Abstract
Background
Vitamin D3 (VitD3) is known to have immunomodulatory functions, and VitD3 deficiency is associated with more severe asthma.
Objective
We aimed to assess the immunoregulatory effects of VitD3 food supplementation on asthma manifestation, with particular focus on T cells and type 2 innate lymphoid cells.
Methods
Preschool children and adult asthmatic cohorts were analyzed in the context of VitD3 supplementation and serum levels. In a murine model of ovalbumin-induced asthma, effects of diet VitD3 sufficiency and deficiency on T cells and type 2 innate lymphoid cells immune mechanisms were investigated.
Results
We found less severe and better-controlled asthma phenotypes along with reduced need for steroid medication in preschool children and asthmatic adults with VitD3 supplementation. VitD3 serum levels correlated with B lymphocyte–induced maturation protein 1 (Blimp-1) expression in blood peripheral mononuclear cells. VitD3-supplement–fed mice showed decreased asthmatic traits, with a decrease in IgE serum levels, reduced airway mucus, and increased IL-10 production by lung cells. Furthermore, we discovered an upregulation of effector T cells and Blimp-1+ lung tissue-resident memory T cells as well as induction of anti-inflammatory Blimp-1+ lung innate lymphoid cells producing IL-10.
Conclusion
Supplementing VitD3 resulted in amelioration of clinical asthma manifestations in human studies as well as in experimental allergic asthma, indicating that VitD3 shifts proinflammatory immune responses to anti-inflammatory immune responses via upregulating Blimp-1 in lung innate lymphoid cells and tissue-resident memory cells.
Key words: Allergic asthma, pediatric asthma, adult asthma, vitamin D3, vitamin D3 supplementation, TRM, ILC2s, Blimp-1, resolution of asthma
Allergic asthma is a chronic inflammatory disease of the airways with heterogenous clinical manifestation, affecting millions of people worldwide and considerably limiting their quality of life. In Germany, approximately 7% of the population has asthma, and worldwide prevalence has increased in the last decades. In most cases, asthma first manifests in early infancy.1,2
The pathophysiology of allergic asthma is highly heterogenous thanks to the different endotypes and/or phenotypes, and it is not fully understood.3 As major mediators in type 2 inflammation–mediated asthma, TH2 cells are chronically activated in the lung of asthmatic subjects, leading to an increase in secretion of type 2 cytokines—for example, IL-5 and IL-13. Additionally, TH2 memory cells have been described to play an important role in the pathogenesis of allergic asthma. TH2 memory cells are antigen-experienced cells that can reside in the lung as tissue-resident memory T (TRM) cells and, on allergen re-exposure, develop into activated effector cells and induce rapid inflammatory immune responses.4, 5, 6, 7
In addition to TH2 cells, group 2 innate lymphoid (ILC2) cells have become a focal point in asthma research in the past years. ILC2 are directly activated by alarmins, such as IL-33, IL-25, or thymic stromal lymphopoietin, secreted by epithelial cells on allergen stimulation or epithelial cell barrier damage without the interaction with antigen-presenting cells.8 ILC2 do not express lineage-specific markers such as CD4, CD8, CD19, or CD11b but express TH2-similar effector transcription factors like GATA3, and they secrete similar proinflammatory cytokines (IL-5, IL-13) on stimulation.9 Studies have described subgroups of ILC2 that respond to different cytokine stimuli and exhibit different functions. Inflammatory ILC2 (iILC2) are specified as the proinflammatory subset, which develops on IL-25 stimulation; natural ILC2 (nILC2) are specified as the tissue-resident homeostatic subset, which is increased by IL-33 stimulation.10,11 Additionally, a novel IL-10–producing ILC subset has been reported to be involved in inflammatory immune responses acting in an immunosuppressive manner and expressing B lymphocyte–induced maturation protein 1 (Blimp-1) as a key transcription factor for IL-10 production.12, 13, 14
Blimp-1, a zinc finger–containing transcriptional factor, is expressed in various cell subsets, including B and T cells. Blimp-1 has been reported to be a key regulator of T-cell homeostasis and survival. The highest transcription of Blimp-1 was found in antigen-experienced cells.15 In regulatory T cells, Blimp-1 has been found to be critically important for the expression of IL-10. Blimp-1 therefore seems to be important for peripheral tolerance. Recent studies have shown that Blimp-1 also mediates TRM cell formation in the lung.16,17
Vitamin D3 (VitD3), also termed cholecalciferol, is a prohormone that is mainly produced by sunlight exposure but can be found in food (eg, fish, eggs, or dairy products) or can be taken as a supplement. 1,25-Dihydroxyvitamin D3, the biologically active form, can bind to the vitamin D receptor, which acts as a nuclear transcription factor and can either enhance or suppress its target genes.18 Vitamin D receptor expression has been found in various immune cells such as mast cells, dendritic cells, and T cells.19 VitD3 in the context of asthma has been extensively discussed in the past decades. Previous studies have shown that VitD3 deficiency is associated with more severe asthma phenotypes, especially in children, as well as with ineffective medication and with increase in exacerbations.20, 21, 22, 23 The mechanism by which VitD3 might modulate the immune response in patients with asthma requires further investigation.
Therefore, in this study, we investigated the importance of sufficient VitD3 serum levels in patients with asthma. To further investigate the mechanisms of the immunoregulatory effects of VitD3, we set up a murine model of allergic asthma to compare the immune response in VitD3 deficiency to VitD3 sufficiency in vivo. We discovered an anti-inflammatory effect of VitD3 supplementation in the diet, resulting in less severe asthma in humans and in a murine model of allergic asthma. The mechanism of interest was found to be associated to a VitD3-induced shift to anti-inflammatory immune responses via upregulating Blimp-1 in lung ILC2 and TRM cells.
Methods
Human study PreDicta
The European PreDicta (Postinfectious immune reprogramming and its association with persistence and chronicity of respiratory allergic diseases) study assessed 2 cohorts of healthy and asthmatic preschool children aged 4 to 6 years. The study was performed in collaboration with the Department of Allergy and Pneumology at the Children’s Hospital in Erlangen. The PreDicta study was approved by the ethics committee of Friedrich-Alexander University Erlangen-Nürnberg, Germany (approval 4435) and is registered in the German Clinical Trials Register (www.germanctr.de; approval DRKS00004914). Cohort recruitment, inclusion and exclusion criteria, and data collection have been described previously.24 Relevant clinical characteristics of the study cohort are presented in Table E1 in this article’s Online Repository at www.jaci-global.org.
Human study AZCRA
The AZCRA (Investigation of the role of cytokines, chemokines, and their receptors in the inflammatory process in asthma patients) study assessed 2 cohorts of adult asthmatic and control patients aged between 18 and 65 years. At the baseline visit, all patients answered a questionnaire about, among other things, their family’s predisposition to asthma, their housing situation, and their clinical manifestations. The asthmatic patient cohort was divided into 2 subgroups, those with and without VitD3 supplementation, according to their answer to the questionnaire at the baseline visit. Additionally, blood was taken, and lung function was measured by spirometry.
The study was performed in collaboration with Medical Clinic 1 at University Hospital Erlangen and was registered in the German Clinical Trials Register (DRKS00023843). The study was approved by the ethics committee of the Friedrich-Alexander-University Erlangen-Nürnberg Germany (approval 315-20B). Relevant clinical characteristics of this study cohorts are presented in Table E2 in this article’s Online Repository at www.jaci-global.org. Characteristics of subjects in asthmatic subgroups are presented in Table E3 in the Online Repository. Finally, recruitment, inclusion and exclusion criteria, and data collection are described in Table E4, also in the Online Repository.
Human PBMC isolation and cell culture
For human peripheral blood mononuclear cell (PBMC) isolation and cell culture, we provide detailed information about the reagents used in the experiments in the Methods section in the Online Repository at www.jaci-global.org.
In the PreDicta study, PBMCs were isolated with Ficoll using density centrifugation from heparinized blood samples. Afterward, cells were cultured at 106 cells/mL for 48 hours in complete culture medium as described above at 37°C and 5% CO2.
