Abstract
Background
Infants with respiratory-syncytial virus bronchiolitis hospitalization are more likely to develop wheezing and subsequent asthma. Reportedly, palivizumab prophylaxis effectively prevents respiratory-syncytial virus hospitalization in high-risk children—such as premature infants or infants with bronchopulmonary dysplasia (BPD).
Objective
We sought to explore the effect of respiratory-syncytial virus immunoprophylaxis on the risk of asthma development in premature infants with BPD in subtropical areas.
Methods
This case-control study included preterm children with BPD born at Mackay Memorial Hospital, Taipei, Taiwan, from 1999 to 2015. Overall, medical records of 616 eligible participants were retrospectively collected from their birth to the time they attained an age of 5 to 20 years. The primary outcome was onset of active asthma.
Results
Overall, 576 consecutive cases met the inclusion criteria. Of these, 306 (53.2%) patients had palivizumab exposure and 191 (33.2%) were diagnosed with asthma. Patients with history of respiratory-syncytial virus bronchiolitis hospitalization had a higher risk of developing asthma in the future (adjusted odds ratio, 3.77; 95% CI, 2.30-6.20, P < .001; hazard ratio, 2.56; 95% CI, 1.81-3.62, P < .001). Palivizumab prophylaxis reduced future asthma development through the inhibition of respiratory-syncytial virus bronchiolitis hospitalization (coefficient, −0.021; 95% CI, −0.031 to −0.011, P = .027). Asthmatic children who received palivizumab immunoprophylaxis had a lesser active asthma duration than those who did not (P = .005).
Conclusions
Children with BPD with hospitalization for respiratory-syncytial virus bronchiolitis had higher risk of developing asthma compared with those without respiratory-syncytial virus infection. Prophylactic palivizumab might reduce later asthma development through inhibition of respiratory-syncytial virus bronchiolitis hospitalization. For those already developing asthma, palivizumab could reduce active asthma duration.
Key words: Asthma, palivizumab, RSV bronchiolitis, bronchopulmonary dysplasia, prematurity
Respiratory-syncytial virus (RSV) infection is common globally and accounts for 20% of respiratory tract infections in children younger than 5 years.1,2 The highest incidence of RSV infection–related hospitalization occurs before the age of 6 months.3
Children with RSV bronchiolitis, severe enough for hospitalization, have a higher risk of developing recurrent wheezing, childhood asthma, or allergic rhinoconjunctivitis.4, 5, 6 The influence of RSV on the future development of asthma was reported for children up to the age of 13 years.7
Palivizumab, a potent RSV-neutralizing mAb, can bind to the fusion protein of RSV and prevent its entry into the host cell and subsequently reduce viral replication.8 Palivizumab has been licensed since 1998 for prophylaxis to reduce severe RSV infection in high-risk infants, such as preterm infants with or without bronchopulmonary dysplasia (BPD) and those with hemodynamically significant congenital heart disease, Down syndrome, or neuromuscular disease.1, 2, 3,9
Palivizumab has also been reported to reduce recurrent wheezing in high-risk groups.3 Preterm birth is a risk factor for recurrent wheezing and future asthma development.10,11 Therefore, the effect of palivizumab on decreasing the risk of subsequent asthma development has mostly been focused on the premature population; however, it is not fully clarified. A previous study reported that palivizumab prophylaxis does not change the onset of atopic asthma in Japanese preterm infants at age 6 years.12 In the Netherlands, a single-blind, randomized, placebo-controlled trial showed that RSV prevention in healthy preterm infants did not have a major effect on current asthma at age 6 years.13 However, in Turkey, palivizumab was reported to reduce the risk of asthma in children between the age of 2 and 5 years.14
The RSV season typically lasts for 5 to 6 months globally.15, 16, 17 In Taiwan, RSV infection is prevalent in all 4 seasons; therefore, palivizumab prophylaxis is administered round the year. Taiwan national health insurance policy supports 6 doses of palivizumab for high-risk groups, which is more than those administered in other countries.18
BPD is the most common form of chronic lung disease of prematurity.19 It was reported to be an independent factor that increases the risk of asthma in schoolchildren and adolescents.20
Taiwan has its own palivizumab prophylaxis policy for high-risk children; however, no study has reported on the impact of palivizumab prophylaxis on the future development of asthmatic symptoms. Therefore, this study focused on exploring the impact of RSV immunoprophylaxis on future asthmatic symptoms developing in preterm infants with BPD in Taiwan. Furthermore, we analyzed some antenatal and postnatal factors that might influence the onset of asthma.
Methods
Study design
In this retrospective study, we reviewed the medical records of the participants from January 1999 to December 2015. In total, 616 eligible participants diagnosed with prematurity and BPD were enrolled from the outpatient department and wards in Mackay Memorial Hospital, Taipei, Taiwan. The medical records of all the participants were retrospectively collected up to the age of 5 to 20 years (from birth date to December 2020). This study design was approved by the appropriate ethics review board.
Primary and secondary end points
The primary end point was the onset of physician-diagnosed active asthma. The secondary end points were the onset of prescription of long-term medication for asthma control, active asthma duration, and long-term medication accumulation days in active asthma duration.
Covariates
We analyzed some factors that might affect the onset of asthma, such as fetal growth, palivizumab prophylaxis, RSV bronchiolitis hospitalization history, and antenatal corticosteroid prescription.21,22
Preterm birth was associated with an increased risk of wheezing disorders. The risk was higher among children born as very preterm.23 Therefore, we included gestational age (GA) as a factor for the logistic regression analysis to adjust the impact on the outcome. We also examined various factors, including sex and delivery method (normal spontaneous delivery or cesarean section), that might influence the risk of asthma.24,25
According to the National Institute of Child Health and Human Development and the National Institutes of Health guidelines, the severity of BPD is defined as mild, moderate, or severe. Children with mild BPD need supplementary oxygen for at least 28 days. In children with a GA of more than 32 weeks, the termination of supplementary oxygen is achieved by 56 days postnatal age or before hospital discharge. In children with a GA of less than 32 weeks, the discontinuation of supplementary oxygen is achieved by 56 days postnatal age or hospital discharge.26 In contrast to mild BPD, children with moderate BPD need oxygen during oxygen necessity assessment, and those with severe BPD need ventilator support or higher oxygen supply (≥30%). Therefore, children with a history of oxygen therapy at home indicate higher severity of BPD. After considering the influence of BPD severity on asthma development, the use of home oxygen was also analyzed in our study.
