Abstract
The hippocampus has a well-established role in spatial and episodic memory but a broader function has been proposed including aspects of perception and relational processing. Neural bases of sound analysis have been described in the pathway to auditory cortex, but wider networks supporting auditory cognition are still being established. We review what is known about the role of the hippocampus in processing auditory information, and how the hippocampus itself is shaped by sound. In examining imaging, recording, and lesion studies in species from rodents to humans, we uncover a hierarchy of hippocampal responses to sound including during passive exposure, active listening, and the learning of associations between sounds and other stimuli. We describe how the hippocampus' connectivity and computational architecture allow it to track and manipulate auditory information – whether in the form of speech, music, or environmental, emotional, or phantom sounds. Functional and structural correlates of auditory experience are also identified. The extent of auditory-hippocampal interactions is consistent with the view that the hippocampus makes broad contributions to perception and cognition, beyond spatial and episodic memory. More deeply understanding these interactions may unlock applications including entraining hippocampal rhythms to support cognition, and intervening in links between hearing loss and dementia.
Keywords: Hippocampus, Hearing, Auditory cognition, Sound, Medial temporal lobe, Auditory, Perception
Highlights
-
•
Hippocampus responds to sound during passive exposure, active listening and learning.
-
•
Auditory experience shapes the structure and function of hippocampus.
-
•
Sound can entrain hippocampal oscillations, with potential clinical implications.
-
•
Recording, imaging and lesion studies are reviewed in species from mouse to human.
1. Introduction and motivation
Given the two most well-known functions of the hippocampus – supporting episodic memory in humans (Scoville and Milner, 1957, Squire and Zola-Morgan, 1991) and spatial navigation in animals (O’Keefe and Dostrovsky, 1971) – a review of this structure in relation to sound may seem an unlikely exercise. In humans, cortical circuits underlying auditory perception and cognition are largely found in lateral, rather than medial temporal lobe structures (Bizley and Cohen, 2013, Griffiths and Warren, 2004, Rauschecker and Tian, 2000, Schnupp et al., 2013). Although the hippocampus has access to highly processed information from all sensory modalities, it is often conceptualized as sitting atop a visual cortical hierarchy (Felleman and Van Essen, 1991, Turk-Browne, 2019) and its role in auditory memory in primates has been challenged on anatomical and functional grounds (Fritz et al., 2005, Munoz-Lopez et al., 2010).
However, theories of hippocampal function have long extended beyond episodic memory and spatial navigation (Kimble, 1968, Papez, 1937). One idea is that the hippocampus is important for the binding of arbitrary relations and mediating their flexible expression (Cohen and Eichenbaum, 1993, Lisman et al., 2017). This extended job description encompasses linking multimodal objects with a spatiotemporal, environmental, or cognitive context to form episodic memories (Yonelinas et al., 2019), supporting short-term memory (Hannula and Ranganath, 2008, Pertzov et al., 2013), associating disparate elements of a scene (Graham et al., 2010, Maguire and Mullally, 2013, Olsen et al., 2012), structuring conceptual knowledge (Behrens et al., 2018), and forming predictions (Stachenfeld et al., 2017). Strong versions of such accounts might allow for involvement of the hippocampus in a range of situations involving auditory information, such as binding acoustic features into a perceptual whole, anticipating the continuation of sentences or melodies, and "mental navigation" along sequences of auditory stimuli. We shall see that the computational circuitry of the hippocampus is well suited for operating on information organized in time - such as that carried by acoustic signals. In light of this extended proposed functional scope, a full account of the auditory system should at least consider the hippocampus.
There are also practical and clinical motivations for understanding interactions between sound and the hippocampus. Auditory stimulation can entrain hippocampal rhythms, with implications for enhancing memory and mitigating cognitive decline (Derner et al., 2018, Harrington and Cairney, 2021, Martorell et al., 2019). Auditory signals interact with hippocampal memories, an effect that could be harnessed to boost learning (Cousins et al., 2016, Crowley et al., 2019) or target pathological memories in a clinical setting (Ressler et al., 2021). Hippocampal structure and function are also shaped by experience, raising the question of how auditory expertise and deprivation affect the hippocampus, for example playing a role in tinnitus (Kraus and Canlon, 2012; L. Zhang et al., 2019) or mediating a link between hearing loss and dementia (Griffiths et al., 2020, Livingston et al., 2017). Addressing these issues and realizing therapeutic potential requires a consolidation of knowledge about pathways that carry information between auditory sites and hippocampus.
Recognizing the relative preservation of hippocampal anatomy and physiology across species, and the advances in understanding function this affords (Buffalo, 2015, Clark and Squire, 2013, Cohen and Eichenbaum, 1993, Witter and Amaral, 2021), we include studies from rodents to primates. Electrophysiological and neuroimaging data are considered alongside neuropsychological and animal lesion work. We begin by outlining the anatomy of the hippocampus and the pathways connecting it with canonical auditory structures. We then characterize hippocampal responses to meaningless sounds, going on to consider how these change as sounds signal value or acquire task-relevance. Circumstances under which the hippocampus supports different types of association are set out, with a focus on interactions between processing of sound, time, and space. This leads to a consideration of how the hippocampus might support the formation and retrieval of objects, scenes, and memories that are purely auditory. We cover the special cases of speech, music, emotional sounds and phantom percepts, then set out how auditory experience affects hippocampal structure and function.
Dominant accounts of hippocampal function, as well as key physiological properties, are briefly introduced as required, but readers are referred to detailed reviews (and for a short primer might consult Knierim, 2015). We describe known computational principles of the hippocampus to the extent that they account for auditory data. A key question throughout is to what degree hippocampal involvement in sound processing is secondary to or dependent on its established roles in episodic memory and spatial navigation. Another is the extent to which the hippocampus automatically processes auditory information as opposed to any requirement for the information to be relevant to behavior. We shall find the concept of the hippocampus as a predictive map useful for drawing together some of the findings. However, rather than attempting an integrated theory of the hippocampus through the prism of sound, our aim is to highlight the range of circumstances under which it processes and is shaped by auditory signals. In essence, we are not "claiming" the hippocampus as an auditory structure so much as examining how its computational architecture might be engaged in and altered by auditory tasks.
2. Anatomy and auditory-hippocampal pathways
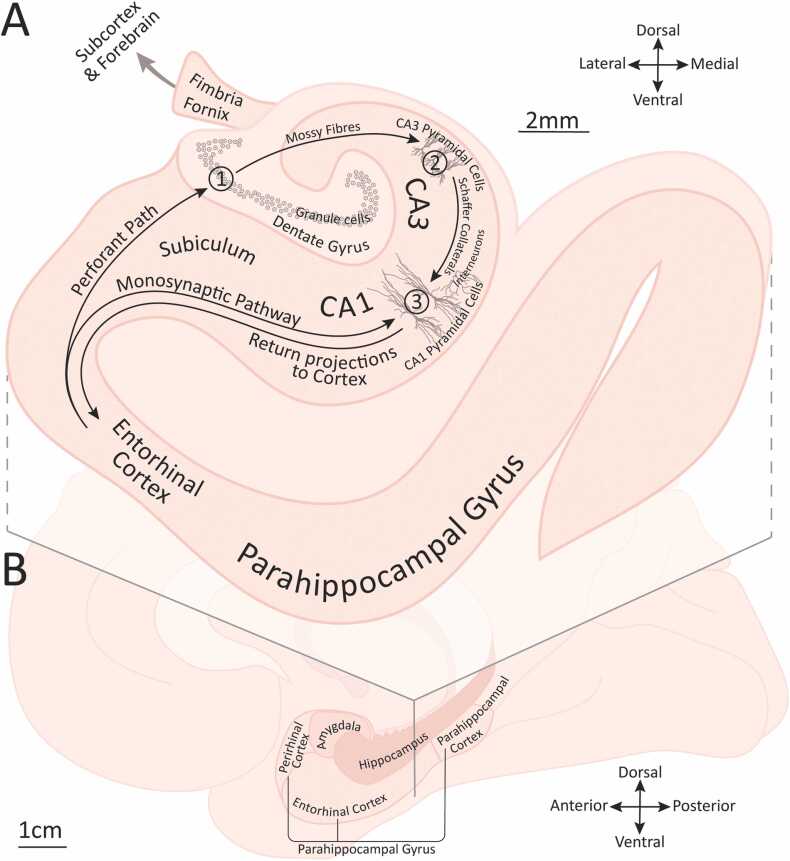
Fig. 1A shows the two interlocking gyri of the hippocampus - the cornu ammonis (including subfields CA1 and CA3) and dentate gyrus (DG) - extending postero-anteriorally in the medial temporal lobe of primates, and dorso-ventrally just below neocortex in rodents. The major cortical input to this bilateral structure is from adjacent entorhinal cortex (ERC), which in primates forms part of the parahippocampal gyrus. A well-described pathway, the trisynaptic loop, projects from ERC through DG, CA3, and CA1 back to ERC, from where output is routed back to neocortex. There are also direct projections from ERC to CA1 (the monosynaptic pathway) and extensive recurrent connections within CA3. The hippocampus is reciprocally connected via the fornix to thalamus, mammillary bodies, and the basal forebrain, as well as to amygdala, basal ganglia, cingulate, and frontal and parietal lobes. We will see later how computations associated with these pathways may be relevant to auditory processing.
Fig. 1.
(A) Coronal cross-section of human medial temporal lobe showing parahippocampal gyrus, entorhinal cortex, hippocampus with primary subfields, and key pathways. Numbers 1–3 indicate synapses of the trisynaptic pathway. Pyramidal cells, interneurons and granule cells not shown to scale. (B) Medial sagittal view of human brain showing medial temporal lobe structures (including amygdala) and indicating the position of the cross-section shown in A.
Along with ERC, the parahippocampal gyrus in primates consists of perirhinal and parahippocampal (postrhinal in rodents) cortices, which connect to ERC from anterior and posterior directions respectively as shown in Fig. 1B (for detailed connections see Burwell and Amaral, 1998; Garcia and Buffalo, 2020; Munoz-Lopez et al., 2010; Nilssen et al., 2019; van Strien et al., 2009 and other anatomical studies in Supplementary Table A). Fellemann & Van Essen (1991) show the hippocampus at the apex of a visual cortical hierarchy, with parahippocampal and perirhinal cortices exchanging information with high-order areas in the ventral visual pathway. The functional anatomy of the primate auditory system is less well mapped than that of vision, at least downstream of primary cortex beyond the lemniscal path from cochlea through the cochlear nucleus, inferior colliculus, and medial geniculate body of the thalamus. A dorsal pathway (sometimes termed a "where" stream due to its role in audiospatial processing) runs from posterior auditory cortex via parietal sites to dorsolateral prefrontal cortex (Rauschecker and Scott, 2016). More ventrally, a "what" stream courses anteriorly along superior temporal gyrus, superior temporal sulcus, and middle temporal gyrus, with features extracted and represented that are increasingly abstract and removed from the acoustic signal. This ventral pathway is often described as terminating in ventrolateral frontal cortex, however, at least in monkeys, additional projections from those anterior temporal sites via the temporal pole reach perirhinal, parahippocampal and entorhinal cortices, which in turn connect to hippocampus (Munoz-Lopez et al., 2015, Munoz-Lopez et al., 2010). Auditory information has multiple opportunities along this series of synapses to be integrated with that from other modalities. There are also somewhat more direct projections from association (belt or parabelt) cortex to entorhinal/perirhinal/parahippocampal cortex in macaques, and from primary cortex to perirhinal and entorhinal cortices in rodents, although these may be sparser for audition than in other sensory modalities (Amaral et al., 1983, Burwell and Amaral, 1998, Munoz-Lopez et al., 2010, Suzuki and Amaral, 1994, Yi et al., 2022). Efferent pathways from the medial temporal lobe trace similar routes back as the afferent connections described (Muñoz and Insausti, 2005, Tranel et al., 1988, Vaudano et al., 1991) but - in rodents at least - are supplemented by others, such as from hippocampus direct to primary auditory cortex (Cenquizca and Swanson, 2007) and even inferior colliculus (Olthof et al., personal communication).
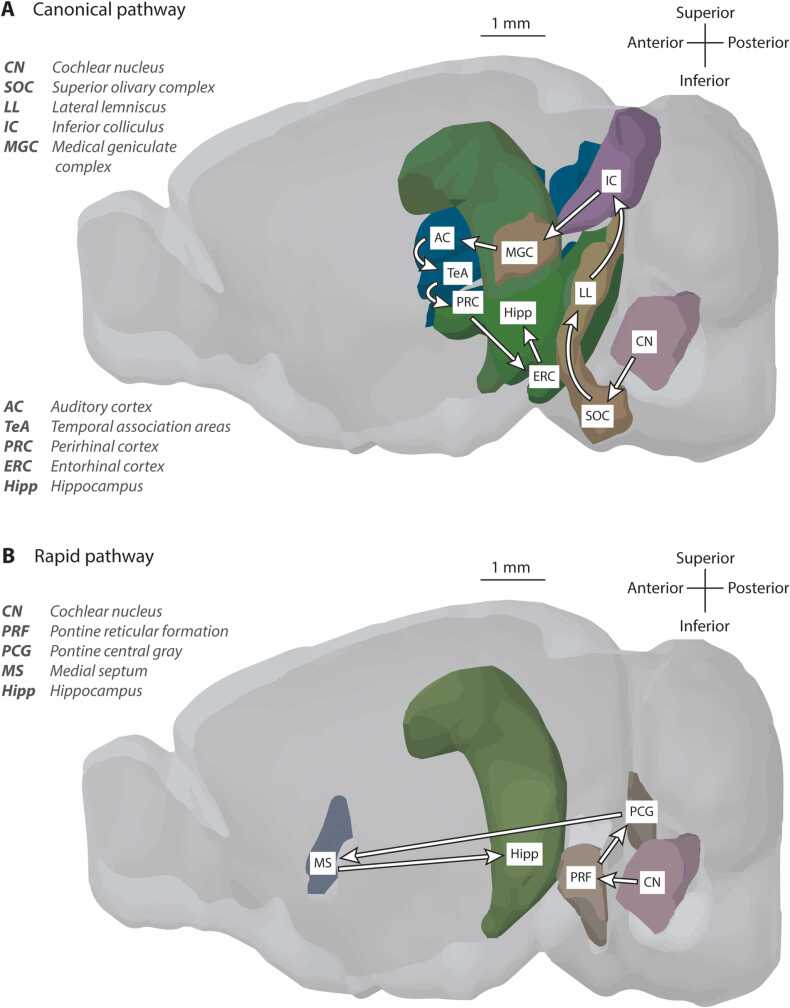
In rodents, subcortical pathways also carry auditory information to the hippocampal formation, including one from cochlear nucleus via pontine nuclei and medial septum (Xiao et al., 2018; G.-W. Zhang et al., 2018), and another from thalamus via basolateral amygdala and entorhinal cortex (Bordi and LeDoux, 1994, LeDoux et al., 1985, Wahlstrom et al., 2018). That these bear auditory information is evidenced by early latency auditory responses at hippocampus and entorhinal cortex, prior to those occurring in auditory cortex, described in Section 3. Anatomical connections from medial septum to entorhinal cortex have also been traced in non-human primates (Insausti et al., 1987). These subcortical pathways may provide fast, indiscriminate communication of the presence of sound, in contrast to slower cortical routes conveying more elaborated representations of a sound and its meaning, including after integrating information from other sensory modalities (Rolls, 1996). See Fig. 2 for two ascending auditory-hippocampus pathways in the mouse, and Kraus and Canlon (2012) for more detail on the interaction between the auditory system and other medial temporal lobe structures.
Fig. 2.
Lateral view of mouse brain showing pathways from auditory brainstem structures to hippocampus. (A) Canonical pathway consisting of at least ten synapses and (B) A rapid five-synapse pathway. Adapted from Allen Reference Atlas - Mouse Brain (available from atlas.brain-map.org).
Establishing the extent to which both these standard and non-canonical pathways in rodents and non-human primates are mirrored in humans is difficult. In the absence of axonal tract tracing or post-mortem studies, indirect measures of structural connectivity such as diffusion tensor imaging (DTI) can be informative. A neurosurgical atlas based on data from the human connectome project highlighted the absence of direct connections between medial and lateral temporal structures, although indirect pathways were functionally established that correspond to some of those outlined in non-human primates, such as from auditory association areas on the superior temporal gyrus to posterior parahippocampal fields then entorhinal cortex (Baker et al., 2018). One ultra-high-resolution DTI study found white matter tracts between hippocampus and both the temporal pole and planum polare, but not low-level auditory cortex (Maller et al., 2019). Another identified connections between hippocampus and a region of interest that included both auditory core and belt areas (Jang and Choi, 2022) in a majority of subjects. Differences across the results of these studies may relate to the thresholds used in the probabilistic tractography procedure.
Complementing structural approaches like DTI that identify anatomical tracts, functional-connectivity analysis defines correlated time series between areas, from which direct or indirect connections can be inferred. Such analysis of resting-state blood-oxygen-level-dependent (BOLD) activity measured with functional magnetic resonance imaging (fMRI) has revealed distinct connectivity between different parts of the hippocampus and neocortical regions. Whereas activity in posterior hippocampus and parahippocampal cortex correlates with activity in lateral parietal cortex and midline sites, activity in anterior hippocampus and perirhinal/entorhinal cortex correlates with that in lateral temporal regions including superior temporal gyrus extending to temporal pole (Kahn et al., 2008; S.-F. Wang et al., 2016). Clustering of more temporally-resolved functional connectivity patterns derived from intracranially recorded high-frequency resting state activity found anterior and medial temporal sites, including hippocampus, to have the strongest coupling with auditory cortex (Banks et al., 2022). While that finding included primary auditory cortex, other resting state intracranial and fMRI studies of connections between medial temporal and sensory cortex emphasize those between hippocampus and association areas in humans, with the possible exception of olfaction, compared to the primary sites that dominate in rodents (Bergmann et al., 2016, Zhou et al., 2021).
Electrophysiological and fMRI studies coupled with electrical or optogenetic stimulation also reveal pathways relevant to auditory processing. The hippocampus orchestrates activity across cortex, propagating theta oscillations (4–7 Hz in rodents) for temporal control of information processing, and sharp-wave ripples for memory consolidation (Buzsáki, 2015, Buzsáki, 2002; see Section 8). For example, neurons in guinea pig inferior colliculus and auditory cortex phase lock to hippocampal theta both during spontaneous firing and in response to sound (Liberman et al., 2009, Pedemonte et al., 2001, Pedemonte et al., 1996). In cats, brief electrical stimulation of dorsal hippocampus at a theta rate enhances auditory cortical responses to subsequent clicks (Parmeggiani and Rapisarda, 1969). Beyond theta, optogenetic stimulation at lower (1 Hz; Chan et al., 2017) and higher (40 Hz; Weitz et al., 2015) rates in rat hippocampus influences BOLD activity in auditory cortex. Electrical stimulation at even faster rates in rabbits led to an increase in the amplitude of click responses in motor cortex (Cazard and Buser, 1963), while other studies in cats found electrical stimulation of hippocampus leading to reduced auditory cortical responses to brief medial geniculate body electrical pulses (Redding, 1967), and reduced click responses in cerebellum (Fox et al., 1967) and hypothalamus (Feldman and Dafny, 1968). Single-pulse electrical stimulation of hippocampus even in the absence of an auditory stimulus elicits rapid responses in the auditory cortex not only of cats (Parmeggiani and Rapisarda, 1969) but also of humans. In the latter case these occur not only in auditory association areas on the lateral temporal lobe (Catenoix et al., 2011, Enatsu et al., 2015) but also primary auditory cortex in Heschl's gyrus, with initial responses as early as 10 ms (Rocchi et al., 2021). These electrical and optogenetic stimulation studies provide further evidence for anatomical and functional links from hippocampus to auditory cortex.
The influence of the hippocampus on auditory processing elsewhere is also revealed by lesion studies in animals, and in patients. For example, the neonatal ventral hippocampal lesion rat, a model for neurodevelopmental aspects of schizophrenia, shows altered responses to sound in inferior colliculus and auditory cortex compared to controls, such as reduced power of the 40-Hz auditory steady state response (ASSR) (Li et al., 2018, Macedo et al., 2010, Vohs et al., 2012, Vohs et al., 2010, Vohs et al., 2009). ASSRs are abnormally lateralized in in medial temporal lobe epilepsy patients (Matsubara et al., 2018, Shigeto, 2021), who also have reduced magnetic evoked responses to pure tones in auditory cortex ipsilateral to the hippocampal sclerosis (Chatani et al., 2016, Matsubara et al., 2018). Finally, pharmacological and chemogenetic shutdown of projections from dorsal CA1 via medial entorhinal cortex affects the amplitude and latency of the mismatch negativity response in mouse auditory cortex (Yi et al., 2022). See Supplementary Tables A and B for other relevant physiological studies.
In sum, multiple pathways are available for auditory information to reach the hippocampus, and for the hippocampus in turn to influence activity at canonical auditory structures. Although the most direct have so far only been anatomically verified in rodents, some electrophysiological studies hint at their presence in humans.
3. Sound responses in the absence of a task
Sounds that hold no meaning for a passively listening animal elicit a number of forms of hippocampal response (see Fig. 3 for a selection and Supplementary Table C for a more complete list). The prominent theta component of hippocampal electroencephalographic (EEG) activity has been variously associated with exploratory movement, memory encoding and retrieval, and arousal, but since the earliest hippocampal recordings in anaesthetized rabbits and cats (Green and Arduini, 1954, Jung and Kornmüller, 1938) as well as in awake animals (Eidelberg et al., 1959, Irmiš et al., 1970), increases in theta power have also been observed in response to meaningless stimuli such as clicks (Fig. 3A). Pure tone presentation can also reset the phase of ongoing hippocampal oscillations (Başar et al., 1979a, Başar et al., 1979b, Başar and Demiralp, 1995, Demiralp et al., 1996, Abe et al., 2014a) in the absence of any task. Such resets may contribute to deflections in evoked hippocampal local field potentials that have been described in anesthetized or passively listening animals (Başar and Özesmi, 1972, Başar and Ungan, 1973, Brankačk and Buzsáki, 1986, Green and Adey, 1956, Hall and Borbely, 1970, Liberson and Cadilhac, 1953, O’Connor et al., 1992, Ungiadze, 1967) and humans (Rosburg et al., 2007).
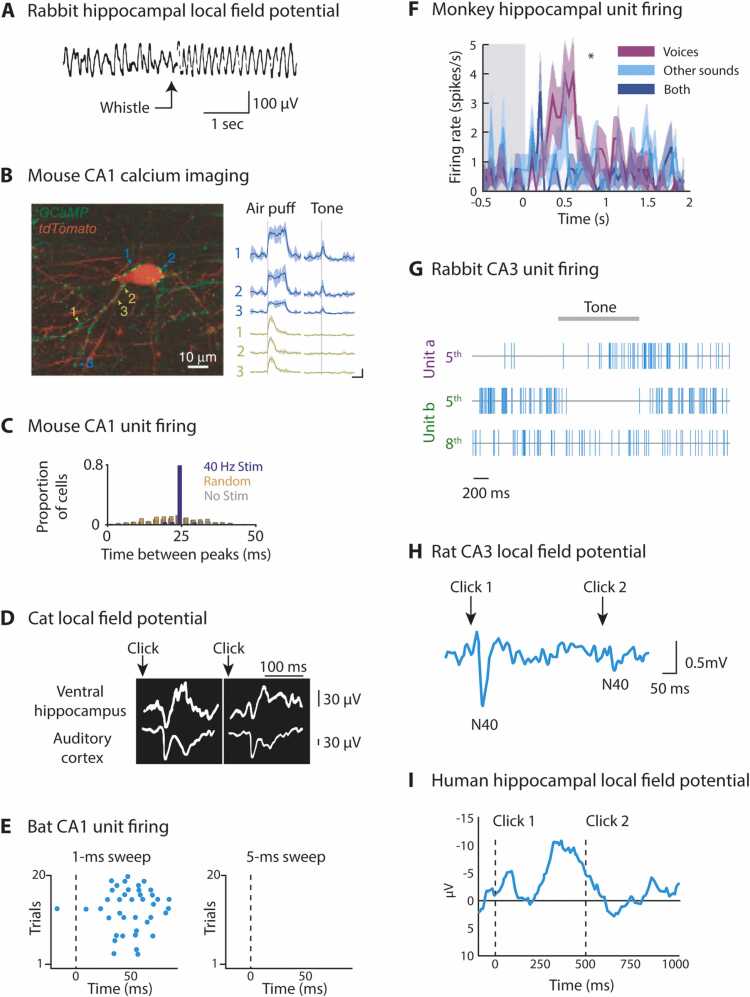
Fig. 3.
Selection of hippocampal responses to sound in the absence of a task. (A) Bilateral LFP responses in rabbit hippocampus to a whistle. Adapted from Green, J.D., Arduini, A.A., 1954. Hippocampal electrical activity in arousal. J. Neurophysiol. 17, 533–557. Green and Arduini (1954). (B) Left: Postsynaptic CA1 interneurons expressing tdTomato (red) and septo-hippocampal GABA axons expressing GCaMP5 (green) in mouse hippocampus with six labelled boutons. Right: Stimulus-triggered Ca2+ averages (+/- SEM) at the same six boutons in response to air-puffs or a 20-s 10-kHz tone. Scale bars show 50% ΔF/F and 3 s. Adapted by permission from Springer Nature Customer Service Centre GmbH: Springer. Nature Neuroscience. Septo-hippocampal GABAergic signaling across multiple modalities in awake mice. Kaifosh, P., Lovett-Barron, M., Turi, G.F., Reardon, T.R., Losonczy, A., 2013. Nat. Neurosci. 16, 1182-1184. Copyright © 2013 Nature America, Inc. Kaifosh et al. (2013). (C) Intervals between firing rate peaks in 338 CA1 cells in 5 mice during 40 Hz click stimulation (blue), random-interval click stimulation (orange) and no stimulation (gray). Reprinted from Cell, 177, Martorell, A.J., Paulson, A.L., Suk, H.-J., Abdurrob, F., Drummond, G.T., Guan, W., Young, J.Z., Kim, D.N.-W., Kritskiy, O., Barker, S.J., Mangena, V., Prince, S.M., Brown, E.N., Chung, K., Boyden, E.S., Singer, A.C., Tsai, L.-H, Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition, 256-271, Copyright © 2019 Elsevier Inc., with permission from Elsevier. Martorell et al. (2019). (D) Similar-latency LFP responses in cat ventral hippocampus and auditory cortex. Responses to two successive clicks separated by 15 s are shown side by side. Reprinted from Electroencephalography and Clinical Neurophysiology, 8, Green, J.D., Adey, W.R., Electrophysiological studies of hippocampal connections and excitability, 245-262, Copyright © 1956 Published by Elsevier Ireland Ltd., with permission from Elsevier. Green and Adey (1956). (E) Spike rasters for a single cell in bat CA1 show selective responses to frequency sweeps of 1-ms duration (left) but not 5-ms duration (right) duration presented at 0 ms. Adapted with permission from Yu, C., Moss, C.F., 2022. Natural acoustic stimuli evoke selective responses in the hippocampus of passive listening bats. Hippocampus 32, 298-309. Copyright © 2022 The Authors. Hippocampus published by Wiley Periodicals LLC. Yu and Moss (2022). (F) Mean firing rate (+/- SEM) of single cell in monkey hippocampus in response to voices and other sounds. Adapted from Sliwa, J., Planté, A., Duhamel, J.-R., Wirth, S. Independent neuronal representation of facial and vocal identity in the monkey hippocampus and inferotemporal cortex. Cerebral Cortex, 2014, 26, 950-966, by permission of Oxford University Press. Sliwa et al. (2014). (G) Spike trains of rabbit CA3 neurons. Top: Activatory response at Unit A to 5th presentation of a 900 Hz tone. Middle/Bottom: Responses at Unit B to 5th and 8th presentations of an 800 Hz tone, showing suppression that habituates over trials. After Vinogradova (1975a). (H) Grand average evoked LFP response from CA3 in 12 rat hippocampi to pairs of clicks presented 500 ms apart. Reprinted from Biological Psychiatry, 27, Bickford-Wimer, P.C., Nagomoto, H., Johnson, R., Adler, L.E., Egan, M., Rose, G.M., Freedman, R., Auditory sensory gating in hippocampal neurons: A model system in the rat, 183-192, Copyright © 1990 Published by Elsevier Inc., with permission from Elsevier. Bickford-Wimer et al. (1990). (I) Grand average evoked response recorded intracranially in 21 human posterior hippocampi to clicks presented at 0 and 500 ms (dashed lines). Adapted with permission from Boutros, N.N., Mears, R., Pflieger, M.E., Moxon, K.A., Ludowig, E., Rosburg, T. Sensory gating in the human hippocampal and rhinal regions: Regional differences. Hippocampus 18, 310-316. Copyright © 2007 Wiley-Liss, Inc. Boutros et al. (2008).