Additionally, whole blood was collected in Tempus Blood RNA Tubes (Life Technologies, Darmstadt, Germany), and RNA was extracted with MagMax (Thermo Fisher Scientific, Waltham, Mass) for the stabilized blood tube RNA isolation kit. Synthesis of cDNA and then real-time reverse transcriptase PCR were performed as described for the murine cells below.
In the AZCRA study, PBMCs were isolated in SepMate tubes (STEMCELL Technologies, Vancouver, British Columbia, Canada) according to the manufacturer’s recommended protocol.
PBMCs were cultured as 1 × 106 viable cells in RPMI 1640 complete culture medium (Thermo Fisher Scientific) supplemented with HEPES 1:40, β-mercaptoethanol 1 mol solution 1:20, penicillin–streptomycin 1:100, l-glutamine 1:100, sodium pyruvate 1:100, Mem Vitamin 1:100, Mem Non Essential Amino Acid 1:100, and FCS 10% (see the Methods section in the Online Repository at www.jaci-global.org) in a 48-well plate for 96 hours at 37°C and 5% CO2 either unstimulated or stimulated with 10 nmol 1,25-OH-VitD3 (Tocris Bioscience, Bristol, United Kingdom). Addition of 1,25-OH-VitD3 to the culture condition was performed under as little light exposure as possible. After 96 hours, all supernatants were removed carefully and stored at −80°C. PBMCs were diluted in QIAzol Lysis Reagent (Qiagen, Venlo, The Netherlands) and stored at −80°C for RNA isolation.
Mice
Five-week-old female BALB/c wild-type mice were obtained from Janvier Labs (Saint-Berthevin, France). The mice were maintained under specific-pathogen–free conditions in our animal facility and had free access to food and water. Mice were continuously fed, starting 3 weeks before the start of the asthma model, with either a VitD3-supplemented (1324 supplemented with 1 g VitD3 per kilogram of food; Altromin, Lage, Germany) diet containing a theoretical level of 5600 IU/kg, or a VitD3-deficient diet (C 1017, Altromin) containing 0 IU/kg VitD3 according to the manufacturer until the animals were humanely killed. In total, all mice were fed with VitD3-deficient or -supplemented diet for 42 days. Actual VitD3 levels in the diet might vary as a result of sensitivity to light, sensitivity to humidity, and/or ambient temperature. The experiments were approved by the government of Mittelfranken, Bavaria (approval Az. 55.2.2-2532.2-633) and were performed in accordance with German and European laws for animal protection.
Murine model of allergic asthma
For murine asthma induction, we used an ovalbumin (OVA)-induced asthma model. Mice were subdivided and fed for 3 weeks either VitD3-deficient or VitD3-supplemented food. After this prefeeding period, animals were sensitized via 2 intraperitoneal injections of 100 μg OVA/alum (500 μg/mL OVA; Sigma-Aldrich, St Louis, Mo) complexed with 10% aluminum potassium sulfate (Sigma-Aldrich) at pH 6.5 and dissolved in NaCl (0.9% solution) on experimental days 0 and 7. On experimental days 18, 19, and 20, mice were challenged intranasally with 50 μg OVA dissolved in PBS (2 mg OVA/mL PBS) after receipt of light isoflurane anaesthesia.
Noninvasive and invasive airway hyperresponsiveness measurement
To measure airway hyperresponsiveness, we used noninvasive whole-body plethysmography with Buxco Electronics apparatus (Buxco Research Systems, Wilmington, NC) at day 20 at 3 hours after allergen challenge. Mice were placed into an exposition chamber and challenged with PBS and increasing doses of aerosolized methacholine (Sigma-Aldrich) dissolved in PBS (0, 5, 10, 25, 50 mg/mL) while enhanced pause was measured. Invasive airway resistance was measured with a FlexiVent FX device (SCIREQ, EMKA Technologies, Paris, France) and FlexiWare software. After injection of 200 μL Pentobarbital solution, a small cannula was inserted into the trachea and attached to the FlexiVent device afterward. Airway resistance was measured during mechanical ventilation with aerosolized PBS and increasing doses of methacholine (0, 5, 10, 25, and 50 mg/mL) dissolved in PBS.
Total murine lung cell isolation
Nine-week-old mice were humanely killed at day 21 for the asthma model, immediately before invasive measurement of airway resistance. Whole lungs were taken, cut into small pieces with a scalpel under sterile conditions, and digested with 300 U/mL collagenase type Ia and 0.015% DNAse, 10 mg/mL in PBS (37°C for 45 min). The digested lung was pressed through a 40 μm cell strainer; the cell strainer was then rinsed with 5 mL RPMI 1640 medium to dissolve any residue. After centrifugation (1500 rpm, 10 minutes, 4°C), the supernatant was removed and the pellet resuspended in 10 mL ACK-Lysis buffer (0.15 mol NH4Cl, 0.1 mmol KHCO3, and 0.1 mmol Na2-EDTA dissolved in ddH2O) and incubated for 2 minutes at room temperature for erythrocyte lysis. After another centrifugation step, cells were washed with PBS and dissolved to 1 Mio cell/mL.
Total murine lung cell culture and ILC2 culture conditions
Total lung cells were adjusted to 1 Mio cell/mL in RPMI 1640 medium containing 100 IU/mL penicillin, 100 μg/mL streptomycin (all from anprotec, Bruckberg, Germany), and 10% FCS (Biochrom, Berlin, Germany) and cultured in 48-well plates at 37°C 5% CO2. For OVA restimulation, total lung cells were stimulated with 500 μg/mL OVA for 48 hours. For cell differentiation and expansion of ILC2 in vitro, total lung cells were either cultured with recombinant murine 20 ng/mL IL-2 (rmIL-2), 20 ng/mL rmIL-7 and 10 ng/mL rmIL-33 or with 20 ng/mL rmIL-2, 20 ng/mL rmIL-7 and 20 ng/mL rmIL-25 (all Immunotools, Friesoythe, Germany) for 120 hours in 48-well plates at 37°C and 5% CO2.
Histologic sections, inflammation score, and periodic acid–Schiff score
Pieces of mice total lungs were fixed in 4% formaldehyde, dehydrated, and embedded in paraffin. Sections were cut 3 μm thick and stained with hematoxylin and eosin (HE) or periodic acid–Schiff (PAS) at the Institute of Pathology, Universitätsklinikum Erlangen. Determination of lung inflammation score was performed on HE-stained lung sections by a pathologist without knowledge of the group affiliation as previously published.25 PAS scoring was performed semiquantitatively; the score was determined by classifying bronchi according to the size of PAS-positive areas.26
Flow cytometric cell staining and analysis
Murine isolated total lung cells, murine cultured total lung cells, and murine cultured cells of ILC2 skewing conditions were washed once with 200 μL FACS-Buffer (PBS + 1% FCS; catalog S0615; Sigma-Aldrich), centrifuged (5 minutes, 1500 rpm), and preincubated with murine anti-CD16/CD32 (1:100, BD Biosciences, Franklin Lakes, NJ) for 5 minutes at 4°C to prevent unspecific binding. Afterward, samples were centrifuged and the supernatants discarded. For surface staining, samples were stained with fluorochrome-conjugated antibodies for 30 minutes at 4°C in FACS-Buffer in the dark. For intracellular staining, cells were then fixed and permeabilized with FoxP3 fixation/permeabilization reagent (catalog 00-5523-00; Thermo Fisher Scientific) according to the manufacturer’s protocol for 35 minutes at 4°C in the dark, followed by intracellular staining in permeabilization buffer for 30 minutes at 4°C in the dark. Finally, cells were washed with FACS-Buffer and measured by a FACS-Canto II device (BD Biosciences) and analyzed by FlowJo v10 software (Treestar, Ashland, Ore). For ILC2 staining, samples were prestained with lineage cocktail (containing biotinylated anti-CD5, anti-CD11b, anti-CD45R, anti–7-4, anti-Gr1, and anti-Ter119, premixed by Miltenyi Biotec, San Diego, Calif). In the surface staining step, additional lineage markers (anti-CD3, anti-CD4, anti-CD11c, anti-SiglecF) conjugated to allophycocyanin (APC) and streptavidin-conjugated APC were applied because they could exclude lineage-associated cell types in further analysis steps. All antibodies used for flow cytometric analyses are listed in Table E5 in the Online Repository at www.jaci-global.org.
