Abstract
We report the case of a patient with eosinophilic granulomatosis with polyangiitis and unexplained thrombocytopenia that culminated in a fatal intracerebral hemorrhage. Because vessel damage associated with thrombocytopenia could be the leading cause of fatal hemorrhage, more urgent treatment of thrombocytopenia should be performed in such cases.
A 26-year-old male had a medical history of allergic rhinitis, unknown history of bronchial asthma, and no history of receiving coronavirus disease 2019 (COVID-19) vaccine. Three days before admission, the patient presented with generalized myalgia, especially in the lower limb muscle groups, arthralgia of the hips and knees, numbness, and quadriparesis. The numbness and weakness of his limbs increased gradually. He was admitted to the hospital in a normal state of consciousness, with muscle pain and a temperature ranging from 37.5°C to 38°C. He had asymmetric bilateral paresis. His numbness and sensory abnormalities were predominantly distal. He had normal bowel function. We detected thrombocytopenia with a platelet count of 127 × 109/L (Table I), and we ordered a wide range of diagnostic tests (Table II).
Table I.
Thrombocytopenia status and results of tests to find causes
| Test index | Result | Normal range | |
|---|---|---|---|
| Platelet count (× 109/L) | Day 1 | 127 | 140-350 |
| Day 3 | 32 | ||
| Day 4 | 39 | ||
| Day 5 | 39 | ||
| Day 6 | 46 | ||
| Day 7 | 46 | ||
| Day 9 (after hemorrhage) | 43 | ||
| Dengue virus status | Nonstructural protein 1 antigen (NS1Ag) | Negative | |
| Dengue IgM | Negative | ||
| Dengue IgG | Positive | ||
| Peripheral blood smear | No red blood cell fragments and percentage of reticulocyte < 1% | ||
| Renal function | Urea level (mmol/L) | 5.42 | 3.3-8.3 |
| Creatinine level (μmol/L) | 48 | 62-106 | |
| d-Dimer level (ng/mL) | 6572 | <500 | |
| Thrombosis status | Abdominal ultrasound | Normal | |
| Lower extremity arteriovenous ultrasound | Normal | ||
| Echocardiogram | Normal | ||
| Chest computed tomography scan | Normal | ||
Table II.
Antibody serostatus and other test results
| Test index | Result | Normal range | |
|---|---|---|---|
| Thyroid function test | Free thyroxine level (pmol/L) | 15.93 | 12-22 |
| Thyroid-stimulating hormone level (μIU/mL) | 4.23 | 0.27-4.2 | |
| Viral infection status | Hepatitis B virus | Negative | |
| Hepatitis C virus | Negative | ||
| HIV | Negative | ||
| Coronavirus PCR | Negative | ||
| Cerebrospinal fluid analysis | Cell count (cells/mm3) | 2 | < 5 |
| Protein level (g/L) | 0.34 | 0.1-0.25 | |
| Glucose level (mmol/L) | 4.8 | ||
| EBV, CMV, HSV, tuberculosis bacilli | Negative | ||
| Antibody serostatus | Antiphospholipid antibodies (IgM, IgG) | Negative | |
| Anticardiolipin antibodies (IgM, IgG) | Negative | ||
| Anti-beta-2 glycoprotein I antibodies (IgM, IgG) | Normal range | ||
| cANCA, pANCA | Negative | ||
| ANA anti-dsDNA | Negative | ||
| Head computed tomography and angiography | Normal | ||
| Nerve conduction studies | Polyneuropathy | ||
ANA, Antinuclear antibody; c-ANCA, cytoplasmic antineutrophil cytoplasmic antibody; CMV, cytomegalovirus; dsDNA, double-stranded DNA; HSV, herpes simplex virus; p-ANCA, perinuclear antineutrophil cytoplasmic antibody.
On the third day after admission, his symptoms tended to worsen. He had a rising temperature, reduced muscle power, more severe myalgia of the extremities, and arthralgia. He had a bronchospasm that was quickly relieved after use of a bronchodilator. Some purpura fulminans appeared on both of his legs. His platelet count was 32 × 109/L. Our initial diagnosis was possible systemic inflammatory disease. He was treated with methylprednisolone, 80 mg per day, beginning on day 3.