Definition
Active asthma was defined as physician’s diagnosis with prescription of associated medications in the previous 12 months. Active asthma duration was calculated from the first outpatient visit date to the last recorded date, and it was understood to be continuous without interruption in excess of 12 months. Long-term medication included inhaled corticosteroids or leukotriene receptor antagonists (montelukast).
Palivizumab prophylaxis was completed before the age of 1 year and before the diagnosis of asthma. Complete vaccination meant at least 6 doses, and incomplete vaccination meant otherwise.
Fetal growth was as follows: small for GA (<10th percentile birth body weight [BBW]), appropriate for GA (10th-90th percentile BBW), and large for GA (>90th percentile BBW).
Serum total IgE and allergen-specific IgE levels were checked and analyzed using the IMMULITE 2000 (Siemens Healthcare Diagnostics, Inc, Deerfield, Ill). Serum allergen-specific IgE levels were analyzed using 2 kinds of allergen-specific IgE assays—the ImmunoCAP (Thermo Fisher Scientific, Uppsala, Sweden) and the Multiple Allergen Simultaneous Test (MAST) Optigen assay (Hitachi Chemical Diagnostics, Mountain View, Calif).
The ImmunoCAP included 8 kinds of allergen-specific IgE (Dermatophagoides pteronyssinus, D farinae, cat dander, dog dander, cockroach, egg white, milk, and codfish). The MAST Optigen allergen-specific assay contained 36 kinds of allergens. The list included 16 food allergens (citrus mix, corn, wheat, vegetable mix, crab, shellfish mix, shrimp, codfish, pork, beef, milk, brewer’s yeast, soybean, peanut, egg yolk, and egg white) and 20 inhaled allergens (pine mix, cottonwood, eucalyptus, mulberry mix, grass mix, Bermuda grass, ragweed mix, pigweed mix, Alternaria, Aspergillus, Candida, Cladosporium, Penicillium, feather mix, cat dander, dog dander, cockroach mix, house dust mite, D pteronyssinus, and D farinae).
Allergen sensitization was defined as at least 1 positive allergen-specific serum IgE test result (allergen-specific IgE ≥ 0.35 kUA/L) to any food or inhalant allergens mentioned in the medical records. Aeroallergen sensitization was defined as any positive allergen-specific IgE test result to inhaled allergens; food sensitization as any positive allergen-specific IgE test result to food allergens; and mite sensitization as any positive allergen-specific IgE test result to mites.
Selection of participants
Inclusion criteria
Patients were included on the basis of the following criteria: (1) GA of the prematurity group was at least 23 weeks but less than 37 weeks; (2) RSV infection was defined as throat virus culture–proved RSV bronchiolitis with hospitalization records and before the diagnosis of asthma; and (3) prophylactic palivizumab injections were completed before the diagnosis of asthma.
Exclusion criteria
Patients with other comorbidities—such as rhinosinusitis, anomaly of airway, malignant neoplasm, cystic fibrosis, gastroesophageal reflux, and bronchiectasis—were excluded.
Statistical analysis
In total, 616 consecutive cases were recruited from the outpatient department and wards. Binary logistic regression and Cox proportional hazards regression models were used to investigate the effects of all variables on the development of asthma and prescription of long-term medication. The primary analysis focused on exploring the impact of all variables on asthma development.
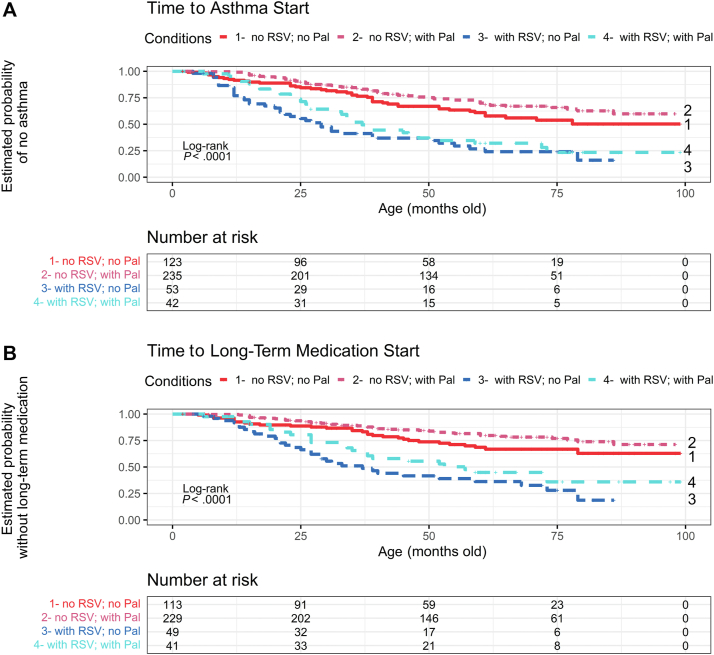
The Cox regression model was used for multivariable analysis. To visualize the time-series events between groups, the Kaplan-Meier curve was used for plotting with the primary end point (onset of asthma) and a secondary end point (onset of long-term medication prescription) as events. The differences between groups were examined using the log-rank test.
For descriptive statistics, the continuous variables were expressed as mean ± SD as appropriate, and the categorical variables were expressed as number of cases and the corresponding percentages.