Statistical analysis
All statistical analyses were performed by GraphPad Prism v8 software for Windows (GraphPad Software, La Jolla, Calif). Statistical significance was calculated by 2-tailed Student t test (Gaussian distribution) or Mann-Whitney test (not normally distributed) for the analysis of 2-group comparisons and 1-way ANOVA (Gaussian distribution), Kruskal-Wallis test (not normally distributed), or 2-way ANOVA for multiple comparisons to generate P value data (∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001). For post hoc analysis, we used the Tukey method test (1-way ANOVA) and the Šídák method test (2-way ANOVA), respectively. P ≤ .05 was considered statistically significant. Unless otherwise indicated, data are presented as means ± SEMs. Statistical details (eg, number of animals or subjects per group) are provided in the figure captions.
Results
Higher VitD3 levels are associated with increased IL-10 production in asthmatic children and higher Blimp-1 expression in controls
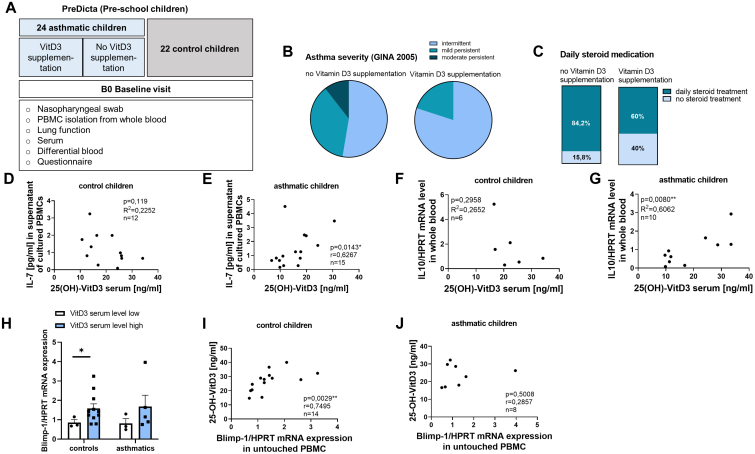
First, we wanted to analyze potential positive effects of VitD3 in pediatric asthma. Therefore, we divided the asthmatic cohort of preschool children in our study PreDicta into 2 subgroups (with and without VitD3 supplementation), then analyzed asthma severity, symptom control, symptom occurrence, daily receipt of steroid treatment, and impairment in activities (Fig 1, A-C, and see Fig E1, A-D, in this article’s Online Repository at www.jaci-global.org). VitD3 supplementation in these children was associated with less severe asthma, fewer symptoms during the day, and better symptom control. Additionally, we found a reduced need for steroid medication in the supplemented asthmatic group (Fig 1, C).
Fig 1.
VitD3 supplementation in asthmatic preschool children leads to less severe asthma and less steroid treatment. High VitD3 serum levels correlate with IL-7 and IL-10 in asthmatic children and Blimp-1 in control children. (A) Experimental design of human study PreDicta of preschool-age cohorts of children analyzed. (B) Asthma severity (GINA 2005) of asthmatic children with and without VitD3 supplementation according to questionnaire answer (n = 19 and 5). (C) Requirement for daily steroid treatment of asthmatic children with and without VitD3 supplementation according to questionnaire answer (n = 19 and 5). (D and E) 1,25-OH-VitD3 serum levels (ng/mL) in correlation to IL-7 levels (pg/mL) in supernatants of cultured PBMCs of control and asthmatic preschool children (n = 12 and 14). (F and G) 1,25-OH-VitD3 serum levels (ng/mL) in correlation to IL-10 mRNA levels in total blood (Tempus tubes) of control and asthmatic preschool children (n = 6 and 10). (H) Blimp-1/HPRT expression in control and asthmatic children divided by VitD3 serum levels ≤20 ng/mL (n = 3, 11, 3, and 5). (I and J) 1,25-OH-VitD3 serum levels (ng/mL) in correlation to Blimp-1/HPRT mRNA levels in untouched PBMCs of control and asthmatic preschool children. Correlations were calculated by Pearson correlation (D, F, and G) and Spearman correlation (E, I, and J). Data are shown as means ± SEMs; ∗P ≤ .05; ∗∗P ≤ .01. HPRT, Hypoxanthine guanine phosphoribosyl transferase.
Next, we wanted to investigate the differences in VitD3-supplemented and -nonsupplemented children in T-cell and ILC2 immune responses. IL-7 is a cytokine important for T-cell maturation and survival, but it also plays a role in ILC2 survival.27 Thus, we wanted to investigate whether high VitD3 serum levels are associated with higher or lower IL-7. We found a positive significant correlation of 25-(OH)-VitD3 serum levels with higher IL-7 levels in the supernatants of cultured, unstimulated PBMCs (Fig 1, D and E). IL-33 is a cytokine known to drive TH2 immune responses, but it also stimulates anti-inflammatory nILC2. We previously reported in a cohort of children a strong positive correlation of 25-(OH)-VitD3 serum levels with IL-33 in nasopharyngeal fluid compared to the control cohort.28 Finally, we measured IL-10 mRNA expression in the children’s whole blood to assess the effect of VitD3 on circulating IL-10, which is known to be a major anti-inflammatory cytokine.29 High VitD3 serum levels were associated with higher IL-10 mRNA expression in the blood of asthmatic children but not in control children (Fig 1, F and G). Therefore, we reasoned that Blimp-1 might be involved in increased IL-10 production with VitD3 treatment because Blimp-1 is a transcription factor driving regulatory T-cell IL-10 production.15 When we measured Blimp-1 mRNA expression, we found that higher VitD3 serum levels in control children were associated with higher Blimp-1 expression in untouched PBMCs (Fig 1, H). In control children, but not in asthmatic children, we observed a positive correlation between Blimp-1 and VitD3 serum levels (Fig 1, I and J).
Taken together, these data led us to confirm that VitD3 supplementation in preschool children plays an asthma-resolving role. Further, we also found a relationship between VitD3 serum levels and Blimp-1 expression.
VitD3 supplementation in asthmatic adults is associated with less severe clinical asthma manifestation and less steroid treatment
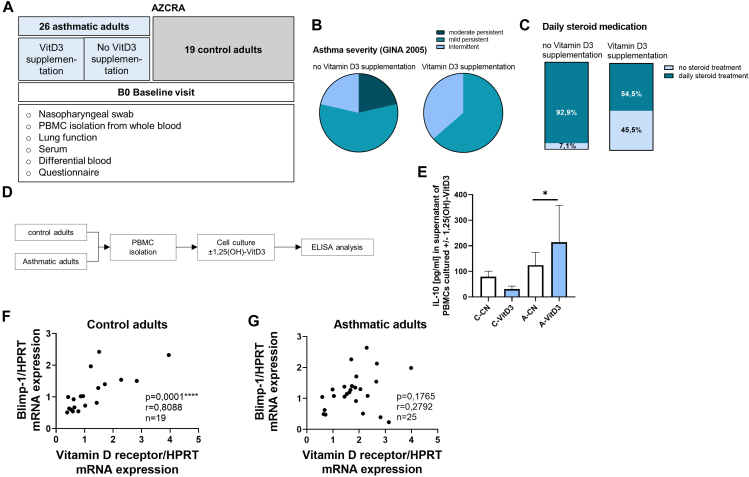
To investigate the role of VitD3 supplementation in adult allergic asthma, we analogously divided the asthmatic cohort of our study AZCRA into 2 subgroups —those with and without VitD3 supplementation, as reported by the subjects—and correlated them with asthma severity, symptom control, occurrence of symptoms, daily receipt of steroid treatment, and impairment in activities (Fig 2, A-C, and see Fig E1, E-I). VitD3 supplementation in the adult cohort was similarly associated with less severe asthma, fewer symptoms during the day, and better symptom control. Additionally, we found a reduced need for steroid medication in the supplemented asthmatic group.
