Abstract
Background
There are no reports on the relationship between food protein–induced enterocolitis syndrome (FPIES) diagnosis and procalcitonin levels.
Objective
Our study sought to demonstrate a correlation between the presence or absence and severity of FPIES symptoms and postemetic procalcitonin levels.
Methods
The subjects were 53 patients with FPIES (44 with hen’s egg allergy, 4 with milk allergy, 4 with wheat allergy, and 3 with soy allergy), who collectively underwent a total of 75 oral food challenges (OFCs). Procalcitonin levels at 5 hours after antigen ingestion were compared between patients with a positive OFC result and those with a negative OFC result and between patients who experienced mild or moderate events and those who experienced severe events.
Results
At 5 hours after ingestion of the causative food, the median procalcitonin levels in patients with a negative OFC result, patients who experienced a mild or moderate event, and patients who experienced a severe event were 0.02, 0.03, and 0.16 ng/mL, respectively. The procalcitonin level was significantly higher in the groups with a positive OFC result than in the groups with a negative OFC result (P < .001), and it was significantly higher in those who experienced severe events than in those who experienced mild or moderate events (P = .012).
Conclusion
Measurement of procalcitonin levels has the potential to provide a quantitative and objective assessment of FPIES diagnosis and severity.
Key words: Food allergy, food protein–induced enterocolitis syndrome, procalcitonin, oral food challenge, pediatrics
Introduction
Food protein–induced enterocolitis syndrome (FPIES) is a non–IgE-mediated allergic disease that manifests exclusively as gastrointestinal symptoms such as emesis and diarrhea.1 The diagnosis of FPIES can be established by fulfilling the diagnostic criteria or performing an oral food challenge test (OFC); nonetheless, a disease-specific serologic test for FPIES is currently unavailable. Hence, the discovery of a disease-specific biomarker of FPIES would be beneficial. Recently, serum thymus- and activation-regulated chemokine (TARC) has been proposed as a potential biomarker, as it has been linked to the presence or absence of symptoms and disease severity.2 The overexpression of TNF-α has been suggested as an etiologic factor for FPIES.3 TNF-α and other cytokines have been shown to trigger procalcitonin production from systemic organs.4 Previous research has demonstrated that procalcitonin is produced by TNF-α and other cytokines after symptom onset.5 Although there are case reports of elevated procalcitonin levels following the onset of FPIES symptoms,6, 7, 8 there have been no case series or reports on the disease's severity. In the present investigation, we assessed the procalcitonin levels of patients with FPIES 5 hours after antigen ingestion of the causative food and examined their correlation with symptoms presence or absence and disease severity.
The study involved subjects who underwent OFC for purposes of diagnosing FPIES or confirming tolerance between November 2020 and July 2022. This study was a prospective study of patients eligible to undergo OFC. Diagnosis of FPIES was based on the diagnostic criteria outlined in the International Consensus Guideline.1
OFC was carried out by using the open and single-serving methods. The allergist determined the food load based on the patient's history and other factors (see Appendix 1 in the Online Repository at www.jaci-global.org). The criteria for positivity and severity of the OFC reaction were in accordance with the International Consensus Guideline.1
The patient characteristics assessed included the antigen, age at initial presentation in months, sex, symptoms (emesis, diarrhea, hematochezia, hypovolemia, pallor, and frequency of emesis), and history of physician-diagnosed IgE-mediated food allergy and atopic dermatitis.
Regarding the blood tests, venous blood samples were obtained both before and 5 hours after loading. A venous catheter was inserted before OFC, and blood was collected before loading after the catheter had been secured. Blood was then collected 5 hours after loading by either using the same catheter or puncturing the vessel again. Procalcitonin levels were determined using LUMIPULSE Presto Brahms procalcitonin (Fujirebio, Tokyo, Japan). Additionally, TARC level, white blood cell count, neutrophil count, eosinophil count, hematocrit level, platelet count, C-reactive protein level, lactate dehydrogenase level, venous blood pH, HCO3– level, and methemoglobin level were also evaluated. Blood test data were compared between patients with positive and negative OFC results. Patients with positive OFC results were further compared between those in the mild or moderate groups and those in the severe group according to disease severity. The results are presented as medians (quartiles), and statistical analysis was performed by using IBM SPSS, version 28.0.0.0 (IBM, Armonk, NY). The Mann-Whitney U test was used to compare blood test data between the 2 groups. A level of P less than .05 was defined as significant. This study was conducted in compliance with the Declaration of Helsinki and approved by the ethics committee at Showa University Hospital (approval number 22-183-B).