For univariate analyses, comparisons of independent samples were assessed using the chi-square test or the Fisher exact test for categorical variables as appropriate. For quantitative variable analyses, comparisons of independent samples were assessed using the 2-way ANOVA for categorical variables as appropriate. For ordinal variables, the Mann-Whitney U test was used. Statistical significance was determined at a P value of .05 for all tests.
A model was made for testing the hypothesized mediation effect of RSV infection between palivizumab exposure and the onset of asthma. The model was fit using the structural equation modeling function of the Lavaan package (v0.6-8) using the R program, version 4.2.0 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria). When analyzing causal relationships between palivizumab exposure, RSV bronchiolitis infection, and the onset of asthma, all participants fulfilled the time sequence of palivizumab exposure occurring before RSV infection and RSV infection happening before onset of asthma.
Results
Patient characteristics
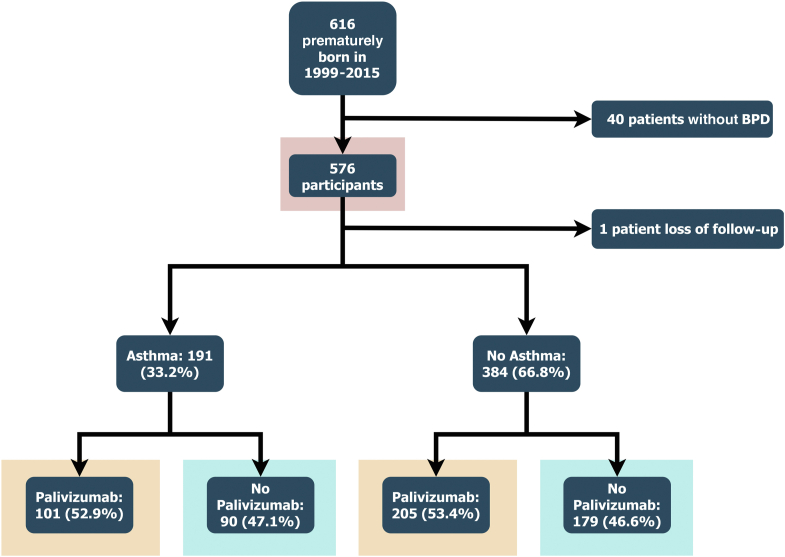
Overall, 616 patients were enrolled initially, and 576 patients were analyzed. Of these, 306 (53.2%) children received palivizumab immunoprophylaxis and 191 (33.2%) patients developed asthma while growing up (Fig 1). Of those diagnosed with asthma, 140 (73.3%) had used long-term medication for asthma control.
Fig 1.
Flow diagram representing the participants enrolled.
Among the 306 children of the palivizumab group, 253 (82.7%) received complete vaccination (at least 6 doses), whereas 53 (17.3%) had incomplete vaccination (1-5 doses). The average palivizumab injection dose was 5.6 per child.
Risk of future asthma development and long-term medication dependence
Children with BPD with histories of RSV bronchiolitis hospitalization had a higher risk of developing asthma after growing up (adjusted odds ratio, 3.77; 95% CI, 2.30-6.20; P < .001; hazard ratio, 2.56; 95% CI, 1.81-3.62; P < .001) (Table I; Fig 2, A). Those with a history of RSV bronchiolitis also had a higher risk of undergoing long-term medication for asthma control (adjusted odds ratio, 4.12; 95% CI, 2.48-6.86; P < .001; hazard ratio, 2.95; 95% CI, 1.98-4.40; P < .001) (see Table E1 in this article’s Online Repository at www.jaci-global.org; Fig 2, B). All these analyses were adjusted by GA, BBW, sex, delivery method, fetal growth, betamethasone prescription history, and home oxygen dependence history.
Table I.
The risk of developing asthma
| Variables | Multiple variables analysis, OR (95% CI) | Adjusted P value | HR (95% CI) | Adjusted P value |
|---|---|---|---|---|
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 0.93 (0.61-1.43) | .745 | 0.92 (0.66-1.28) | .632 |
| GA (wk) | ||||
| <28 | Reference | Reference | ||
| 28-31 | 0.80 (0.44-1.43) | .449 | 0.93 (0.59-1.48) | .771 |
| ≥32 | 0.35 (0.10-1.27) | .111 | 0.52 (0.18-1.55) | .242 |
| BBW (mg) | ||||
| <1000 | Reference | Reference | ||
| ≥1000 | 1.25 (0.70-2.24) | .450 | 1.27 (0.81-1.99) | .299 |
| Fetal growth | ||||
| AGA | Reference | Reference | ||
| SGA | 1.72 (0.90-3.27) | .100 | 1.56 (0.95-2.55) | .078 |
| LGA | 0.83 (0.30-2.29) | .712 | 0.97 (0.45-2.10) | .937 |
| Delivery method | ||||
| CS | Reference | Reference | ||
| NSD | 1.02 (0.64-1.62) | .948 | 0.97 (0.68-1.39) | .876 |
| RSV bronchiolitis hospitalization | ||||
| No | Reference | Reference | ||
| Yes | 3.77 (2.30-6.20) | <.001 | 2.56 (1.81-3.62) | <.001 |
| Palivizumab dose | ||||
| 0 | Reference | Reference | ||
| 1-5 | 1.31 (0.65-2.66) | .453 | 1.19 (0.70-2.03) | .511 |
| ≥6 | 0.87 (0.53-1.43) | .584 | 0.98 (0.67-1.44) | .916 |
| Betamethasone injection | ||||
| No | Reference | Reference | ||
| Yes | 0.83 (0.47-1.48) | .532 | 0.81 (0.52-1.24) | .329 |
| Home oxygen therapy history | ||||
| No | Reference | Reference | ||
| Yes | 1.38 (0.83-2.29) | .217 | 1.31 (0.87-1.97) | .190 |
AGA, Appropriate for gestational age (10th-90th percentile); CS, cesarean section; HR, hazard ratio; LGA, large for gestational age (>90th percentile); NSD, normal spontaneous delivery; OR, odds ratio; SGA, small for gestational age (<10th percentile). Boldface indicates P < .05.