Fig 2.
VitD3 supplementation in asthmatic adults leads to less severe clinical asthma manifestation and less steroid treatment. (A) Experimental design of AZCRA human study of adult cohorts analyzed in this study. (B) Asthma severity (GINA 2005) of asthmatic adults with and without VitD3 supplementation according to questionnaire answer (n = 14 and 10). (C) need for daily steroid treatment of asthmatic adults with and without VitD3 supplementation according to questionnaire answer (n = 14 and 10). (D) Experimental design of PBMC analysis of human study AZCRA. (E) ELISA analysis of IL-10 (pg/mL) levels in supernatant of cultured PBMCs of asthmatic (A) and healthy control (C) adults treated with (VitD3) and without (CN) 1,25-OH-VitD3 for 4 days (n = 18, 19, 16, and 21; ∗P = .0435; Kruskal-Wallis test). (F and G) Vitamin D receptor/HPRT mRNA levels in correlation (Spearman correlation) to Blimp-1/HPRT mRNA levels in untouched PBMCs of control and asthmatic preschool children (n = 19 and 25). Data are shown as means ± SEMs; ∗P ≤ .05, ∗∗∗∗P ≤ .0001. HPRT, Hypoxanthine guanine phosphoribosyl transferase.
Next, we wanted to investigate the direct effect of VitD3 on the inflammatory immune response that drives the airway inflammation in asthmatic adults. Therefore, we stimulated cultured PBMCs of asthmatic cohorts and controls with 1,25-OH-VitD3 for 4 days (Fig 2, D). We found a significant induction of IL-10 in the supernatant of cultured PBMCs of asthmatic adults on 1,25-OH-VitD3 stimulation compared to the unstimulated cells of the respective group (Fig 2, E). When we further analyzed the effects of VitD3 on the transcription factor Blimp-1, we found a significant correlation of vitamin D receptor with Blimp-1 expression in freshly isolated PBMCs from controls (Fig 2, F). In contrast, asthmatic adults did not show this correlation (Fig 2, G), thus supporting the findings in our children cohort. Taken together, these results support the notion of an immunosuppressive and resolving function of VitD3 supplementation in asthma that might involve regulation of immune cells via Blimp-1.
Food supplementation with VitD3 resulted in ameliorated allergic trait in a murine model of OVA-induced asthma
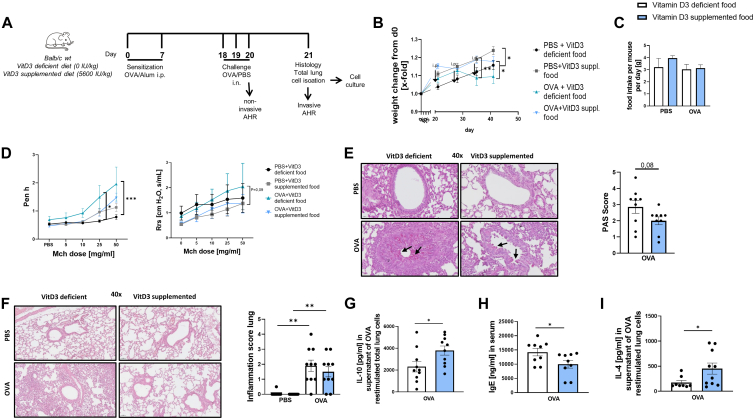
To investigate the mechanism of how supplemented VitD3 alters the immune response resulting in improved clinical asthma, we next set up a model of experimental OVA-induced asthma. To simulate the state of VitD3 deficiency and sufficiency in asthma, BALB/c wild-type mice were fed with either VitD3-supplemented or VitD3-deficient food starting 3 weeks before asthma induction protocol (Fig 3, A). All mice had age-appropriate weight development (Fig 3, B). After intraperitoneal sensitization, OVA-treated mice fed a VitD3-deficient diet exhibited significant weight loss compared to the respective group fed a VitD3-supplemented diet, despite the presence of other comparable nutrient composition (see Fig E2, A, in the Online Repository at www.jaci-global.org). In order to check whether the weight loss was caused by lower food intake, the average food intake per cage was quantified. We found no significant differences in food intake between the groups (Fig 3, C). To exclude the possibility of VitD3 hypervitaminosis and, because calcium has important functions in cellular signal transduction pathways, hyper-/hypocalcemia due to the special different food, we measured 25-(OH)-VitD3 and calcium serum levels. We found low or not measurable 25-(OH)-VitD3 serum levels in the deficient groups and no significant differences in calcium levels in all groups (Fig E2, B and C). Next, we wanted to assess the severity of asthma by measuring lung function. We performed noninvasive whole-body plethysmography for evaluating airway hyperresponsiveness of the upper respiratory tract and invasive measurement for evaluating airway hyperresponsiveness of the lower respiratory tract. We observed a trend to less airway resistance in upper and lower respiratory tract in asthmatic mice fed a VitD3-supplemented diet compared to the deficient group (Fig 3, D). In histologic PAS-stained lung sections, we found a trend to reduced mucus production in the lungs of VitD3-supplemented asthmatic mice but no differences in inflammatory cell infiltration in HE-stained lung parts (Fig 3, E and F).
Fig 3.
VitD3 supplementation leads to higher IL-10 levels in allergen-restimulated cells and reduces serum IgE levels in a murine model of OVA-induced asthma. (A) Experimental design of murine OVA-induced asthma model. (B) X-fold weight change of OVA- and PBS-treated mice fed VitD3-deficient or -supplemented diet from day 0 (day of diet change). (C) Food intake per mouse per day, calculated as average intake per cage (n = 2, 2, 2, and 2). (D) Lung function, measured as enhanced pause (Penh) by noninvasive plethysmography, on increasing doses of methacholine (n = 5, 4, 5, and 5; ∗P = .0135, ∗∗∗P = .0003; 2-way ANOVA). Lung function, measured as resistance (Rrs) invasively, on increasing doses of methacholine (n = 5, 4, 5, and 5; P = .0890; 2-way ANOVA). (E) PAS score of PAS-stained histologic sections of total lungs of OVA-treated mice with VitD3-deficient or -supplemented diet (n = 9 and 9; P = .0837, unpaired t test). Representative PAS-stained histologic sections of total lungs of respective groups and respective PBS-treated control group; black arrow indicates mucus. (F) Lung inflammation score calculated with HE staining (n = 9, 10, 10, and 10; ∗∗P = .0013, ∗∗P = .0033; Kruskal-Wallis test). Representative HE-stained histologic sections of total lungs of respective groups. (G) ELISA analysis of IL-10 (pg/mL) levels in supernatant of OVA-restimulated total lung cells (n = 9 and 9; ∗P = .0337, unpaired t test). (H) ELISA analysis of serum IgE levels (ng/mL) (n = 9 and 9; ∗P = .0400, unpaired t test). (I) ELISA analysis of IL-4 (pg/mL) levels in supernatant of OVA-restimulated total lung cells (n = 8 and 10; ∗P = .0434, Mann-Whitney test). Data are shown as means ± SEMs; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001.
We next reasoned that a VitD3-rich diet might reduce inflammatory immune responses or that much more would promote anti-inflammatory cytokines that suppress potential harmful immune responses, so we measured IL-10 levels. We found that on allergen restimulation, total lung cells secreted more IL-10 in the supernatants of VitD3-supplemented, OVA-treated mice compared to the deficient group (Fig 3, G). We next measured serum IgE levels and found it reduced in VitD3-supplemented asthmatic mice compared to their VitD3-deficient counterparts (Fig 3, H). Because IL-4, as opposed to IL-10, is known to induce IgE, we measured IL-4 in the supernatants of VitD3-supplemented, OVA-treated mice compared to the deficient group (Fig 3, I).30 However, IL-4 was found to be upregulated in the OVA group fed with high VitD3. These data, taken together, show that VitD3 in food induced IL-10, thus suppressing the allergic IgE response in mice.