His platelet count reached its lowest value (32 × 109/L) on day 3 and gradually increased in the following days (Table I). A platelet count less than 20 × 109/L has been proved to be indicative of a major risk of bleeding in immunologic diseases.1 Because we did not find any appropriate causes of his thrombocytopenia, we delayed platelet transfusion and treatment for that condition.
A skin biopsy was ordered at the site of purpura fulminans on day 4. After the result (Fig 1) had been obtained, the patient was diagnosed as having eosinophilic granulomatosis with polyangiitis (EGPA) according to the 2022 diagnostic criteria2; and his Birmingham Vasculitis Activity Score (version 3)3 indicated that the disease was in an active phase.
Fig 1.
Biopsy specimen of purpura fulminans on the lower limb showed the eosinophilic infiltrates (A) and fibrinoid necrosis of vessel wall with eosinophilic infiltrates in the dermis and subcutaneous fat magnification (B). Hematoxylin and eosin staining; original magnification, ×20.
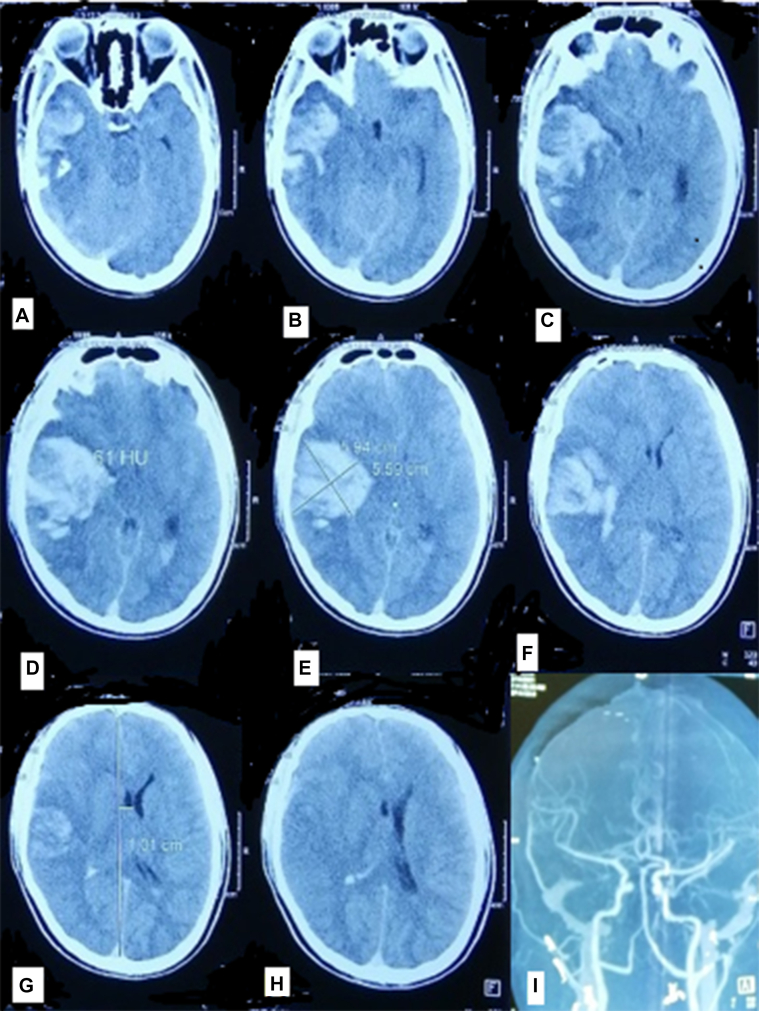
From day 4 to day 8, the patient's fever and myalgia improved and he was able to walk with a walker. On day 9, he suddenly fell into a coma. Brain computed tomography showed massive intracerebral hemorrhage in the left temporal lobe and extravasation of blood into the subarachnoid space and ventricles (Fig 2). He died on day 10 with a platelet count of 46 × 109/L.
Fig 2.
