older and patients aged 18 years or older who had at least 1 additional risk factor for severe COVID-19 and were infected with SARS-CoV-2.
For patients enrolled in the study, once-daily oral obefazimod, 50 mg, had no benefit over placebo when added to the SoC; as a result, the DSMB recommended stopping enrollment for futility after 509 patients (339 in the SoC-ABX464 group and 170 in the SoC-placebo group) had undergone randomization. The reasons for the lack of benefit for 50 mg of obefazimod in this trial are unknown, but the results should be interpreted in the context of a preliminary study assessing only 1 dose, which is clearly not appropriate for this population.
The negative efficacy readout of the study may be linked to a flaw in the protocol because at the time of the study's design, there was no information on when obefazimod treatment should be initiated to maximize its potential efficacy. As is the case with dexamethasone,17 the efficacy of obefazimod might be dependent on time of the initial treatment intervention. The patients in the miR-AGE study might have been receiving obefazimod too late during the infection. Obefazimod is a compound known to dampen chronic inflammation, and it does not completely suppress the immune/inflammation response. Such a mechanism of action may not be adequate to tackle an ongoing acute respiratory syndrome.
There was no safety concern at the time of the interim analysis, so the safety evaluation was performed at the end of the study; 505 patients were included in the SAF. Obefazimod showed a good safety profile in patients who have SARS-CoV-2 infection and are at risk for severe COVID-19. The incidences of treatment-emergent SAEs, death, and treatment discontinuation were similar among the SoC-ABX464 and SoC-placebo groups. The most frequently reported AEs in the SoC-ABX464 group, occurring in 5% or more of patients, were headache, abdominal or back pain, diarrhea, and nausea; no treatment-related changes in laboratory parameters were reported. No new safety signal of obefazimod was identified versus in the previous obefazimod trials, all of which have shown a consistent safety profile. The nature of these AEs was consistent with what has been observed in the more than 850 subjects who have thus far been treated in other clinical trials with obefazimod across different indications. Thus, phase 1 studies in healthy volunteers and a phase 2 study in patients with HIV infection have shown obefazimod to be well tolerated, with no treatment-limiting AEs10,11,18; the most common AEs in patients treated for rheumatoid arthritis or UC were abdominal pain, headache, nausea, diarrhea, and nasopharyngitis.5,19,20 No treatment-related changes in laboratory parameters demonstrated clinically significant abnormal values.
The study has limitations. Enrollment into both arms was halted prematurely, so the rather small sample size is a limitation of the current study; however, the signal observed in the small number of patients suggests a high probability of inefficiency.
In conclusion, among patients who have SARS-CoV-2 infection and are at risk for severe COVID-19, once-daily oral obefazimod in a dose of 50 mg provided no benefit over placebo when added to the SoC, although it had a good safety profile, comparable to that reported in many therapeutic areas.
Study approval
The study was approved by the responsible independent ethics committee/institutional review board at each participating center, and it was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All participants provided written informed consent.
Disclosure statement
Supported by Abivax. The authors received no direct compensation related to the development of this article. Financing for this trial, as well as manufacturing scale-up and additional clinical and development costs were granted by the French investment bank Bpifrance, which supports entrepreneurial activities that are in the public interest (reference no. PSPC-AAP-COVID19). This study has been labeled as a National Research Priority by the National Orientation Committee for Therapeutic Trials and other researches on Covid-19 (CAPNET). The investigators would like to acknowledge ANRS/Emerging infectious diseases for their scientific support, the French Ministry of Health and Prevention, and the French Ministry of Higher Education, Research and Innovation for their funding and support.
Disclosure of potential conflict of interest: P. Giavina-Bianchi and J. Kalil received financial compensation from Abivax for miR-AGE study development. P, Gineste, A. Vissian, C. Joie, and H. Ehrlich are employees at Abivax. P. Pouletty is a member of the board of directors of ABIVAX, chief executive officer of Truffle Capital, and an ABIVAX shareholder. The rest of the authors declare that they have no relevant conflicts of interest.
Key messages.
-
•
Obefazimod, a small molecule that modulates inflammation through the selective upregulation of miR-124 in immune cells, was administered for 28 days (50 mg once daily) to patients who had SARS-CoV-2 infection and were at risk for severe COVID-19.
-
•
When added to the SoC, oral obefazimod (50 mg once daily) provided no benefit over placebo.
-
•
The safety profile of obefazimod was good (comparable to that reported in other therapeutic areas).
Acknowledgments
We thank the patients, investigators, and study teams who were involved in the trial.