VitD3-rich diet promotes effector T cells, CD103 expression in CD4+ T cells, and TRM cells
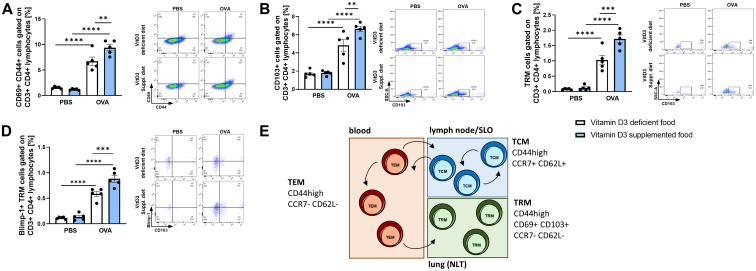
As a result of the reduced IL-10 levels in VitD3-deficient mice, we wanted to determine whether regulatory T cells (here defined as CD4+CD25+Foxp3+ T cells) or regulatory B cells (here defined as CD19+IL-10high B cells), known to be typically IL-10–secreting cells, are reduced in this group.31 We found no difference between OVA-treated groups with VitD3-enriched or -deficient diet (Fig E2, D and F). Next we wanted to investigate the effect of VitD3 supplementation on T cells. We asked whether VitD3 deficiency is associated with an increased type 2 inflammatory immune response. Because GATA3+ TH2 cells mainly drive type 2 immune responses, we assessed GATA3+CD4+ T cells in the lung, and found no differences in the numbers of TH2 cells between the OVA-treated groups (Fig E2, E). Next, we analyzed differences in effector CD4+ T cells and found significant induction of activated effector T cells (CD3+CD4+CD44+CD69+) in VitD3-supplemented, OVA-treated mice (Fig 4, A). Additionally, we found an induction of CD103+ T cells, a marker of mucosa associated lymphocytes and activated T cells (Fig 4, B). Because CD103 is also expressed on TRM lung cells, and TRM cells are known to be rapid inductors of proinflammatory immune response, we hypothesized that TRM cells might be enhanced in VitD3-deficient mice.32 We thus analyzed this CD103+ CD4 T-cell population for TRM cell markers. We found significant induction of lung TRM cells and their Blimp-1 expression (Blimp-1 expression is a major driver of TRM cells) in the VitD3-supplemented, OVA-treated group (Fig 4, C and D). Because TRM cells derive from central T memory (TCM) cells from lymph nodes or secondary lymphoid organs, we analyzed mesenteric lymph node cells for TCM cell characteristic markers. We found a significant induction of central memory cells in the OVA-treated, VitD3-supplemented group compared to the respective control group; this induction was not be observed in VitD3-deficient groups (Fig E2, G). We additionally found no significant differences in effector T memory (TEM) cell proportions and TEM/TCM cell ratios (Fig E2, H).
Fig 4.
VitD3 supplementation promotes CD103 in T cells as well as development of effector T cells and Blimp-1+ TRM cells in lungs of asthmatic mice. (A) Flow cytometric analysis of CD69+CD44+ cells gated on CD3+CD4+ lymphocytes in total lung cells. (B) Flow cytometric analysis of CD103+ cells gated on CD3+CD4+ lymphocytes in total lung cells. (C) Flow cytometric analysis of CD44+CD69+CD103+ cells gated on CD3+CD4+ lymphocytes in total lung cells. (D) Flow cytometric analysis of Blimp-1+CD44+CD69+CD103+ cells gated on CD3+CD4+ lymphocytes in total lung cells. Representative dot plot is provided for each group. (E) Schematic illustration of TRM, TEM, and TCM cell development and circulation. Statistical significances in (A, B, C, and D) (n = 5, 5, 5, and 5) were calculated by 2-way ANOVA. Data are shown as means ± SEMs; ∗P ≤ .05; ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
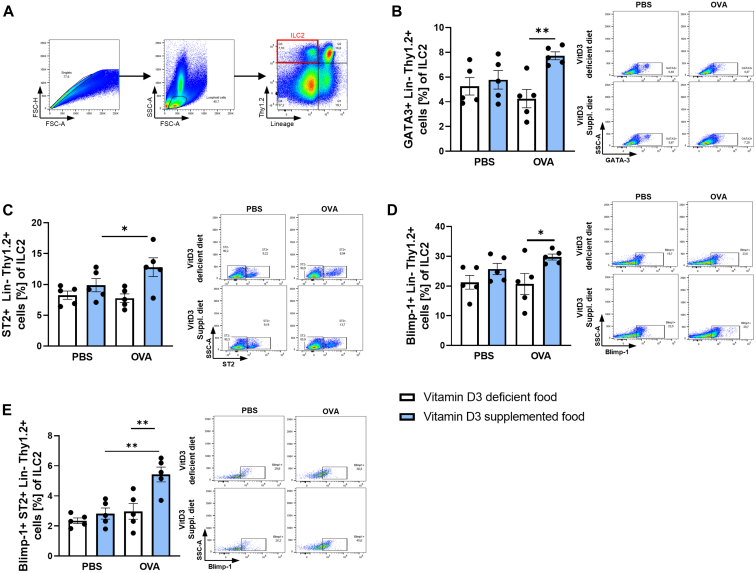
VitD3-supplemented diet increases GATA3 and Blimp-1 expression in lung ILC2
The past 2 decades have seen ILC2 become a focal point of interest in asthma research. Therefore, we analyzed the effect of VitD3 on lung ILC2 phenotypes in a murine model of experimental asthma. Because ILC2 do not express lineage-specific markers, we stained isolated total lung cells with lineage markers in the same fluorochrome (lineage markers: CD5, CD11b, CD45R, anti–7-4, anti–Gr-1 [Ly6G/C], anti–Terr-119, CD3, CD4, CD11c, SiglecF) for flow cytometric analysis to be able to exclude them (Fig 5, A). Lineage-negative and Thy1.2-positive cells, known to be surface markers for ILC2, were defined as ILC2. GATA3 is known as a characteristic transcription factor of ILC2 important for survival and development.33, 34, 35 We found significant induction of GATA3 in the VitD3-supplemented asthma group (Fig 5, B). GATA3 is additionally known to regulate the IL-25R and IL-33R/ST2 expression on the surface of ILC2.36, 37, 38 Thus, we also examined ST2 expression on ILC2. We found a significant induction of ST2 on ILC2 in the VitD3-supplemented food of the asthmatic group compared to the respective counterparts (Fig 5, C).
Fig 5.
VitD3 supplementation increases GATA3 and Blimp-1 expression in ST2+ lung ILC2. (A) Flow cytometric gating strategy for lung ILC2 (here defined as Lin−Thy1.2+). (B) Flow cytometric analysis of GATA3+Lin−Thy1.2+ cells in total lung cells. (C) Flow cytometric analysis of ST2+Lin−Thy1.2+ cells in total lung cells. (D) Flow cytometric analysis of Blimp-1+Lin−Thy1.2+ cells in total lung cells. (E) Flow cytometric analysis of Blimp-1+ST2+Lin−Thy1.2+ cells in total lung cells. Representative dot plot is provided for each group. Statistical significances in (B, C, D, and E) (n = 5, 5, 5, and 5) were calculated by 2-way ANOVA. Data are shown as means ± SEMs; ∗P ≤ .05; ∗∗P ≤ .01. Lin, Lineage.
Studies described 2 subgroups of ILC2. iILC2 were specified as the proinflammatory subset, which develops on IL-25 stimulation, and nILC2 were specified as the natural/homeostatic subset, which is increased by IL-33 stimulation, is able to secrete IL-10, and has recently been described to expresses Blimp-1 as key transcription factor necessary for IL-10 production.10, 11, 12,14 To differentiate between the pro- and anti-inflammatory ILC2 subsets, we analyzed Blimp-1 expression in lung ILC2. We observed significantly increased Blimp-1 expression in the supplemented asthma group (Fig 5, D). We additionally wanted to know whether the IL-33R (ST2)+Blimp-1+ ILC2 subset is increased in VitD3-supplemented asthmatic mice, and this was indeed the case (Fig 5, E). Taken together, these data indicate that VitD3 modulated ILC fate and promoted the development of anti-inflammatory IL-33–responsive and Blimp-1–expressing nILC2.