Results and discussion
Of the 130 OFC results from 65 patients with diagnosed or suspected FPIES, 55 without accompanying blood sampling data were excluded. In all, 75 OFC results from 53 patients were included in the analysis (Table I). Among the cohort of 53 patients, 31 individuals received a definitive diagnosis in accordance with the International Consensus Guideline before OFC, whereas the remaining 24 were categorized as suspected of having FPIES. In 55 cases, blood samples were not collected for various reasons. Specifically, 45 instances corresponded to the second or subsequent OFC, and considering the negative outcome and small dose of the challenge, the attending physician deemed the likelihood of induction of symptoms to be low, thereby rendering blood sampling unnecessary. In addition, in 7 cases there was no parental consent, whereas procedural challenges were encountered in 3 cases. Of the 55 cases, 7 (13%) exhibited a positive outcome in the OFCs. Of that total, the results of 26 OFCs were positive and the results of 49 were negative. The median age of the patients was 12 months (interquartile range [IQR] = 10-17 months) at the time of OFC. Of the cases with a positive result, 12 were mild, 1 was moderate, and 13 were severe.
Table I.
Patient characteristics
| Characteristic | All patients (n = 53) |
All OFC results (n = 75) | Negative OFC results (n = 49) | Positive OFC results (n = 26) | P value |
|---|---|---|---|---|---|
| Male sex, no. (%) | 26 (49) | 37 (49) | 21 (43) | 16 (62) | .124 |
| Age at onset (mo), median (IQR) | 7 (7-8) | ||||
| Age at OFC (mo), median (IQR) | 12 (10-17) | 12 (10-17) | 12 (9-20) | 1.000 | |
| Causative food, no. (%) | .808 | ||||
| Egg | 44 (83) | 60 (80) | 38 (78) | 22 (85) | |
| Milk | 4 (8) | 6 (8) | 5 (10) | 1 (4) | |
| Wheat | 4 (8) | 6 (8) | 4 (8) | 2 (7) | |
| Soy | 3 (6) | 3 (4) | 2 (4) | 1 (4) | |
| Total IgE level (IU/mL), median (IQR) | 24 (9-59) | 27 (10-64) | 22 (10-50) | 59 (22-75) | .084 |
| Allergen-specific IgE level (UA/mL), median (IQR) | 0.37 (0.1-3.89) | 0.6 (0.1-6.63) | 0.34 (0.1-3.62) | 2.16 (0.1-12.4) | .213 |
| History of IgE-dependent food allergy, no. (%) | 3 (6) | 4 (5) | 2 (4) | 2(8) | |
| History of atopic dermatitis, no. (%) | 1 (2) | 1 (1) | 1 (2) | 0 (0) |
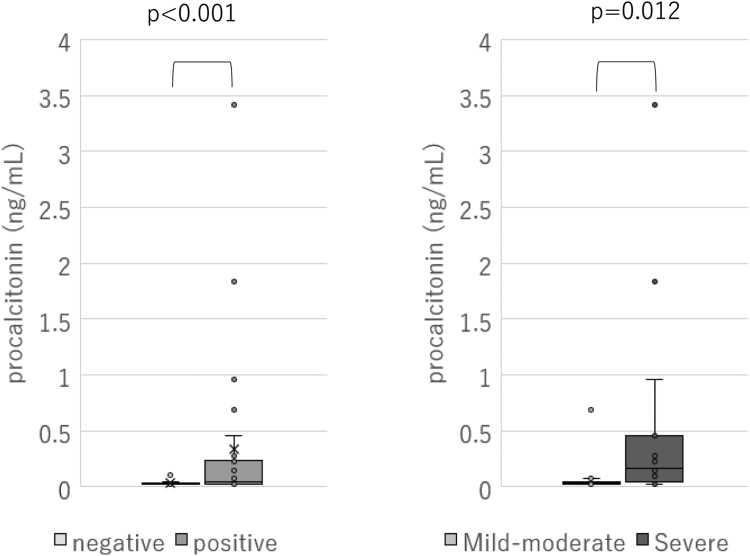
The test results for both groups are presented in Tables II and III. The positive group exhibited significantly higher levels of procalcitonin (ng/mL), with a median level of 0.04 ng/mL (IQR = 0.02-0.21 ng/mL) in the positive group and 0.02 ng/mL (IQR = 0.02-0.03 ng/mL) in the negative group (P < .001) (Fig 1). Moreover, the positive group had significantly higher TARC levels, neutrophil counts, and eosinophil counts, along with lower venous blood pH. However, no significant differences in other parameters were observed between the 2 groups.