Different classes of hippocampal cells can be distinguished based on anatomy and physiology, including the relationship of their firing to ongoing hippocampal theta oscillations (Ranck, 1973). Inhibitory interneurons tend to fire with a consistent theta phase at a rate that can increase or decrease in response to meaningless sound (Miller and Freedman, 1995, Vinogradova, 2001). Such responses have been imaged at the level of individual synaptic boutons in mouse CA1 that receive GABAergic projections from the septum (Kaifosh et al., 2013; Fig. 3B). In contrast, principal pyramidal cells show occasional burst firing with no fixed relationship to theta phase. Although their most famous behavioral correlate is physical location (“place cells”, O’Keefe and Dostrovsky, 1971; see Section 8), hippocampal pyramidal cells also respond to pure tones, artificial vowels, and noise, even in the absence of a task (Miller and Freedman, 1995, Vinnik et al., 2012).
Hippocampal responses to sound can be brief, persist throughout or beyond the stimulus, or be phasically modulated by its temporal structure (Lidsky et al., 1974, Martorell et al., 2019; Fig. 3C). Although responses in hippocampus typically follow in auditory cortex, this is not always the case (Green and Adey, 1956; L. Zhang et al., 2019; Fig. 3D). This indicates that subcortical hippocampal afferents described earlier may convey the presence of sound, a proposition further supported by the fact that responses of some medial septal neurons to behaviorally irrelevant sounds precede periods of elevated hippocampal and theta and gamma power (Zhang et al., 2011) and that medial septal inactivation reduces auditory responses in certain hippocampal subfields (Xiao et al., 2018). The medial septal pathway may be particularly responsive to high-intensity sounds in mice (Abe et al., 2014b, Kaifosh et al., 2013). Given that temporal windows of integration increase along the lemniscal pathway to primary auditory cortex and thence through the cortical hierarchy (Baumann et al., 2015, Dheerendra et al., 2021, Joris et al., 2004, Nourski et al., 2009), the alternate subcortical routes may also be those that carry rapid temporal modulations to hippocampus (Arnal et al., 2019, Chan et al., 2021, Martorell et al., 2019).
Many of the hippocampal responses described above, whether at the single cell or evoked potential level, are not specific to particular sounds but rather scale with intensity. Because the hippocampus is involved in a range of cognitive and spatial processing, such unselective responses are hard to attribute to auditory processing per se rather than to general arousal or orienting. However, there are exceptions to this lack of selectivity during passive exposure: Brown and Buchwald (1973) describe stable hippocampal tuning to tone frequency in cats, Yu and Moss (2022; Fig. 3E) report duration tuning in bat CA1, and Sliwa et al. (2014; Fig. 3F) find voice-selective responses in the hippocampus of monkeys - in all cases the animals were listening passively. Most single cell and evoked potential recordings during passive listening will have been insensitive to any fine-grained population coding. Calcium imaging allowing simultaneous mapping of activity of large numbers of neurons has identified robust and stable but sparse responses to passively presented odors in mouse dentate gyrus (Woods et al., 2020), but has rarely been used to detect auditory hippocampal responses.
A key factor influencing the magnitude of hippocampal responses to sound in the absence of behavior is stimulus history. Although habituation to a repeated auditory stimulus is a widespread neural phenomenon (Bickford et al., 1993, Miller and Freedman, 1993, Moxon et al., 1999, Picton et al., 1976), it is particularly pronounced in hippocampus, at least for behaviorally irrelevant input in rodents and rabbits (Bickford-Wimer et al., 1990, Vinogradova, 1975b). Both firing rate changes (Vinogradova et al., 1970; Fig. 3G; Vinogradova, 1975b, Vinogradova, 2001) and evoked responses (Bickford-Wimer et al., 1990, Ehlers et al., 1994, Kaneko et al., 1993, Ruusuvirta et al., 1996; Fig. 3H) reduce in magnitude with each repetition. In these studies, a striking reduction in sound responses occurs after only one sound. Many rodent studies have focused on the neurochemical basis of the reduced hippocampal response to the second in a pair of stimuli separated by a 500-ms interval, because impairment of such “sensory gating” at the scalp is associated with various human psychiatric disorders (for a review see Cromwell et al., 2008). An intact hippocampus is also important for pre-pulse inhibition of the acoustic startle response - an important behavioral correlate of sensory gating (Inta et al., 2014, Kemble and Ison, 1971; see also Supplementary Table H). But habituation effects can also extend for much longer than the intervals in these gating studies – for several seconds in rats (Mays and Best, 1975) or even minutes in rabbits (Vinogradova et al., 1970).
Selective habituation to identical stimuli gives rise to a neural indicator of the presence of novelty. In many species, any perceptible change in stimulus after repetition can be sufficient to restore a large hippocampal response, with a greater effect for larger differences and more rarely occurring sounds (Başar-Eroglu et al., 1991, Csépe et al., 1989, Ruusuvirta et al., 2013, Vinogradova, 2001). Although differences between passively heard repetitive auditory standards and deviants have been detected in human hippocampus (Fuhrer et al., 2021, Herdener et al., 2010, Rosburg et al., 2007, Zevin and McCandliss, 2005; Fig. 3I) these diminish over the course of an experimental session, possibly reflecting that at the level of hippocampal processing deviant sounds become less unexpected (Rosburg et al., 2007). Gross hippocampal novelty responses during passive listening are unlikely to directly contribute to the human mismatch negativity response recorded at the scalp, which has predominantly superior temporal and inferior frontal generators (Näätänen, 1990). However, the amplitude and latency of the mismatch negativity in mouse auditory cortex have recently been found to depend on a circuit via entorhinal cortex and the hippocampal trisynaptic loop (Yi et al., 2022). In the context of vision, Kumaran and Maguire (2007) identified that the novelty not of an individual stimulus but rather of associations between stimuli is particularly important in driving hippocampal activity. We will come to the role of hippocampus in representing or forming associations between sounds and other sounds, images, and locations in subsequent sections. But first we consider how this structure is involved when animals learn to associate a sound with value in appetitive or aversive conditioning.
4. Activity during conditioning to sound
Training animals to associate a particular sound (conditioned stimulus) with a subsequent reward or punishment (unconditioned stimulus) elicits changes in hippocampal activity over the course of learning (see Supplementary Table D). Different hippocampal synapses are modified in strength through long term potentiation (for a review see Gruart et al., 2015), leading to changes in firing rates that differentiate sounds that have been associated with value from those that have not (Berger et al., 1976, Disterhoft and Segal, 1978, Klee et al., 2021, Olds and Hirano, 1969). Such differential responses can arise between tones of different frequencies, or between more complex sounds such as artificial vowels with different formant structure (Itskov et al., 2012). Conditioning correlates are observed not only in firing rates of hippocampal cells, but also in local field potentials. For example, in rat dentate gyrus the amplitude of a late sustained component increases as the animal learns the association between a tone and water reward (Deadwyler et al., 1985). This contrasts with an earlier component, the magnitude of which depends on the identity of multiple preceding stimuli (whether reinforced or not), consistent with habituation effects described in Section 3. Theta activity also accompanies different phases of learning (Adey et al., 1960, Berry and Seager, 2001, Grastyán et al., 1959, Hoffmann et al., 2015) and can itself affect the success of conditioning (Berry and Seager, 2001, Hoffmann et al., 2015).
The temporal pattern of firing that develops during conditioning may reveal something of what is learned. During eyeblink conditioning, animals learn to associate a tone with a subsequent air-puff (Berger et al., 1976). As the animal begins to acquire the conditioned response – blinking just prior to the air-puff – the timecourse of hippocampal pyramidal cell firing comes to resemble the motor response, occurring progressively earlier than it as conditioning progresses (Berger et al., 1980). However, hippocampal responses and motor behavior do not always correspond; during extinction or learning of new associations, firing patterns in some units may reflect stimulus contingencies that are not shown in behavior (Berger and Thompson, 1982, Hoehler and Thompson, 1979, Laroche et al., 1987). Nor does the appearance of hippocampal firing require there to be an overt conditioned movement (Hirano and Yamaguchi, 1985).
Although most hippocampal recordings during conditioning with auditory stimuli have been made in rats and rabbits, some of the principal findings have been replicated in cats (Patterson et al., 1977). Humans show increased metabolic and hemodynamic activity in regions including hippocampus during eyeblink conditioning compared to when sounds and air-puffs are presented unpaired – this activity correlates weakly with learning (Blaxton et al., 1996, Cheng et al., 2008, Logan and Grafton, 1995).
Despite these extensive learning-related changes in neural activity, animals with hippocampal damage are not impaired in many conditioning settings (Berger and Orr, 1983, Berger and Orr, 1982, Brady et al., 1954, Brady and Hunt, 1955, Ross et al., 1984, Schwartzbaum et al., 1964; see Supplementary Table H). Multiple brain areas are involved during learning, and when a conditioned stimulus is sufficiently loud (Wu et al., 2013) and the unconditioned stimulus sufficiently close in time (Beylin et al., 2001), the essential neural circuitry lies elsewhere. For example the cerebellum is critical for eye-blink conditioning (Daum et al., 1993, McCormick et al., 1982, Thompson, 2005), the amygdala for fear conditioning (Phillips and LeDoux, 1994), and the striatum for appetitive conditioning (Cole et al., 2017). An intact hippocampus appears most important when there is a silent interval to be bridged between conditioned and unconditioned stimulus (Clark and Squire, 1998, Solomon et al., 1986; see Supplementary Table H), as well as when conditioned responses are to be constrained, such as by spatial context or configurations of cues - especially if such configurations are to be learned incidentally and rapidly (Rudy, 2009). Subsequent sections build on some of these points to detail the role of hippocampus in temporal, sequential and spatial aspects of auditory processing. First, we consider some further aspects of hippocampal responses to sounds that have been associated with value or become pertinent to a task.
5. Activity during task-based listening
Once sounds have acquired behavioral relevance, hippocampal activity can be examined as animals perform tasks relating to them (see Supplementary Table E). For example, in rodents trained to press a lever when they detect rare frequency deviants in a train of standard sounds, late (250–500 ms post onset) differences in local field potentials to targets versus standards are particularly pronounced (Brankačk et al., 1996, Ehlers et al., 1994, Hattori et al., 2010, Shin, 2011, Shinba, 1999, Shinba et al., 1996). Other signatures of target detection include induced theta and gamma power increases (Shin, 2011) as well as more complex firing patterns in pyramidal cells than arise during standard tones (Gao et al., 2010). In one study, late firing increases occurred for targets but not standards, regardless of target intensity. This was in contrast to an earlier (~40 ms) peak that occurred for all tones and scaled with intensity (Shinba, 1999) and the dominance of stimulus intensity as a determinant of firing rate in the passive listening studies described earlier. For some units in the study by Shinba (1999), the degree of late firing activity correlated with both the amplitude of the late LFP response and how quickly behavioral responses were made.
In human subjects, behavioral relevance can be instilled in the absence of explicit reward through task instructions, such as to count particular target sounds or to make certain judgments. By having subjects report target counts at the end of a trial or block, explicit motor confounds are removed. In contrast to the passive listening case in humans described earlier, active detection of rare frequency (Altafullah et al., 1986, Halgren et al., 1995, Halgren et al., 1980, Kropotov et al., 2000, Kropotov et al., 1995, McCarthy et al., 1989, Smith et al., 1990, Smith et al., 1986, Stapleton et al., 1987) or intensity (Velasco et al., 1986) targets generates large hippocampal LFP deflections with peaks between 260 and 500 ms (Fig. 4A). These are also accompanied by changes in local unit activity (Halgren et al., 1980, Heit et al., 1990) and may contribute to the P3/P300 scalp component (Fonken et al., 2019, Sutton et al., 1965). Some fMRI studies also show increased hippocampal BOLD for rare target sounds (Crottaz-Herbette et al., 2005, Yoshiura et al., 1999) or when listening to changes in pitch rather than identifying particular pitches (Schwenzer and Mathiak, 2011).
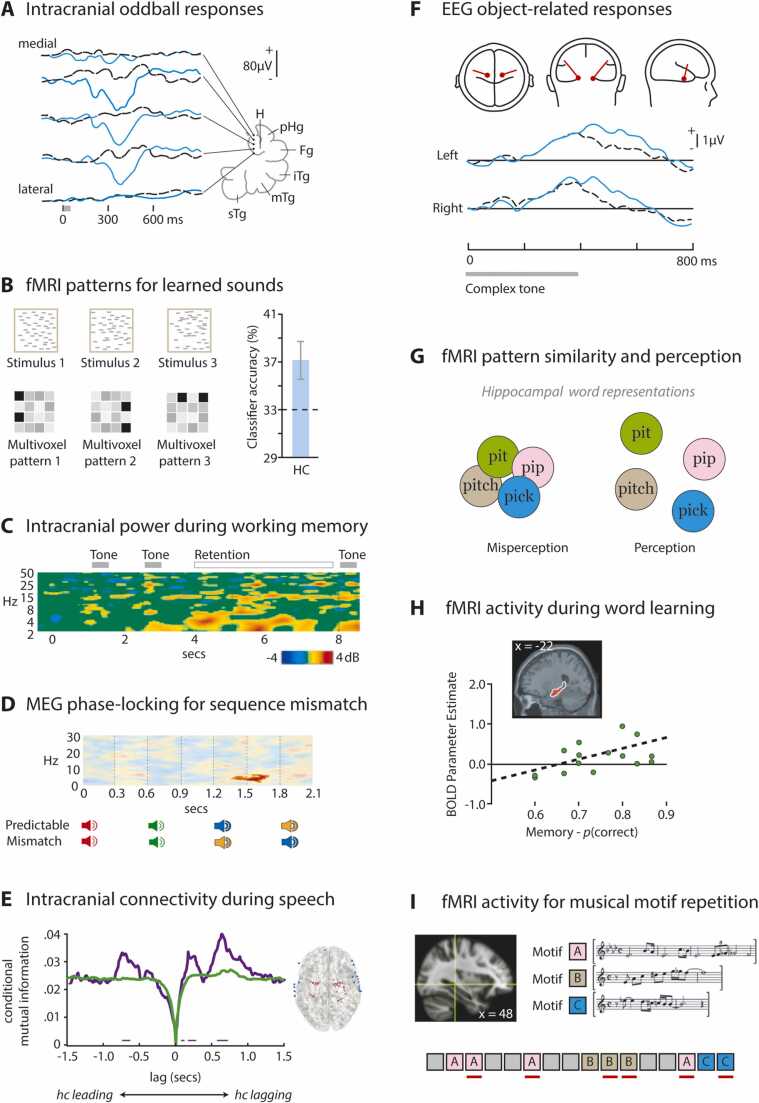
Fig. 4.
Selection of human studies with attentive or task-based listening. (A) Left: Intracranial potentials elicited by frequent (dashed black traces) and rare (solid blue traces) pure tones of different frequencies in medial temporal lobe of a single patient. Right: Electrode locations indicated on line drawing of brain, lateral surface at bottom. H=hippocampus, pHg=parahippocampal gyrus, Fg=fusiform gyrus, iTg=inferior temporal gyrus, mTg=medial temporal gyrus, sTg=superior temporal gyrus. Reprinted from Electroencephalography and Clinical Neurophysiology, 76, Smith, M.E., Halgren, E., Sokolik, M., Baudena, P., Musolino, A., Liegeois-Chauvel, C., Chauvel, P., The intracranial topography of the P3 event-related potential elicited during auditory oddball, 235-248, Copyright © 1990 Elsevier Scientific Publishers Ireland, Ltd, with permission from Elsevier. Smith et al. (1990). (B) Top: Cartoon time-frequency spectrograms of three 1.5-s complex noise stimuli consisting of overlapping pure-tone pips. Bottom: Cartoon hippocampal multivoxel BOLD activity patterns elicited by each of these stimuli after implicit learning through repeated exposure in a stream of other noise stimuli. Right: Above-chance decoding of the same stimuli from the multivoxel patterns in 7 subjects (mean and standard error). Adapted from Fig. 4 in Kumar S., Bonnici H.M., Teki, S., Agus, T.R., Pressnitzer, D., Maguire, E.A., Griffiths, T.D., 2014. Representations of specific acoustic patterns in the auditory cortex and hippocampus. Proc. R. Soc. B 281: 20141000. Licensed under CC-BY. Kumar et al. (2014). (C) Event-related spectral perturbation at a hippocampal electrode during a working memory task. Subjects heard tones of two frequencies (first two gray lines) then received a retro-cue advising which to hold in mind over a delay period until comparing to a target tone (third gray line). A pronounced increase in delta-theta power is apparent during the delay period compared to a pre-trial baseline. Adapted from Fig. 3 in Kumar S., Gander, P.E., Berger, J.I., Billig, A.J., Nourski, K.V., Oya, H., Kawasaki, H., Howard, M.A., Griffiths, T.D., 2021. Oscillatory correlates of auditory working memory examined with human electrocorticography. Neuropsychologia, 150. Licensed under CC-BY. Kumar et al. (2021). (D) Source-localized hippocampal inter-trial phase coherence of MEG recordings during implicit comparison of pure tone sequences. Saturated red region reflects significantly greater theta coherence when frequencies of third and fourth tones in the sequence mismatch implicit predictions compared to when they match. Adapted from Figs. 1, 3 in Recasens, M., Gross, J., Uhlhaas, P.J., 2018. Low-frequency oscillatory correlates of auditory predictive processing in cortical-subcortical networks: A MEG-study. Sci. Rep., 8, 14007. Licensed under CC-BY. Recasens et al. (2018). (E) Nine participants heard two repetitions of a story. Intracranial electrode sites were identified where 70–200 Hz activity showed signs of predictive recall during the second repetition; these included auditory cortex. Left: Connectivity was assessed between these sites and either hippocampus (purple trace) or all other sites (green trace) at moments of peak predictive recall. Mutual information in the neural time series (y-axis) is shown at different lags (x-axis) (excluding influences at zero lag). Purple horizontal bars indicate lags for which mutual information between hippocampus and predictive recall sites was significantly greater than chance. Left-most bar and peak indicates information flow from hippocampus to sites including auditory cortex 720 ms prior to predictive recall. Right: Ventral view of brain showing hippocampal electrode sites (red) and neocortical predictive recall sites (blue) included in the analysis. Adapted from Figs. 3, 5 in Michelmann, S., Price, A.R., Aubrey, B., Strauss, C.K., Doyle, W.K., Friedman, D., Dugan, P.C., Devinsky, O., Devore, S., Flinker, A., Hasson, U., Norman, K.A., 2021. Moment-by-moment tracking of naturalistic learning and its underlying hippocampo-cortical interactions. Nat Commun. 12, 5394. Licensed under CC-BY. Michelmann et al. (2021). (F) Subjects listened to a complex tone, which sometimes contained a mistuned harmonic, and reported whether they heard one or two sounds. Top: Location (red dots) and orientation (red lines) of pair of equivalent current dipoles in medial temporal lobes contributing to EEG scalp activity during the task. Bottom: Activity projected to left and right hemisphere dipoles when the complex tone (gray bar) did (solid blue traces) or did not (dashed black traces) contain a mistuned harmonic. From Alain, C., Arnott, S. R., & Picton, T. W. (2001). Bottom-up and top-down influences on auditory scene analysis: Evidence from event-related brain potentials. Journal of Experimental Psychology: Human Perception and Performance, 27(5), 1072-1089. Copyright © 2001 American Psychological Association. Reproduced and adapted with permission. Alain et al. (2001). (G) Cartoon of hippocampal representations of candidate words during a degraded speech task. Closer circles reflect more overlapping representations, as assessed by similarity of multivoxel BOLD activity patterns. When 24 participants heard a degraded word preceded by a partially mismatching visual cue, those whose hippocampal representations of mismatching candidate words were more distinct were more likely to perceive the correct spoken word. Based on results from Blank et al. (2018). (H) Univariate BOLD activity in anterior and middle hippocampus (top) during exposure to novel pseudo-words correlates positively across 16 participants with their ability to subsequently recognize the stimuli (bottom). Copyright © 2008 Massachusetts Institute of Technology. Adapted with permission. Davis, M.H., Di Betta., A.M., Macdonald, M.J.E., Gaskell, M.G., 2009. Learning and consolidation of novel spoken words. J. Cogn. Neurosci. 21, 803-820. Davis et al. (2009). (I) 11 participants listened to a live recording of a multi-instrumental piece of tango music, containing a number of repeating motifs (right). After key acoustic features were modelled out from the univariate BOLD signal, repetitions (but not first occurrences) of the motifs activated regions including hippocampus (left). Bottom: Indicative illustration of motifs with repeats underlined in red, and intervening non-motivic material in gray. Reprinted from Cortex, 57, Burunat, I., Alluri, V., Toiviainen, P., Numminen, J., Brattico, E. Dynamics of brain activity underlying working memory for music in a naturalistic condition, 254-269, Copyright © 2014 Elsevier Ltd., with permission from Elsevier. Burunat et al. (2014).
Even if a subject is not aware of what distinguishes a particular sound from others, previous exposure can result in the formation of distinct hippocampal representations that are revealed during subsequent active listening. For example, Kumar et al. (2014) found exemplar-specific patterns of multivoxel hippocampal BOLD activity for complex noise stimuli during a repetition detection task (Fig. 4B). Although subjects had been exposed to these specific exemplars multiple times during an earlier training session, they did not typically recognize their recurrence across the experiment. It may be that some degree of pre-exposure is important for hippocampal representations of sounds to form: in another study with no pre-exposure, Liang et al. (2013) were unable to decode particular environmental sounds or spoken words from multivoxel patterns in hippocampus during target detection, even though these stimulus classes could be distinguished from others (such as faces and visual words) in such patterns. Further cases of hippocampal responses during actively attended sounds will be discussed in later sections on space, sequences, and the special cases of speech and music. But first we consider hippocampal involvement in temporal aspects of sound, including during the silence between a behaviorally relevant sound and the reward, punishment, or task that follows.
6. Time and working memory for sound
As mentioned earlier, whereas animals with hippocampal lesions are able to learn the association between a conditioned stimulus and unconditioned stimulus if these are presented in an overlapping or abutting fashion (“delay conditioning”), the insertion of a silent interval between the two (“trace conditioning”) renders these animals impaired. In rodents, persistent firing of individual hippocampal neurons over the trace interval is rare (Gilmartin and McEchron, 2005, McEchron et al., 2003, McEchron and Disterhoft, 1997, Weiss et al., 1996). However, calcium imaging reveals subsets of mouse CA1 neurons that together selectively encode the conditioned stimulus identity as they span this interval (Ahmed et al., 2020, Modi et al., 2014).
This bridging of a silent interval by assemblies of hippocampal cells, each with its own temporal firing field, is not limited to conditioning paradigms in which a sound is to be associated with a subsequent appetitive or aversive stimulus. Hippocampal “time cells” (Manns et al., 2007, Pastalkova et al., 2008) are also active when animals have to retain stimulus-specific information in memory over a short silent interval, for example to compare an odor to a probe in a delayed-match-to-sample task (MacDonald et al., 2013). The population of cells encodes the retained stimulus with a fidelity that predicts subsequent task performance. Time cells have also been identified in human hippocampus during a free recall task (Umbach et al., 2020) as have cells selective for particular visual stimuli that fire at fixed phases of low-frequency (1–7 Hz) oscillations when they are held in mind over a delay period (Kamiński et al., 2017, Kornblith et al., 2017). Comparable findings linking unit activity to oscillatory phase or particular timepoints are yet to be reported for auditory memoranda, however the degree of synchrony among groups of neurons in rat CA1 can distinguish a tone frequency held in memory from another, even when such information is not carried in the firing rate of individual neurons (Takahashi and Sakurai, 2009). In humans, increases in hippocampal BOLD activity (Kumar et al., 2016) and low frequency oscillatory power (Kumar et al., 2021) emerge when human subjects keep a tone frequency in mind for comparison to a probe (Fig. 4C). It has been argued that the hippocampus is involved in retention over a few seconds only when the stimuli require complex high-resolution binding (Yonelinas, 2013) or additional demands are placed on working memory (Jeneson and Squire, 2011), but neither were the case in the Kumar et al. (2021) study.
Does the presence of activity in these cases reflect a critical role for hippocampus in maintaining sound features over a temporal interval, outside of a conditioning setting? Lesioning the fimbria-fornix input to rat hippocampus impairs their ability to remember the presentation rate of click trains, as well as their duration (which is consistently underestimated; Meck et al., 1984). Medial temporal lesions in monkeys impair short-term retention of sound identity although it has been argued that this is an artifact of damage during surgery to auditory-prefrontal cortical pathways (Fritz et al., 2005). Dogs with medial temporal lobe lesions can retain sound identities for over a minute (Kowalska et al., 2001), although their memory for tone locations over a 10-s delay is impaired (Kowalska, 1999). Human patients with hippocampal damage can struggle to hold sounds in mind for several seconds, at least if the material cannot be rehearsed sub-vocally (Cave and Squire, 1992, Chao and Knight, 1995, Keane et al., 1995, Milner, 1972, Milner and Teuber, 1968, Penfield and Milner, 1958, Squire et al., 2001, Stefanacci et al., 2000, Wickelgren, 1968). It seems that the criticality of the hippocampus depends on species and the auditory feature to be maintained (see Supplementary Tables H, I).