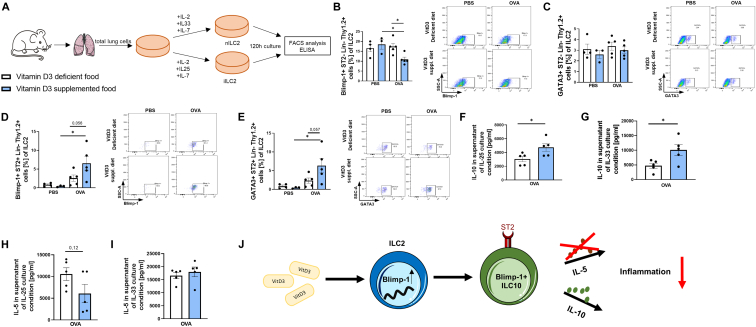
VitD3 food supplementation enhances IL-10 production and Blimp-1+ ILC2 under ILC2 skewing conditions
Next we asked whether a VitD3-supplemented diet has an effect on ILC fate during ILC2 skewing conditions. To do so, we isolated lung cells and differentiated them under 2 different ILC2 skewing conditions to promote either nILC2 or iILC2 (Fig 6, A). We performed flow cytometric analysis to assess the differences in ILC fate between VitD3-deficient and -sufficient mice. We found a significant decrease in Blimp-1+ST2− ILC2, but no difference in GATA3 expression in ST2− ILC2 between VitD3-deficient and -sufficient asthmatic mice in nILC2 skewing condition (Fig 6, B and C). We additionally found that VitD3-supplemented animals have a strong trend to increased Blimp-1+ and GATA3+ST2+ ILC2 in the IL-33–stimulated ILC2 culture condition (Fig 6, D and E).
Fig 6.
Asthmatic mice with VitD3 supplementation have increased GATA3 and Blimp-1 expression, as well as IL-10 secretion in IL-33–dependent lung ILC2. (A) Experimental design for analysis and differentiation of ILC2 into IL-25–dependent iILC2 and IL-33–dependent nILC2. (B) Flow cytometric analysis of Blimp-1+ST2−Lin−Thy1.2+ total lung cells cultured in IL-25–stimulated ILC2 culture conditions. (C) Flow cytometric analysis of GATA+ST2−Lin−Thy1.2+ total lung cells cultured in IL-25–stimulated ILC2 culture conditions. (D) Flow cytometric analysis of Blimp-1+ ST2+Lin−Thy1.2+ total lung cells cultured in IL-33–stimulated ILC2 culture conditions. (E) Flow cytometric analysis of GATA3+ST2+Lin−Thy1.2+ total lung cells cultured in IL-33–stimulated ILC2 culture conditions. (F) ELISA analysis of IL-10 in supernatant total lung cells cultured in IL-25–stimulated ILC2 culture conditions. (G) ELISA analysis of IL-10 in supernatant total lung cells cultured in IL-33–stimulated ILC2 culture conditions. (H) ELISA analysis of IL-5 in supernatant total lung cells cultured in IL-25–stimulated ILC2 culture conditions. (I) ELISA analysis of IL-5 in supernatant total lung cells cultured in IL-33–stimulated ILC2 culture conditions (n = 5 and 5; P = .5356, not significant [ns], unpaired t test). (J) Schematic illustration of effect of VitD3 on ILC2 fate. Representative dot plot is provided for each group. Statistical significances in (B, C, D, and E) (n = 4, 3, 5, and 5) were calculated by 2-way ANOVA and (F, G, H, and I) (n = 5 and 5) Student t test. Data are shown as means ± SEMs; ∗P ≤ .05. Lin, Lineage.
Furthermore, we collected the supernatant and found significantly increased IL-10 levels in both nILC2 and iILC2 conditions in the supplemented asthmatic group, supporting the hypothesis that VitD3 promotes anti-inflammatory response and may promote ILC fate toward IL-10–secreting nILC2 (also referred to as ILC10) (Fig 6, F and G). We also hypothesized that the typical proinflammatory cytokine secreted by ILC2, IL-5, should be decreased in the supplemented group in both conditions, but this was the case only by trend in the iILC2 condition (Fig 6, H and I).
This indicates that VitD3 sufficiency leads to more Blimp-1 expressing ILC2, IL-10 on stimulation, and slightly reduced IL-5 levels in IL-25–stimulated ILC2 culture conditions.
Discussion
The role of VitD3 in inflammatory and autoimmune diseases has been extensively and controversially discussed. In asthma disease, injection of 25-(OH)-VitD3 improved forced expiratory volume in 1 second and reduced symptom severity.39 Furthermore, it has already been described that low VitD3 serum levels are associated with more severe asthma manifestation.20, 21, 22,40 Although this has been shown by several studies, there is no clear VitD3-related additional therapeutic recommendation. In our study, we aimed to better understand the immunoregulatory roles and potential benefits of VitD3 sufficiency in asthma disease.
In our human studies, we clearly found less severe asthma manifestations regarding occurrence of symptoms, asthma control, and regular need for steroid medication in adults as well as in children with VitD3 supplementation. Our study confirms similar findings reported in previous studies.41 To make clear recommendations, a larger study population might be needed.
IL-7 and IL-33 might have proinflammatory potential, as IL-33 drives TH2, but, consistent with our murine data, IL-33 has also been described as a driver of nILC2 and therefore of anti-inflammatory immune responses.12,14,38,42,43 In our previous studies, in asthmatic preschool children of the PreDicta cohort, we observed increased levels of IL-33 in nasopharyngeal fluid, where ILC2 reside, in direct correlation with enhanced 25-(OH)-VitD3 serum levels.28 Consistently, in that study we also reported that the anti-inflammatory form of ST2, soluble ST2, directly correlated with IL-33 and with previous reports indicating that VitD3 induces soluble ST2.44 Investigating ILC2 immune responses in human studies in relation to asthma disease is generally difficult because they are mainly located in nasopharyngeal and lung tissue, and only progenitor cells are circulating within the blood.45 For this reason, we measured ILC2-related cytokines in patient sera.
To understand the mechanism causing overall improved asthma in patients with VitD3 as a supplement, we investigated the effect of supplemented VitD3 as a VitD3-enriched diet on inflammatory immune responses in a murine model of asthma. An effect of VitD3 on T cells in asthma has already been described because activated T cells express vitamin D receptor, although it remains unclear whether it has a positive or negative effect on T-cell–mediated inflammation.19 We thus wanted to understand the effect of VitD3 as a supplement in T-cell and ILC2 immune responses.
In our experimental asthma model, we clearly found reduced allergy markers such as IgE serum levels or airway mucus production. In contrast to former findings, we could not see any effect of VitD3-enriched diet on TH2 cells. In earlier studies, contradictory findings regarding induction or inhibition of TH2 cells by VitD3 were also described, as studies showed either induction or inhibition of TH2 immune response in combination with enhanced IgE levels.46,47 Here it might be considered that additional factors like mouse strain, allergen used to induce experimental asthma, or method of sensitization and/or challenge might be reasons for the results’ variation.