Supplementary data
References
- 1.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vautrin A., Manchon L., Garcel A., Campos N., Lapasset L., Laaref A.M., et al. Both anti-inflammatory and antiviral properties of novel drug candidate ABX464 are mediated by modulation of RNA splicing. Sci Rep. 2019;9:792. doi: 10.1038/s41598-018-37813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tazi J., Begon-Pescia C., Campos N., Apolit C., Garcel A., Scherrer D. Specific and selective induction of miR-124 in immune cells by the quinoline ABX464: a transformative therapy for inflammatory diseases. Drug Discov Today. 2021;26:1030–1039. doi: 10.1016/j.drudis.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Vermeire S., Sands B.E., Tilg H., Tulassay Z., Kempinski R., Danese S., et al. ABX464 (obefazimod) for moderate to severe active ulcerative colitis: a randomised, placebo controlled phase 2b induction trial and 48 week extension. Lancet Gastroenterol Hepatol. 2022;7:1024–1035. doi: 10.1016/S2468-1253(22)00233-3. [DOI] [PubMed] [Google Scholar]
- 6.ABIVAX. Investigator's brochure (7th ed) ABX464. Inflammatory bowel diseases 2022. p 1-133.
- 7.Apolit C., Campos N., Vautrin A., Begon-Pescia C., Lapasset L., Scherrer D., et al. ABX464 (obefazimod) up-regulates miR-124 to reduce pro-inflammatory markers in inflammatory bowel diseases. Clin Transl Gastroenterol. 2023;14 doi: 10.14309/ctg.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin Z., Wang P.Y., Su D.F., Liu X. miRNA-124 in immune system and immune disorders. Front Immunol. 2016;7:406. doi: 10.3389/fimmu.2016.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campos N., Myburgh R., Garcel A., Vautrin A., Lapasset L., Nadal E.S., et al. Long lasting control of viral rebound with a new drug ABX464 targeting Rev-mediated viral RNA biogenesis. Retrovirology. 2015;12:30. doi: 10.1186/s12977-015-0159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherrer D., Rouzier R., Noel Barrett P., Steens J.M., Gineste P., Murphy R.L., et al. Pharmacokinetics and tolerability of ABX464, a novel first-in-class compound to treat HIV infection, in healthy HIV-uninfected subjects. J Antimicrob Chemother. 2017;72:820–828. doi: 10.1093/jac/dkw458. [DOI] [PubMed] [Google Scholar]
- 11.Steens J.M., Scherrer D., Gineste P., Barrett P.N., Khuanchai S., Winai R., et al. Safety, pharmacokinetics, and antiviral activity of a novel HIV antiviral, ABX464, in treatment-naive HIV-infected subjects in a phase 2 randomized, controlled study. Antimicrob Agents Chemother. 2017;61:e00545. doi: 10.1128/AAC.00545-17. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutsaert S., Steens J.M., Gineste P., Cole B., Kint S., Barrett P.N., et al. Safety, tolerability and impact on viral reservoirs of the addition to antiretroviral therapy of ABX464, an investigational antiviral drug, in individuals living with HIV-1: a phase IIa randomised controlled study. J Virus Erad. 2019;5:10–22. doi: 10.1016/S2055-6640(20)30273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano S., Nakamachi Y. miR-124a as a key regulator of proliferation and MCP-1 secretion in synoviocytes from patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(suppl 1):i88–i91. doi: 10.1136/ard.2010.138669. [DOI] [PubMed] [Google Scholar]
- 14.Veremeyko T., Siddiqui S., Sotnikov I., Yung A., Ponomarev E.D. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chebli K., Papon L., Paul C., Garcel A., Campos N., Scherrer D., et al. The anti-Hiv candidate Abx464 dampens intestinal inflammation by triggering Il-22 production in activated macrophages. Sci Rep. 2017;7:4860. doi: 10.1038/s41598-017-04071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manchon L., Chebli K., Papon L., Paul C., Garcel A., Campos N., et al. RNA sequencing analysis of activated macrophages treated with the anti-HIV ABX464 in intestinal inflammation. Sci Data. 2017;4 doi: 10.1038/sdata.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherrer D., Rouzier R., Cardona M., Barrett P.N., Steens J.M., Gineste P., et al. Randomized trial of food effect on pharmacokinetic parameters of ABX464 administered orally to healthy male subjects. Antimicrob Agents Chemother. 2017;61:e01288. doi: 10.1128/AAC.01288-16. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeire S., Hebuterne X., Tilg H., De Hertogh G., Gineste P., Steens J.M., et al. Induction and long-term follow-up with ABX464 for moderate-to-severe ulcerative colitis: results of phase IIa trial. Gastroenterology. 2021;160:2595–2598. doi: 10.1053/j.gastro.2021.02.054. e3. [DOI] [PubMed] [Google Scholar]
- 20.Daien C., Krogulec M., Gineste P., Steens J.M., Desroys du Roure L., Biguenet S., et al. Safety and efficacy of the miR-124 upregulator ABX464 (obefazimod, 50 and 100 mg per day) in patients with active rheumatoid arthritis and inadequate response to methotrexate and/or anti-TNFα therapy: a placebo-controlled phase II study. Ann Rheum Dis. 2022;81:1076–1084. doi: 10.1136/annrheumdis-2022-222228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.