The relative contributions of prefrontal cortex and hippocampus to working memory remain a matter of debate (Jin and Maren, 2015, Sreenivasan and D’Esposito, 2019, Tang et al., 2021). Prefrontal cortex receives input from auditory cortex (Plakke and Romanski, 2014, Rocchi et al., 2021, Romanski et al., 1999) and activity there can encode the frequency of a tone held in mind (Kumar et al., 2016). Lesioning or disrupting medial prefrontal cortex in rats (Rodgers and DeWeese, 2014) and lateral prefrontal cortex in non-human primates (Gross and Weiskrantz, 1962, Plakke et al., 2015) impairs auditory working memory, suggesting a critical role. Hippocampus and prefrontal cortex, which are connected via direct and indirect pathways (for a review, see Eichenbaum, 2017a), may work together to support short-term maintenance of auditory material, but such interactions have not yet been directly tested.
The involvement of hippocampus in temporal processing - beyond bridging silent gaps described above - has been extensively reviewed by Banquet et al. (2021). With respect to sound, neurons in rodent hippocampus can be tuned to specific durations (e.g. 2 vs. 8 s) when these are relevant to auditory behavior (McEchron et al., 2003, Onoda et al., 2003). However, duration may be subordinate to pitch in hippocampal coding according to results from Sakurai (2002), who trained rats to perform both pitch and duration discrimination on the same stimulus set. Some hippocampal neurons were tuned to pitch alone and others to both pitch and duration, but none to duration alone. In contrast to the task-based findings in rodents, some neurons in bat CA1 are tuned to the duration (e.g. 1 vs 3 ms) of frequency sweeps, even during passive listening (Yu and Moss, 2022). As these resemble calls used for echolocation, it might be argued that such tuning relates to hippocampal spatial function (see also Section 8). However, no tuning was found in the same study to the delay between call and echo, a more relevant feature for navigation.
Longer time intervals related to auditory content can also be read out from hippocampus. The similarity of hippocampal multivoxel BOLD patterns associated with clips from different times in a spoken narrative is correlated with the perceived elapsed time between the clips (Lositsky et al., 2016). This is consistent with the idea that the medial temporal lobe provides a slowly shifting mental context that acts as a temporal tag for memories of items and episodes (Howard et al., 2005, Yonelinas et al., 2019; see also Section 13). At the other end of the temporal scale, while processing of sub-second intervals between sounds draws largely on extra-hippocampal structures, such as cerebellum and striatum (Nani et al., 2019, Teki and Griffiths, 2016), there is some indirect evidence for hippocampal involvement at these shorter timeframes (see Supplementary Table I). Patients with medial temporal lobe epilepsy including hippocampal sclerosis have problems identifying patterns of durations of hundreds of milliseconds (Han et al., 2011), making anisochrony judgments on the order of tens of milliseconds (Lavasani et al., 2013), and detecting gaps in noise at below 10 ms (Ehrlé, 2001, Rabelo et al., 2015). Replicating these findings in other groups with circumscribed hippocampal damage will be valuable. As we shall see in Section 9, the hippocampus may be critically important when it comes to storing the order of stimuli in time.
7. Sound context
The hippocampus plays an important role in learning the constraints and contexts under which reward contingencies and behaviors should apply (see Supplementary Table H). Animals with hippocampal lesions fail to inhibit responses to uninformative or salient but unconditioned sounds (Freeman et al., 1973, Loechner and Weisz, 1987, Micco and Schwartz, 1971, Niki, 1967, Rickert et al., 1979, Rickert et al., 1978, Solomon, 1977, Solomon and Moore, 1975, Swanson and Isaacson, 1967) and take much longer to learn to extinguish a conditioned response to sound than control animals (Berger and Orr, 1983, Berger and Orr, 1982, Schmaltz and Theios, 1972). Neocortical sites including perirhinal cortex can also support the learning of conditional dependencies, but only with sufficient exposure and when those associations are important for current behavior. The hippocampus learns more rapidly – even after a single exposure - and importantly can associate stable background elements that occur together into a context, even if they are not associated with reward or punishment at the time (Rudy, 2009, Rudy and O’Reilly, 2001). Once a context representation has been formed, pattern completion mechanisms supported by auto-associative networks in CA3 may allow a full context to be retrieved from a partial cue (Marr, 1971, McNaughton and Morris, 1987, O’Reilly and McClelland, 1994). The neocortical and hippocampal learning systems likely operate in parallel (Hebscher et al., 2019, McClelland and O’Reilly, 1995), but the dominance of the hippocampal system is illustrated by impaired context retrieval when hippocampal lesions are made after but not before learning (Lehmann et al., 2009). A broader definition of context is adopted in the contextual binding theory of human episodic memory (Yonelinas et al., 2019; see also Section 13). It holds that not only background elements of the physical environment, but also slowly changing cognitive state or mood as well as temporal context, are bound together in the hippocampus with items and events as they are encountered or experienced. The theory accounts for a range of experimental data, including interference in memory between items encountered in similar places or times, or under similar behavioral states.
The dependence of context learning on hippocampus matters for sound processing in a number of ways. First, hippocampally lesioned rats fail to show context-specific expression or extinction of auditory fear conditioning - instead the conditioned stimulus can trigger the fear behavior even in inappropriate contexts (Corcoran and Maren, 2001, Holt and Maren, 1999, Hunsaker and Kesner, 2008). Second, a context may itself include auditory elements. For example, Grau-Perales et al. (2019) investigated the role of hippocampus in contextual control of habituation of taste neophobia, whereby mice are initially reluctant to drink novel-tasting solutions but become less averse over days. Whereas a change in the auditory context (the presence of a pure tone versus white noise) resets this attenuation in control animals, this is not the case for lesioned animals. Sounds forming part of a context can also be presented as a partial cue to reactivate hippocampus-dependent memories. In rats, such reactivation has allowed the ensemble of hippocampal cells encoding a specific memory to be reactivated and subsequently targeted through inhibition of protein synthesis (Ressler et al., 2021). Interfering with memory reconsolidation in this way may be relevant for future clinical interventions that rely on indirect retrieval of traumatic memories.
Whether the hippocampus is involved in combining purely auditory cues into a context has not been investigated. It would be informative to establish whether hippocampally lesioned animals can automatically form a context representation on the basis of - for example - the level of reverberation and type of background noise in an environment, to constrain conditioned behavior.
8. Interactions between sound and space
The automatic learning of associations in the absence of reward, highlighted with respect to context learning in the previous section, is at the heart of the “cognitive map” concept originally proposed by Tolman (1948). He observed that rats learn the structure of a maze - subsequently enabling them to retrieve alternative routes - even when such (“latent”) learning is not driven by immediate reward. Hippocampal cells that are selectively active during the exploration of space (“place cells”) discovered by O’Keefe and Dostrovsky (1971) offered a biological basis for such a map. We outline a few key features of place cells before discussing their interaction with sound.
Place cells with adjoining place fields fire in sequence as an animal traverses an environment. The formation of place fields is driven by path integration, a computation that transforms motion into a sense of location, supplemented with landmark perception (Savelli and Knierim, 2019). These processes depend on cells that track head direction, speed, boundaries and other environmental properties (Lever et al., 2009, O’Keefe and Burgess, 1996, Taube et al., 1990). Place cells can remap – that is change their firing rate or even firing field - in the presence of changes of context – whether physical, such as a new cage, or task-related (Anderson and Jeffery, 2003, Jackson and Redish, 2007, Leutgeb et al., 2004, Wills et al., 2005). “Grid cells” in entorhinal cortex fire at multiple locations arranged in a hexagonal grid, tiling the environment between them (Hafting et al., 2005). Grid spacing increases from dorsal/posterior to ventral/anterior entorhinal cortex (Brun et al., 2008) but generally remains fixed across environments (Fyhn et al., 2007); these properties enable grid cells to provide a stable coordinate and metric system. There is a systematic change in the phase of theta oscillations at which a place cell fires as the animal moves through its firing field (“phase precession”; O’Keefe and Recce, 1993 in rodents; Qasim et al., 2021 in humans). This results in each theta cycle containing a representation of the recent past (places visited), present (current location) and future (planned trajectory). Such sequences are further compressed during “sharp-wave ripple” events (Buzsáki et al., 1983). In rodents these happen during pauses in navigation, and can be decoded as reflecting both recently experienced ("replay") and future ("preplay") trajectories (Diba and Buzsáki, 2007). They can occur forward or in reverse, may reflect credit assignment and planning (Foster and Wilson, 2006, Pfeiffer and Foster, 2013), and are associated with memory consolidation during sleep (see Section 13; for a general review of sharp-wave ripples see Buzsáki, 2015).
Sound and place cells interact in a variety of ways. Although path integration and use of visual landmarks are key for forming spatial maps in most species, sound provides an important signal for place-cell coding during echolocation in the bat (Ulanovsky and Moss, 2011, Ulanovsky and Moss, 2008, Ulanovsky and Moss, 2007, Yu and Moss, 2022). In rats, changes that induce place cell remapping include auditory fear conditioning in a particular location (Donzis et al., 2013, Moita et al., 2004) and high-intensity sound exposure (Goble et al., 2009). Even the introduction of a behaviorally-irrelevant sound can influence aspects of spatial coding, such as the relationship between locomotion speed and hippocampal theta (Long et al., 2014).
Synaptic change in CA1 facilitates learning the association between locations with behaviorally-salient ultrasonic signals (Dietz and Manahan-Vaughan, 2017), just as with visual cues. Firing of dentate gyrus cell assemblies reflects learning of the mapping between particular tone frequencies and reward location, and silencing these assemblies impairs such learning (J. Shen et al., 2021). In rodents, hippocampus is critical for incidental learning of the association between a sound and its location, and for auditory fear conditioning that is specific to the spatial or spatiotemporal context (Iordanova et al., 2009, Talk et al., 2002). Another case in which the absence of hippocampus prevents incidental learning of associations is provided by Talk et al. (2016). Hippocampally-lesioned rats failed to incidentally learn the association between a noise stimulus and a particular location, demonstrated by their failure to avoid the location in the absence of the sound, after that sound had been paired with a shock. This was despite their learning to associate the sound with the shock, and control animals avoiding the location in the absence of the sound. Memory of auditory space is also impaired in dogs (Kowalska, 1999) and humans (Lancelot et al., 2005, Lancelot et al., 2003) with hippocampal damage (see also Supplementary Tables H, I). In humans, hippocampal lesions - particularly when coupled with superior temporal lobe damage - are associated with under-estimation of the length of sound trajectories in space (Kotelenko et al., 2013). This is somewhat consistent with the attenuation of boundary extension in visual space in patients with hippocampal amnesia (Mullally et al., 2012), and with the underestimation of temporal extent in rats with diminished hippocampal input (Meck et al., 1984).
Space, or spatial context, also has a strong influence over coding of sound in the hippocampus. For example, the firing rate of hippocampal units in response to a conditioned noise stimulus is gated by location-specific tuning (Moita et al., 2003). In another experiment some units in rat CA1 fired selectively in response to one of several rewarded artificial vowel sounds regardless of location, but this mapping only persisted as long as the spatial environment was fixed (Itskov et al., 2012). Some monkey hippocampal neurons are selective for particular types of sound (e.g. human voice over pure tone), but respond only when sounds come from a particular direction, typically behind the animal and out of its visual field (Tamura et al., 1992, Tamura et al., 1990). Eichenbaum and colleagues have demonstrated the mixed selectivity of hippocampal neurons more generally (Eichenbaum, 2017b). Representational similarity analysis over populations of neurons reveals multiplexed coding of context, location, reward, and object – often in that order of precedence (McKenzie et al., 2014). Although position-related firing is normally present from the outset of exposure to a new environment, the extent to which object and reward information are encoded increases based on their relevance to behavior (Lee and Kim, 2010, Muzzio et al., 2009).
9. Auditory sequences and predictions
Might the phase precession described in the last section allow for the maintenance in hippocampus of not only spatial trajectories but also auditory sequences? In this way recent, past, current, and predicted or planned sounds (e.g. in a sentence or melody) could be linked based on the phase in a theta cycle at which corresponding neural assemblies fire. Direct experimental support for this kind of auditory phase precession is currently lacking. We know that in their firing rates CA1 cells code sequences of non-spatial events, such as the presentation of different odors to rats (Terada et al., 2017) or images to humans (Reddy et al., 2021). Additionally, the hippocampus is certainly involved in human auditory sequence learning, including when sounds are encountered incidentally. Patients with hippocampal lesions show severe impairment in learning probabilistic relationships between successive pure tones and syllables (Covington et al., 2018, Schapiro et al., 2014), mirroring results in vision (Schapiro et al., 2012). Neuroimaging work in healthy subjects provides further support. Jablonowski et al. (2018). exposed healthy subjects to tone sequence regularities during a learning phase in which they performed an orthogonal sensorimotor task. During a subsequent test phase, they had to decide whether the next tone in a sequence would be higher or lower. Despite subjects having no explicit knowledge of the underlying regularities, accuracy was high and correlated positively with bilateral hippocampal BOLD activity during the learning phase. Relatedly, in a magnetoencephalography (MEG) study, subjects were presented with tones in rapid succession while engaged in an irrelevant visual task. A slow shift in magnetic field strength with a generator in hippocampus (as well as auditory and inferior frontal cortex) occurred from the point at which repetitive structure (recurring frequency patterns) occurred (Barascud et al., 2016).
In addition to these univariate markers of increased activity during sequence learning, representations of specific sound sequences emerge in hippocampus over the course of exposure. In one fMRI experiment, multivariate patterns of activity in left hippocampus came to encode the identity of ordered sequences of spoken letters that repeated over the experiment, even though the individual elements were shared across all sequences (Kalm et al., 2013). Another study exposed subjects to continuous syllable streams, in which particular syllable triplets always occurred in the same order (Henin et al., 2021). While the subjects’ task was to detect the repetition of individual syllables, they implicitly learned the hidden regularities. This was reflected not only in faster reaction times during these structured sequences compared to unstructured ones, but also in patterns of intracranially recorded hippocampal activity that became more similar over time for syllables belonging to the same triplet. In a similar intracranial study, hippocampal activity contained greater power at the (three-syllable) word repetition rate than did auditory cortical activity, with the reverse being true at the syllable repetition rate (Ramos-Escobar et al., 2022). The words that had been implicitly learned subsequently elicited reduced hippocampal evoked responses than did syllable combinations that had not been presented.
Some have argued that sequences of sensory content (or spatial paths) become associated with pre-existing hippocampal cell assemblies that fire in a particular order, while the sensory elements themselves are represented in neocortex (Dragoi and Tonegawa, 2013, Friston and Buzsáki, 2016). Related is the idea of the hippocampus as a predictive (not merely spatial) map (Gershman, 2018, Stachenfeld et al., 2017) that encodes successor representations, namely predictions of future states (discounted future occupancy) given an animal’s current state (Dayan, 1993, Momennejad, 2020). These representations are thought to allow for learning of relational structure – in physical space or otherwise – separate from sensory content or reward contingency, facilitating generalization across environments that share the same relational structure (Geerts et al., 2020, Whittington et al., 2020). A large range of hippocampal findings can be accounted for in this predictive framework, including the modulation of place-cell firing fields by reward locations (Hollup et al., 2001) and barriers (Alvernhe et al., 2011, Muller and Kubie, 1987), and the asymmetric form of place fields during motion along a linear track (Mehta et al., 2000).
Indirect evidence that the hippocampus predicts future auditory content comes from responses to violation of learned rules about sound sequences. Such violations can be considered a form of associative novelty, described in the visual modality by Kumaran and Maguire (2007) and contrasting with the simple stimulus novelty covered at the end of Section 3. Violations of auditory-sequence order elicit scalp components in EEG (Takakura et al., 2003) and MEG (Recasens et al., 2018) that have been localized to hippocampus. In the latter these were accompanied by greater hippocampal theta power and phase locking (Fig. 4D). In probabilistic sound sequences, violation is not all-or-nothing. Instead, time-varying continuous measures of uncertainty (entropy) and surprise can be derived based on learned statistics. Cheung et al. (2019) trained a Markov model on harmonic progressions in pop songs and compared its estimates of uncertainty and surprise during novel progressions to BOLD activity. Anterior hippocampus (along with amygdala and auditory cortex) reflected the interaction between these factors, being elevated when chords deviated substantially from strong expectations, or when they met relatively imprecise ones. Interestingly, these were the same conditions that elicited the greatest pleasure ratings in listeners. Other fMRI work has been more equivocal as to whether hippocampus tracks uncertainty in auditory sequences (Tobia et al., 2012), and in one study the hippocampal BOLD signal was reduced in tone sequences in which simple or hierarchical rules concerning pitch and duration were violated compared to when they were met (Martins et al., 2020). Disparate findings may relate to functional heterogeneity of hippocampal fields, position of activity along the long axis, or subtle task differences.
Violation and surprise responses in hippocampus are consistent with it acting as a comparator, with predictions passed from CA3 to CA1 where they are combined with sensory input from entorhinal cortex (Hasselmo and Wyble, 1997, Lisman and Grace, 2005, Vinogradova, 1975b, Vinogradova, 2001). However, there is also evidence for hippocampus sending predictions to sensory cortex. In the study by Recasens et al. (2018), predictable tone sequences elicited elevated effective connectivity (based on alpha-band Granger causality) from right hippocampus to Heschl's gyrus, compared to unpredictable sequences. In other work with perfectly predictable intervals of sound and silence, a spatial independent component of the BOLD signal that included hippocampus led the auditory cortex signal (Langers and Melcher, 2011). In a more specific and naturalistic demonstration of information exchange between hippocampus and sensory cortex, Michelmann et al. (2021) presented a spoken story to intracranially implanted epilepsy patients, repeating the material a second time. Auditory cortical sites were identified where the high-gamma (70–200 Hz) time series showed signs of predictive recall during the second run. At peaks of this predictive measure, mutual information between the auditory cortical high-gamma activity and lower frequency activity in hippocampus was maximal, with hippocampus leading cortical activity by an average of 740 ms (Fig. 4E). In Section 2 (see also Supplementary Table A) we noted pathways from hippocampus via entorhinal, parahippocampal and lateral temporal sites, along which such predictions could be conveyed to auditory cortex. Any error signals resulting from a mismatch between predicted and actually heard sounds could be passed in the reverse direction to update corresponding hippocampal models (Barron et al., 2020).
A missing piece in the puzzle is evidence of specific predicted auditory content being decodable in hippocampus. A number of studies have decoded visual content that is predicted on the basis of simple auditory cues from hippocampal BOLD patterns (Aitken and Kok, 2022, Ekman et al., 2022, Kok et al., 2020, Kok and Turk-Browne, 2018). Predicted visual content can also be decoded from hippocampus when that prediction is triggered by auditory cues on the basis of semantic knowledge. In human intracranial recordings, when spoken words primed a particular semantically-related image, high frequency (50–250 Hz) hippocampal activity that was more similar across periods prior to and during the image predicted faster response times (Jafarpour et al., 2017). Furthermore, such activity showed similarity structure across stimuli that reflected the similarity of the predicted objects in semantic space. Another study with a similar task found the most pronounced hippocampal theta activity during sentences and words that set a strong semantic context for the subsequently presented picture (Piai et al., 2016). It remains to be determined whether sounds predicted on the basis of associations learned in the short-term (such as the next note in a melody learned in an experiment) or through semantic context (such as the sound of a bark following a picture of a dog) can be decoded from hippocampal activity before they are heard.
10. Navigating frequency space
We have seen that rodent grid cells can provide a basis for spatial navigation (Hafting et al., 2005). In humans, there is evidence that entorhinal grid cells support navigation in virtual (Doeller et al., 2010, Jacobs et al., 2013), imagined (Bellmund et al., 2016) and visual (Julian et al., 2018, Killian et al., 2012, Meister and Buffalo, 2018, Nau et al., 2018) space, as well as time (Ezzyat and Davachi, 2014). Other dimensions of experience can be represented in a similar way; these include social hierarchies spanned by affiliation and power (Tavares et al., 2015), a two dimensional space of body part lengths (Constantinescu et al., 2016), an imagined two dimensional odor space (Bao et al., 2019), and semantic spaces of written words (Solomon et al., 2019). Most of these studies indirectly measure the presence of grid cells by virtue of hexadirectional symmetry of the BOLD response with respect to navigation direction. In some cases, a neural correlate of distance has also been identified in abstract spaces. The evidence is growing that computational circuitry in the hippocampus and entorhinal cortex can facilitate “navigation” through and memory of any arbitrary space.
An important auditory example has been described by Aronov et al. (2017), who trained rats to depress a lever while a tone increased in frequency and to release it in a target frequency range for a reward. They identified CA1 cells that had particular frequency firing fields during this task and others that fired preferentially at the start or end of a trial. The number of tuned cells decreased when the animal was no longer responsible for releasing the lever but was still rewarded when the tone reached the target frequency. When the tone changed in a block without involvement of the animal and in the absence of reward, no such tuning existed. During the active task, cells in the entorhinal cortex could have multiple firing fields. Notably, some of these same hippocampal and entorhinal cells also had place and grid fields when the animal was instead foraging in an open arena. Of all CA1 cells recorded, approximately a quarter had auditory and place fields, a half one or the other, and a quarter neither. This example is different from those described earlier in which discrete individual sounds have acquired behavioral significance (e.g. conditioning studies, Sakurai, 2002) - here the continuous range of presented frequencies is represented. Another important point is that the trial duration and the rate at which the tone frequency changed was varied throughout the experiment. Units retained their frequency tuning across this variability, meaning that they were not simply tuned to the absolute time elapsed in a trial. However subsequent research has established that ensembles of CA1 and entorhinal cells in rats and humans can carry temporal information on a range of scales and individual time cells can stretch their tuning in accordance with the demands of a task (Mau et al., 2018, Reddy et al., 2021, Shimbo et al., 2021, Tsao et al., 2018). It is therefore possible that the units in Aronov et al. (2017) reflected relative time in task (“retime cells” in MacDonald et al., 2011) or relative “distance” to the target sound. The work of Aronov et al. therefore raises intriguing possibilities about the representation of an acoustic dimension in hippocampus during an active task that require critical reappraisal in further experiments.
11. Auditory objects and scenes
9, 10 showed that the hippocampus tracks sequences of sound and may map one of its most salient dimensions, frequency. In Section 3 we gave examples of intensity and amplitude modulation rate affecting hippocampal responses and also described how changes in sound features can drive hippocampal responses through release from habituation. In cluttered acoustic scenes, the rate of change of sound features is among the key determinants of which sequential elements should be grouped into auditory streams or objects (Bregman, 1990, van Noorden, 1975). An auditory perceptual object, like its visual counterparts, is defined by the binding of multiple sensory features, represents a source distinct from others in the scene, and is invariant over different sensory instances (Bizley and Cohen, 2013, Griffiths and Warren, 2004, Kubovy and Van Valkenburg, 2001). The medial temporal lobe is important in visual object processing and although the hippocampus itself is not critical here, it does support the construction of scenes – configurations of objects in space (Barense et al., 2009, Bussey and Saksida, 2005, Chadwick et al., 2013, Hassabis et al., 2007, Lee et al., 2012, McCormick et al., 2021, Mullally et al., 2012, Smith et al., 2014, Zeidman et al., 2015).
Does the medial temporal lobe contribute to the formation of auditory objects or their collection into scenes? Much of the abstraction of different auditory features and their combination into object representations is accomplished in the ascending subcortical auditory pathway and auditory cortex. For example, adaptation occurs in the auditory nerve for grouping of harmonics by common onset (Holmes and Roberts, 2011) and tuning for combinations of auditory features is present in primary auditory cortex (Bizley et al., 2009). Representations in auditory association cortex demonstrate object-level intensity gain control (Simon, 2015) and correlate with object-level perception (Billig et al., 2018) and attention (O’Sullivan et al., 2019). The extraction of timbre, defined by sound features other than intensity and pitch, involves higher auditory-associated cortex including planum temporale and anterior superior temporal sulcus (Kumar et al., 2007, Warren et al., 2005). Selectivity for phonemes defined by particular combinations of spectrotemporal features is found on the superior temporal gyrus (Mesgarani et al., 2014) and illusory percepts of vowels based on apparent object continuity have correlates in superior temporal sulcus and middle temporal gyrus (Heinrich et al., 2008). Although the medial temporal lobe could provide top-down object information to these earlier sites, unlike in vision (Devlin and Price, 2007) evidence for its involvement in auditory object formation, perception, and scene analysis is scarce (Bizley and Cohen, 2013, Christison-Lagay et al., 2015, Griffiths and Warren, 2004, Snyder and Elhilali, 2017). Patients with Alzheimer’s disease show impairment in auditory segregation and scene analysis tasks, but structural and functional correlates of these deficits are reported in lateral temporal and parietal cortices, rather than in the medial temporal lobe (Golden et al., 2015, Goll et al., 2012). However there is some support for the number of perceptual objects in a simple acoustic scene being tracked there. A human intracranial study found that hippocampal activity distinguished between perceptual interpretations of bistable tone triplets that could be heard as one or two streams (Curtu et al., 2019). Another human study found a medial temporal source for a late P400-like scalp potential associated with successful detection of a mistuned harmonic in a tone complex, which gives rise to the percept of two concurrent auditory objects (Alain et al., 2001; Fig. 4F). In rodents, damage to perirhinal cortex (hippocampus was not tested) impaired rats in binding discontinuous temporal vocalization elements into an object to act as a conditioned stimulus in fear conditioning (Bang and Brown, 2009) and we have already described involvement of hippocampus in bridging temporal gaps, both during auditory working memory and trace conditioning, and in representing auditory sequences in memory. Less direct evidence for hippocampal involvement in auditory scene segregation is its elevated activity in subjects performing a verbal working memory task in noise compared to in quiet (Manan et al., 2012).
Two important ingredients to successful parsing of an auditory scene are the ability to distinguish an object of interest from the background, and to restore a partially masked sound on the basis of prior knowledge. These requirements to "separate" and "complete" auditory representations under different circumstances bring to mind two terms describing particular computations in support of memory, thought to involve hippocampus (Marr, 1971, McNaughton and Morris, 1987, Rolls, 2013). Pattern separation refers to the storing of distinct activity patterns for memories that share similar features. Modeling suggests that the large number of granule cells in dentate gyrus can support the sparse coding necessary to transform the overlapping representations from entorhinal cortex and project the result to CA3 and beyond for storage and consolidation. In the case of an unfolding and spectrotemporally overlapping acoustic scene, might this separation act rapidly enough for the results to be read out during online perception? The hippocampus can certainly guide perceptual sampling of a cluttered visual scene (Kragel et al., 2021) and the same may be true of audition. Impaired perceptual discrimination of complex visual objects in a patient with relatively selective dentate gyrus lesions also supports the idea that pattern separation in this hippocampal subfield is relevant not only for memory, but also online perception (Mitchnick et al., 2022).