Additionally, regulatory T cells were described to be induced by VitD3. Unlike the current literature, we could not confirm these findings.48 To determine the potential source of enhanced IL-10 levels in our murine model, we focused on investigating lung ILC2. We found high expressions of Blimp-1 as well as GATA3 in ILC2, which was associated with higher IL-10 levels in cell culture supernatants. Why this is remains poorly understood, but it has been previously described that Blimp-1+ ILC2 produce IL-10 and contribute to anti-inflammatory immune responses in asthma.12 In ILC2, GATA3 was described to be a major transcription factor, one essential for ILC2 survival and development. GATA3 regulates the expression of IL-25R and IL-33R but was described to be the key transcription factor of pro-inflammatory ILC2. However, other studies have described GATA3 as indispensable for ILC2 survival.37,38,49
We found IL-4 to be increased; the literature suggests that TH2 cell–derived IL-4 drives IL-10 production in pulmonary ILC2s and represents an important cytokine to stimulate IL-10 secretion in nILC2. Interestingly, Howard et al12 report that costimulation of IL-4 and IL-33 generates the highest numbers of ILC10, which is consistent with our finding in our experimental asthma model.14 IL-4 is additionally known to promote Blimp-1 expression, indicating that there may exist an IL-4/Blimp-1/IL-10 axis in nILC2 that is affected by VitD3 and that might also induce Blimp-1 in T cells, therefore driving TRM cells.15
However, to further investigate the exact effect of VitD3 supplementation on ILC2 and ILC2 interaction with other pro- or anti-inflammatory cell subsets, further experiments with sorted ILC2 or coculture conditions might be necessary.
CD4+ TRM cells are known to have proinflammatory functions, inducing rapid immune response on allergen stimulation and expressing Blimp-1 as a major transcription factor to drive TRM cell development.5, 6, 7,15 In this study, we showed an upregulation of this cell type, but without a negative impact on asthmatic burden; instead, we observed an upregulation of IL-10 in total allergen-restimulated lung cells. Additionally, enhanced IL-4 levels in restimulated lung cells might be consistent with these findings, as IL-4 is a cytokine that promotes T-cell activation, upregulates Blimp-1 expression, and results in TRM cell differentiation.6,15,50 This indicates that there might be a protective subset or mechanism suppressing the following proinflammatory immune responses by VitD3. To our knowledge, the effect of VitD3 on TRM cells in experimental asthma has not yet been described. In human studies, higher 25-(OH)-VitD3 levels have been found to be associated with higher numbers of TEM lymphocytes in asthmatic children.51 Because our knowledge about TRM cells in asthma is still in its early stages, this might be an important focus for further studies.
Our results, taken together, indicate that supplementing food with VitD3 resulted in less severe clinical asthma manifestations in human studies as well as in a murine model of allergic asthma, indicating that VitD3 alters proinflammatory immune responses to anti-inflammatory immune responses in asthma and upregulates Blimp-1 in ILC2 cells compared to vitamin D deficiency. Our study contributes to a better understanding of the complex interaction of different transcription factors and cytokines in the context of VitD3 in asthma disease. A larger cohort of asthmatic subjects would be needed in the future to extend our findings and to investigate if the VitD3 anti-inflammatory action is observed across the spectrum of asthma severity, even as asthma phenotype may change from predominance of eosinophils to neutrophils in more intractable steroid-refractory asthma.
Surprisingly, VitD3 sufficiency also upregulated TRM lung cells, but this had no negative effect on the asthma manifestation, indicating that there might be TRM cells that do not secrete proinflammatory cytokines and/or suppress further proinflammatory responses, but this observation needs further investigation.
Our findings underline the importance of sufficient VitD3 serum levels in asthma patients and provide a new perspective on the immunoregulatory role of VitD3 in allergic asthma. We demonstrate the potential additional therapeutic benefit of VitD3 for patients with allergen-induced asthma. Further studies will be needed to clarify the involved pathways so we can make clear potential therapeutic recommendations.
Clinical implication.
Patients with asthma benefit from VitD3 supplementation because it helps regulate ILC2 and T cells in the airways.
Acknowledgments
We thank the whole team at the Department of Molecular Pneumology, especially Susanne Mittler, Sonja Trump, Adriana Geiger, and Elvedina Nendel for their technical support. Furthermore, we thank all children and their parents/guardians and adults who took part in our PreDicta and AZCRA study. Moreover, we want to thank team that enabled us to run the AZCRA study, especially Markus F. Neurath, Cristina Sicorschi Gutu, the lung function team (Klaudia Hinz, Simone Seidemann), and the private ambulance team (Nina Eger, Katharina Kreissl, Laura Seitz) from the Department of Medicine 1, Universitätsklinikum Erlangen. We are grateful to the Pediatric Pneumology-Allergology Department of Pediatrics and Adolescent Medicine, University Hospital Erlangen, for support in the PreDicta study.
Footnotes
This work was funded by Deutsche Forschungsgemeinschaft grant CRC1181/SFB1181-261193037 (TPB08). It was further supported by European Grant PreDicta (Collaborative Project grant 260895, WP1) awarded to S.F. in Erlangen, and in the other European centers to N.G.P. in Athens. J.C.G. was supported by a SFB1181 MD thesis scholarship in Erlangen and by the molecular pneumology department in Erlangen.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
The present work was performed in partial fulfillment of the requirements for obtaining the “Dr med” degree for J. C. Grund.
Supplementary data
References
- 1.Bateman E.D., Hurd S.S., Barnes P.J., Bousquet J., Drazen J.M., FitzGerald J.M., et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 2.Akmatov M.K., Holstiege J., Steffen A., Bätzing J. Trends and regional distribution of outpatient claims for asthma, 2009-2016, Germany. Bull World Health Organ. 2020;98:40–51. doi: 10.2471/BLT.19.229773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenzel S.E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 4.Raphael I., Joern R.R., Forsthuber T.G. Memory CD4+ T cells in immunity and autoimmune diseases. Cells. 2020;9:531. doi: 10.3390/cells9030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahimi R.A., Nepal K., Cetinbas M., Sadreyev R.I., Luster A.D. Distinct functions of tissue-resident and circulating memory Th2 cells in allergic airway disease. J Exp Med. 2020;217 doi: 10.1084/jem.20190865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hondowicz B.D., An D., Schenkel J.M., Kim K.S., Steach H.R., Krishnamurty A.T., et al. Interleukin-2–dependent allergen-specific tissue-resident memory cells drive asthma. Immunity. 2016;44:155–166. doi: 10.1016/j.immuni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan R., Yu J., Jiao Z., Li J., Wu F., Yan R., et al. The roles of tissue-resident memory T cells in lung diseases. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.710375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang D., Han Z., Oppenheim J.J. Alarmins and immunity. Immunol Rev. 2017;280:41–56. doi: 10.1111/imr.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klose C.S., Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y., Guo L., Qiu J., Chen X., Hu-Li J., Siebenlist U., et al. IL-25–responsive, lineage-negative KLRG1hi cells are multipotential “inflammatory” type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch S., Knipfer L., Kölle J., Mirzakhani H., Graser A., Zimmermann T., et al. Targeted deletion of NFAT-interacting-protein-(NIP) 45 resolves experimental asthma by inhibiting innate lymphoid cells group 2 (ILC2) Sci Rep. 2019;9 doi: 10.1038/s41598-019-51690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard E., Lewis G., Galle-Treger L., Hurrell B.P., Helou D.G., Shafiei-Jahani P., et al. IL-10 production by ILC2s requires Blimp-1 and cMaf, modulates cellular metabolism, and ameliorates airway hyperreactivity. J Allergy Clin Immunol. 2021;147:1281–1295.e5. doi: 10.1016/j.jaci.