Brain computed tomography scan (A-H) showed massive intracerebral hemorrhage in the left temporal lobe and blood in the subarachnoid space and ventricles at day 9 and brain angiogram (I) showed no abnormalities at day 1.
Discussion
EGPA or Churg-Strauss syndrome is a rare systemic necrotizing vasculitis that affects small- to medium-size vessels and involves the respiratory tract. It is usually associated with antineutrophil cytoplasmic autoantibodies.2
Peripheral nerve damage due to the disease is quite common, affecting 50% to 98% of patients, whereas central nervous system (CNS) involvement is rare, with an prevalence of only 4% to 17%.4 The lesions predominantly present as cerebral infarction, reversible posterior circulation syndrome, spinal cord, or medulla involvement, and ischemic stroke is the most prevalent type. Intracerebral hemorrhage is extremely, rare with some isolated case reports; however, it is the leading cause of death due to CNS lesions in EGPA.4
The pathogenesis of hemorrhage is not fully understood.4 In 1 research study, 26 patients in France and 62 patients in other countries were found to have lesions in the CNS.5 Of those patients, 9 had intracerebral hemorrhage and 13 had intracranial hemorrhage, mainly subarachnoid hemorrhage. There was only 1 case related to cerebral aneurysm and 1 case related to infarction, but no detailed information was provided. The study's authors suggested that vasculitis appeared to be the major cause of bleeding.5 Another study indicated that almost all of the EGPA cases with intracerebral hemorrhage presented in the active phase of the disease with vasculitis.6 Patients with EGPA also had general risk factors for primary or secondary intracranial hemorrhage such as vascular abnormalities, use of anticoagulants or antiplatelet agents for treatment or prevention of thrombosis, and hypertension after using fibrinolytic drugs or hemorrhagic transformation of intracerebral infarction.6
Our patient had a previous normal cerebral angiogram that excluded aneurysm or cerebral arteriovenous malformation. He did not use anticoagulation or antiplatelet agents before or during the admission. His coagulation function (Table III) and blood pressure were normal. Therefore, we ruled out those risk factors and suggest that vasculitis might be the cause of the hemorrhage.
Table III.
Complete blood count and coagulation test results
| Test index | Day 1-2 | Day 3 | Day 6 | Day 9 (after hemorrhage) | Normal range |
|---|---|---|---|---|---|
| White blood cell count (× 109/L) | 10.28 | 10.6 | 14.5 | 22.6 | 4-10 |
| Neutrophils (%) | 61.4 | 74.8 | 64.2 | 86.5 | 40-74 |
| Lymphocytes (%) | 9.2 | 9.1 | 14.0 | 4.3 | 19-48 |
| Eosinophils (%) | 17.2 | 10.2 | 12.3 | 0.2 | 0-7 |
| Red blood cell count (L) | 5.18 | 5.1 | 4.73 | 4.93 | 4.2-6 |
| Hemoglobin level (g/L) | 127 | 160 | 147 | 150 | 130-170 |
| Prothrombin time (s) | 16.6 | 16.1 | 10-14 | ||
| Prothrombin (%) | 70 | 73 | 70-140 | ||
| Fibrinogen level (g/L) | 5.31 | 2.92 | 2-4 |
Thrombocytopenia is not a symptom of EGPA, but it is mentioned in some isolated reports.7 The causes may be related to thrombotic microangiopathies,8 hemolytic uremic syndrome,9 heparin-associated thrombocytopenia, or thrombosis.7 Thrombocytopenia due to Dengue virus was ruled out in our patient despite his living in endemic areas. A peripheral blood smear showed no red blood cell fragments and a reticulocyte count less than 1% (Table I); therefore, thrombotic microangiopathies were excluded. Tests for ADAMST13 were not performed because of their unavailability in our country. A diagnosis of hemolytic-uremic syndrome was also deemed inappropriate because of the patient's normal renal function. He had not taken heparin before.
Thrombocytopenia has also been reported in patients with EGPA with elevated d-dimer levels.7 Our patient had an increased d-dimer index (Table I), but examination of his lower extremity vasculature did not reveal any abnormalities associated with venous thrombosis. No clinical symptoms of thrombosis in the hepatic vein, pulmonary artery, or cerebral venous sinus were recorded. Therefore, EGPA could have been the cause of the patient's thrombocytopenia.