The other concept, pattern completion, refers to the retrieval of a memory on the basis of a partial cue, thought to be supported by auto-associative or attractor networks in CA3 (Rolls, 2013). Such a completion process could potentially retrieve features of known sources (e.g. the vocal characteristics of a known conversation partner) or anticipate likely continuations of interrupted sentences based on semantic knowledge or prior exposure. In this vein, one study found theta synchrony between medial temporal lobe (only parahippocampal gyrus was available for analysis) and auditory cortex during the illusory continuation of familiar music when interrupted by noise (Müller et al., 2013). The passing of predictions from hippocampus to auditory cortex based on learned sequences and discussed in Section 9 would also constitute a form of pattern completion. These ideas remain mostly speculative, and a critical role for hippocampus in auditory object formation has not been demonstrated. Alternatively, it is possible that hippocampus is only required when incorporating auditory elements into scenes primarily determined by visual objects defined in a spatial framework. In the next section we consider auditory-visual and other crossmodal interactions in hippocampus.
12. Multi- and supra-modal representations and associations
The intrinsic circuitry and external connectivity of the hippocampus allow it to bind sensory experience across modalities. Patients with hippocampal lesions have impaired memory for associations between simultaneously presented faces and voices (Mayes et al., 2004, Vargha-Khadem et al., 1997), and between other sounds and scenes (Mayes et al., 2004) or abstract images (Borders et al., 2017). At the same time, transcranial magnetic stimulation of parietal sites identified in individual subjects to be functionally connected to hippocampus boosts memory for word-face pairs (Wang et al., 2014). Hippocampal BOLD activity during encoding of an object in memory scales with the number of features to be integrated, with location making a greater contribution than color or sound (Cooper and Ritchey, 2020). Activity is also elevated for successful encoding or retrieval of cross-modal associations compared to within-modality pairs (Butler and James, 2011, Gottlieb et al., 2010, Joassin et al., 2011, Persson et al., 2011) and demonstrates functional connectivity with cortical sites including superior temporal gyrus during such multimodal associations (Cooper and Ritchey, 2019, Griffiths and Fuentemilla, 2019, Joassin et al., 2011, Love et al., 2011). In an experiment involving memory for text-sound associations, pairings with the greatest hippocampal activity at encoding were recalled most accurately and showed the most similar neocortical patterns across encoding and retrieval (Danker et al., 2016). In these experiments, learning the association was explicitly required as part of the task. However, elevated hippocampal BOLD has also been demonstrated when images are combined with emotionally congruent music compared to when they are presented alone during an emotion-rating task without an explicit memory component (Baumgartner et al., 2006).
Tone frequency can combine with a non-auditory dimension to define a semantic space that is represented in the medial temporal lobe. Viganò and Piazza (2020) trained participants to associate particular quadrants in a two-dimensional space of visual shape and tone frequency with four different non-word labels. After training, right entorhinal cortex showed tuning to direction of navigation through this space during a one-back task that used both the audiovisual objects and the written semantic labels. Auditory stimuli can also trigger or be subsumed into super-modal conceptual representations in the hippocampus. For example, Quiroga and colleagues have identified human hippocampal units that respond selectively to famous people, whether in photograph form, or as a written or spoken name (Quiroga, 2020, Quiroga et al., 2009). Twice as many neurons respond to the image than to the sound in these studies, and while there are neurons that respond to the image but not the sound, the reverse is not true (however this bias may be a result of the distribution of stimuli used). To establish the extent to which auditory representations are subordinate in the hippocampus it would be valuable to establish whether such concept tuning can be identified based on multiple auditory instances only, such as the spoken word "dog" and the sound of a dog barking, or a person's spoken name and their voice.
13. Auditory elements of episodic memories and their consolidation
The hippocampus not only binds across sensory modalities, but situates these multimodal objects in a spatiotemporal context to form memories of particular episodes (Gelbard-Sagiv et al., 2008, Paz et al., 2010). We note the apparent contradiction that under its proposed role in statistical learning the hippocampus generalizes to learn the probabilities of transitions between sounds over multiple presentations, but it is also able to form discrete memories for individual episodes. Modeling work suggests that statistical learning could rely more on the monosynaptic connection between entorhinal cortex and CA1, where inhibition and sparsity are less pronounced, than along the trisynaptic pathway from entorhinal cortex through dentate gyrus and CA3, likely important for memory of individual episodes (Schapiro et al., 2017).
The extent and nature of the hippocampus' ongoing involvement in maintaining and retrieving episodic memories has been controversial. The standard consolidation model (Squire and Zola-Morgan, 1991, Squire and Alvarez, 1995) holds that episodic memories that initially depend on hippocampus to index distributed content in neocortex (Teyler and DiScenna, 1986) become consolidated over time, with direct links between those neocortical sites strengthening, and hippocampal dependence declining. Such a model accounts for the graded retrograde memory deficit observed in amnesic patients such as H.M., whereby older memories are relatively preserved - however, it has been argued that these are of more of a semantic nature than vividly episodic. Alternative accounts – such as multiple trace, trace transformation, and contextual binding theories - propose that hippocampus continues to be involved, either in indexing or reconstructing distributed cortical content, or maintaining such content itself (Nadel et al., 2000, Winocur and Moscovitch, 2011, Yonelinas et al., 2019).
Sound-related evidence for ongoing hippocampal involvement in supporting rich memories (rather than memories that are given a semantic label), comes from findings that BOLD activity there scales with the vividness of a retrieved episodic memory, including its auditory content (Sekeres et al., 2018). Furthermore, patients with medial temporal lobe damage report fewer perceptual (including auditory) details when retrieving episodic memories than controls (St-Laurent et al., 2014). Hippocampal BOLD activity is elevated when hearing a recording of one's own autobiographical memories (Svoboda and Levine, 2009) or a melody previously associated with a particular object and location (Prabhakar et al., 2018), in both cases after several days. When listening to familiar music, subjects show greater hippocampal BOLD activity as they retrieve specific autobiographical episodes associated with the music than more general ongoing events or personal knowledge from the relevant period in their lives (Ford et al., 2011). Hippocampus is also more active during such retrieval than when attending to structural features of the music (Kubit and Janata, 2018).
Which memories get consolidated in humans during sleep can be biased by presentation of relevant sounds. In one study, subjects learned object-location pairs while presented with an object-specific sound, then slept during a scanning session (van Dongen et al., 2012). Greater hippocampal activity when previously heard sounds were repeated during sleep was associated with better retention of object-location pairs as tested the following day. In another study, participants learned motor patterns that were associated with different tones. Presentation of one of those tones during sleep led to faster execution of the cued compared to the uncued pattern the following day, with a corresponding difference in bilateral hippocampal activity and hippocampal connectivity to motor areas (Cousins et al., 2016). In rodents, hippocampal involvement in consolidation of auditory memories during sleep was demonstrated by Bendor and Wilson (2012), who first paired spatial trajectories with auditory cues in behaving rats. Presenting one of these sounds during subsequent sleep increased the probability that the place cell sequence encoding the related trajectory would be reactivated. In another study, auditory cortical patterns that occurred when rats approached a location associated with a sound were recapitulated during sleep, both spontaneously and when cued by the auditory stimulus (Rothschild et al., 2017). This activity predicted subsequent hippocampal sharp-wave ripples, which in turn predicted subsequent auditory cortical activity, suggesting a bidirectional exchange of information. Content-specific sharp-wave ripple playback from hippocampus to sensory cortex has also been detected in humans, but so far only during awake visual recall (Norman et al., 2019). The fact that auditory signals, unlike visual information, can trigger hippocampal activity during sleep points to possible overnight learning applications (Harrington and Cairney, 2021), discussed further in Section 18.
14. Perception and memory of speech and music
Speech (Supplementary Tables F, J) and music (Supplementary Tables G, K) are two classes of sound particularly important in human communication. A small proportion of hippocampal neurons respond selectively to specific spoken words (Urgolites et al., 2022), and in challenging listening conditions the distinctness of candidate word representations in left hippocampus is positively correlated with speech understanding (Blank et al., 2018; Fig. 4G). Intelligible speech elicits greater hippocampal activity than unintelligible speech, regardless of whether that greater intelligibility is due to acoustic clarity or provision of prior information (Clos et al., 2014, Davis et al., 2011, Davis and Johnsrude, 2003). The hippocampus is also important in resolving syntactic or semantic ambiguity based on information from earlier in a sentence or exchange (Kurczek et al., 2013, Rubin et al., 2011). Another potential role of hippocampus during conversation is in monitoring one's own speech, perhaps through comparing predictions of motor commands with resulting auditory feedback (van de Ven et al., 2020; see also Rummell et al., 2016 for self-generated sounds in mice).
Beyond online speech perception there is substantial neuropsychological evidence for left hippocampus in particular playing a key role in the learning and recall of verbal material (Barbeau et al., 2005, Boon et al., 2011, Cavazzuti et al., 1980, Coras et al., 2014, Dulay et al., 2004, Frisk and Milner, 1990, Gadian et al., 2000, Goldstein et al., 1988, Helmstaedter et al., 1997, Helmstaedter and Elger, 1996, Huijgen et al., 2015, Jayakar et al., 2015, McMillan et al., 1987, Meyer and Yates, 1955, Mueller et al., 2012, O’Brien et al., 2003, Rausch and Crandall, 1982, Squire et al., 2001, Tachibana et al., 1999, Vargha-Khadem et al., 1997, Witt et al., 2014). Neuroimaging data support this lateralization, with left hippocampal BOLD signal during exposure to novel pseudo-words correlating across subjects with subsequent recall (Davis et al., 2009; Fig. 4H). Not only the level of activity in the hippocampus (Kato et al., 1998, Park and Rugg, 2009, Petersson et al., 1999, Schmithorst et al., 2006, Urgolites et al., 2020) but also its degree of functional connectivity with lateral temporal cortex is associated with successful encoding of speech in memory (Babiloni et al., 2009, Gagnepain et al., 2011). During continuous speech, such encoding may occur during moments of increased connectivity at perceived event boundaries (Michelmann et al., 2021). This is consistent with hippocampus segmenting ongoing experience, such as when it marks moments of narrative shift in movies (Baldassano et al., 2017, Ben-Yakov and Henson, 2018) or identifies recurring words in a continuous stream of syllables during statistical learning (Henin et al., 2021, Ramos-Escobar et al., 2022). The novelty of both a word (Davis et al., 2009) and its category (Dolan and Fletcher, 1997) can drive hippocampal activity during memory encoding. However single unit recordings from human hippocampus reveal neurons that respond preferentially to repeated words alongside those driven by word novelty (Urgolites et al., 2022).
While the above studies point to a role for hippocampus in the encoding of memory for words, deficits in consolidation have been identified in groups with presumed hippocampal damage. Patients with transient epileptic amnesia (Hoefeijzers et al., 2013) and with presymptomatic autosomal dominant Alzheimer's disease (Weston et al., 2018) show accelerated long-term forgetting, such that word memory is impaired after a week, but not after 30 min. This suggests that verbal acquisition itself may not be impaired when the hippocampus is compromised, but rather the durability of the memories that form. A role of hippocampus in speech memory consolidation is also supported by a study of healthy subjects, in whom hippocampal volume correlated positively with post-training overnight change in non-native speech sound discrimination (Fuhrmeister and Myers, 2022).
Whereas verbal memory deficits are associated particularly with left hippocampal damage, memory for melody may depend to a greater extent on right-hemisphere structures. However, although right hemisphere damage has been linked to selective impaired memory for melody versus lyrics (Samson and Zatorre, 1992, Samson and Zatorre, 1991), and to a reduced “mere exposure” effect where previously heard melodies are usually judged as more likeable than novel ones (Samson and Peretz, 2005), lesions in these studies extended to extra-hippocampal temporal regions important in music perception and discrimination (Milner, 1965, Samson and Zatorre, 1994, Zatorre, 1984 but see Koike and Ishijima, 1996). Indeed, a number of neuropsychological cases indicate that intact hippocampi are not necessary for a range of musical perceptual abilities relating to timing and pitch (Esfahani-Bayerl et al., 2019), nor for learning to play new music (Cavaco et al., 2012, Valtonen et al., 2014) or discriminating pieces from closely matched ones heard a short time earlier (Finke et al., 2012). These findings are consistent with intact musical memory in patients with Alzheimer’s disease, in whom other hippocampal-dependent memories are impaired (Baird and Samson, 2009, Cuddy et al., 2015).
However, as we have seen in earlier sections, the hippocampus not being critical for a task does not prevent it from tracking relevant stimulus or behavioral variables. For example, during listening to a rich naturalistic stimulus in the absence of a task, hippocampal BOLD activity was associated with repeated occurrences of musical motifs after regressing out acoustic predictors (Burunat et al., 2014; Fig. 4I), and showed functional connectivity with regions involved in holding melodies in mind, including dorsolateral prefrontal cortex, cerebellum, and the supplementary motor area. Schmithorst (2005) also identified melody-specific activity in hippocampus using an independent components analysis of fMRI data during passive listening, and in a study of memory for newly-learned melodies retrieval success was associated with greater right hippocampal BOLD signal (Watanabe et al., 2008). As with speech there is electrophysiological evidence for greater hippocampal processing at musical phrase boundaries (Knösche et al., 2005).
Hippocampal activity and connectivity has been detected during a range of tasks during music listening, including tone detection (Lehne et al., 2014), timbre and tonality deviant detection (Janata, 2002), temporal order judgments (Mueller et al., 2015), spontaneity judgments (Engel and Keller, 2011), and memory encoding (Bonetti et al., 2021). Hippocampus is also activated during passive music listening, compared to a silent or scrambled baseline (Brown et al., 2004, Mueller et al., 2015, Mueller et al., 2011, Mutschler et al., 2010). We will see in 15, 16 that familiarity and emotional aspects of music may partly drive these responses.
15. Long-term familiarity
4, 5 covered responses to sounds that had recently acquired behavioral relevance, for example through aversive conditioning or prior exposure in a target detection task. Longer-term familiarity with an auditory stimulus also affects the magnitude of the hippocampal activity it drives. The hippocampus of rabbits, cats, and monkeys can show greater responses to familiar sounds, such as hisses, than to louder but unfamiliar synthetic sounds, such as tones and clicks (Grastyán et al., 1959, Green and Arduini, 1954, Tamura et al., 1990). Note that greater responses to familiar than unfamiliar sounds contrast with the novelty responses described in 3, 14, which presumably arise through different mechanisms operating over a shorter timescale.
While in the above studies not only the familiarity but also the gross acoustical features of the contrasted sounds differed, other work has attempted to control the latter. For example, when children listened to their mother’s voice, fMRI connectivity across hippocampus, reward- and voice-sensitive regions was greater than when listening to other female voices (Abrams et al., 2016). Long-term familiarity with verbal expressions correlated with posterior hippocampal BOLD activity; this was not the case for musical melodies presented to the same subjects (Gagnepain et al., 2017, Groussard et al., 2010b). There are other indications that the hippocampus is relatively unimportant in familiarity processing of music. Using multivoxel pattern analysis, long-term familiar songs could be distinguished from songs heard on the same day or novel songs in anterior cingulate and pre-supplementary motor area, but not in the medial temporal lobe (Jacobsen et al., 2015). Subjects listening to their favorite song showed less auditory-hippocampus connectivity than when listening to other songs in the same genre (Wilkins et al., 2014). Additionally, a study of patients with medial temporal lobe resections were impaired in verbal learning and recall but had a spared feeling of familiarity when hearing short excerpts of well-known music (Huijgen et al., 2015).
These negative findings are consistent with visual work identifying a greater dependence on hippocampus (and connected diencephalic structures) for explicit recollection of an episode, compared to general familiarity likely to be supported more by parahippocampal/perirhinal circuits (Aggleton and Brown, 2006, Brown and Aggleton, 2001, Tsivilis et al., 2008). However, for audition the story is mixed, with some studies finding greater responses in hippocampus for familiar compared to unfamiliar music (Pereira et al., 2011, Plailly et al., 2007) and deficits in recognizing well-known songs in patients with hippocampal damage (Papp et al., 2014). In addition, it is not always straightforward to isolate processes relating to familiarity from those involved in explicit recollection, as familiar sounds can elicit the recollection of particular events.
16. Emotion and sound
Also hard to dissociate from sound familiarity are sound preference and other emotional drivers of hippocampal activity, both due to “mere exposure” effects of repetition (Hunter et al., 2010, Samson and Peretz, 2005) and the fact that people choose to listen to music they enjoy. In some experiments that show greater hippocampal BOLD activity for familiar than unfamiliar music, stimulus manipulations affect both long-term familiarity and pleasantness ratings (Koelsch et al., 2006, Mueller et al., 2015, Mueller et al., 2011, Pereira et al., 2011). Particularly intense emotional responses to music can be evoked by familiar music that listeners select for themselves. Blood and Zatorre (2001) reported reduced hippocampal blood flow when listening to self-selected music experienced as intensely pleasurable, eliciting “chills” and other physiological responses, in comparison to control music selected by other subjects. This reduced response is thought to arise through inhibitory projections from the nucleus accumbens, part of the dopaminergic reward system in which activity increases during chills. These two regions demonstrate increased BOLD functional connectivity during music to which listeners assign increasing value - even new music with which they are not familiar (Salimpoor et al., 2013).
Medial temporal sites - and the focus is more parahippocampal gyrus than hippocampus proper - are involved in processing emotion in music, beyond the familiarity effects described above. Lesion (Gosselin, 2006) and neuroimaging (Blood et al., 1999, Ferri et al., 2014) data link the rating of dissonant harmonies as unpleasant to parahippocampal gyrus, paralleling work with unpleasant images (Lane et al., 1997). Diffuse medial temporal lesions affect more general estimates of pleasantness and arousal in music (Gosselin et al., 2005, Khalfa et al., 2008) and impair recognition of musical emotion (Dellacherie et al., 2008, Gosselin et al., 2011, Gosselin et al., 2005, Omar et al., 2011, Papp et al., 2014) (but see Dellacherie et al., 2011), and posterior hippocampal gray matter volume is greater in frontotemporal dementia patients with musicophilia, (an abnormal craving for and delight in music) than in those without (Fletcher et al., 2013).
Not only simple musical emotional contrasts (such as happy versus sad, joyful versus fearful) but also subtler differences involving arousal or valence alone modulate (para)hippocampal BOLD activity and its relationship with that in auditory and reward networks (Flores-Gutiérrez et al., 2007, Koelsch et al., 2018, Koelsch et al., 2013, Koelsch and Skouras, 2014, Trost et al., 2012), and individuals with high alexithymia scores show less such modulation (Koelsch et al., 2007). Some of these effects may arise through the uncertainty and surprise associated with harmonic progressions, which jointly predict pleasantness ratings and the BOLD response in regions including hippocampus as described previously (Cheung et al., 2019).
Section 12 considered hippocampal correlates of multimodal stimulus processing; these are also apparent with respect to emotionally-charged material. For example, combining emotional music and pictures elicits greater (para)hippocampal BOLD than the pictures alone (Baumgartner et al., 2006) and the presence of narrative action in a neutral film increases modulation of hippocampal activity by emotional music (Eldar et al., 2007). However, shutting off uninformative input by closing the eyes leads to more extreme valence ratings and elevated hippocampal BOLD in response to emotional music (Lerner et al., 2009). Not only the structural elements of a musical piece but also expressive performance determine its emotional profile; this too affects hippocampal activity (Chapin et al., 2010a, Engel and Keller, 2011). The involvement of hippocampus in processing emotional music occurs regardless of expertise and although it can habituate over the course of listening it does not generally require active engagement (Brown et al., 2004, Chapin et al., 2010b, Mutschler et al., 2010, Pallesen et al., 2009).
Music is not the only means of expressing emotion through sound. (Para)hippocampal areas are also sensitive to different types of laughter (Szameitat et al., 2010), as well as to prosodic (Wiethoff et al., 2008) and semantic (Bellace et al., 2012, Mitchell et al., 2003) emotion cues in speech. These sites are more sensitive to natural than to computer-generated speech (Beaucousin et al., 2006), and respond to or support processing of vocal fear, anger, happiness, surprise, disgust (Bonora et al., 2011, Fowler et al., 2006, Kotz et al., 2013, Leitman, 2010, Phillips et al., 1998, Sander et al., 2005), as well as more complex auditory emotional expression such as pride, guilt, and boredom (Alba-Ferrara et al., 2011). Several studies describe functional connectivity signatures of auditory emotion processing. For example, alarm sounds rated as highly unpleasant are associated with reduced hippocampal BOLD activity and functional connectivity consistent with suppression by the amygdala (Hirano et al., 2006). This coincides with worse encoding of a concurrent visual stimulus, in line with findings in animal models showing that stress and fear impair learning. Subjects with misophonia, for whom everyday sounds evoke strong negative emotional responses, show elevated functional connectivity between anterior insula and a salience network that includes hippocampus (Kumar et al., 2017). Individual differences in responses to aversive sounds may also have structural correlates – in one large study, subjects’ sensitivity to a noise stimulus correlated with hippocampal gray matter volume (Kliuchko et al., 2018). In another study, synthetic sounds rated as more aversive generated elevated cerebral blood flow in (para)hippocampus and amygdala (Mirz et al., 2000). Negative reactions to certain sounds may relate to the energy in the “roughness” range of 30–150 Hz; click trains presented at this rate are rated as highly salient and aversive and bring about neuronal synchronization at the presentation rate, particularly in the hippocampus and insula (Arnal et al., 2019).
17. Phantom percepts
Aversive auditory experiences do not require an external stimulus. Tinnitus (see Supplementary Table L) usually takes the form of a low-intensity, high-frequency ringing or white noise that is typically readily masked by environmental sounds but can be chronic and emotionally distressing (Jastreboff, 1990). It tends to co-occur with hearing loss, and many theories of its generation are based on maladaptive change to deafferentation, or deficient noise-canceling (Jastreboff, 1990, Noreña, 2011, Rauschecker et al., 2010, Schaette and Kempter, 2006). In-depth reviews and integrative accounts are available elsewhere (De Ridder et al., 2014, Sedley et al., 2016); here we focus on correlates in (para)hippocampus but note that comorbidity of tinnitus with hearing loss, distress, depression, cognitive dysfunction and insomnia presents a challenge in establishing specific neural signatures (Adjamian et al., 2014, Bhatt et al., 2017, Crönlein et al., 2007, Hallam et al., 2004, Hwang et al., 2009, Melcher et al., 2013). At the molecular level, damage to rat inner hair cells leads to tinnitus-like behavior only for those animals whose hippocampus and auditory cortex fail to mobilize Arc (Singer et al., 2013), a protein involved in long-term potentiation and adjusting synaptic transmission following sensory deprivation (Korb and Finkbeiner, 2011). Tinnitus-like behavior is also present in rats with noise-induced disruption to neurogenesis and cholinergic and GABAergic pathways in hippocampus (Kraus et al., 2010; L. Zhang et al., 2019; Zhang et al., 2021). In terms of gross structure, the volume of left hippocampus is smaller in patients with tinnitus than in controls matched for hearing loss (Boyen et al., 2013, Landgrebe et al., 2009) and its surface area correlates negatively with tinnitus handicap inventory scores (Tae et al., 2018). Hippocampal gray matter abnormalities have been identified in tinnitus sufferers using diffusion tensor imaging (Gunbey et al., 2015), and tinnitus symptoms have been reported after hippocampus damage or resection (Corkin et al., 1997, Kreyberg et al., 1992, Paquette et al., 2017, Rey et al., 1984).
These negative links between hippocampal structure and tinnitus stand in distinction to functional studies that often (but not always, Shulman, 1995; Shulman et al., 1995; Simonetti et al., 2022) report a positive association between hippocampal activity and tinnitus loudness or incidence (De Ridder et al., 2006, Lockwood et al., 1998). Resting state fMRI indicates greater connectivity of the hippocampus with auditory cortex and beyond for louder tinnitus percepts and longer tinnitus durations (Chen et al., 2017b, Ueyama et al., 2013), and highlights left hippocampus as a key node in functional networks of chronic tinnitus patients compared to controls (Lan et al., 2022). In contrast, functional connectivity between hippocampus and subcortical auditory nuclei is reduced in rats with salicylate-induced tinnitus and hyperacusis symptoms (Chen et al., 2015); these animals also show elevated hippocampal local field potential responses to noise-bursts compared to controls (Chen et al., 2014). Distinct neural correlates of salicylate- and noise exposure have been described and these complicate the search for a unified signature of tinnitus (Eggermont, 2013). In humans, regional blood flow in other medial temporal structures, including amygdala and parahippocampal gyrus, also differs across suppressed versus active tinnitus states (Mirz, 2000) and levels of distress (Schecklmann et al., 2013), as well as between participants with tinnitus compared to hearing-impaired and normal-hearing controls (Carpenter-Thompson et al., 2014, Laureano et al., 2014). Indeed, resting state activity in parahippocampal gyrus more consistently differentiates these groups than does activity in hippocampus proper (Chen et al., 2017a, Song et al., 2012) and may form an important tinnitus hub (De Ridder et al., 2014, Sedley et al., 2015).
Auditory hallucinations (see Supplementary Table M) are another example of sound perception in the absence of a stimulus. These have been associated with hippocampal lesions in a number of reports, but rarely isolating the hallucinations from comorbid symptoms, for example in patients with schizophrenia (Maller et al., 2012, Suzuki et al., 2003, Takebayashi et al., 2002). A similar caveat holds for early imaging studies that reported increased medial temporal lobe activity in patients with schizophrenia featuring auditory hallucinations (DeLisi et al., 1989, Friston et al., 1992, Liddle et al., 1992, Medoff et al., 2001, Volkow et al., 1987) although some studies are more specific, with greater medial temporal cerebral blood flow for auditory compared to tactile hallucinations (Musalek et al., 1989) or with longer durations of auditory hallucination (Copolov et al., 2003). Within-subjects comparisons have revealed greater (para)hippocampal activity for periods with auditory hallucinations compared to those without (Dierks et al., 1999, Raij et al., 2009, Shergill, 2001, Shergill et al., 2000, Silbersweig et al., 1995). As with tinnitus, compared to hippocampus proper (Bentaleb et al., 2002, Jardri et al., 2009, Jardri et al., 2007, Lennox et al., 2000, McGuire et al., 1993, Sommer et al., 2008), the involvement of parahippocampal gyrus is most reliable across studies (Diederen et al., 2010, Hoffman et al., 2008, Jardri et al., 2011). In both cases parahippocampal gyrus may convey aberrant predictions originating in hippocampus to auditory cortex, but the relative lack of corresponding hippocampus activity remains to be explained. The presence of auditory auras in a subset of patients with mesial temporal lobe epilepsy is also relevant (Asadi-Pooya et al., 2016, Ferrari-Marinho et al., 2012). One striking case is that of a patient with a hippocampal seizure focus, who experienced ringing in both ears prior to seizures. Electrical stimulation of the hippocampus generated the same percept, which did not recur once the tissue was resected (Kumar et al., 2022).