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Kim H.S., Jang J.H., Lee M.B., Jung I.D., Kim Y.M., Park Y.M., et al. A novel IL-10–producing innate lymphoid cells (ILC10) in a contact hypersensitivity mouse model. BMB Rep. 2016;49:293–296. doi: 10.5483/BMBRep.2016.49.5.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H., Wu Y., Zhang Y., Ni B. IL-10–producing ILCs: molecular mechanisms and disease relevance. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu S.H., Yeh L.T., Chu C.C., Yen B.L., Sytwu H.K. New insights into Blimp-1 in T lymphocytes: a divergent regulator of cell destiny and effector function. J Biomed Sci. 2017;24:49. doi: 10.1186/s12929-017-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behr F.M., Kragten N.A.M., Wesselink T.H., Nota B., van Lier R.A.W., Amsen D., et al. Blimp-1 rather than Hobit drives the formation of tissue-resident memory CD8+ T cells in the lungs. Front Immunol. 2019;10:400. doi: 10.3389/fimmu.2019.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benevides L., Costa R.S., Tavares L.A., Russo M., Martins G.A., da Silva L.L.P., et al. B lymphocyte–induced maturation protein 1 controls TH9 cell development, IL-9 production, and allergic inflammation. J Allergy Clin Immunol. 2019;143:1119–1130.e3. doi: 10.1016/j.jaci.2018.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange N.E., Litonjua A., Hawrylowicz C.M., Weiss S. Vitamin D, the immune system and asthma. Expert Rev Clin Immunol. 2009;5:693–702. doi: 10.1586/eci.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maes K., Serré J., Mathyssen C., Janssens W., Gayan-Ramirez G. Targeting vitamin D deficiency to limit exacerbations in respiratory diseases: utopia or strategy with potential? Calcif Tissue Int. 2020;106:76–87. doi: 10.1007/s00223-019-00591-4. [DOI] [PubMed] [Google Scholar]
- 21.Jolliffe D.A., Greenberg L., Hooper R.L., Griffiths C.J., Camargo C.A., Jr., Kerley C.P., et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881–890. doi: 10.1016/S2213-2600(17)30306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turkeli A., Ayaz O., Uncu A., Ozhan B., Bas V.N., Tufan A.K., et al. Effects of vitamin D levels on asthma control and severity in pre-school children. Eur Rev Med Pharmacol Sci. 2016;20:26–36. [PubMed] [Google Scholar]
- 23.Jartti T., Liimatainen U., Xepapadaki P., Vahlberg T., Bachert C., Finotto S., et al. Clinical correlates of rhinovirus infection in preschool asthma. Allergy. 2021;76:247–254. doi: 10.1111/all.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hentschke I., Graser A., Melichar V.O., Kiefer A., Zimmermann T., Kroß B., et al. IL-33/ST2 immune responses to respiratory bacteria in pediatric asthma. Sci Rep. 2017;7 doi: 10.1038/srep43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doganci A., Karwot R., Maxeiner J.H., Scholtes P., Schmitt E., Neurath M.F., et al. IL-2 receptor beta-chain signaling controls immunosuppressive CD4+ T cells in the draining lymph nodes and lung during allergic airway inflammation in vivo. J Immunol. 2008;181:1917–1926. doi: 10.4049/jimmunol.181.3.1917. [DOI] [PubMed] [Google Scholar]
- 26.Krammer S., Yang Z., Zimmermann T., Xepapadaki P., Geppert C.I., Papadopoulos N.G., et al. An immunoregulatory role of interleukin-3 in allergic asthma. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.821658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barata J.T., Durum S.K., Seddon B. Flip the coin: IL-7 and IL-7R in health and disease. Nat Immunol. 2019;20:1584–1593. doi: 10.1038/s41590-019-0479-x. [DOI] [PubMed] [Google Scholar]
- 28.Haag P., Sharma H., Rauh M., Zimmermann T., Vuorinen T., Papadopoulos N.G., et al. Soluble ST2 regulation by rhinovirus and 25(OH)-vitamin D3 in the blood of asthmatic children. Clin Exp Immunol. 2018;193:207–220. doi: 10.1111/cei.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang W., Rutz S., Crellin N.K., Valdez P.A., Hymowitz S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 30.Lambrecht B.N., Hammad H., Fahy J.V. The cytokines of asthma. Immunity. 2019;50:975–991. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Boonpiyathad T., Satitsuksanoa P., Akdis M., Akdis C.A. IL-10 producing T and B cells in allergy. Semin Immunol. 2019;44 doi: 10.1016/j.smim.2019.101326. [DOI] [PubMed] [Google Scholar]
- 32.Zundler S., Becker E., Spocinska M., Slawik M., Parga-Vidal L., Stark R., et al. Hobit- and Blimp-1–driven CD4+ tissue-resident memory T cells control chronic intestinal inflammation. Nat Immunol. 2019;20:288–300. doi: 10.1038/s41590-018-0298-5. [DOI] [PubMed] [Google Scholar]
- 33.Vivier E., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Moro K., Ealey K.N., Kabata H., Koyasu S. Isolation and analysis of group 2 innate lymphoid cells in mice. Nat Protoc. 2015;10:792–806. doi: 10.1038/nprot.2015.047. [DOI] [PubMed] [Google Scholar]
- 35.Vivier E., van de Pavert S.A., Cooper M.D., Belz G.T. The evolution of innate lymphoid cells. Nat Immunol. 2016;17:790–794. doi: 10.1038/ni.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griesenauer B., Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:475. doi: 10.3389/fimmu.2017.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J. GATA3 regulates the development and functions of innate lymphoid cell subsets at multiple stages. Front Immunol. 2017;8:1571. doi: 10.3389/fimmu.2017.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yagi R., Zhong C., Northrup D.L., Yu F., Bouladoux N., Spencer S., et al. The transcription factor GATA3 is critical for the development of all IL-7Rα–expressing innate lymphoid cells. Immunity. 2014;40:378–388. doi: 10.1016/j.immuni.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Einisadr A., Rajabi M., Moezzi H., Bakhshandeh H. Impact of rapid correction of vitamin D deficiency in asthmatic patients. Wien Klin Wochenschr. 2022;134:18–23. doi: 10.1007/s00508-021-01975-z. [DOI] [PubMed] [Google Scholar]
- 40.Forno E., Bacharier L.B., Phipatanakul W., Guilbert T.W., Cabana M.D., Ross K., et al. Effect of vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low vitamin D levels: the VDKA randomized clinical trial. JAMA. 2020;324:752–760. doi: 10.1001/jama.2020.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solidoro P., Bellocchia M., Aredano I., Mattei A., Pivetta E., Patrucco F., et al. Asthmatic patients with vitamin D deficiency have decreased exacerbations after vitamin replacement. Nutrients. 2017;9:1234. doi: 10.3390/nu9111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan B.C.L., Lam C.W.K., Tam L.S., Wong C.K. IL33: roles in allergic inflammation and therapeutic perspectives. Front Immunol. 2019;10:364. doi: 10.3389/fimmu.2019.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De la Fuente M., MacDonald T.T., Hermoso M.A. The IL-33/ST2 axis: role in health and disease. Cytokine Growth Factor Rev. 2015;26:615–623. doi: 10.1016/j.cytogfr.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Pfeffer P.E., Chen Y.H., Woszczek G., Matthews N.C., Chevretton E., Gupta A., et al. Vitamin D enhances production of soluble ST2, inhibiting the action of IL-33. J Allergy Clin Immunol. 2015;135:824–827.e3. doi: 10.1016/j.jaci.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drake L.Y., Kita H. Group 2 innate lymphoid cells in the lung. Adv Immunol. 2014;124:1–16. doi: 10.1016/B978-0-12-800147-9.00001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantorna M.T., Zhu Y., Froicu M., Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80(6 suppl):1717s–1720s. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 47.Matheu V., Bäck O., Mondoc E., Issazadeh-Navikas S. Dual effects of vitamin D–induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112:585–592. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 48.Agrawal T., Gupta G.K., Agrawal D.K. Vitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine model. Clin Exp Allergy. 2013;43:672–683. doi: 10.1111/cea.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou L. Striking similarity: GATA-3 regulates ILC2 and Th2 cells. Immunity. 2012;37:589–591. doi: 10.1016/j.immuni.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caminati M., Pham D.L., Bagnasco D., Canonica G.W. Type 2 immunity in asthma. World Allergy Organ J. 2018;11:13. doi: 10.1186/s40413-018-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Looman K.I.M., Jansen M.A.E., Voortman T., van den Heuvel D., Jaddoe V.W.V., Franco O.H., et al. The role of vitamin D on circulating memory T cells in children: the Generation R study. Pediatr Allergy Immunol. 2017;28:579–587. doi: 10.1111/pai.12754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.