Table II.
Comparison of laboratory findings in the positive and negative OFC results
| Indicator | Negative OFC result, median (IQR) (n = 49) |
Positive OFC result, median (IQR) (n = 26) |
P value |
|---|---|---|---|
| Procalcitonin level (ng/mL) | 0.02 (0.02-0.03) | 0.04 (0.02-0.21) | <.001 |
| TARC level (pg/mL) | 787 (585-1032) | 1051(836-1651) | .021 |
| Neutrophil count (/μL) | 3930 (2470-4940) | 6665 (5435-9158) | <.001 |
| Eosinophil count (/μL) | 150 (100-210) | 100 (97-154) | .011 |
| Hematocrit level (%) | 34.9 (32.2-36.8) | 34.3 (33.5-35.8) | .760 |
| Platelet count (104/mm3) | 39.75 (35.0-45.9) | 33.1 (28.1-38.1) | .173 |
| CRP level (mg/dL) | 0.04 (0.04-0.04) | 0.04 (0.04-0.04) | .716 |
| LDH level (U/L) | 299 (275-334) | 299 (272-315) | .388 |
| pH | 7.424 (7.404-7.45) | 7.382 (7.375-7.413) | <.001 |
| HCO3– level (mEq/L) | 20.1 (19.1-21.6) | 18.35 (16.6-21.9) | .074 |
| Methemoglobin level (%) | 0.6 (0.5-0.7) | 0.6 (0.5-0.7) | .425 |
CRP, C-reactive protein; LDH, lactate dehydrogenase.
Table III.
Comparison of laboratory findings between mild or moderate and severe events
| Indicator | Severe, median (IQR) (n = 13) | Mild or moderate, median (IQR) (n = 13) |
P value |
|---|---|---|---|
| Procalcitonin level (ng/mL) | 0.16 (0.04-0.46) | 0.03 (0.02-0.04) | .012 |
| TARC level (pg/mL) | 1623 (1362-1999) | 892 (544-989) | .009 |
| Neutrophil count (/μL) | 6840 (5390-10710) | 6795 (5740-9448) | .551 |
| Eosinophil count (/μL) | 100 (51-152) | 100 (99-208) | .443 |
| Hematocrit level (%) | 35.3 (34.2-37.7) | 33.7 (33.2-34.3) | .039 |
| Platelet count (×104/mm3) | 33.5 (25.9-46.5) | 31.7 (28.8-35.7) | .755 |
| CRP level (mg/dL) | 0.04 (0.04-0.05) | 0.04 (0.04-0.04) | .247 |
| LDH level (U/L) | 278 (267-314) | 302 (298-321) | .060 |
| pH | 7.3785 (7.374-7.393) | 7.396 (7.38-7.42) | .180 |
| HCO3– level (mEq/L) | 18.2 (16.5-20.4) | 19.75 (17.2-22.5) | .539 |
| Methemoglobin level (%) | 0.6 (0.5-0.7) | 0.6 (0.6-0.7) | .346 |
CRP, C-reactive protein; LDH, lactate dehydrogenase.
Fig 1.
Comparison of procalcitonin between OFC-positive and OFC-negative events and between mild or moderate and severe events.
Five hours after ingestion of the causative food, the median procalcitonin level was 0.03 ng/mL (IQR = 0.02-0.04) in the mild or moderate group and 0.16 ng/mL (IQR = 0.04-0.46 ng/mL) in the severe group, with the severe group exhibiting a significantly higher level (P = .012) (Fig 1). Moreover, the positive group had significantly lower levels of hematocrit.
The results of the receiver operating characteristic analysis of procalcitonin levels 5 hours after ingestion of the causative food are illustrated in Fig 2. The area under the curve and cutoff values for procalcitonin were 0.755 and 0.035 ng/mL, respectively. The positive and negative predictive values were 63.0% and 81.2%, respectively.
Fig 2.
Receiver operating characteristic analysis of procalcitonin levels 5 hours after ingestion. AUC, Area under the curve.
Although the pathogenesis of FPIES remains unclear, excessive expression of TNF-α is hypothesized to be one of the etiologic factors.3 There have been reports of heightened levels of TNF-α following the onset of symptoms in patients with FPIES.5 Additionally, this study has shown a correlation between the presence or absence and severity of FPIES symptoms and postemetic procalcitonin levels.