Fig 2.
A and B, Kaplan-Meier plot of time to the onset of physician-diagnosed active asthma (Fig 2, A) or long-term medication prescription (Fig 2, B). Children in condition 1 had no RSV bronchiolitis hospitalization history and did not receive palivizumab. Children in condition 2 had no RSV bronchiolitis hospitalization history but received palivizumab. Children in condition 3 had RSV bronchiolitis hospitalization history but did not receive palivizumab. Children in condition 4 had RSV bronchiolitis hospitalization history and received palivizumab. Participants’ age (months) is indicated on the x-axis. The 4 conditions are statistically different (P < .0001). Pal, Palivizumab.
Causal relationship between palivizumab exposure, RSV bronchiolitis infection, and asthma onset
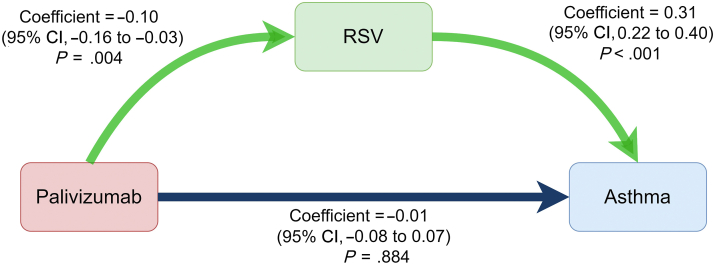
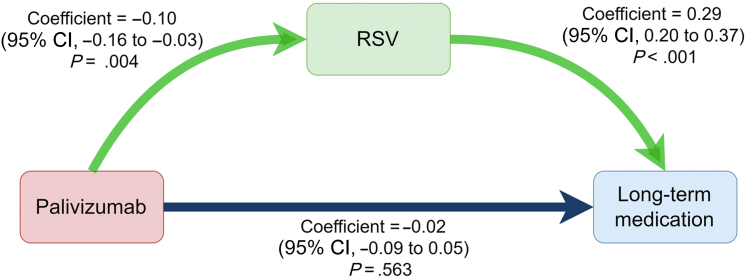
Because palivizumab could decrease RSV bronchiolitis infection rates (coefficient, −0.10; 95% CI, −0.16 to −0.03; P = .004), we found that RSV bronchiolitis infection increased the development of asthma (coefficient, 0.31; 95% CI, 0.22-0.40; P < .001) using linear regression models. Therefore, we used mediation analysis to examine the mediation effect of RSV bronchiolitis infection between palivizumab infusion and asthma development. We found that receiving palivizumab could reduce asthma development through the inhibition of RSV bronchiolitis (coefficient, −0.02; 95% CI, −0.03 to −0.01; P = .027) (Fig 3). We also found that receiving palivizumab could decrease long-term medication use for asthma control by inhibition of RSV infection (coefficient, −0.02; 95% CI, −0.03 to −0.01; P = .023) (see Fig E1 in this article’s Online Repository at www.jaci-global.org).
Fig 3.
The causal relationship between palivizumab exposure, RSV infection, and asthma development. Linear regression models showed that receiving palivizumab could decrease the incidence of RSV bronchiolitis hospitalization (coefficient, −0.10; P = .004) and RSV bronchiolitis hospitalization could increase future asthma development (coefficient, 0.31; P < .001). The direct influence of palivizumab on asthma development is nonsignificant (coefficient, −0.01; P = .884). Mediation analysis showed that palivizumab prophylaxis could decrease the onset of asthma through inhibition of RSV bronchiolitis hospitalization (coefficient, −0.02; P = .027).
Asthma prevalence between palivizumab-unexposed and palivizumab-exposed groups
The prevalence of asthma between the palivizumab-unexposed and the palivizumab-exposed groups showed no significant difference in the participants, the non–RSV hospitalization group, the RSV hospitalization group, and those who needed intensive care (Table II).
Table II.
The relative risk of asthma onset in different groups
| Group | Palivizumab unexposed, asthma/all (%) | Palivizumab exposed asthma/all (%) | RR (95% CI) | P value |
|---|---|---|---|---|
| All participants | 90/268 (33.6%) | 101/306 (33%) | 0.99 (0.82-1.2) | .477 |
| Non–RSV hospitalization group | 51/197 (25.9%) | 70/255 (27.5%) | 1.02 (0.91-1.14) | .396 |
| RSV hospitalization group | 39/71 (54.9%) | 31/51 (60.8%) | 1.15 (0.75-1.76) | .324 |
| RSV hospitalization and ventilator support group | 5/10 (50%) | 2/5 (40%) | 0.83 (0.32-2.15) | .573 |
| RSV hospitalization and intensive care group | 9/17 (53%) | 3/6 (50%) | 0.94 (0.37-2.42) | .635 |
RR, Relative risk.
Comparison of asthmatic children with and without palivizumab exposure
For those who developed asthma later, children had lower RSV bronchiolitis admission rates (P = .048), shorter hospitalization stays (P = .035), lesser need of intensive care (P = .044), and lower allergic rhinitis incidence (P = .028) after palivizumab prophylactic therapy (Table III). Serum total and allergen-specific IgE levels were compared between asthmatic children with and without palivizumab exposure, and no significant difference was found. However, 61 asthmatic children with specific IgE tests showed a lower risk of IgE sensitization to inhaled allergen post–palivizumab exposure (Table IV). The comparison of lung function tests by spirometry in asthmatic children with and without palivizumab administration also showed no significant difference (data not shown).
Table III.