18. Short-term effects of sound on non-auditory tasks and hippocampal activity
We have described immediate responses to sound in the hippocampus and its possible involvement in a range of auditory tasks, functions and percepts. In Section 2 we also outlined effects of electrically or optogenetically stimulating hippocampus on auditory brain regions. Here we cover cases in which sound stimulation instigates, boosts or entrains hippocampal oscillations to affect non-auditory cognitive function. We consider oscillations over three timescales, as shown in Fig. 5. First, slow (< 1 Hz) oscillations reflect global fluctuations in cellular excitability - alternating phases of hyperpolarization and depolarization across large populations of neurons. In humans these oscillations are likely driven by prefrontal cortex but propagate via parahippocampal and entorhinal cortex to hippocampus (Nir et al., 2011) where they can synchronize ripple events (Born and Wilhelm, 2012). Introducing short bursts of pink noise at the peak of the up state of these oscillations during sleep increases their amplitude and leads to improved memory encoding the following day (Ngo et al., 2013; Fig. 5A). It has not been possible to directly record from hippocampus during overnight auditory stimulation, however in one study the pink noise protocol was applied during a nap, and a picture encoding task was performed subsequently in a scanner (Ong et al., 2018). The magnitude of slow oscillation enhancement during the nap correlated with picture encoding success and with hippocampal BOLD during encoding. This may indicate that boosting slow oscillations allowed previous memories to be consolidated to cortex, freeing up hippocampal resources for subsequent encoding.
Fig. 5.
Auditory entrainment of hippocampal rhythms (see Section 18 of main text for related behavioral outcomes). (A) Suitably timed white noise bursts (black) boost widespread cortical slow (< 1 Hz) oscillations (red), which propagate to hippocampus and synchronize sharp-wave ripples (not shown) in humans (Ngo et al., 2013). (B) Dichotically presented pure tones separated in frequency by 5 Hz generate binaural beats (black) and boost hippocampal theta oscillations (red) in humans (Derner et al., 2018). (C) 40 Hz click trains (black) affect firing of hippocampal CA1 units (red dots), increasing phase synchrony (blue arrows) at the same (gamma) frequency in mice (Martorell et al., 2019).
The second timescale of oscillations at which auditory entrainment may boost hippocampal function is the theta range. Roberts et al. (2018) presented audiovisual stimuli at either 5.5 Hz or 14 Hz, or white noise, between training and testing of verbal memory. In the 5.5-Hz condition only, theta power recorded at the scalp was enhanced both during entrainment and subsequent retrieval, and hippocampus-dependent source memory was selectively boosted. Other studies have generated binaural beats in the theta range by presenting pure tones of frequencies differing by 5 Hz to separate ears, then directly measuring hippocampal activity in neurosurgical patients (Derner et al., 2021, Derner et al., 2020, Derner et al., 2018; Fig. 5B). Binaural beats were associated with improved source and item memory along with increased phase synchrony and unit firing in human (para)hippocampus, with firing rate differences between monaural and binaural beat conditions correlating across subjects with differences in memory performance.
Third, 40-Hz click trains or stimuli amplitude-modulated at this rate have long been known to elicit a strong steady state response in human auditory cortex (ASSR, Galambos et al., 1981). However they also modulate unit activity in mouse hippocampus (as well as auditory cortex and medial prefrontal cortex) such that firing tends to cluster at fixed phases of the 40-Hz cycle (Martorell et al., 2019; Fig. 5C). Remarkably, click trains presented at this rate (but not with random timing) reduced signs of Alzheimer's pathology (amyloid load and tau phosphorylation) in the hippocampus of this mouse model and boosted memory for the identity and location of objects. Effects were larger when the auditory stimulus was paired with visual flicker at the same rate - this combined stimulation also induced an increase in 40 Hz power and a clustering effect of microglia around amyloid deposits. The implications for dementia treatment are considerable, and work is ongoing to test the same approach in human trials (Chan et al., 2021). The mechanism is not yet understood but networks of inhibitory interneurons generate peaks at this low-gamma frequency, which may also be involved in coupling CA3 and CA1 during memory retrieval (Colgin et al., 2009, Mably and Colgin, 2018).
19. Long-term experience with sound and its absence
Having considered effects of short-term auditory exposure we turn now to longer-term experience with sound and its positive and negative effects on hippocampal structure and function (see Supplementary Table N and Fig. 6). If the hippocampus maps out non-spatiotemporal dimensions as suggested in Section 10 then experts at navigating such dimensions might be expected to demonstrate gross anatomical differences, given the finding of Maguire et al. (2000) that London taxi drivers have enlarged posterior hippocampi. Such associations have been found, with positive relationships between years of training and anterior hippocampal gray matter volume in musicians (Groussard et al., 2014, Groussard et al., 2010a) and piano-tuners (Teki et al., 2012). For spatial navigation and other hippocampus-dependent tasks, the generalizability of a volume-function relationship to a non-expert population has not been demonstrated (Clark et al., 2020, Weisberg and Ekstrom, 2021). However with respect to music, the volume of parahippocampal gyrus (hippocampus was not tested) was found to correlate positively with an index of musical sophistication in a group of 73 older adults after controlling for intracranial volume (Chaddock-Heyman et al., 2021). Longitudinal studies, similar to those in taxi drivers (Woollett and Maguire, 2011), will be important to establish causality in any of the above relationships.
Fig. 6.
Examples of processes at molecular, synaptic, neuronal and gross structural hippocampal levels on which auditory experience (such as music listening or training, noise exposure, and auditory deprivation) can act. See Section 19 of main text and Supplementary Table N for details and references. The causal pathways underlying such effects are largely yet to be established.
Functional differences in hippocampus of musicians and non-musicians have been identified through task-related BOLD activity and connectivity (Alluri et al., 2017, Alluri et al., 2015, Burunat et al., 2018, Chapin et al., 2010a, Gagnepain et al., 2017) as well as in scalp EEG responses, for example to incongruous harmonic endings to phrases (James et al., 2008). These cross-sectional studies again may to some extent reflect pre-existing group differences in personality traits, socio-economic status or cognitive factors (Corrigall et al., 2013, Orsmond and Miller, 1999). In one longitudinal study, right hippocampal BOLD activity during music listening and imagery was positively correlated with success in a subsequent six-week piano training course; this marker of predisposition contrasted with cerebellar and fronto-parietal activity that increased over the course of that training (Herholz et al., 2016). In another study, tone patterns containing rhythmic deviants were presented to musicians before and after two semesters of university-level musical training, and to a control group of musicians receiving no such additional training (Herdener et al., 2010). Greater left anterior hippocampal responses to deviants occurred in the second session, only for the group receiving training. A positive relationship between musical aptitude and degree of activity in that same region was also found in cross-sectional analysis of a separate group. Taken together, the results suggest that at least some music-related differences in hippocampal function arise from training rather than innate ability.
The eye-catching finding that listening to a Mozart sonata improves spatial reasoning (Rauscher et al., 1993), an effect later established to be a rather non-specific consequence of arousal (Pietschnig et al., 2010, Thompson et al., 2001), led to interest in whether passive music exposure brings about hippocampal changes in animal models. Such exposure, especially prenatally or in development, can result in differential gene expression and regulation, elevated markers of neurogenesis, and changes in synaptic density and regulation in rodent (Angelucci et al., 2007, Chikahisa et al., 2006, Kim et al., 2006, Lee et al., 2016, Meng et al., 2009, Xing et al., 2016a) and avian (Chaudhury et al., 2010, Chaudhury et al., 2008, Chaudhury et al., 2006, Chaudhury and Wadhwa, 2009) hippocampus (but see Rizzolo et al., 2021). These effects are often accompanied by functional improvements, including in spatial (e.g. Xing et al., 2016a) and fear learning (e.g. Meng et al., 2009) tasks. Findings are mixed as to whether particular musical elements, such as rhythmic structure, versus more general auditory arousal, are responsible for the behavioral and neuronal changes (Angelucci et al., 2007, Chaudhury et al., 2008, Chaudhury et al., 2006, Field et al., 2007, Hu et al., 2014, Kirste et al., 2015, Sanyal et al., 2013a, Sanyal et al., 2013b, Xing et al., 2016b, Yang et al., 2014b, Yang et al., 2014a). Understanding how and why levels of the protein brain-derived neurotrophic factor increase through music exposure and engagement may be key to this question (Brattico et al., 2021).
In contrast to positive effects of music listening, noise exposure elevates hippocampal stress hormone levels (Barzegar et al., 2015, Britton et al., 1992, Campeau and Watson, 1997, Ferrarese et al., 1991, Gai et al., 2017, Jáuregui-Huerta et al., 2011, Jin et al., 2017) and markers of oxidative stress (Ambrosini et al., 2005, Cheng et al., 2016, Cheng et al., 2011, Manikandan et al., 2006, Uran et al., 2014), often resulting in accelerated cell death and reduced neurogenesis (Cui et al., 2009, Cui et al., 2013, Frenzilli et al., 2017, Gonzalez-Perez et al., 2011; B.-K. Kim et al., 2013; Kim et al., 2006; Kraus et al., 2010; Liu et al., 2010; Manikandan et al., 2006; Säljö et al., 2000, Säljö et al., 2002; Uran et al., 2012). Noise-induced reductions in NMDA receptors and related protein expression (Cui et al., 2013, Cui et al., 2009, Kapolowicz and Thompson, 2016, Singer et al., 2013) impair hippocampal long term potentiation (Barzegar et al., 2015, Cunha et al., 2018, Cunha et al., 2015, de Deus et al., 2017) and learning that depends on it (Barzegar et al., 2015, Cheng et al., 2011, Cui et al., 2009, Cui et al., 2012a, Cunha et al., 2015, Cunha et al., 2018, de Deus et al., 2017, de Deus et al., 2021, Di and Qin, 2018, Haider et al., 2012; B.-K. Kim et al., 2013; Kim et al., 2006; Manikandan et al., 2006; Uran et al., 2010, Uran et al., 2012, Uran et al., 2014). Spatial memory deficits may also result through destabilization of place cell receptive fields (Goble et al., 2009). A range of neurotransmitters and other markers of neural activity in the hippocampus are sensitive to noise exposure (Campeau and Watson, 1997, Cui et al., 2009, Cui et al., 2012a, de Deus et al., 2021, Di and Qin, 2018, Fernandes and File, 1993, Haider et al., 2012, Lai, 1987, Lai, 1988, Lai et al., 1989, Lai and Carino, 1990, Manikandan et al., 2006), as is DNA integrity (Frenzilli et al., 2017). Abnormalities in glial cells and levels of their activating proteins have also been described following noise presentation (Cui et al., 2015, Frenzilli et al., 2017, Huet-Bello et al., 2017) as have signatures of Alzheimer’s disease pathology, including tau hyperphosphorylation (Cheng et al., 2011, Cui et al., 2015, Cui et al., 2013, Cui et al., 2012a, Cui et al., 2012b, Frenzilli et al., 2017, Gai et al., 2017) and elevated amyloid-β and pro-inflammatory protein levels (Cui et al., 2015, Jafari et al., 2019).
Some of these changes depend on the duration (Barzegar et al., 2015, Cheng et al., 2011) or level (Hosseini-Sharifabad and Sabahi, 2008, Matt et al., 2018, Singer et al., 2013) of the noise, others can reverse over time (Cui et al., 2012a, Di and Qin, 2018, Frenzilli et al., 2017) or be protected against by various compounds (Abousetta et al., 2014, Alinaghipour et al., 2022, Azman et al., 2016, Li et al., 2014, Sundaramahalingam et al., 2013; S. Wang et al., 2016) or exercise (T.-W. Kim et al., 2013). Many are not specific to sound, arising due to a range of stressors (Chen et al., 2010; B.-K. Kim et al., 2013; Kim and Diamond, 2002). Given rodent work showing that rapid subcortical auditory pathways are more important for conveying noise than tone presence to hippocampus (Zhang et al., 2019), it will be important to establish whether such pathways also exist in humans to judge the clinical relevance of these findings. More in-depth reviews of the effects of noise on hippocampus are available elsewhere (Kraus and Canlon, 2012, Manukyan, 2022, Nadhimi and Llano, 2021, Zhang et al., 2022).
While noise exposure can cause deafness, most acquired hearing loss occurs gradually over the lifetime. Age-related hearing loss (presbycusis) is an independent risk factor for dementia, estimated to account for 9% of cases (Livingston et al., 2017). The hippocampus and entorhinal cortex are among the earliest sites showing dysfunction and atrophy in Alzheimer's disease (Braak and Braak, 1991, Khan et al., 2014), and poorer hearing in midlife is associated with steeper volumetric declines in these regions later in life (Armstrong et al., 2019). A large longitudinal study also found that individuals developing a hearing loss between scans an average of two years apart had greater decline in left hippocampal gray matter volume than those developing no such hearing loss (Fitzhugh and Pa, 2022). This group also showed a greater decrease in functional connectivity over time between auditory cortex and hippocampus (see also Andin, Holmer, 2022 for comparable results in individuals who are deaf from birth compared to controls). Connectivity in subjects with presbycusis is also disrupted between hippocampus and the inferior parietal lobule, to an extent that correlates with the degree of working memory impairment (Chen et al., 2020). The nature of any causal link between age-related hearing loss (or other listening impairments) and the hippocampal pathology and cognitive decline associated with dementia has not been fully established (Griffiths et al., 2020, Nadhimi and Llano, 2021, Tuwaig et al., 2017) but recent animal work offers some clues. The C57BL/6 mouse exhibits progressive hearing impairment and is widely used as a model for presbycusis. These animals, which also demonstrate impaired spatial behavior, suffer synaptic degeneration, decreased cell numbers and abnormal morphology in hippocampal CA1 and CA3 as well as altered neurotransmitter receptor expression (Beckmann et al., 2020, Dong et al., 2018, Yu et al., 2011). In studies with otherwise healthy mice, occluding ears to simulate conductive hearing loss also impairs hippocampal neurogenesis and increases microglial invasion and stress responses (Kurioka et al., 2021). Temporary conductive hearing loss can also be induced through ear drum perforation; in rats this interferes with NMDA receptor-mediated currents in CA1, reducing local field potentials and impairing spatial learning (Zhao et al., 2018). Finally, administration of ototoxic drugs to induce sensorineural hearing loss leads to hippocampal degeneration, impaired spatial learning, and tau phosphorylation (Y. Shen et al., 2021). Whether similar effects occur in humans is not yet known.
20. Synthesis and outstanding questions
We have reviewed imaging, recording, lesion and neuropsychological work across species, charting diverse interactions between sound and the hippocampus. These include a hierarchy of responses, from reacting non-selectively during passive listening, through tracking associations between particular sounds and rewards, to mapping out auditory dimensions during behavior. We identified hippocampal involvement in linking sounds with each other, with stimuli in other sensory modalities, and with spatiotemporal context to form episodic memories. We also detailed roles of the hippocampus in processing music, speech, emotional sound, and aberrant auditory percepts, as well as how hippocampal structure and function can be shaped by auditory experience. We described how the machinery that supports spatial navigation and memory may be harnessed in more general sequential processing, including that relating to sound. Questions remain as to the extent to which the hippocampus helps build representations of auditory objects and scenes, which are structured in time, frequency and space. However, the synthesis of auditory-hippocampal interaction that emerges does not support an adequate account of hippocampal function based on spatial navigation and episodic memory. The auditory work requires an explanation based on broader aspects of perception and cognition.
What does it mean to say the hippocampus is “involved in” a function? It may not be critical for a given task, but the stimulus and behavioral information it tracks becomes available either for an optimal solution to the current problem, or as part of a more complex representation than is available upstream and/or that may be drawn on subsequently. Although single-neuron tuning to specific auditory features is most pronounced when they are behaviorally relevant (Aronov et al., 2017, Itskov et al., 2012) we have also identified plenty of cases of hippocampal responses to sound during passive listening. Determining the critical dependency of certain auditory computations and functions on the hippocampus will require a combination of optogenetic techniques in animal models to temporarily shut down pathways, invasive and non-invasive stimulation in humans, and administering standardized batteries of auditory cognition (e.g. those previously applied to dementia patients, Hardy et al., 2020) to groups with particularly circumscribed hippocampal damage (e.g. autoimmune limbic encephalitis, Lad et al., 2019).
What characteristics of hippocampal circuits might lend themselves to auditory cognition? A non-exhaustive list could include: theta phase precession to track past, present and future in sound sequences; a monosynaptic pathway that can learn probabilistic contingencies between sounds and other stimuli; the means to separate patterns of signals arising from distinct auditory objects or in different contexts through sparseness and inhibition in dentate gyrus; the means to compare stored representations in CA3 with, or to complete, or predict from, current auditory input; connectivity with subcortical and cortical sites that provide auditory information processed with respect to distinct features or to different extents.
A number of steps can be taken to further test the idea that circuits in hippocampus and entorhinal cortex that support memory and navigation in physical space can be harnessed for auditory processing. Neuroimaging work that has indirectly found evidence for grid-cell like structures supporting non-spatial navigation (e.g. of semantic spaces) could be extended to cover auditory dimensions. Human intracranial recordings may provide more direct evidence for tuning to auditory features if coupled with the right task. Methodological advances in magnetic sensory technology, headcasts and source localization may facilitate temporally resolved magnetoencephalographic hippocampal recordings during listening (Alberto et al., 2021, Hill et al., 2020, Meyer et al., 2017, Pizzo et al., 2019, Recasens et al., 2018, Tierney et al., 2021).
An important outstanding question is the extent to which spatial coding and processing in the hippocampus dominates over other dimensions of experience. O'Keefe & Krupic (2021) argue that cells responding selectively to non-spatial stimuli are in fact feature-in-place cells, at least in non-humans; for humans they argue the spatial cognitive map has been enhanced by language and its metaphorical use. That is, responses that appear to be determined by stimulus characteristics are in fact primarily driven by the animal's spatial location - but this is masked because animals are not typically tested in different locations. This argument finds support in the Itskov et al. (2012) result that tuning to a particular sound only held when the animal's location was fixed. Also relevant is a finding that hippocampal responses during eyeblink trace conditioning only occurred in cells in whose place field the animal was situated, with conditioning leading to an arousal-related enhancement in firing (Shan et al., 2016). However, Mount et al. (2021) identified different hippocampal populations responding during acquisition versus extinction of trace conditioning. This suggests that arousal-modulated place tuning is unlikely alone to account for selective responses to particular sounds in individual cells.
Relatedly, is the hippocampus only engaged in forming auditory scenes or objects when there is an element of spatial variation rather than when spectral and/or temporal information alone are present? Experiments are needed in which subjects have to perceive, remember or mentally construct combinations of sounds composed of different feature conjunctions (such as a sound with a high amplitude modulation rate and low carrier frequency, followed by one with the reverse configuration). Comparable experiments with visual objects by Maguire and colleagues (Dalton et al., 2018, Zeidman et al., 2015) have been important in establishing the nature of hippocampal involvement in visual scene processing.
More straightforward analogs of experiments in other sensory modalities can answer pressing questions that have already been addressed for olfaction (in rodents) and vision (in humans and non-human primates), and that might be even more relevant for audition for which stimulation always unfolds over time. For example, does the phase at which rat hippocampal neurons fire for sequential sounds precess as the sequence unfolds (c.f. Terada et al., 2017)? Can calcium imaging reveal sparse encoding of behaviorally irrelevant auditory stimuli in mouse dentate gyrus (c.f. Woods et al., 2020)? Do sharp wave ripples in human hippocampus during auditory memory tasks reflect content-specific encoding, retrieval, replay and pre-play, in partnership with cortex (c.f. Norman et al., 2019)? Can predicted auditory content be decoded from human hippocampus and does this depend on stimulus complexity (c.f. Kok et al., 2020)? In addition, some proposed hippocampal coding schemes, based on spatiotemporal similarity (Turk-Browne, 2019), or modality/exemplar-invariant concepts (Quiroga et al., 2009) have not yet been tested against substantial auditory data.
We have seen how rhythmically structured sound at a range of timescales provides a non-invasive means of driving or entraining neural oscillations, which hippocampus plays a role in orchestrating across cortex. There are promising signs that such auditory interventions can improve memory and even disrupt pathology in animal models of disease. Compared to electrical, magnetic and invasive stimulation, an acoustic approach is accessible, inexpensive and generally non-intrusive/intimidating. To harness its full potential it will be important to understand how the auditory pathway and hippocampus work together with other regions including prefrontal cortex in bringing about these changes. More broadly, although we have focused on the hippocampus throughout this piece, considering such a richly connected structure in isolation will have only given part of the picture - whether with respect to conditioning, sound sequences, spatial and temporal aspects of sound, auditory memory, musical emotion, or phantom percepts.
If the findings from animal models concerning links between hippocampus, hearing loss, tinnitus and dementia are to translate into to clinical advances in humans it is important that we understand the degree of overlap between species in terms of anatomy, connectivity, physiology, and function. Recent results are reassuring in this regard. For example the identification of human hippocampal place cells, time cells, and phase precession - originally discovered in rodents - indicate that at least some of the coding and computational principles overlap. At the same time, post-mortem anatomical data on human auditory-hippocampal pathways is almost non-existent. We also acknowledge that different species evolved in the context of unique environmental constraints, with a range of sensory and higher-level capabilities (Basile et al., 2020). Despite these varied contexts under which the hippocampus operates, there are considerable areas of convergence in the auditory results we have reported. Another caveat is that we have surveyed literature linking sound and hippocampus as comprehensively as possible, but have not systematically reviewed each sub-topic in a meta-analytical manner, nor included every study in which no hippocampal involvement was reported. Findings should be interpreted accordingly and we hope that this review provides a launching point for further study.
In conclusion, the hippocampus receives all manner of auditory information regardless of its behavioral relevance at the time. Any tuning to acoustic features or criticality of involvement is strongest when there is a requirement to associate sounds with locations, rewards or punishments separated in time, other sounds, or stimuli in other sensory modalities - either for perception or memory. The structural, synaptic and biochemical components that facilitate such processing are themselves sensitive to the organism's auditory experience - both in the short and long-term.
Acknowledgments
This work was supported by the Wellcome Trust, United Kingdom (WT106964MA), Medical Research Council, United Kingdom (MR/T032553/1), and the National Institutes of Health, United States of America (5P50DC000242-35).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pneurobio.2022.102326.
Appendix A. Supplementary material
Supplementary material
.