Currently, there is no definitive biomarker for the diagnosis of FPIES, and evaluation of acute-phase symptoms following antigen ingestion by a medical professional is necessary for diagnosis and severity assessment. However, there may be situations in which precise evaluation of acute symptoms is not possible, such as in emergency room or clinic settings, where a physician without specialization in pediatric allergies is handling the case, or when the patient visits his or her family doctor the following day. In these instances, measurement of procalcitonin levels has the potential to provide a quantitative and objective assessment of FPIES diagnosis and severity. However, distinguishing other inflammatory conditions (eg, sepsis, acute gastroenteritis, and other infections) from FPIES by using only a procalcitonin level outside OFC setting would be difficult. Procalcitonin levels should be assessed in conjunction with a meticulous patient interview and comprehensive documentation of the patient's clinical course. Conversely, procalcitonin could serve as a valuable biomarker in the determination of OFC outcomes.
Several limitations of this study must be noted. Procalcitonin levels were measured 5 hours after loading, and it is unclear whether this measurement time is appropriate, as procalcitonin is known to peak 24 hours after a stimulus such as infection.9 Therefore, the measurement time of 5 hours after ingestion of the causative food may have captured the timing of the onset of the rise. Nevertheless, the fact that the groups with a positive OFC result and severe illness exhibited significantly higher values even at 5 hours after ingestion of the causative food does not diminish the significance of the present results. Furthermore, this study's target antigen was limited to chicken eggs, and whether the observed trends hold true for all causative antigens is uncertain. Given the increasing number of FPIES cases in recent years, further case accumulation and prospective studies are warranted in the future.
Disclosure Statement
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Clinical implications.
A correlation between the presence or absence and severity of FPIES symptoms and postemetic procalcitonin levels has been demonstrated. Measurement of procalcitonin levels has the potential to provide a quantitative and objective assessment of FPIES diagnosis and severity.
Supplementary data
References
- 1.Nowak-Węgrzyn A., Chehade M., Groetch M.E., Spergel J.M., Wood R.A., Allen K., et al. International consensus guidelines for the diagnosis and management of food protein–induced enterocolitis syndrome: executive summary—workgroup report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2017;139:1111–1126.e4. doi: 10.1016/j.jaci.2016.12.966. [DOI] [PubMed] [Google Scholar]
- 2.Makita E., Kuroda S., Itabashi K., Sugawara D., Ichihashi K. Evaluation of the diagnostic accuracy of thymus and activation-regulated chemokine to discriminate food protein-induced enterocolitis syndrome from infectious gastroenteritis. Int Arch Allergy Immunol. 2021;182:229–233. doi: 10.1159/000510723. [DOI] [PubMed] [Google Scholar]
- 3.Chung H.L., Hwang J.B., Park J.J., Kim S.G. Expression of transforming growth factor β1, transforming growth factor type I and II receptors, and TNF-α in the mucosa of the small intestine in infants with food protein–induced enterocolitis syndrome. J Allergy Clin Immunol. 2002;109:150–154. doi: 10.1067/mai.2002.120562. [DOI] [PubMed] [Google Scholar]
- 4.Linscheid P., Seboek D., Nylen E.S., Langer I., Schlatter M., Becker K.L., et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology. 2003;144:5578–5584. doi: 10.1210/en.2003-0854. [DOI] [PubMed] [Google Scholar]
- 5.Kimura M., Ito Y., Shimomura M., Morishita H., Meguro T., Adachi Y., et al. Cytokine profile after oral food challenge in infants with food protein-induced enterocolitis syndrome. Allergol Int. 2017;66:452–457. doi: 10.1016/j.alit.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro A., Moreira D., Costa C., Pinto Pais I. Food protein-induced enterocolitis syndrome: a challenging diagnosis. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-222822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jędrzejczyk M., Bartnik K., Funkowicz M., Toporowska-Kowalska E. FPIES induced by locust bean gum in an infant. J Investig Allergol Clin Immunol. 2020;30:197–199. doi: 10.18176/jiaci.0475. [DOI] [PubMed] [Google Scholar]
- 8.Kono I., Okamoto M., Inoue S., Tanaka Y. Markedly elevated procalcitonin in food protein induced enterocolitis Syndrome. Kobe J Med Sci. 2021;67:E7–E9. [PMC free article] [PubMed] [Google Scholar]
- 9.Dandona P., Nix D., Wilson M.F., Aljada A., Love J., Assicot M., et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–1608. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.