Comparison of asthmatic children with and without palivizumab exposure
| Demographic factors (n = 191) | Palivizumab unexposed (n = 90) | Palivizumab exposed (n = 101) | P value |
|---|---|---|---|
| GA (wk), mean ± SD | 27.23 ± 2.46 | 26.89 ± 2.20 | .314 |
| BBW (mg), mean ± SD | 1046.02 ± 353.45 | 920.62 ± 301.47 | .01∗ |
| Male (%) | 57 of 90 (63) | 53 of 101 (52.48) | .085 |
| Normal spontaneous delivery (%) | 16 of 58 (28) | 37 of 100 (37) | .151 |
| Betamethasone injection (%) | 38 of 50 (76) | 86 of 100 (86) | .099 |
| RSV bronchiolitis hospitalization (%) | 39 of 90 (43) | 31 of 101 (31) | .048∗ |
| Length of RSV hospitalization stays (d), mean ± SD | 9.82 ± 6.35 | 6.50 ± 7.57 | .035∗ |
| RSV hospitalization and intensive care (%) | 9 of 90 (10) | 3 of 101 (3) | .044∗ |
| RSV hospitalization and ventilator use (%) | 5 of 90 (6) | 2 of 101 (2) | .178 |
| Asthma-diagnosed age (mo), mean ± SD | 35.80 ± 30.40 | 37.07 ± 20.74 | .740 |
| Long-term medicine prescription for asthma control (%) | 68 of 90 (76) | 71 of 101 (70) | .258 |
| Allergic rhinitis (%) | 57 of 90 (63) | 49 of 101 (49) | .028∗ |
| Serum total IgE level (IU/mL), mean ± SD | 216.23 ± 343.52 | 171.40 ± 285.35 | .608 |
| Active asthma duration in whole asthmatic children (d), mean ± SD | 895.19 ± 932.46 | 564.93 ± 627.74 | .005∗ |
| Active asthma duration in asthmatic children with RSV bronchiolitis hospitalization history (d), mean ± SD | 999 ± 998 | 595 ± 651 | .046∗ |
| Active asthma duration in asthmatic children without RSV bronchiolitis hospitalization history (d), mean ± SD | 815 ± 880 | 551 ± 621 | .07 |
| Long-term medication prescription days in whole asthmatic children (d), mean ± SD | 560.93 ± 892.31 | 331.76 ± 530.23 | .035∗ |
| Long-term medication prescription days in asthmatic children with RSV bronchiolitis hospitalization history (d), mean ± SD | 671 ± 948 | 428 ± 558 | .186 |
| Long-term medication prescription days in asthmatic children without RSV bronchiolitis hospitalization history (d), mean ± SD | 476 ± 846 | 289 ± 515 | .165 |
P < .05.
Table IV.
Risk of palivizumab to allergen-specific IgE sensitization in asthmatic children
| Sensitization items | OR (95% CI) | P value |
|---|---|---|
| 61 children evaluated by ImmunoCAP or MAST assays | ||
| Any allergen sensitization | 0.74 (0.25-2.17) | .392 |
| Aeroallergen sensitization | 0.51 (0.18-1.46) | .158 |
| Food allergen sensitization | 0.79 (0.27-2.28) | .434 |
| Mite sensitization | 0.64 (0.22-1.81) | .279 |
| 33 children evaluated by MAST assays | ||
| Any allergen sensitization | 0.37 (0.08-1.75) | .183 |
| Aeroallergen sensitization | 0.65 (0.16-2.72) | .410 |
| Food allergen sensitization | 0.40 (0.09-1.75) | .196 |
| Mite sensitization | 0.62 (0.15-2.58) | .381 |
| 30 children evaluated by ImmunoCAP test | ||
| Any allergen sensitization | 0.89 (0.20-3.91) | .590 |
| Aeroallergen sensitization | 0.64 (0.15-2.78) | .410 |
| Food allergen sensitization | 2.73 (0.44-16.75) | .250 |
| Mite sensitization | 0.64 (0.15-2.78) | .410 |
OR, Odds ratio.
Comparison of active asthma duration and long-term medication use in different groups
Children who received palivizumab immunoprophylaxis had a shorter active asthma duration than those who did not receive palivizumab (P = .005), especially post–RSV infection (P = .046). Children with palivizumab exposure had shorter long-term medication accumulation days than those without palivizumab exposure (P = .035) (Table III). When asthmatic children with a history of RSV bronchiolitis grew up to the age of 3, 6, 9, and 12 years, the palivizumab treatment group also had a shorter active asthma duration than the nontreatment group (Table V). We also compared the influence of RSV and palivizumab on active asthma duration using the 2-way ANOVA. Palivizumab significantly reduced the active asthma period (P = .006) in 3-, 6-, 9-, and 12-year-old children, but RSV infection did not. The same comparison was performed for long-term medication accumulation days. Palivizumab significantly decreased the long-term medication dependence days in 3- and 12-year-old children (data not shown).
Table V.
Active asthma duration (d, mean ± SD) in asthmatic children with RSV bronchiolitis hospitalization history with and without palivizumab exposure
| Group | Palivizumab unexposed | Palivizumab exposed | P value |
|---|---|---|---|
| All ages (n = 70) | 999 ± 998 | 595 ± 651 | .046∗ |
| 3 y old (n = 59) | 700 ± 395 | 330 ± 334 | <.001∗ |
| 6 y old (n = 68) | 899 ± 731 | 553 ± 575 | .032∗ |
| 9 y old (n = 70) | 965 ± 898 | 578 ± 616 | .037∗ |
| 12 y old (n = 70) | 999 ± 999 | 595 ± 651 | .046∗ |
P < .05.