References
- Abe, R., Sakaguchi, T., Kitajo, K., Ishikawa, D., Matsumoto, N., Matsuki, N., Ikegaya, Y., 2014a. Sound-induced modulation of hippocampal θ oscillations.: NeuroReport 25 2014a 1368 1374 doi: 10.1097/WNR.0000000000000274. [DOI] [PubMed]
- Abe, R., Sakaguchi, T., Matsumoto, N., Matsuki, N., Ikegaya, Y., 2014b. Sound-induced hyperpolarization of hippocampal neurons: NeuroReport 25, 1013–1017. https://doi.org/10.1097/WNR.0000000000000206. [DOI] [PubMed]
- Abousetta A., Makhlouf N.A., El-Beshbishy R.A. The effects of concomitant Ginkgo intake on noise induced Hippocampus injury. Possible auditory clinical correlate. Egypt. J. Ear Nose Throat Allied Sci. 2014;15:231–239. doi: 10.1016/j.ejenta.2014.05.003. [DOI] [Google Scholar]
- Abrams D.A., Chen T., Odriozola P., Cheng K.M., Baker A.E., Padmanabhan A., Ryali S., Kochalka J., Feinstein C., Menon V. Neural circuits underlying mother’s voice perception predict social communication abilities in children. Proc. Natl. Acad. Sci. U.S.A. 2016;113:6295–6300. doi: 10.1073/pnas.1602948113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adey W.R., Dunlop C.W., Hendrix C.E. Hippocampal slow waves: distribution and phase relationships in the course of approach learning. Arch. Neurol. 1960;3:74. doi: 10.1001/archneur.1960.00450010074007. [DOI] [PubMed] [Google Scholar]
- Adjamian P., Hall D.A., Palmer A.R., Allan T.W., Langers D.R.M. Neuroanatomical abnormalities in chronic tinnitus in the human brain. Neurosci. Biobehav. Rev. 2014;45:119–133. doi: 10.1016/j.neubiorev.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton J.P., Brown M.W. Interleaving brain systems for episodic and recognition memory. Trends Cogn. Sci. 2006;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Ahmed M.S., Priestley J.B., Castro A., Stefanini F., Solis Canales A.S., Balough E.M., Lavoie E., Mazzucato L., Fusi S., Losonczy A. Hippocampal network reorganization underlies the formation of a temporal association memory. Neuron. 2020;107(283–291) doi: 10.1016/j.neuron.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken F., Kok P. Hippocampal representations switch from errors to predictions during acquisition of predictive associations. Nat. Commun. 2022;13:3294. doi: 10.1038/s41467-022-31040-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C., Arnott S.R., Picton T.W. Bottom-up and top-down influences on auditory scene analysis: evidence from event-related brain potentials. J. Exp. Psychol.: Hum. Percept. Perform. 2001;27:1072–1089. doi: 10.1037//0096-1523.27.5.1072. [DOI] [PubMed] [Google Scholar]
- Alba-Ferrara L., Hausmann M., Mitchell R.L., Weis S. The neural correlates of emotional prosody comprehension: disentangling simple from complex emotion. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberto G.E., Stapleton-Kotloski J.R., Klorig D.C., Rogers E.R., Constantinidis C., Daunais J.B., Godwin D.W. MEG source imaging detects optogenetically-induced activity in cortical and subcortical networks. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-25481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alinaghipour A., Ashabi G., Riahi E., Soheili M., Salami M., Nabavizadeh F. Effects of nano-curcumin on noise stress-induced hippocampus-dependent memory impairment: behavioral and electrophysiological aspects. Pharmacol. Rep. 2022;74:461–469. doi: 10.1007/s43440-022-00354-3. [DOI] [PubMed] [Google Scholar]
- Alluri V., Brattico E., Toiviainen P., Burunat I., Bogert B., Numminen J., Kliuchko M. Musical expertise modulates functional connectivity of limbic regions during continuous music listening. Psychomusicology: Music Mind Brain. 2015;25:443–454. doi: 10.1037/pmu0000124. [DOI] [Google Scholar]
- Alluri V., Toiviainen P., Burunat I., Kliuchko M., Vuust P., Brattico E. Connectivity patterns during music listening: evidence for action-based processing in musicians: Connectivity Patterns During Music Listening. Hum. Brain Mapp. 2017;38:2955–2970. doi: 10.1002/hbm.23565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altafullah I., Halgren E., Stapleton J.M., Crandall P.H. Interictal spike-wave complexes in the human medial temporal lobe: Typical topography and comparisons with cognitive potentials. Electroencephalogr. Clin. Neurophysiol. 1986;63:503–516. doi: 10.1016/0013-4694(86)90138-0. [DOI] [PubMed] [Google Scholar]
- Alvernhe A., Save E., Poucet B. Local remapping of place cell firing in the Tolman detour task. Eur. J. Neurosci. 2011;33:1696–1705. doi: 10.1111/j.1460-9568.2011.07653.x. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Insausti R., Cowan W.M. Evidence for a direct projection from the superior temporal gyrus to the entorhinal cortex in the monkey. Brain Res. 1983;275:263–277. doi: 10.1016/0006-8993(83)90987-3. [DOI] [PubMed] [Google Scholar]
- Ambrosini M.V., Mariucci G., Tantucci M., Van Hooijdonk L., Ammassari-Teule M. Hippocampal 72-kDa heat shock protein expression varies according to mice learning performance independently from chronic exposure to stress. Hippocampus. 2005;15:413–417. doi: 10.1002/hipo.20069. [DOI] [PubMed] [Google Scholar]
- Anderson M.I., Jeffery K.J. Heterogeneous modulation of place cell firing by changes in context. J. Neurosci. 2003;23:8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andin J., Holmer E. Reorganization of large-scale brain networks in deaf signing adults: The role of auditory cortex in functional reorganization following deafness. Neuropsychologia. 2022;166 doi: 10.1016/j.neuropsychologia.2021.108139. [DOI] [PubMed] [Google Scholar]
- Angelucci, F., Fiore, M., Ricci, E., Padua, L., Sabino, A., Tonali, P.A., 2007. Investigating the neurobiology of music: brain-derived neurotrophic factor modulation in the hippocampus of young adult mice: Behavioural Pharmacology 18, 491–496. https://doi.org/10.1097/FBP.0b013e3282d28f50. [DOI] [PubMed]
- Armstrong N.M., An Y., Doshi J., Erus G., Ferrucci L., Davatzikos C., Deal J.A., Lin F.R., Resnick S.M. Association of midlife hearing impairment with late-life temporal lobe volume loss. JAMA Otolaryngol. Neck Surg. 2019;145:794. doi: 10.1001/jamaoto.2019.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal L.H., Kleinschmidt A., Spinelli L., Giraud A.-L., Mégevand P. The rough sound of salience enhances aversion through neural synchronisation. Nat. Commun. 2019:10. doi: 10.1038/s41467-019-11626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D., Nevers R., Tank D.W. Mapping of a non-spatial dimension by the hippocampal–entorhinal circuit. Nature. 2017;543:719–722. doi: 10.1038/nature21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi-Pooya A.A., Nei M., Sharan A., Sperling M.R. Auras in patients with temporal lobe epilepsy and mesial temporal sclerosis. J. Neurol. Sci. 2016;364:24–26. doi: 10.1016/j.jns.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Azman K.F., Zakaria R., Abdul Aziz C.B., Othman Z. Tualang honey attenuates noise stress-induced memory deficits in aged rats. Oxid. Med. Cell. Longev. 2016;2016:1–11. doi: 10.1155/2016/1549158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C., Vecchio F., Mirabella G., Buttiglione M., Sebastiano F., Picardi A., Di Gennaro G., Quarato P.P., Grammaldo L.G., Buffo P., Esposito V., Manfredi M., Cantore G., Eusebi F. Hippocampal, amygdala, and neocortical synchronization of theta rhythms is related to an immediate recall during rey auditory verbal learning test. Hum. Brain Mapp. 2009;30:2077–2089. doi: 10.1002/hbm.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A., Samson S. Memory for music in Alzheimer’s disease: unforgettable? Neuropsychol. Rev. 2009;19:85–101. doi: 10.1007/s11065-009-9085-2. [DOI] [PubMed] [Google Scholar]
- Baker C.M., Burks J.D., Briggs R.G., Milton C.K., Conner A.K., Glenn C.A., Sali G., McCoy T.M., Battiste J.D., O’Donoghue D.L., Sughrue M.E. A connectomic atlas of the human cerebrum—chapter 6: the temporal lobe. Oper. Neurosurg. 2018;15:S245–S294. doi: 10.1093/ons/opy260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano C., Chen J., Zadbood A., Pillow J.W., Hasson U., Norman K.A. Discovering event structure in continuous narrative perception and memory. Neuron. 2017;95(709–721) doi: 10.1016/j.neuron.2017.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S.J., Brown T.H. Perirhinal cortex supports acquired fear of auditory objects. Neurobiol. Learn. Mem. 2009;92:53–62. doi: 10.1016/j.nlm.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks M.I., Krause B.M., Berger D.G., Campbell D.I., Boes A.D., Bruss J.E., Kovach C.K., Kawasaki H., Steinschneider M., Nourski K.V. Functional geometry of auditory cortical resting state networks derived from intracranial electrophysiology. bioRxiv. 2022 doi: 10.1101/2022.02.06.479292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banquet J.-P., Gaussier P., Cuperlier N., Hok V., Save E., Poucet B., Quoy M., Wiener S.I. Time as the fourth dimension in the hippocampus. Prog. Neurobiol. 2021;199 doi: 10.1016/j.pneurobio.2020.101920. [DOI] [PubMed] [Google Scholar]
- Bao X., Gjorgieva E., Shanahan L.K., Howard J.D., Kahnt T., Gottfried J.A. Grid-like neural representations support olfactory navigation of a two-dimensional odor space. Neuron. 2019;102(1066–1075) doi: 10.1016/j.neuron.2019.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barascud, N., Pearce, M.T., Griffiths, T.D., Friston, K.J., Chait, M., 2016. Brain responses in humans reveal ideal observer-like sensitivity to complex acoustic patterns. Proceedings of the National Academy of Sciences 113, E616–E625. 10.1073/pnas.1508523113. [DOI] [PMC free article] [PubMed]
- Barbeau E.J., Felician O., Joubert S., Sontheimer A., Ceccaldi M., Poncet M. Preserved visual recognition memory in an amnesic patient with hippocampal lesions. Hippocampus. 2005;15:587–596. doi: 10.1002/hipo.20079. [DOI] [PubMed] [Google Scholar]
- Barense M.D., Henson R.N.A., Lee A.C.H., Graham K.S. Medial temporal lobe activity during complex discrimination of faces, objects, and scenes: effects of viewpoint. Hippocampus. 2009;20:389–401. doi: 10.1002/hipo.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron H.C., Auksztulewicz R., Friston K. Prediction and memory: a predictive coding account. Prog. Neurobiol. 2020;192 doi: 10.1016/j.pneurobio.2020.101821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar M., Sajjadi F.S., Talaei S.A., Hamidi G., Salami M. Prenatal exposure to noise stress: anxiety, impaired spatial memory, and deteriorated hippocampal plasticity in postnatal life: prenatal sound stress and cognition. Hippocampus. 2015;25:187–196. doi: 10.1002/hipo.22363. [DOI] [PubMed] [Google Scholar]
- Başar E., Demir N., Gönder A., Ungan P. Combined dynamics of EEG and evoked potentials I. Studies of simultaneously recorded EEG-EPograms in the auditory pathway, reticular formation, and hippocampus of the cat brain during the waking stage. Biol. Cybern. 1979;34:1–19. doi: 10.1007/BF00336852. [DOI] [PubMed] [Google Scholar]
- Başar E., Demiralp T. Fast rhythms in the hippocampus are a part of the diffuse gamma-response system. Hippocampus. 1995;5:240–241. doi: 10.1002/hipo.450050311. [DOI] [PubMed] [Google Scholar]
- Başar E., Durusan R., Gönder A., Ungan P. Combined dynamics of EEG and evoked potentials II. Studies of simultaneously recorded EEG-EPograms in the auditory pathway, reticular formation, and hippocampus of the cat brain during sleep. Biol. Cybern. 1979;34:21–30. doi: 10.1007/BF00336853. [DOI] [PubMed] [Google Scholar]
- Başar E., Özesmi Ç. The hippocampal EEG-activity and a systems analytical interpretation of averaged evoked potentials of the brain. Kybernetik. 1972;12:45–54. doi: 10.1007/BF00289236. [DOI] [PubMed] [Google Scholar]
- Başar E., Ungan P. A component analysis and principles derived for the understanding of evoked potentials of the brain: studies in the hippocampus. Kybernetik. 1973;12:133–140. doi: 10.1007/BF00289165. [DOI] [PubMed] [Google Scholar]
- Başar-Eroglu C., Başar E., Schmielau F. P300 in freely moving cats with intracranial electrodes. Int. J. Neurosci. 1991;60:215–226. doi: 10.3109/00207459109080641. [DOI] [PubMed] [Google Scholar]
- Basile B.M., Templer V.L., Gazes R.P., Hampton R.R. Preserved visual memory and relational cognition performance in monkeys with selective hippocampal lesions. Sci. Adv. 2020 doi: 10.1126/sciadv.aaz0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann S., Joly O., Rees A., Petkov C.I., Sun L., Thiele A., Griffiths T.D. The topography of frequency and time representation in primate auditory cortices. eLife. 2015;4 doi: 10.7554/eLife.03256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T., Lutz K., Schmidt C.F., Jäncke L. The emotional power of music: How music enhances the feeling of affective pictures. Brain Res. 2006;1075:151–164. doi: 10.1016/j.brainres.2005.12.065. [DOI] [PubMed] [Google Scholar]
- Beaucousin V., Lacheret A., Turbelin M.-R., Morel M., Mazoyer B., Tzourio-Mazoyer N. FMRI study of emotional speech comprehension. Cereb. Cortex. 2006;17:339–352. doi: 10.1093/cercor/bhj151. [DOI] [PubMed] [Google Scholar]
- Beckmann D., Feldmann M., Shchyglo O., Manahan-Vaughan D. Hippocampal synaptic plasticity, spatial memory, and neurotransmitter receptor expression are profoundly altered by gradual loss of hearing ability. Cereb. Cortex. 2020 doi: 10.1093/cercor/bhaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T.E.J., Muller T.H., Whittington J.C.R., Mark S., Baram A.B., Stachenfeld K.L., Kurth-Nelson Z. What is a cognitive map? Organizing knowledge for flexible behavior. Neuron. 2018;100:490–509. doi: 10.1016/j.neuron.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Bellace M., Williams J.M., Mohamed F.B., Faro S.H. An fMRI study of the activation of the hippocampus by emotional memory. Int. J. Neurosci. 2012;123:121–127. doi: 10.3109/00207454.2012.742894. [DOI] [PubMed] [Google Scholar]
- Bellmund J.L., Deuker L., Navarro Schröder T., Doeller C.F. Grid-cell representations in mental simulation. eLife. 2016:5. doi: 10.7554/eLife.17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor D., Wilson M.A. Biasing the content of hippocampal replay during sleep. Nat. Neurosci. 2012;15:1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentaleb L.A., Beauregard M., Liddle P., Stip E. Cerebral activity associated with auditory verbal hallucinations: a functional magnetic resonance imaging case study. J. Psychiatry. 2002;27:6. [PMC free article] [PubMed] [Google Scholar]
- Ben-Yakov A., Henson R.N. The hippocampal film editor: sensitivity and specificity to event boundaries in continuous experience. J. Neurosci. 2018:10057–10068. doi: 10.1523/JNEUROSCI.0524-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T., Alger B., Thompson R. Neuronal substrate of classical conditioning in the hippocampus. Science. 1976;192:483–485. doi: 10.1126/science.1257783. [DOI] [PubMed] [Google Scholar]
- Berger T.W., Laham R.I., Thompson R.F. Hippocampal unit-behavior correlations during classical conditioning. Brain Res. 1980;193:229–248. doi: 10.1016/0006-8993(80)90960-9. [DOI] [PubMed] [Google Scholar]
- Berger T.W., Orr W.B. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behav. Brain Res. 1983;8:49–68. doi: 10.1016/0166-4328(83)90171-7. [DOI] [PubMed] [Google Scholar]
- Berger, T.W., Orr, W.B., 1982. Role of the hippocampus in reversal learning of the rabbit nictitating membrane response, in: Woody (Ed.), Conditioning: Representation of Involved Neural Functions. p. 12.
- Berger T.W., Thompson R.F. Hippocampal cellular plasticity during extinction of classically conditioned nictitating membrane behavior. Behav. Brain Res. 1982;4:63–76. doi: 10.1016/0166-4328(82)90165-6. [DOI] [PubMed] [Google Scholar]
- Bergmann E., Zur G., Bershadsky G., Kahn I. The organization of mouse and human cortico-hippocampal networks estimated by intrinsic functional connectivity. Cereb. Cortex. 2016;26:4497–4512. doi: 10.1093/cercor/bhw327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S.D., Seager M.A. Hippocampal theta oscillations and classical conditioning. Neurobiol. Learn. Mem. 2001;76:298–313. doi: 10.1006/nlme.2001.4025. [DOI] [PubMed] [Google Scholar]
- Beylin A.V., Gandhi C.C., Wood G.E., Talk A.C., Matzel L.D., Shors T.J. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol. Learn. Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Bhatt J.M., Bhattacharyya N., Lin H.W. Relationships between tinnitus and the prevalence of anxiety and depression: tinnitus and mood disorders. Laryngoscope. 2017;127:466–469. doi: 10.1002/lary.26107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford P.C., Luntz-Leybman V., Freedman R. Auditory sensory gating in the rat hippocampus: modulation by brainstem activity. Brain Res. 1993;607:33–38. doi: 10.1016/0006-8993(93)91486-C. [DOI] [PubMed] [Google Scholar]
- Bickford-Wimer P.C., Nagamoto H., Johnson R., Adler L.E., Egan M., Rose G.M., Freedman R. Auditory sensory gating in hippocampal neurons: a model system in the rat. Biol. Psychiatry. 1990;27:183–192. doi: 10.1016/0006-3223(90)90648-L. [DOI] [PubMed] [Google Scholar]
- Billig A.J., Davis M.H., Carlyon R.P. Neural decoding of bistable sounds reveals an effect of intention on perceptual organization. J. Neurosci. 2018;38:2844–2853. doi: 10.1523/JNEUROSCI.3022-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley J.K., Cohen Y.E. The what, where and how of auditory-object perception. Nat. Rev. Neurosci. 2013;14:693–707. doi: 10.1038/nrn3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley J.K., Walker K.M.M., Silverman B.W., King A.J., Schnupp J.W.H. Interdependent encoding of pitch, timbre, and spatial location in auditory cortex. J. Neurosci. 2009;29:2064–2075. doi: 10.1523/JNEUROSCI.4755-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank H., Spangenberg M., Davis M.H. Neural prediction errors distinguish perception and misperception of speech. J. Neurosci. 2018;38:6076–6089. doi: 10.1523/JNEUROSCI.3258-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxton T.A., Zeffiro T.A., Gabrieli J.D.E., Bookheimer S.Y., Carrillo M.C., Theodore W.H., Disterhoft J.F. Functional mapping of human learning: a positron emission tomography activation study of eyeblink conditioning. J. Neurosci. 1996;16:4032–4040. doi: 10.1523/JNEUROSCI.16-12-04032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood A.J., Zatorre R.J. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood A.J., Zatorre R.J., Bermudez P., Evans A.C. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat. Neurosci. 1999;2:382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- Bonetti L., Brattico E., Carlomagno F., Donati G., Cabral J., Haumann N.T., Deco G., Vuust P., Kringelbach M.L. Rapid encoding of musical tones discovered in whole-brain connectivity. NeuroImage. 2021;245 doi: 10.1016/j.neuroimage.2021.118735. [DOI] [PubMed] [Google Scholar]
- Bonora A., Benuzzi F., Monti G., Mirandola L., Pugnaghi M., Nichelli P., Meletti S. Recognition of emotions from faces and voices in medial temporal lobe epilepsy. Epilepsy Behav. 2011;20:648–654. doi: 10.1016/j.yebeh.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Boon M.E., Melis R.J.F., Rikkert M.O., Kessels R.P.C. Atrophy in the medial temporal lobe is specifically associated with encoding and storage of verbal information in MCI and Alzheimer patients. J. Neurol. Res. 2011 doi: 10.4021/jnr18w. [DOI] [Google Scholar]
- Borders A.A., Aly M., Parks C.M., Yonelinas A.P. The hippocampus is particularly important for building associations across stimulus domains. Neuropsychologia. 2017;99:335–342. doi: 10.1016/j.neuropsychologia.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F., LeDoux J.E. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. Exp. Brain Res. 1994;98:275–286. doi: 10.1007/BF00228415. [DOI] [PubMed] [Google Scholar]
- Born J., Wilhelm I. System consolidation of memory during sleep. Psychol. Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros N.N., Mears R., Pflieger M.E., Moxon K.A., Ludowig E., Rosburg T. Sensory gating in the human hippocampal and rhinal regions: regional differences. Hippocampus. 2008;18:310–316. doi: 10.1002/hipo.20388. [DOI] [PubMed] [Google Scholar]
- Boyen K., Langers D.R.M., de Kleine E., van Dijk P. Gray matter in the brain: differences associated with tinnitus and hearing loss. Hear. Res. 2013;295:67–78. doi: 10.1016/j.heares.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brady J.V., Hunt H.F. An experimental approach to the analysis of emotional behavior. J. Psychol. 1955;40:313–324. doi: 10.1080/00223980.1955.9712986. [DOI] [Google Scholar]
- Brady J.V., Schreiner L., Geller I., Kling A. Subcortical mechanisms in emotional behavior: the effect of rhinencephalic injury upon the acquisition and retention of a conditioned avoidance response in cats. J. Comp. Physiol. Psychol. 1954 doi: 10.1037/h0055426. [DOI] [PubMed] [Google Scholar]
- Brankačk J., Buzsáki G. Hippocampal responses evoked by tooth pulp and acoustic stimulation: depth profiles and effect of behavior. Brain Res. 1986;378:303–314. doi: 10.1016/0006-8993(86)90933-9. [DOI] [PubMed] [Google Scholar]
- Brankačk J., Seidenbecher T., Muller-Gartner H.-W. Task-relevant late positive component in rats: Is it related to hippocampal theta rhythm? Hippocampus. 1996;6:475–482. doi: 10.1002/(SICI)1098-1063(1996)6:5<475::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Brattico E., Bonetti L., Ferretti G., Vuust P., Matrone C. Putting cells in motion: advantages of endogenous boosting of BDNF production. Cells. 2021;10:183. doi: 10.3390/cells10010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman A.S. Analysis: The Perceptual Organization of Sound. MIT Press; Cambridge: 1990. Auditory scene. [Google Scholar]
- Britton K.T., Segal S., Kuczenski D., Hauger, R R. Dissociation between in vivo hippocampal norepinephrine response and behavioral/neuroendocrine responses to noise stress in rats. Brain Res. 1992;574:125–130. doi: 10.1016/0006-8993(92)90808-M. [DOI] [PubMed] [Google Scholar]
- Brown K.A., Buchwald J.S. Acoustic responses and plasticity of limbic units in cats. Exp. Neurol. 1973;40:608–631. doi: 10.1016/0014-4886(73)90099-X. [DOI] [PubMed] [Google Scholar]
- Brown M.W., Aggleton J.P. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown S., Martinez M.J., Parsons L.M. Passive music listening spontaneously engages limbic and paralimbic systems. NeuroReport. 2004;15:2033–2037. doi: 10.1097/00001756-200409150-00008. [DOI] [PubMed] [Google Scholar]
- Brun V.H., Solstad T., Kjelstrup K.B., Fyhn M., Witter M.P., Moser E.I., Moser M.-B. Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus. 2008;18:1200–1212. doi: 10.1002/hipo.20504. [DOI] [PubMed] [Google Scholar]
- Buffalo E.A. Bridging the gap between spatial and mnemonic views of the hippocampal formation. Hippocampus. 2015;25:713–718. doi: 10.1002/hipo.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burunat I., Alluri V., Toiviainen P., Numminen J., Brattico E. Dynamics of brain activity underlying working memory for music in a naturalistic condition. Cortex. 2014;57:254–269. doi: 10.1016/j.cortex.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Burunat I., Brattico E., Hartmann M., Vuust P., Särkämö T., Toiviainen P. Musical training predicts cerebello-hippocampal coupling during music listening. Psychomusicol.: Music Mind Brain. 2018;28:152–163. doi: 10.1037/pmu0000215. [DOI] [Google Scholar]
- Burwell R.D., Amaral D.G. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J. Comp. Neurol. 1998;398:179–205. doi: 10.1002/(SICI)1096-9861(19980824)398:2<179::AID-CNE3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bussey T.J., Saksida L.M. Object memory and perception in the medial temporal lobe: an alternative approach. Curr. Opin. Neurobiol. 2005;15:730–737. doi: 10.1016/j.conb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Butler A.J., James K.H. Cross-modal versus within-modal recall: differences in behavioral and brain responses. Behav. Brain Res. 2011 doi: 10.1016/j.bbr.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Lai-Wo S., Vanderwolf, C.H L. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. Rev. 1983;6:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Campeau S., Watson S.J. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J. Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Carpenter-Thompson J.R., Akrofi K., Schmidt S.A., Dolcos F., Husain F.T. Alterations of the emotional processing system may underlie preserved rapid reaction time in tinnitus. Brain Res. 2014;1567:28–41. doi: 10.1016/j.brainres.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Catenoix H., Magnin M., Mauguière F., Ryvlin P. Evoked potential study of hippocampal efferent projections in the human brain. Clin. Neurophysiol. 2011;122:2488–2497. doi: 10.1016/j.clinph.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Cavaco S., Feinstein J.S., van Twillert H., Tranel D. Musical memory in a patient with severe anterograde amnesia. J. Clin. Exp. Neuropsychol. 2012;34:1089–1100. doi: 10.1080/13803395.2012.728568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzuti V., Winston K., Baker R., Welch K. Psychological changes following surgery for tumors in the temporal lobe. J. Neurosurg. 1980;53:618–626. doi: 10.3171/jns.1980.53.5.0618. [DOI] [PubMed] [Google Scholar]
- Cave C.B., Squire L.R. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 1992;2:151–163. doi: 10.1002/hipo.450020207. [DOI] [PubMed] [Google Scholar]
- Cazard P., Buser P. Modification des réponses sensorielles corticales par stimulation de l’hippocampe dorsal chez le lapin. Electroencephalogr. Clin. Neurophysiol. 1963;15:413–425. doi: 10.1016/0013-4694(63)90063-4. [DOI] [PubMed] [Google Scholar]
- Cenquizca L.A., Swanson L.W. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res. Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock-Heyman L., Loui P., Weng T.B., Weisshappel R., McAuley E., Kramer A.F. Musical training and brain volume in older adults. Brain Sci. 2021;11:50. doi: 10.3390/brainsci11010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick M.J., Mullally S.L., Maguire E.A. The hippocampus extrapolates beyond the view in scenes: an fMRI study of boundary extension. Cortex. 2013;49:2067–2079. doi: 10.1016/j.cortex.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D., Suk H.-J., Jackson B., Milman N.P., Stark D., Klerman E.B., Kitchener E., Fernandez Avalos V.S., Banerjee A., Beach S.D., Blanchard J., Stearns C., Boes A., Uitermarket B., Gander P., Howard M., Sternberg E.J., Nieto-Castanon A., Anteraper S., Whitfield-Gabrieli S., Brown E.N., Boyden E.S., Dickerson B., Tsai L.-H. Gamma frequency sensory stimulation in probable mild alzheimer’s dementia patients: results of a preliminary clinical trial. Available at SSRN. 2021 doi: 10.2139/ssrn.3846540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R.W., Leong A.T.L., Ho L.C., Gao P.P., Wong E.C., Dong C.M., Wang X., He J., Chan Y.-S., Lim L.W., Wu E.X. Low-frequency hippocampal–cortical activity drives brain-wide resting-state functional MRI connectivity. Proc. Natl. Acad. Sci. U.S.A. 2017;114:E6972–E6981. doi: 10.1073/pnas.1703309114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L.L., Knight R.T. Human prefrontal lesions increase distractibility to irrelevant sensory inputs. NeuroReport. 1995;6:1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Chapin H., Jantzen K., Scott Kelso J.A., Steinberg F., Large E. Dynamic emotional and neural responses to music depend on performance expression and listener experience. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin H., Zanto T., Jantzen K.J., Kelso S.J.A., Steinberg F., Large E.W. Neural responses to complex auditory rhythms: the role of attending. Front. Psychol. 2010:1. doi: 10.3389/fpsyg.2010.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatani H., Hagiwara K., Hironaga N., Ogata K., Shigeto H., Morioka T., Sakata A., Hashiguchi K., Murakami N., Uehara T., Kira J., Tobimatsu S. Neuromagnetic evidence for hippocampal modulation of auditory processing. NeuroImage. 2016;124:256–266. doi: 10.1016/j.neuroimage.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Chaudhury S., Jain S., Wadhwa S. Expression of synaptic proteins in the hippocampus and spatial learning in chicks following prenatal auditory stimulation. Dev. Neurosci. 2010;32:114–124. doi: 10.1159/000279758. [DOI] [PubMed] [Google Scholar]