The fatal intracerebral hemorrhage in our patient with EGPA suggested that there might be a relationship between intracranial hemorrhage and the condition of thrombocytopenia and vasculitis. Thrombocytopenia could be the major risk of bleeding in patients with vessel damage due to vasculitis. The vessel impairment could increase the risk of hemorrhage in patients with thrombocytopenia even though low platelet count (>20 × 109/L) has been proved to not be a major risk factor in bleeding. In addition, the low platelet count also hindered the emergency surgery to save our patient.
To our knowledge, our patient may be the first reported patient with EGPA accompanied by thrombocytopenia and fatal massive intracerebral hemorrhage. The causes and consequences of thrombocytopenia in EGPA require evaluation in further studies. Thrombocytopenia may be warning signs for early management of thrombocytopenia and could be considered as the risk factors of severe hemorrhage to guide the initial selection of therapy in patients with EGPA to reduce the severe hemorrhage.
Disclosure statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Footnotes
The caregiver understood the purpose of the case report, the potential risks and benefits of publication, and any risks of harm or embarrassment that may result from the publication. The caregiver granted permission for the case report and accompanying visual elements to be published. We have written entirely original works, and the work and words of others have been appropriately cited.
References
- 1.Piel-Julian M.L., Mahevas M., Germain J., Languille L., Comont T., Lapeyre-Mestre M., et al. Risk factors for bleeding, including platelet count threshold, in newly diagnosed immune thrombocytopenia adults. J Thromb Haemost. 2018;16:1830–1842. doi: 10.1111/jth.14227. [DOI] [PubMed] [Google Scholar]
- 2.Grayson P.C., Ponte C., Suppiah R., Robson J.C., Craven A., Judge A., et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81:309–314. doi: 10.1136/annrheumdis-2021-221794. [DOI] [PubMed] [Google Scholar]
- 3.Mukhtyar C., Lee R., Brown D., Carruthers D., Dasgupta B., Dubey S., et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3) Ann Rheum Dis. 2009;68:1827–1832. doi: 10.1136/ard.2008.101279. [DOI] [PubMed] [Google Scholar]
- 4.Liu S., Guo L., Fan X., Zhang Z., Zhou J., Tian X., et al. Clinical features of central nervous system involvement in patients with eosinophilic granulomatosis with polyangiitis: a retrospective cohort study in China. Orphanet J Rare Dis. 2021;16:152. doi: 10.1186/s13023-021-01780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andre R., Cottin V., Saraux J.L., Blaison G., Bienvenu B., Cathebras P., et al. Central nervous system involvement in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): report of 26 patients and review of the literature. Autoimmun Rev. 2017;16:963–969. doi: 10.1016/j.autrev.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Mencacci N.E., Bersano A., Cinnante C.M., Ciammola A., Corti S., Meroni P.L., et al. Intracerebral haemorrhage, a possible presentation in Churg-Strauss syndrome: case report and review of the literature. J Neurol Sci. 2011;301:107–111. doi: 10.1016/j.jns.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Wolf F., Glick K., Elias M., Mader R. Portal Vein Thrombosis and thrombocytopenia in eosinophilic granulomatosis with polyangiitis: a paradox? Eur J Case Rep Intern Med. 2018;5 doi: 10.12890/2018_000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukui S., Iwamoto N., Tsuji S., Umeda M., Nishino A., Nakashima Y., et al. Eosinophilic granulomatosis with polyangiitis with thrombotic microangiopathy: is simultaneous systemic lupus erythematosus associated with clinical manifestations?: A case report and review of the literature. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao M., Ferreiro T., Leite B.N., Pita F., Bolanos L., Valdes F., et al. Two cases of atypical hemolytic uremic syndrome (aHUS) and eosinophilic granulomatosis with polyangiitis (EGPA): a possible relationship. CEN Case Rep. 2017;6:91–97. doi: 10.1007/s13730-017-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]