Discussion
In the past 40 years, the global prevalence and morbidity associated with childhood asthma have increased.27 From 2008 to 2018, the asthma prevalence in Taiwanese children aged 6 to 12 years was 9% to 11%.28 BPD in infancy is associated with higher respiratory morbidity and increased risk of asthma development in school-age children and adolescents.20 A retrospective cohort study in Taiwan conducted from 1995 to 2001 reported that 51.6% of infants with BPD had childhood asthma at the age of 4.8 to 5.6 years.29 In Hong Kong, from 1987 to 1995, 44% of children who had BPD in infancy developed asthma at the age of 5 years.30 After surfactant administration and also with advanced mechanical ventilator and neonatal care, survival rates of BPD have increased in the past 20 years. The prevalence of asthma in the BPD group has changed. Children with a history of BPD had an asthma prevalence of 31.57% in Korea from 2002 to 2018. The assessed age of asthma was before 18 years.31 This study’s incidence of asthma in the BPD population was similar to that in our report. In our study, 33.2% of participants were found to develop asthma before the age of 20 years from 1999 to 2020.
There are various RSV immunoprophylaxis regimens for preterm infants in the first RSV season. Palivizumab has been licensed for infants born with a GA less than or equal to 35 weeks, with or without BPD. The first guideline for palivizumab immunoprophylaxis was reported by the American Academy of Pediatrics in 1998.32 For preterm infants born with a GA of less than or equal to 28 weeks, palivizumab was recommended for prophylaxis up to 12 months. For those born at 29 to 32 weeks, palivizumab was recommended for up to 6 months.1 According to the latest American Academy of Pediatrics guidelines, palivizumab prophylaxis is suggested for preterm infants with chronic lung disease in the RSV season during the first year of life. In the second year, palivizumab prophylaxis is recommended only for 6 months in preterm infants with chronic lung disease and under medical support during the RSV season.33
RSV infection can be found all year round in Taiwan. This is different from the situation in Europe and America, where RSV prevalence is usually in winter.34 The incidence of RSV infection–related hospitalization is the highest before the age of 6 months, and the peak incidence in Taiwanese is at the age of 1 to 2 months.3 Therefore, Taiwan’s national health insurance policy provides 6 doses of palivizumab for high-risk infants after birth and recommends complete vaccination before the age of 1 year, irrespective of the season. Because of the high cost of palivizumab, most infants receive a maximum of 6 doses of palivizumab usually before the age of 1 year. The number of doses of palivizumab in Europe and the United States is usually fewer than 5 and administered before the child attains the age of 2 years. According to our study, the average number of palivizumab doses received was 5.64 in Taiwan, which is higher than the average number of palivizumab doses in Denmark (5.4) and Sweden (4.2).18 Regardless of the differing conditions in other countries, by using binary logistic regression and Cox proportional hazards regression models, our study showed that palivizumab prophylaxis did not exert a significant impact on future asthma development. The results were similar to those obtained by the studies conducted in Denmark, Sweden, the Netherlands, and the United States.13,18,35 However, several studies have shown that RSV bronchiolitis hospitalization rates were reduced after palivizumab injection and children with a history of RSV bronchiolitis hospitalization had a higher risk of developing asthma in the future.5,6,9,36 In our study, we obtained similar results; therefore, we considered the causal relationship between palivizumab, RSV infection, and asthma development by using the mediation model. We found that palivizumab decreased asthma development via inhibition of RSV bronchiolitis infection in the Taiwanese BPD population. We also found that palivizumab could reduce long-term medication use for asthma control via inhibition of RSV bronchiolitis infection in the same population. Long-term medication dependence in asthmatic children implies higher asthma severity; therefore, RSV bronchiolitis infection plays an important role as a mediator between palivizumab prophylaxis and the development of asthmatic symptoms. Furthermore, a 6-dose regimen of palivizumab prophylaxis might prevent asthma development by inhibition of RSV infection in Taiwan. Taiwan has no obvious RSV seasonality. A 6-dose monthly palivizumab prophylaxis regimen has been implemented for high-risk preterm babies since December 2010. Lin et al37 reported that BPD population with palivizumab prophylaxis had a lower RSV hospitalization rate, shorter hospitalization stays, lesser need of intensive care, and lower mechanical ventilation rate than that without RSV immunoprophylaxis in Taiwan.37 Similarly, our study revealed that asthmatic children with a BPD history post–palivizumab prophylaxis had a lower RSV admission rate, shorter hospitalization stay, and lesser need of intensive care. Ventilator use rate was lower but did not reach statistical significance; therefore, the 6-dose RSV prophylaxis regimen can also help asthmatic children in reducing severity and cost of RSV infection.
Airway epithelial cells of RSV infection could release thymic stromal lymphopoietin, IL-33, high-mobility group box 1, and IL-25, which activate group 2 innate lymphoid cells to produce type 2 cytokine, including the production of IL-5 and IL-13. This type 2 cytokine might promote mucus production and responsiveness of airway.38 IL-5 plays an important role of maturation and survival of eosinophils and basophils. Accumulation of eosinophils in tissue results in edema and bronchoconstriction.39 Pulmonary expression of IL-13 results in mucus hypersecretion, airway obstruction, and nonspecific airway hyperresponsiveness.40 TH2 immune response, airway hyperresponsiveness, and airway narrowing might result in wheezing or increase the severity of asthma. Palivizumab can reduce disease severity and shorten the course of RSV infection. In our study, asthmatic children with previous palivizumab immunoprophylaxis had shorter active asthma duration and lower long-term medication dependence, especially those with a history of RSV infection. Researchers in Korea and Germany have reported that people carrying some variants of IL4/IL13 genes are predisposed to type 2 immune response and highly associated with severe RSV infection and subsequent asthma.41,42 By reducing the RSV infection rate using palivizumab, TH2 cytokines and airway inflammation could possibly be reduced. Therefore, the necessity of acute and chronic medication for wheezing decreased subsequently; however, more studies are needed to validate this.
As a retrospective and single-center study, there were some limitations. There were insufficient lung function data to follow the changes in airway inflammation or enough allergen-specific IgE data to observe kinetic differences in IgE levels.