- Chaudhury S., Nag T.C., Wadhwa S. Calbindin D-28K and parvalbumin expression in embryonic chick hippocampus is enhanced by prenatal auditory stimulation. Brain Res. 2008;1191:96–106. doi: 10.1016/j.brainres.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Chaudhury S., Nag T.C., Wadhwa S. Prenatal acoustic stimulation influences neuronal size and the expression of calcium-binding proteins (calbindin D-28K and parvalbumin) in chick hippocampus. J. Chem. Neuroanat. 2006;32:117–126. doi: 10.1016/j.jchemneu.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Chaudhury S., Wadhwa S. Prenatal auditory stimulation alters the levels of CREB mRNA, p-CREB and BDNF expression in chick hippocampus. Int. J. Dev. Neurosci. 2009;27:583–590. doi: 10.1016/j.ijdevneu.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Chen G.-D., Radziwon K.E., Kashanian N., Manohar S., Salvi R. Salicylate-induced auditory perceptual disorders and plastic changes in nonclassical auditory centers in rats. Neural Plast. 2014;2014:1–18. doi: 10.1155/2014/658741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Rex C.S., Rice C.J., Dube C.M., Gall C.M., Lynch G., Baram T.Z. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc. Natl. Acad. Sci. U.S.A. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-C., Li X., Liu L., Wang J., Lu C.-Q., Yang M., Jiao Y., Zang F.-C., Radziwon K., Chen G.-D., Sun W., Krishnan Muthaiah V.P., Salvi R., Teng G.-J. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. eLife. 2015 doi: 10.7554/eLife.06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-C., Wang F., Wang J., Bo F., Xia W., Gu J.-P., Yin X. Resting-state brain abnormalities in chronic subjective tinnitus: a meta-analysis. Front. Hum. Neurosci. 2017:11. doi: 10.3389/fnhum.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-C., Xia W., Chen H., Feng Y., Xu J.-J., Gu J.-P., Salvi R., Yin X. Tinnitus distress is linked to enhanced resting-state functional connectivity from the limbic system to the auditory cortex: altered effective connectivity in tinnitus. Hum. Brain Mapp. 2017;38:2384–2397. doi: 10.1002/hbm.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-C., Yong W., Xing C., Feng Y., Haidari N.A., Xu J.-J., Gu J.-P., Yin X., Wu Y. Directed functional connectivity of the hippocampus in patients with presbycusis. Brain Imaging Behav. 2020;14:917–926. doi: 10.1007/s11682-019-00162-z. [DOI] [PubMed] [Google Scholar]
- Cheng D.T., Disterhoft J.F., Power J.M., Ellis D.A., Desmond J.E. Neural substrates underlying human delay and trace eyeblink conditioning. Proc. Natl. Acad. Sci. U.S.A. 2008;105:8108–8113. doi: 10.1073/pnas.0800374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Wang S.-H., Chen Q.-C., Liao X.-M. Moderate noise induced cognition impairment of mice and its underlying mechanisms. Physiol. Behav. 2011;104:981–988. doi: 10.1016/j.physbeh.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Cheng L., Wang S.-H., Huang Y., Liao X.-M. The hippocampus may be more susceptible to environmental noise than the auditory cortex. Hear. Res. 2016;333:93–97. doi: 10.1016/j.heares.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Cheung V.K.M., Harrison P.M.C., Meyer L., Pearce M.T., Haynes J.-D., Koelsch S. Uncertainty and surprise jointly predict musical pleasure and amygdala, hippocampus, and auditory cortex activity. Curr. Biol. 2019:29. doi: 10.1016/j.cub.2019.09.067. [DOI] [PubMed] [Google Scholar]
- Chikahisa S., Sei H., Morishima M., Sano A., Kitaoka K., Nakaya Y., Morita Y. Exposure to music in the perinatal period enhances learning performance and alters BDNF/TrkB signaling in mice as adults. Behav. Brain Res. 2006;169:312–319. doi: 10.1016/j.bbr.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Christison-Lagay K.L., Gifford A.M., Cohen Y.E. Neural correlates of auditory scene analysis and perception. Int. J. Psychophysiol. 2015;95:238–245. doi: 10.1016/j.ijpsycho.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I.A., Monk A.M., Hotchin V., Pizzamiglio G., Liefgreen A., Callaghan M.F., Maguire E.A. Does hippocampal volume explain performance differences on hippocampal-dependant tasks. NeuroImage. 2020;221 doi: 10.1016/j.neuroimage.2020.117211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.E., Squire Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Clark R.E., Squire L.R. Similarity in form and function of the hippocampus in rodents, monkeys, and humans. Proc. Natl. Acad. Sci. U.S.A. 2013;110:10365–10370. doi: 10.1073/pnas.1301225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M., Langner R., Meyer M., Oechslin M.S., Zilles K., Eickhoff S.B. Effects of prior information on decoding degraded speech: an fMRI study. Hum. Brain Mapp. 2014;35:61–74. doi: 10.1002/hbm.22151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N.J., Eichenbaum H. MIT Press; Cambridge: 1993. Memory, Amnesia, and the Hippocampal System. [Google Scholar]
- Cole S., Stone A.D., Petrovich G.D. The dorsomedial striatum mediates Pavlovian appetitive conditioning and food consumption. Behav. Neurosci. 2017;131:447–453. doi: 10.1037/bne0000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin L.L., Denninger T., Fyhn M., Hafting T., Bonnevie T., Jensen O., Moser M.-B., Moser E.I. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Constantinescu A.O., OReilly J.X., Behrens T.E.J. Organizing conceptual knowledge in humans with a gridlike code. Science. 2016;352:1464–1468. doi: 10.1126/science.aaf0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R.A., Ritchey M. Progression from feature-specific brain activity to hippocampal binding during episodic encoding. J. Neurosci. 2020;40:1701–1709. doi: 10.1523/JNEUROSCI.1971-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R.A., Ritchey M. Cortico-hippocampal network connections support the multidimensional quality of episodic memory. eLife. 2019:8. doi: 10.7554/eLife.45591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolov D.L., Seal M.L., Maruff P., Ulusoy R., Wong M.T.H., Tochon-Danguy H.J., Egan G.F. Cortical activation associated with the experience of auditory hallucinations and perception of human speech in schizophrenia: a PET correlation study. Psychiatry Res.: Neuroimaging. 2003;122:139–152. doi: 10.1016/S0925-4927(02)00121-X. [DOI] [PubMed] [Google Scholar]
- Coras R., Pauli E., Li J., Schwarz M., Rössler K., Buchfelder M., Hamer H., Stefan H., Blumcke I. Differential influence of hippocampal subfields to memory formation: insights from patients with temporal lobe epilepsy. Brain. 2014;137:1945–1957. doi: 10.1093/brain/awu100. [DOI] [PubMed] [Google Scholar]
- Corcoran K.A., Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J. Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S., Amaral D.G., González R.G., Johnson K.A., Hyman B.T. H. M.’s medial temporal lobe lesion: findings from magnetic resonance imaging. J. Neurosci. 1997;17:3964–3979. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall K.A., Schellenberg E.G., Misura N.M. Music training, cognition, and personality. Front. Psychol. 2013:4. doi: 10.3389/fpsyg.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins J.N., El-Deredy W., Parkes L.M., Hennies N., Lewis P.A. Cued reactivation of motor learning during sleep leads to overnight changes in functional brain activity and connectivity. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington N.V., Brown-Schmidt S., Duff M.C. The necessity of the hippocampus for statistical learning. J. Cogn. Neurosci. 2018;30:680–697. doi: 10.1162/jocn_a_01228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell H.C., Mears R.P., Wan L., Boutros N.N. Sensory gating: a translational effort from basic to clinical science. Clin. EEG Neurosci. 2008;39:69–72. doi: 10.1177/155005940803900209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crönlein T., Langguth B., Geisler P., Hajak G. Progress in Brain Research. Elsevier; 2007. Tinnitus and insomnia; pp. 227–233. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S., Lau K.M., Glover G.H., Menon V. Hippocampal involvement in detection of deviant auditory and visual stimuli. Hippocampus. 2005;15:132–139. doi: 10.1002/hipo.20039. [DOI] [PubMed] [Google Scholar]
- Crowley R., Bendor D., Javadi A.-H. A review of neurobiological factors underlying the selective enhancement of memory at encoding, consolidation, and retrieval. Prog. Neurobiol. 2019;179 doi: 10.1016/j.pneurobio.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Csépe V., Karmos G., Molnár M. Brain Dynamics: Progress and Perspectives. Springer; Berlin: 1989. Subcortical evoked potential correlates of early information processing: mismatch negativity in cats. [Google Scholar]
- Cuddy L.L., Sikka R., Vanstone A. Preservation of musical memory and engagement in healthy aging and Alzheimer’s disease: musical memory in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2015;1337:223–231. doi: 10.1111/nyas.12617. [DOI] [PubMed] [Google Scholar]
- Cui B., Li K., Gai Z., She X., Zhang N., Xu C., Chen X., An G., Ma Q., Wang R. Chronic noise exposure acts cumulatively to exacerbate Alzheimer’s disease-like amyloid-β pathology and neuroinflammation in the rat hippocampus. Sci. Rep. 2015:5. doi: 10.1038/srep12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B., Wu M., She X. Effects of chronic noise exposure on spatial learning and memory of rats in relation to neurotransmitters and NMDAR2B alteration in the hippocampus. J. Occup. Health. 2009;51:152–158. doi: 10.1539/joh.L8084. [DOI] [PubMed] [Google Scholar]
- Cui B., Wu M., She X., Liu H. Impulse noise exposure in rats causes cognitive deficits and changes in hippocampal neurotransmitter signaling and tau phosphorylation. Brain Res. 2012;1427:35–43. doi: 10.1016/j.brainres.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Cui B., Wu M.Q., Zhu L.X., She X.J., Qiang M.A., Liu H.T. Effect of chronic noise exposure on expression of N-Methyl-D-Aspartic acid receptor 2B and tau phosphorylation in hippocampus of rats. Biomed. Environ. Sci. 2013;26:163–168. doi: 10.3967/0895-3988.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Cui B., Zhu L., She X., Wu M., Ma Q., Wang T., Zhang N., Xu C., Chen X., An G., Liu H. Chronic noise exposure causes persistence of tau hyperphosphorylation and formation of NFT tau in the rat hippocampus and prefrontal cortex. Exp. Neurol. 2012;238:122–129. doi: 10.1016/j.expneurol.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Cunha A.O.S., Ceballos C.C., de Deus J.L., Leão R.M. Long-term high-intensity sound stimulation inhibits h current ( Ih) in CA1 pyramidal neurons. Eur. J. Neurosci. 2018;47:1401–1413. doi: 10.1111/ejn.13954. [DOI] [PubMed] [Google Scholar]
- Cunha A.O.S., de Oliveira J.A.C., Almeida S.S., Garcia-Cairasco N., Leão R.M. Inhibition of long-term potentiation in the schaffer-CA1 pathway by repetitive high-intensity sound stimulation. Neuroscience. 2015;310:114–127. doi: 10.1016/j.neuroscience.2015.09.040. [DOI] [PubMed] [Google Scholar]
- Curtu R., Wang X., Brunton B.W., Nourski K.V. Neural signatures of auditory perceptual bistability revealed by large-scale human intracranial recordings. J. Neurosci. 2019;39:6482–6497. doi: 10.1523/JNEUROSCI.0655-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton M.A., Zeidman P., McCormick C., Maguire E.A. Differentiable processing of objects, associations, and scenes within the hippocampus. J. Neurosci. 2018;38:8146–8159. doi: 10.1523/JNEUROSCI.0263-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker J.F., Tompary A., Davachi L. Trial-by-trial hippocampal encoding activation predicts the fidelity of cortical reinstatement during subsequent retrieval. Cereb. Cortex. 2016 doi: 10.1093/cercor/bhw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum I., Schugens M.M., Ackermann H., Lutzenberger W., Dichgans J., Birbaumer N. Classical conditioning after cerebellar lesions in humans. Behav. Neurosci. 1993;107:748–756. doi: 10.1037//0735-7044.107.5.748. [DOI] [PubMed] [Google Scholar]
- Davis M.H., Di Betta A.M., Macdonald M.J.E., Gaskell M.G. Learning and consolidation of novel spoken words. J. Cogn. Neurosci. 2009;21:803–820. doi: 10.1162/jocn.2009.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H., Ford M.A., Kherif F., Johnsrude I.S. Does semantic context benefit speech understanding through “top–down” processes? Evidence from time-resolved Sparse fMRI. J. Cogn. Neurosci. 2011;23:3914–3932. doi: 10.1162/jocn_a_00084. [DOI] [PubMed] [Google Scholar]
- Davis M.H., Johnsrude I.S. Hierarchical processing in spoken language comprehension. J. Neurosci. 2003;23:3423–3431. doi: 10.1523/JNEUROSCI.23-08-03423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P. Improving generalization for temporal difference learning: the successor representation. Neural Comput. 1993;5:613–624. doi: 10.1162/neco.1993.5.4.613. [DOI] [Google Scholar]
- de Deus J.L., Amorim M.R., Ribeiro A.B., Barcellos-Filho P.C.G., Ceballos C.C., Branco L.G.S., Cunha A.O.S., Leão R.M. Loss of brain-derived neurotrophic factor mediates inhibition of hippocampal long-term potentiation by high-intensity sound. Cell Mol. Neurobiol. 2021;41:751–763. doi: 10.1007/s10571-020-00881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Deus J.L., Cunha A.O.S., Terzian A.L., Resstel L.B., Elias L.L.K., Antunes-Rodrigues J., Almeida S.S., Leão R.M. A single episode of high intensity sound inhibits long-term potentiation in the hippocampus of rats. Sci. Rep. 2017:7. doi: 10.1038/s41598-017-14624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D., Fransen H., Francois O., Sunaert S., Kovacs S., Van De Heyning P. Amygdalohippocampal involvement in tinnitus and auditory memory. Acta Oto-Laryngol. 2006;126:50–53. doi: 10.1080/03655230600895580. [DOI] [PubMed] [Google Scholar]
- De Ridder D., Vanneste S., Weisz N., Londero A., Schlee W., Elgoyhen A.B., Langguth B. An integrative model of auditory phantom perception: Tinnitus as a unified percept of interacting separable subnetworks. Neurosci. Biobehav. Rev. 2014;44:16–32. doi: 10.1016/j.neubiorev.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Deadwyler S.A., West M.O., Christian E.P., Hampson R.E., Foster T.C. Sequence-related changes in sensory-evoked potentials in the dentate gyrus: A mechanism for item-specific short-term information storage in the hippocampus. Behav. Neural Biol. 1985;44:201–212. doi: 10.1016/S0163-1047(85)90198-0. [DOI] [PubMed] [Google Scholar]
- DeLisi L.E., Buchsbaum S., Holcomb M., Langston H.H., King K.C., Kessler A.C., Pickar R., Carpenter D., Morihisa W.T., Margolin J.M., Weinberger, D.R R. Increased temporal lobe glucose use in chronic schizophrenic patients. Biol. Psychiatry. 1989;25:835–851. doi: 10.1016/0006-3223(89)90263-1. [DOI] [PubMed] [Google Scholar]
- Dellacherie D., Bigand E., Molin P., Baulac M., Samson S. Multidimensional scaling of emotional responses to music in patients with temporal lobe resection. Cortex. 2011;47:1107–1115. doi: 10.1016/j.cortex.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Dellacherie D., Ehrlé N., Samson S. Is the neutral condition relevant to study musical emotion in patients? Music Percept.: Interdiscip. J. 2008;25:285–294. doi: 10.1525/mp.2008.25.4.285. [DOI] [Google Scholar]
- Demiralp T., Başar-Eroglu C., Başar E. Distributed gamma band responses in the brain studied in cortex, reticular formation, hippocampus and cerebellum. Int. J. Neurosci. 1996;84:1–13. doi: 10.3109/00207459608987246. [DOI] [PubMed] [Google Scholar]
- Derner M., Chaieb L., Dehnen G., Reber T.P., Borger V., Surges R., Staresina B.P., Mormann F., Fell J. Auditory beat stimulation modulates memory-related single-neuron activity in the human medial temporal lobe. Brain Sci. 2021;11:364. doi: 10.3390/brainsci11030364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derner M., Dehnen G., Chaieb L., Reber T.P., Borger V., Surges R., Staresina B.P., Mormann F., Fell J. Patterns of single-neuron activity during associative recognition memory in the human medial temporal lobe. NeuroImage. 2020;221 doi: 10.1016/j.neuroimage.2020.117214. [DOI] [PubMed] [Google Scholar]
- Derner M., Jahanbekam A., Bauckhage C., Axmacher N., Fell J. Prediction of memory formation based on absolute electroencephalographic phases in rhinal cortex and hippocampus outperforms prediction based on stimulus-related phase shifts. Eur. J. Neurosci. 2018;47:824–831. doi: 10.1111/ejn.13878. [DOI] [PubMed] [Google Scholar]
- Devlin J.T., Price C.J. Perirhinal contributions to human visual perception. Curr. Biol. 2007;17:1484–1488. doi: 10.1016/j.cub.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheerendra P., Baumann S., Joly O., Balezeau F., Petkov C.I., Thiele A., Griffiths T.D. The representation of time windows in primate auditory cortex. Cereb. Cortex. 2021 doi: 10.1093/cercor/bhab434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di G.-Q., Qin Z.-Q. Influences of combined traffic noise on the ability of learning and memory in mice. Noise Health. 2018;20:9–15. doi: 10.4103/nah.NAH_27_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba K., Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat. Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederen K.M.J., Neggers S.F.W., Daalman K., Blom J.D., Goekoop R., Kahn R.S., Sommer I.E.C. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am. J. Psychiatry. 2010;167:427–435. doi: 10.1176/appi.ajp.2009.09040456. [DOI] [PubMed] [Google Scholar]
- Dierks T., Linden D.E.J., Jandl M., Formisano E., Goebel R., Lanfermann H., Singer W. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22:615–621. doi: 10.1016/S0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Dietz B., Manahan-Vaughan D. Hippocampal long-term depression is facilitated by the acquisition and updating of memory of spatial auditory content and requires mGlu5 activation. Neuropharmacology. 2017;115:30–41. doi: 10.1016/j.neuropharm.2016.02.026. [DOI] [PubMed] [Google Scholar]
- Disterhoft J.F., Segal M. Neuron activity in rat hippocampus and motor cortex during discrimination reversal. Brain Res. Bull. 1978;3:583–588. doi: 10.1016/0361-9230(78)90003-5. [DOI] [PubMed] [Google Scholar]
- Doeller C.F., Barry C., Burgess N. Evidence for grid cells in a human memory network. Nature. 2010;463:657–661. doi: 10.1038/nature08704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan R.J., Fletcher P.C. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Dong Y., Guo C.-R., Chen D., Chen S.-M., Peng Y., Song H., Shi J.-R. Association between age‑related hearing loss and cognitive decline in C57BL/6J mice. Mol. Med. Rep. 2018;18:1726–1732. doi: 10.3892/mmr.2018.9118. [DOI] [PubMed] [Google Scholar]
- Donzis E.J., Rennaker R.L., Thompson L.T. Fear conditioning alters neuron-specific hippocampal place field stability via the basolateral amygdala. Brain Res. 2013;1525:16–25. doi: 10.1016/j.brainres.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Dragoi G., Tonegawa S. Distinct preplay of multiple novel spatial experiences in the rat. Proc. Natl. Acad. Sci. 2013;110:9100–9105. doi: 10.1073/pnas.1306031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulay M.F., Schefft B.K., Fargo J.D., Privitera M.D., Yeh H. Severity of depressive symptoms, hippocampal sclerosis, auditory memory, and side of seizure focus in temporal lobe epilepsy. Epilepsy Behav. 2004;5:522–531. doi: 10.1016/j.yebeh.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Eggermont J.J. Hearing loss, hyperacusis, or tinnitus: What is modeled in animal research. Hear. Res. 2013;295:140–149. doi: 10.1016/j.heares.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Ehlers C.L., Kaneko W.M., Robledo P., Lopez A.L. Long-latency event-related potentials in rats: Effects of task and stimulus parameters. Neuroscience. 1994;62:759–769. doi: 10.1016/0306-4522(94)90474-X. [DOI] [PubMed] [Google Scholar]
- Ehrlé N. Processing of rapid auditory information in epileptic patients with left temporal lobe damage. Neuropsychologia. 2001;39:525–531. doi: 10.1016/S0028-3932(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Prefrontal–hippocampal interactions in episodic memory. Nat. Rev. Neurosci. 2017;18:547–558. doi: 10.1038/nrn.2017.74. [DOI] [PubMed] [Google Scholar]
- Eichenbaum, H., 2017b Elements of Information Processing in Hippocampal Neuronal Activity: Space, Time, and Memory Hippocampus Cells Syst. Springe Int. Publ., Cham, pp. 69–94 doi: 10.1007/978-3-319-50406-3_3.
- Eidelberg E., White J.C., Brazier M.A.B. The hippocampal arousal pattern in rabbits. Exp. Neurol. 1959;1:483–490. doi: 10.1016/0014-4886(59)90045-7. [DOI] [PubMed] [Google Scholar]
- Ekman M., Gennari G., de Lange F.P. Probabilistic forward replay of anticipated stimulus sequences in human primary visual cortex and hippocampus. Neuroscience. 2022 doi: 10.1101/2022.01.26.477907. [DOI] [Google Scholar]
- Eldar E., Ganor O., Admon R., Bleich A., Hendler T. Feeling the real world: limbic response to music depends on related content. Cereb. Cortex. 2007;17:2828–2840. doi: 10.1093/cercor/bhm011. [DOI] [PubMed] [Google Scholar]
- Enatsu R., Gonzalez-Martinez J., Bulacio J., Kubota Y., Mosher J., Burgess R.C., Najm I., Nair D.R. Connections of the limbic network: a corticocortical evoked potentials study. Cortex. 2015;62:20–33. doi: 10.1016/j.cortex.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Engel A., Keller P.E. The perception of musical spontaneity in improvised and imitated jazz performances. Front. Psychol. 2011:2. doi: 10.3389/fpsyg.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani-Bayerl N., Finke C., Kopp U., Moon D.-U., Ploner C.J. Musical memory and hippocampus revisited: evidence from a musical layperson with highly selective hippocampal damage. Cortex. 2019;119:519–527. doi: 10.1016/j.cortex.2018.12.023. [DOI] [PubMed] [Google Scholar]
- Ezzyat Y., Davachi L. Similarity breeds proximity: pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron. 2014;81:1179–1189. doi: 10.1016/j.neuron.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S., Dafny N. Acoustic responses in the hypothalamus. Electroencephalogr. Clin. Neurophysiol. 1968;25:150–159. doi: 10.1016/0013-4694(68)90139-9. [DOI] [PubMed] [Google Scholar]
- Felleman D.J., Van Essen D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1. [DOI] [PubMed] [Google Scholar]
- Fernandes C., File S.E. Beware the builders: construction noise changes [14C]GABA release and uptake from amygdaloid and hippocampal slices in the rat. Neuropharmacology. 1993;32:1333–1336. doi: 10.1016/0028-3908(93)90028-2. [DOI] [PubMed] [Google Scholar]
- Ferrarese C., Mennini T., Pecora N., Gobbi M., Appollonio I., Bernasconi P., Frigo M., Regondi C., Pierpaoli C., Frattola L., Garattini S. Acute noise stress in rats increases the levels of diazepam binding inhibitor (DBI) in hippocampus and adrenal gland. Psychopharmacology. 1991;103:339–342. doi: 10.1007/BF02244287. [DOI] [PubMed] [Google Scholar]
- Ferrari-Marinho T., Caboclo L.O.S.F., Marinho M.M., Centeno R.S., Neves R.S.C., Santana M.T.C.G., Brito F.S., Junior H.C., Yacubian E.M.T. Auras in temporal lobe epilepsy with hippocampal sclerosis: Relation to seizure focus laterality and post surgical outcome. Epilepsy Behav. 2012;24:120–125. doi: 10.1016/j.yebeh.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Ferri S., Meini C., Guiot G., Tagliafico D., Gilli G., Di Dio C. The effect of simple melodic lines on aesthetic experience: brain response to structural manipulations. Adv. Neurosci. 2014;2014:1–9. doi: 10.1155/2014/482126. [DOI] [Google Scholar]
- Field S.E., Rickard N.S., Toukhsati S.R., Gibbs M.E. Maternal hen calls modulate memory formation in the day-old chick: the role of noradrenaline. Neurobiol. Learn. Mem. 2007;88:321–330. doi: 10.1016/j.nlm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Finke C., Esfahani N.E., Ploner C.J. Preservation of musical memory in an amnesic professional cellist. Curr. Biol. 2012;22:R591–R592. doi: 10.1016/j.cub.2012.05.041. [DOI] [PubMed] [Google Scholar]
- Fitzhugh M.C., Pa J. Longitudinal changes in resting-state functional connectivity and gray matter volume are associated with conversion to hearing impairment in older adults. JAD. 2022:1–14. doi: 10.3233/JAD-215288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.D., Downey L.E., Witoonpanich P., Warren J.D. The brain basis of musicophilia: evidence from frontotemporal lobar degeneration. Front. Psychol. 2013:4. doi: 10.3389/fpsyg.2013.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Gutiérrez E.O., Díaz J.-L., Barrios F.A., Favila-Humara R., Guevara M.Á., del Río-Portilla Y., Corsi-Cabrera M. Metabolic and electric brain patterns during pleasant and unpleasant emotions induced by music masterpieces. Int. J. Psychophysiol. 2007;65:69–84. doi: 10.1016/j.ijpsycho.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Fonken Y.M., Kam J.W.Y., Knight R.T. A differential role for human hippocampus in novelty and contextual processing: Implications for P300. Psychophysiology. 2019 doi: 10.1111/psyp.13400. [DOI] [PubMed] [Google Scholar]
- Ford J.H., Addis D.R., Giovanello K.S. Differential neural activity during search of specific and general autobiographical memories elicited by musical cues. Neuropsychologia. 2011;49:2514–2526. doi: 10.1016/j.neuropsychologia.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D.J., Wilson M.A. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Fowler H.L., Baker G.A., Tipples J., Hare D.J., Keller S., Chadwick D.W., Young A.W. Recognition of emotion with temporal lobe epilepsy and asymmetrical amygdala damage. Epilepsy Behav. 2006;9:164–172. doi: 10.1016/j.yebeh.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Fox S.S., Liebeskind J.C., O’Brien J.H., Dingle R.D.H. Progress in Brain Research. Elsevier; 1967. Mechanisms for Limbic Modification of Cerebellar and Cortical Afferent Information; pp. 254–280. [DOI] [PubMed] [Google Scholar]
- Freeman F., Kramarcy N., Lee J. Discrimination learning and stimulus generalization in rats with hippocampal lesions. Physiol. Behav. 1973;11:273–275. doi: 10.1016/0031-9384(73)90362-4. [DOI] [PubMed] [Google Scholar]
- Frenzilli G., Ryskalin L., Ferrucci M., Cantafora E., Chelazzi S., Giorgi F.S., Lenzi P., Scarcelli V., Frati A., Biagioni F., Gambardella S., Falleni A., Fornai F. Loud noise exposure produces DNA, neurotransmitter and morphological damage within specific brain areas. Front. Neuroanat. 2017:11. doi: 10.3389/fnana.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk V., Milner B. The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia. 1990;28:349–359. doi: 10.1016/0028-3932(90)90061-R. [DOI] [PubMed] [Google Scholar]
- Friston K., Buzsáki G. The functional anatomy of time: what and when in the brain. Trends Cogn. Sci. 2016;20:500–511. doi: 10.1016/j.tics.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Liddle P.F., Frith C.D., Hirsch S.R., Frackowiak R.S.J. The left medial temporal region and schizophrenia: a PET study. Brain. 1992;115:367–382. doi: 10.1093/brain/115.2.367. [DOI] [PubMed] [Google Scholar]
- Fritz J., Mishkin M., Saunders R.C. In search of an auditory engram. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9359–9364. doi: 10.1073/pnas.0503998102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer, J., Glette, K., Ivanovic, J., Larsson, P.G., Bekinschtein, T., Kochen, S., Knight, R.T., Tørresen, J., Solbakk, A.-K., Endestad, T., Blenkmann, A., 2021. Direct brain recordings reveal continuous encoding of structure in random stimuli. bioRxiv, 10.1101/2021.10.01.462295. [DOI]