In our study, we compared IgE sensitization conditions in asthmatic children with a BPD history. Children post–palivizumab exposure had a lower risk of IgE sensitization to inhaled allergens. Although not reaching statistical significances, the trend of decreasing sensitization was similar in the ImmunoCAP test and the MAST assays. We defined asthma by clinical presentation, which may include TH2-high and TH2-low endotypes. Asthma is a heterogeneous disease with various phenotypes. In this study, the number of participants was small and so was not enough for subgroup analysis when analyzing IgE sensitization. We hope more studies are conducted to explore the influence of palivizumab prophylaxis on type 2 immune response, which may help to realize the pathogenesis of RSV infection in asthma.
Because of the use of antenatal corticosteroids and advancement of ventilators, the survival rate of children with BPD increased from 42.3% to 72.6% in infants with extremely low BBW.43,44 Clinicians are likely to observe more asthmatic children with a BPD background. Understanding the benefits and defects of RSV prophylaxis in such high-risk children is critical and important, which might help clinicians in providing care for these children.
Among children with preterm birth and BPD, those hospitalized for RSV bronchiolitis had a higher risk of developing asthma during early childhood and adolescence compared with those without RSV infection. A 6-dose regimen of RSV immunoprophylaxis could protect against asthma development through the inhibition of RSV bronchiolitis infection in Taiwan. RSV immunoprophylaxis reduced the duration of active asthma symptoms and long-term medication use in asthmatic children. Accordingly, RSV immunoprophylaxis might change the onset of asthma through the inhibition of RSV infection and could reduce the severity of asthma.
Having obtained a thorough understanding of RSV immunoprophylaxis, we hope to gain further understanding of whether severe RSV bronchiolitis infection causes the development of asthma in future or that children who develop childhood asthma originally have the tendency to get RSV infection. Larger prospective studies might be needed to ascertain this.
Clinical implications.
Prophylactic palivizumab might reduce later asthma development through the inhibition of RSV bronchiolitis in a high-risk population.
Disclosure statement
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Acknowledgments
We thank Chi-Ling Chen and Wen-Yi Shau of the Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, for statistical counseling.
Supplementary data
fig.
e1
References
- 1.Resch B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum Vaccin Immunother. 2017;13:2138–2149. doi: 10.1080/21645515.2017.1337614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocca A., Biagi C., Scarpini S., Dondi A., Vandini S., Pierantoni L., et al. Passive immunoprophylaxis against respiratory syncytial virus in children: where are we now? Int J Mol Sci. 2021;22:3703. doi: 10.3390/ijms22073703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi H., Chang I.S., Tsai F.Y., Huang L.M., Shao P.L., Chiu N.C., et al. Epidemiological study of hospitalization associated with respiratory syncytial virus infection in Taiwanese children between 2004 and 2007. J Formos Med Assoc. 2011;110:388–396. doi: 10.1016/S0929-6646(11)60057-0. [DOI] [PubMed] [Google Scholar]
- 4.Jartti T., Gern J.E. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140:895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu P., Hartert T.V. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9:731–745. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigurs N., Gustafsson P.M., Bjarnason R., Lundberg F., Schmidt S., Sigurbergsson F., et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 7.Stein R.T., Sherrill D., Morgan W.J., Holberg C.J., Halonen M., Taussig L.M., et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 8.Johnson S., Oliver C., Prince G.A., Hemming V.G., Pfarr D.S., Wang S.C., et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 9.Del Vecchio A., Franco C., Del Vecchio K., Umbaldo A., Capasso L., Raimondi F. RSV prophylaxis in premature infants. Minerva Pediatr. 2018;70:579–588. doi: 10.23736/S0026-4946.18.05300-8. [DOI] [PubMed] [Google Scholar]
- 10.Leps C., Carson C., Quigley M.A. Gestational age at birth and wheezing trajectories at 3-11 years. Arch Dis Child. 2018;103:1138–1144. doi: 10.1136/archdischild-2017-314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaakkola J.J., Ahmed P., Ieromnimon A., Goepfert P., Laiou E., Quansah R., et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–830. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 12.Mochizuki H., Kusuda S., Okada K., Yoshihara S., Furuya H., Simoes E.A.F., et al. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. Six-year follow-up study. Am J Respir Crit Care Med. 2017;196:29–38. doi: 10.1164/rccm.201609-1812OC. [DOI] [PubMed] [Google Scholar]
- 13.Scheltema N.M., Nibbelke E.E., Pouw J., Blanken M.O., Rovers M.M., Naaktgeboren C.A., et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respir Med. 2018;6:257–264. doi: 10.1016/S2213-2600(18)30055-9. [DOI] [PubMed] [Google Scholar]
- 14.Igde M., Kabasakal H., Ozturk O., Karatekin G., Aygun C. Palivizumab prophylaxis, respiratory syncytial virus and subsequent development of asthma. Minerva Pediatr. 2018;70:252–259. doi: 10.23736/S0026-4946.16.04368-1. [DOI] [PubMed] [Google Scholar]
- 15.Stensballe L.G., Devasundaram J.K., Simoes E.A. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22:S21–S32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 16.Haynes A.K., Manangan A.P., Iwane M.K., Sturm-Ramirez K., Homaira N., Brooks W.A., et al. Respiratory syncytial virus circulation in seven countries with Global Disease Detection Regional Centers. J Infect Dis. 2013;208:S246–S254. doi: 10.1093/infdis/jit515. [DOI] [PubMed] [Google Scholar]
- 17.Bloom-Feshbach K., Alonso W.J., Charu V., Tamerius J., Simonsen L., Miller M.A., et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haerskjold A., Stokholm L., Linder M., Thomsen S.F., Bergman G., Berglind I.A., et al. Palivizumab exposure and the risk of atopic dermatitis, asthma and allergic rhinoconjunctivitis: a cross-national, population-based cohort study. Paediatr Drugs. 2017;19:155–164. doi: 10.1007/s40272-017-0215-7. [DOI] [PubMed] [Google Scholar]
- 19.Perez Perez G., Navarro Merino M. Bronchopulmonary dysplasia and prematurity. Short- and long-term respiratory changes [in Spanish] An Pediatr (Barc) 2010;72:79.e1-16. doi: 10.1016/j.anpedi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Perez Tarazona S., Solano Galan P., Bartoll Alguacil E., Alfonso Diego J. Bronchopulmonary dysplasia as a risk factor for asthma in school children and adolescents: a systematic review. Allergol Immunopathol (Madr) 2018;46:87–98. doi: 10.1016/j.aller.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Hung Y.L., Hsieh W.S., Chou H.C., Yang Y.H., Chen C.Y., Tsao P.N. Antenatal steroid treatment reduces childhood asthma risk in very low birth weight infants without bronchopulmonary dysplasia. J Perinat Med. 2010;38:95–102. doi: 10.1515/jpm.2010.002. [DOI] [PubMed] [Google Scholar]
- 22.Tedner S.G., Ortqvist A.K., Almqvist C. Fetal growth and risk of childhood asthma and allergic disease. Clin Exp Allergy. 2012;42:1430–1447. doi: 10.1111/j.1365-2222.2012.03997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Been J.V., Lugtenberg M.J., Smets E., van Schayck C.P., Kramer B.W., Mommers M., et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Yassen A.Q., Al-Asadi J.N., Khalaf S.K. The role of Caesarean section in childhood asthma. Malays Fam Physician. 2019;14:10–17. [PMC free article] [PubMed] [Google Scholar]
- 25.Chowdhury N.U., Guntur V.P., Newcomb D.C., Wechsler M.E. Sex and gender in asthma. Eur Respir Rev. 2021;30 doi: 10.1183/16000617.0067-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naeem A., Ahmed I., Silveyra P. Bronchopulmonary dysplasia: an update on experimental therapeutics. Eur Med J (Chelmsf) 2019;4:20–29. [PMC free article] [PubMed] [Google Scholar]
- 27.Serebrisky D., Wiznia A. Pediatric asthma: a global epidemic. Ann Glob Health. 2019;85:6. doi: 10.5334/aogh.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W.Y., Lin C.W., Lee J., Chen P.S., Tsai H.J., Wang J.Y. Decreasing ten-year (2008-2018) trends of the prevalence of childhood asthma and air pollution in Southern Taiwan. World Allergy Organ J. 2021;14 doi: 10.1016/j.waojou.2021.100538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung Y.L., Hsieh W.S., Chou H.C., Yang Y.H., Chen C.Y., Tsao P.N. Very low birth weight infants with bronchopulmonary dysplasia have higher risk to develop childhood asthma. Clin Neonatol. 2005;12:45–50. [Google Scholar]
- 30.Islam J.Y., Keller R.L., Aschner J.L., Hartert T.V., Moore P.E. Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:134–156. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K., Lee J.Y., Kim Y.M., Kim G., Kim E.H., Lee B.K., et al. Prevalence of asthma in preterm and associated risk factors based on prescription data from the Korean National Health Insurance database. Sci Rep. 2023;13:4484. doi: 10.1038/s41598-023-31558-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 33.American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 34.Obando-Pacheco P., Justicia-Grande A.J., Rivero-Calle I., Rodriguez-Tenreiro C., Sly P., Ramilo O., et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217:1356–1364. doi: 10.1093/infdis/jiy056. [DOI] [PubMed] [Google Scholar]
- 35.Carroll K.N., Gebretsadik T., Escobar G.J., Wu P., Li S.X., Walsh E.M., et al. Respiratory syncytial virus immunoprophylaxis in high-risk infants and development of childhood asthma. J Allergy Clin Immunol. 2017;139:66–71.e3. doi: 10.1016/j.jaci.2016.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garegnani L., Styrmisdottir L., Roson Rodriguez P., Escobar Liquitay C.M., Esteban I., Franco J.V. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst Rev. 2021;11:CD013757. doi: 10.1002/14651858.CD013757.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Y.J., Chung C.H., Chi H., Lin C.H. Six-monthly palivizumab prophylaxis effectively reduced RSV-associated hospitalization rates of preterm infants in a subtropical area: a population-based cohort study. Pediatr Res. 2019;86:628–634. doi: 10.1038/s41390-019-0492-7. [DOI] [PubMed] [Google Scholar]
- 38.Norlander A.E., Peebles R.S., Jr. Innate type 2 responses to respiratory syncytial virus infection. Viruses. 2020;12:521. doi: 10.3390/v12050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenfeder S., Umland S.P., Cuss F.M., Chapman R.W., Egan R.W. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir Res. 2001;2:71–79. doi: 10.1186/rr41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Z., Homer R.J., Wang Z., Chen Q., Geba G.P., Wang J., et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi E.H., Lee H.J., Yoo T., Chanock S.J. A common haplotype of interleukin-4 gene IL4 is associated with severe respiratory syncytial virus disease in Korean children. J Infect Dis. 2002;186:1207–1211. doi: 10.1086/344310. [DOI] [PubMed] [Google Scholar]
- 42.Puthothu B., Krueger M., Forster J., Heinzmann A. Association between severe respiratory syncytial virus infection and IL13/IL4 haplotypes. J Infect Dis. 2006;193:438–441. doi: 10.1086/499316. [DOI] [PubMed] [Google Scholar]
- 43.Latini G., De Felice C., Giannuzzi R., Del Vecchio A. Survival rate and prevalence of bronchopulmonary dysplasia in extremely low birth weight infants. Early Hum Dev. 2013;89:S69–S73. doi: 10.1016/S0378-3782(13)70020-3. [DOI] [PubMed] [Google Scholar]
- 44.Stensvold H.J., Klingenberg C., Stoen R., Moster D., Braekke K., Guthe H.J., et al. Neonatal morbidity and 1-year survival of extremely preterm infants. Pediatrics. 2017;139 doi: 10.1542/peds.2016-1821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.