- Fuhrmeister P., Myers E.B. Structural variation in the temporal lobe predicts learning and retention of non-native speech sounds. Lang. Cogn. Neurosci. 2022;37:63–79. doi: 10.1080/23273798.2021.1944658. [DOI] [Google Scholar]
- Fyhn M., Hafting T., Treves A., Moser M.-B., Moser E.I. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- Gadian D.G., Aicardi J., Watkins K.E., Porter D.A., Mishkin M., Vargha-Khadem F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain. 2000;123:499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- Gagnepain P., Fauvel B., Desgranges B., Gaubert M., Viader F., Eustache F., Groussard M., Platel H. Musical expertise increases top–down modulation over hippocampal activation during familiarity decisions. Front. Hum. Neurosci. 2017:11. doi: 10.3389/fnhum.2017.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnepain P., Henson R., Chételat G., Desgranges B., Lebreton K., Eustache F. Is neocortical–hippocampal connectivity a better predictor of subsequent recollection than local increases in hippocampal activity? New insights on the role of priming. J. Cogn. Neurosci. 2011;23:391–403. doi: 10.1162/jocn.2010.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai Z., Su D., Wang Y., Li W., Cui B., Li K., She X., Wang R. Effects of chronic noise on the corticotropin-releasing factor system in the rat hippocampus: relevance to Alzheimer’s disease-like tau hyperphosphorylation. Environ. Health Prev. Med. 2017:22. doi: 10.1186/s12199-017-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos R., Makeig S., Talmachoff P.J. A 40-Hz auditory potential recorded from the human scalp. Proc. Natl. Acad. Sci. 1981;78:2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Wu Y., Zhu Z., Yang C., Cheng P., Liu L., Sui J. Neuronal firing activity of hippocampal pyramidal cells during an auditory discrimination task in conscious guinea pigs. Behav. Brain Res. 2010;212:35–40. doi: 10.1016/j.bbr.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Garcia A.D., Buffalo E.A. Anatomy and function of the primate entorhinal cortex. Annu. Rev. Vis. Sci. 2020;6:411–432. doi: 10.1146/annurev-vision-030320-041115. [DOI] [PubMed] [Google Scholar]
- Geerts J.P., Chersi F., Stachenfeld K.L., Burgess N. A general model of hippocampal and dorsal striatal learning and decision making. Proc. Natl. Acad. Sci. U.S.A. 2020;117:31427–31437. doi: 10.1073/pnas.2007981117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard-Sagiv H., Mukamel R., Harel M., Malach R., Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman S.J. The successor representation: its computational logic and neural substrates. J. Neurosci. 2018;38:7193–7200. doi: 10.1523/JNEUROSCI.0151-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin M.R., McEchron M.D. Single neurons in the dentate gyrus and CA1 of the hippocampus exhibit inverse patterns of encoding during trace fear conditioning. Behav. Neurosci. 2005;119:164–179. doi: 10.1037/0735-7044.119.1.164. [DOI] [PubMed] [Google Scholar]
- Goble T.J., Møller A.R., Thompson L.T. Acute high-intensity sound exposure alters responses of place cells in hippocampus. Hear. Res. 2009;253:52–59. doi: 10.1016/j.heares.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Golden H.L., Agustus J.L., Goll J.C., Downey L.E., Mummery C.J., Schott J.M., Crutch S.J., Warren J.D. Functional neuroanatomy of auditory scene analysis in Alzheimer’s disease. NeuroImage: Clin. 2015;7:699–708. doi: 10.1016/j.nicl.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L.H., Canavan A.G.M., Polkey C.E. Verbal and abstract designs paired associate learning after unilateral temporal lobectomy. Cortex. 1988;24:41–52. doi: 10.1016/S0010-9452(88)80016-9. [DOI] [PubMed] [Google Scholar]
- Goll J.C., Kim L.G., Ridgway G.R., Hailstone J.C., Lehmann M., Buckley A.H., Crutch S.J., Warren J.D. Impairments of auditory scene analysis in Alzheimer’s disease. Brain. 2012;135:190–200. doi: 10.1093/brain/awr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez O., Chavez-Casillas O., Jauregui-Huerta F., Lopez-Virgen V., Guzman-Muniz J., Moy-Lopez N., Gonzalez-Castaneda R.E., Luquin S. Stress by noise produces differential effects on the proliferation rate of radial astrocytes and survival of neuroblasts in the adult subgranular zone. Neurosci. Res. 2011;70:243–250. doi: 10.1016/j.neures.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Gosselin N. Emotional responses to unpleasant music correlates with damage to the parahippocampal cortex. Brain. 2006;129:2585–2592. doi: 10.1093/brain/awl240. [DOI] [PubMed] [Google Scholar]
- Gosselin N., Peretz I., Hasboun D., Baulac M., Samson S. Impaired recognition of musical emotions and facial expressions following anteromedial temporal lobe excision. Cortex. 2011;47:1116–1125. doi: 10.1016/j.cortex.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Gosselin N., Peretz I., Noulhiane M., Hasboun D., Beckett C., Baulac M., Samson S. Impaired recognition of scary music following unilateral temporal lobe excision. Brain. 2005;128:628–640. doi: 10.1093/brain/awh420. [DOI] [PubMed] [Google Scholar]
- Gottlieb L.J., Uncapher M.R., Rugg M.D. Dissociation of the neural correlates of visual and auditory contextual encoding. Neuropsychologia. 2010;48:137–144. doi: 10.1016/j.neuropsychologia.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K.S., Barense M.D., Lee A.C.H. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Grastyán E., Lissák K., Madarász I., Donhoffer H. Hippocampal electrical activity during the development of conditioned reflexes. Electroencephalogr. Clin. Neurophysiol. 1959;11:409–430. doi: 10.1016/0013-4694(59)90040-9. [DOI] [PubMed] [Google Scholar]
- Grau-Perales A.B., Levy E.R.J., Fenton A.A., Gallo M. Dorsal hippocampal damage disrupts the auditory context-dependent attenuation of taste neophobia in mice. Neurobiol. Learn. Mem. 2019;157:121–127. doi: 10.1016/j.nlm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J.D., Adey W.R. Electrophysiological studies of hippocampal connections and excitability. Electroencephalogr. Clin. Neurophysiol. 1956;8:245–262. doi: 10.1016/0013-4694(56)90117-1. [DOI] [PubMed] [Google Scholar]
- Green J.D., Arduini A.A. Hippocampal electrical activity in arousal. J. Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Griffiths B.J., Fuentemilla L. Event conjunction: How the hippocampus integrates episodic memories across event boundaries. Hippocampus. 2019 doi: 10.1002/hipo.23161. [DOI] [PubMed] [Google Scholar]
- Griffiths T.D., Lad M., Kumar S., Holmes E., McMurray B., Maguire E.A., Billig A.J., Sedley W. How can hearing loss cause dementia? Neuron. 2020;108:401–412. doi: 10.1016/j.neuron.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths T.D., Warren J.D. What is an auditory object? Nat. Rev. Neurosci. 2004;5:887–892. doi: 10.1038/nrn1538. [DOI] [PubMed] [Google Scholar]
- Gross C.G., Weiskrantz L. Evidence for dissociation of impairment on auditory discrimination and delayed response following lateral frontal lesions in monkeys. Exp. Neurol. 1962;5:453–476. doi: 10.1016/0014-4886(62)90057-2. [DOI] [PubMed] [Google Scholar]
- Groussard M., La Joie R., Rauchs G., Landeau B., Chételat G., Viader F., Desgranges B., Eustache F., Platel H. When music and long-term memory interact: effects of musical expertise on functional and structural plasticity in the hippocampus. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groussard M., Rauchs G., Landeau B., Viader F., Desgranges B., Eustache F., Platel H. The neural substrates of musical memory revealed by fMRI and two semantic tasks. NeuroImage. 2010;53:1301–1309. doi: 10.1016/j.neuroimage.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Groussard M., Viader F., Landeau B., Desgranges B., Eustache F., Platel H. The effects of musical practice on structural plasticity: the dynamics of grey matter changes. Brain Cogn. 2014;90:174–180. doi: 10.1016/j.bandc.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Gruart A., Leal-Campanario R., López-Ramos J.C., Delgado-García J.M. Functional basis of associative learning and its relationships with long-term potentiation evoked in the involved neural circuits: lessons from studies in behaving mammals. Neurobiol. Learn. Mem. 2015;124:3–18. doi: 10.1016/j.nlm.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Gunbey H.P., Gunbey E., Aslan K., Bulut T., Unal A., Incesu L. Limbic-auditory interactions of tinnitus: an evaluation using diffusion tensor imaging. Clin. Neuroradiol. 2015;27:221–230. doi: 10.1007/s00062-015-0473-0. [DOI] [PubMed] [Google Scholar]
- Hafting T., Fyhn M., Molden S., Moser M.-B., Moser E.I. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Haider S., Naqvi F., Batool Z., Tabassum S., Perveen T., Saleem S., Haleem D.H. Decreased hippocampal 5-HT and DA levels following sub-chronic exposure to noise stress: impairment in both spatial and recognition memory in male rats. Sci. Pharm. 2012;80:1001–1011. doi: 10.3797/scipharm.1207-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E., Baudena P., Clarke J.M., Heit G., Marinkovic K., Devaux B., Vignal J.-P., Biraben A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II Media, lateral posterior tempo lobe. Electroencephalogr. Clin. Neurophysiol. 1995;94:229–250. doi: 10.1016/0013-4694(95)98475-N. [DOI] [PubMed] [Google Scholar]
- Halgren E., Squires N., Wilson C., Rohrbaugh J., Babb T., Crandall P. Endogenous potentials generated in the human hippocampal formation and amygdala by infrequent events. Science. 1980;210:803–805. doi: 10.1126/science.7434000. [DOI] [PubMed] [Google Scholar]
- Hall R.D., Borbely A.A. Acoustically evoked potentials in the rat during sleep and waking. Exp. Brain Res. 1970:11. doi: 10.1007/BF00234203. [DOI] [PubMed] [Google Scholar]
- Hallam R.S., Mckenna L., Shurlock L. Tinnitus impairs cognitive efficiency. Int. J. Audiol. 2004;43:218–226. doi: 10.1080/14992020400050030. [DOI] [PubMed] [Google Scholar]
- Han M.W., Ahn J.H., Kang J.K., Lee E.M., Lee J.H., Bae J.H., Chung J.W. Central auditory processing impairment in patients with temporal lobe epilepsy. Epilepsy Behav. 2011;20:370–374. doi: 10.1016/j.yebeh.2010.12.032. [DOI] [PubMed] [Google Scholar]
- Hannula D.E., Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. J. Neurosci. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy C.J.D., Yong K.X.X., Goll J.C., Crutch S.J., Warren J.D. Impairments of auditory scene analysis in posterior cortical atrophy. Brain. 2020;143:2689–2695. doi: 10.1093/brain/awaa221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington M.O., Cairney S.A. Sounding it out: auditory stimulation and overnight memory processing. Curr. Sleep. Med. Rep. 2021 doi: 10.1007/s40675-021-00207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D., Kumaran D., Vann S.D., Maguire E.A. Patients with hippocampal amnesia cannot imagine new experiences. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo M.E., Wyble B.P. Free recall and recognition in a network model of the hippocampus: simulating effects of scopolamine on human memory function. Behav. Brain Res. 1997;89:1–34. doi: 10.1016/S0166-4328(97)00048-X. [DOI] [PubMed] [Google Scholar]
- Hattori M., Onoda K., Sakata S. Identification of rat P3-like processes in the anterior cingulate cortex and hippocampus. Neurosci. Lett. 2010;472:43–46. doi: 10.1016/j.neulet.2010.01.052. [DOI] [PubMed] [Google Scholar]
- Hebscher M., Wing E., Ryan J., Gilboa A. Rapid cortical plasticity supports long-term memory formation. Trends Cogn. Sci. 2019;23:989–1002. doi: 10.1016/j.tics.2019.09.009. [DOI] [PubMed] [Google Scholar]
- Heinrich A., Carlyon R.P., Davis M.H., Johnsrude I.S. Illusory vowels resulting from perceptual continuity: a functional magnetic resonance imaging study. J. Cogn. Neurosci. 2008;20:1737–1752. doi: 10.1162/jocn.2008.20069. [DOI] [PubMed] [Google Scholar]
- Heit G., Smith M.E., Halgren E. Neuronal activity in the human medial temporal lobe during recognition memory. Brain. 1990;113:1093–1112. doi: 10.1093/brain/113.4.1093. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C., Elger C.E. Cognitive consequences of two-thirds anterior temporal lobectomy on verbal memory in 144 patients: a three-month follow-up study. Epilepsia. 1996;37:171–180. doi: 10.1111/j.1528-1157.1996.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C., Grunwald Th, Lehnertz K., Gleißner U., Elger C.E. Differential involvement of left temporolateral and temporomesial structures in verbal declarative learning and memory: evidence from temporal lobe epilepsy. Brain Cogn. 1997;35:110–131. doi: 10.1006/brcg.1997.0930. [DOI] [PubMed] [Google Scholar]
- Henin S., Turk-Browne N.B., Friedman D., Liu A., Dugan P., Flinker A., Doyle W., Devinsky O., Melloni L. Learning hierarchical sequence representations across human cortex and hippocampus. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abc4530. eabc4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdener M., Esposito F., di Salle F., Boller C., Hilti C.C., Habermeyer B., Scheffler K., Wetzel S., Seifritz E., Cattapan-Ludewig K. Musical training induces functional plasticity in human hippocampus. J. Neurosci. 2010;30:1377–1384. doi: 10.1523/JNEUROSCI.4513-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz S.C., Coffey E.B.J., Pantev C., Zatorre R.J. Dissociation of neural networks for predisposition and for training-related plasticity in auditory-motor learning. Cereb. Cortex. 2016;26:3125–3134. doi: 10.1093/cercor/bhv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R.M., Boto E., Rea M., Holmes N., Leggett J., Coles L.A., Papastavrou M., Everton S.K., Hunt B.A.E., Sims D., Osborne J., Shah V., Bowtell R., Brookes M.J. Multi-channel whole-head OPM-MEG: Helmet design and a comparison with a conventional system. NeuroImage. 2020;219 doi: 10.1016/j.neuroimage.2020.116995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yamaguchi M. Hippocampal unit response during temporal single alternation of classical conditioning with rewarding brain stimulation in the rat. Physiol. Psychol. 1985;13:7–14. doi: 10.3758/BF03326488. [DOI] [Google Scholar]
- Hirano Y., Fujita M., Watanabe K., Niwa M., Takahashi T., Kanematsu M., Ido Y., Tomida M., Onozuka M. Effect of unpleasant loud noise on hippocampal activities during picture encoding: an fMRI study. Brain Cogn. 2006;61:280–285. doi: 10.1016/j.bandc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Hoefeijzers S., Dewar M., Della Sala S., Zeman A., Butler C. Accelerated long-term forgetting in transient epileptic amnesia: an acquisition or consolidation deficit. Neuropsychologia. 2013;51:1549–1555. doi: 10.1016/j.neuropsychologia.2013.04.017. [DOI] [PubMed] [Google Scholar]
- Hoehler F.K., Thompson R.F. The effect of temporal single alternation on learned increases in hippocampal unit activity in classical conditioning of the rabbit nictitating membrane response. Physiol. Psychol. 1979;7:345–351. doi: 10.3758/BF03326655. [DOI] [Google Scholar]
- Hoffman R.E., Anderson A.W., Varanko M., Gore J.C., Hampson M. Time course of regional brain activation associated with onset of auditory/verbal hallucinations. Br. J. Psychiatry. 2008;193:424–425. doi: 10.1192/bjp.bp.107.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann L.C., Cicchese J.J., Berry S.D. Harnessing the power of theta: natural manipulations of cognitive performance during hippocampal theta-contingent eyeblink conditioning. Front. Syst. Neurosci. 2015;9:50. doi: 10.3389/fnsys.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollup S.A., Molden S., Donnett J.G., Moser M.-B., Moser E.I. Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J. Neurosci. 2001;21:1635–1644. doi: 10.1523/JNEUROSCI.21-05-01635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S.D., Roberts B. The influence of adaptation and inhibition on the effects of onset asynchrony on auditory grouping. J. Exp. Psychol.: Hum. Percept. Perform. 2011;37:1988–2000. doi: 10.1037/a0025642. [DOI] [PubMed] [Google Scholar]
- Holt W., Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J. Neurosci. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Sharifabad M., Sabahi A. Exposure to chronic noise reduces the volume of hippocampal subregions in rats. Iran. J. Basic Med. Sci. 2008;11:18–24. doi: 10.22038/IJBMS.2008.5192. [DOI] [Google Scholar]
- Howard M.W., Fotedar M.S., Datey A.V., Hasselmo M.E. The temporal context model in spatial navigation and relational learning: toward a common explanation of medial temporal lobe function across domains. Psychol. Rev. 2005;112:75–116. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Yang J., Song T., Hou N., Liu Y., Zhao X., Zhang D., Wang L., Wang T., Huang C. A new stress model, a scream sound, alters learning and monoamine levels in rat brain. Physiol. Behav. 2014;123:105–113. doi: 10.1016/j.physbeh.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Huet-Bello O., Ruvalcaba-Delgadillo Y., Feria-Velasco A., Gonzalez-Castaneda R.E., Garcia-Estrada J., Macias-Islas M.A., Jauregui-Huerta F., Luquin S. Environmental noise exposure modifies astrocyte morphology in hippocampus of young male rats. Noise Health. 2017;19:239–244. doi: 10.4103/nah.NAH_97_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijgen J., Dellacherie D., Tillmann B., Clément S., Bigand E., Dupont S., Samson S. The feeling of familiarity for music in patients with a unilateral temporal lobe lesion: a gating study. Neuropsychologia. 2015;77:313–320. doi: 10.1016/j.neuropsychologia.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Hunsaker M.R., Kesner R.P. Dissociations across the dorsal–ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol. Learn. Mem. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P.G., Schellenberg E.G., Schimmack U. Feelings and perceptions of happiness and sadness induced by music: Similarities, differences, and mixed emotions. Psychol. Aesthet. Creat. Arts. 2010;4:47–56. doi: 10.1037/a0016873. [DOI] [Google Scholar]
- Hwang J.-H., Chou P.-H., Wu C.-W., Chen J.-H., Liu T.-C. Brain activation in patients with idiopathic hyperacusis. Am. J. Otolaryngol. 2009;30:432–434. doi: 10.1016/j.amjoto.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Insausti R., Amaral D.G., Cowan W.M. The entorhinal cortex of the monkey: III. Subcortical afferents. J. Comp. Neurol. 1987;264:396–408. doi: 10.1002/cne.902640307. [DOI] [PubMed] [Google Scholar]
- Inta D., Vogt M.A., Elkin H., Weber T., Lima-Ojeda J.M., Schneider M., Luoni A., Riva M.A., Gertz K., Hellmann-Regen J., Kronenberg G., Meyer-Lindenberg A., Sprengel R., Gass P. Phenotype of mice with inducible ablation of GluA1 AMPA receptors during late adolescence: Relevance for mental disorders. Hippocampus. 2014;24:424–435. doi: 10.1002/hipo.22236. [DOI] [PubMed] [Google Scholar]
- Iordanova M.D., Burnett D.J., Aggleton J.P., Good M., Honey R.C. The role of the hippocampus in mnemonic integration and retrieval: complementary evidence from lesion and inactivation studies. Eur. J. Neurosci. 2009;30:2177–2189. doi: 10.1111/j.1460-9568.2009.07010.x. [DOI] [PubMed] [Google Scholar]
- Irmiš F., Radil-Weiss T., Lát J., Krekule I. Inter-individual differences in hippocampal theta activity during habituation. Electroencephalogr. Clin. Neurophysiol. 1970;28:24–31. doi: 10.1016/0013-4694(70)90004-0. [DOI] [PubMed] [Google Scholar]
- Itskov P.M., Vinnik E., Honey C., Schnupp J., Diamond M.E. Sound sensitivity of neurons in rat hippocampus during performance of a sound-guided task. J. Neurophysiol. 2012;107:1822–1834. doi: 10.1152/jn.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonowski J., Taesler P., Fu Q., Rose M. Implicit acoustic sequence learning recruits the hippocampus. PLOS ONE. 2018;13 doi: 10.1371/journal.pone.0209590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J., Redish A.D. Network dynamics of hippocampal cell-assemblies resemble multiple spatial maps within single tasks. Hippocampus. 2007;17:1209–1229. doi: 10.1002/hipo.20359. [DOI] [PubMed] [Google Scholar]
- Jacobs J., Weidemann C.T., Miller J.F., Solway A., Burke J.F., Wei X.-X., Suthana N., Sperling M.R., Sharan A.D., Fried I., Kahana M.J. Direct recordings of grid-like neuronal activity in human spatial navigation. Nat. Neurosci. 2013;16:1188–1190. doi: 10.1038/nn.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J.-H., Stelzer J., Fritz T.H., Chételat G., La Joie R., Turner R. Why musical memory can be preserved in advanced Alzheimer’s disease. Brain. 2015;138:2438–2450. doi: 10.1093/brain/awv135. [DOI] [PubMed] [Google Scholar]
- Jafari Z., Okuma M., Karem H., Mehla J., Kolb B.E., Mohajerani M.H. Prenatal noise stress aggravates cognitive decline and the onset and progression of beta amyloid pathology in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2019;77:66–86. doi: 10.1016/j.neurobiolaging.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Jafarpour A., Piai V., Lin J.J., Knight R.T. Human hippocampal pre-activation predicts behavior. Sci. Rep. 2017:7. doi: 10.1038/s41598-017-06477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C.E., Britz J., Vuilleumier P., Hauert C.-A., Michel C.M. Early neuronal responses in right limbic structures mediate harmony incongruity processing in musical experts. NeuroImage. 2008;42:1597–1608. doi: 10.1016/j.neuroimage.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Janata P. The cortical topography of tonal structures underlying western music. Science. 2002;298:2167–2170. doi: 10.1126/science.1076262. [DOI] [PubMed] [Google Scholar]
- Jang S.H., Choi E.B. Evaluation of structural neural connectivity between the primary auditory cortex and cognition-related brain areas using diffusion tensor tractography in 43 normal adults. Med. Sci. Monit. 2022:28. doi: 10.12659/MSM.936131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R., Lucas B., Delevoye-Turrell Y., Delmaire C., Delion P., Thomas P., Goeb J.-L. An 11-year-old boy with drug-resistant schizophrenia treated with temporo-parietal rTMS. Mol. Psychiatry. 2007;12 doi: 10.1038/sj.mp.4001968. 320–320. [DOI] [PubMed] [Google Scholar]
- Jardri R., Pins D., Bubrovszky M., Lucas B., Lethuc V., Delmaire C., Vantyghem V., Despretz P., Thomas P. Neural functional organization of hallucinations in schizophrenia: Multisensory dissolution of pathological emergence in consciousness. Conscious. Cogn. 2009;18:449–457. doi: 10.1016/j.concog.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Jardri R., Pouchet A., Pins D., Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Jastreboff P.J. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci. Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Jáuregui-Huerta F., García-Estrada J., Ruvalcaba-Delgadillo Y., Trujillo X., Huerta M., Feria-Velasco A., Gonzalez-Perez O., Luquín S. Chronic exposure of juvenile rats to environmental noise impairs hippocampal cell proliferation in adulthood. Noise Health. 2011;13:286. doi: 10.4103/1463-1741.82961. [DOI] [PubMed] [Google Scholar]
- Jayakar R., King T.Z., Morris R., Na S. Hippocampal volume and auditory attention on a verbal memory task with adult survivors of pediatric brain tumor. Neuropsychology. 2015;29:303–319. doi: 10.1037/neu0000183. [DOI] [PubMed] [Google Scholar]
- Jeneson A., Squire L.R. Working memory, long-term memory, and medial temporal lobe function. Learn. Mem. 2011;19:15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Maren S. Prefrontal-hippocampal interactions in memory and emotion. Front. Syst. Neurosci. 2015;9:170. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S.G., Kim M.J., Park S.Y., Park S.N. Stress hormonal changes in the brain and plasma after acute noise exposure in mice. Auris Nasus Larynx. 2017;44:272–276. doi: 10.1016/j.anl.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Joassin F., Pesenti M., Maurage P., Verreckt E., Bruyer R., Campanella S. Cross-modal interactions between human faces and voices involved in person recognition. Cortex. 2011;47:367–376. doi: 10.1016/j.cortex.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Joris P.X., Schreiner C.E., Rees A. Neural processing of amplitude-modulated sounds. Physiol. Rev. 2004;84:541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Julian J.B., Keinath A.T., Frazzetta G., Epstein R.A. Human entorhinal cortex represents visual space using a boundary-anchored grid. Nat. Neurosci. 2018;21:191–194. doi: 10.1038/s41593-017-0049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung R., Kornmüller A.E. Eine Methodik der Ableitung Iokalisierter Potentialschwankungen aus subcorticalen Hirngebieten. Arch. für Psychiatr. und Nervenkrankh. 1938;109:1–30. doi: 10.1007/BF02157817. [DOI] [Google Scholar]
- Kahn I., Andrews-Hanna J.R., Vincent J.L., Snyder A.Z., Buckner R.L. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaifosh P., Lovett-Barron M., Turi G.F., Reardon T.R., Losonczy A. Septo-hippocampal GABAergic signaling across multiple modalities in awake mice. Nat. Neurosci. 2013;16:1182–1184. doi: 10.1038/nn.3482. [DOI] [PubMed] [Google Scholar]
- Kalm K., Davis M.H., Norris D. Individual sequence representations in the medial temporal lobe. J. Cogn. Neurosci. 2013;25:1111–1121. doi: 10.1162/jocn_a_00378. [DOI] [PubMed] [Google Scholar]
- Kamiński J., Sullivan S., Chung J.M., Ross I.B., Mamelak A.N., Rutishauser U. Persistently active neurons in human medial frontal and medial temporal lobe support working memory. Nat. Neurosci. 2017;20:590–601. doi: 10.1038/nn.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko W.M., Riley E.P., Ehlers C.L. Electrophysiological and behavioral findings in rats prenatally exposed to alcohol. Alcohol. 1993;10:169–178. doi: 10.1016/0741-8329(93)90099-A. [DOI] [PubMed] [Google Scholar]
- Kapolowicz M.R., Thompson L.T. Acute high-intensity noise induces rapid Arc protein expression but fails to rapidly change GAD expression in amygdala and hippocampus of rats: Effects of treatment with D-cycloserine. Hear. Res. 2016;342:69–79. doi: 10.1016/j.heares.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Kato T., Erhard P., Takayama Y., Strupp J., Le T.H., Ogawa S., Ugurbil K. Human hippocampal long-term sustained response during word memory processing. NeuroReport. 1998;9:1041–1047. doi: 10.1097/00001756-199804200-00016. [DOI] [PubMed] [Google Scholar]
- Keane M.M., Gabrieli J.D.E., Mapstone H.C., Johnson K.A., Corkin S. Double dissociation of memory capacities after bilateral occipital-lobe or medial temporal-lobe lesions. Brain. 1995;118:1129–1148. doi: 10.1093/brain/118.5.1129. [DOI] [PubMed] [Google Scholar]
- Kemble E.D., Ison J.R. Limbic lesions and the inhibition of startle reactions in the rat by conditions of preliminary stimulation. Physiol. Behav. 1971;7:925–928. doi: 10.1016/0031-9384(71)90068-0. [DOI] [PubMed] [Google Scholar]
- Khalfa S., Guye M., Peretz I., Chapon F., Girard N., Chauvel P., Liégeois-Chauvel C. Evidence of lateralized anteromedial temporal structures involvement in musical emotion processing. Neuropsychologia. 2008;46:2485–2493. doi: 10.1016/j.neuropsychologia.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Khan U.A., Liu L., Provenzano F.A., Berman D.E., Profaci C.P., Sloan R., Mayeux R., Duff K.E., Small S.A. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat. Neurosci. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian N.J., Jutras M.J., Buffalo E.A. A map of visual space in the primate entorhinal cortex. Nature. 2012;491:761–764. doi: 10.1038/nature11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.-K., Ko I.-G., Kim S.-E., Kim C.-J., Yoon J.-S., Baik H.-H., Jin B.-K., Lee C.-Y., Baek S.-B., Shin M.-S. Impact of several types of stresses on short-term memory and apoptosis in the hippocampus of rats. Int. Neurourol. J. 2013;17:114–120. doi: 10.5213/inj.2013.17.3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Lee M.-H., Chang H.-K., Lee T.-H., Lee H.-H., Shin M.-C., Shin M.-S., Won R., Shin H.-S., Kim C.-J. Influence of prenatal noise and music on the spatial memory and neurogenesis in the hippocampus of developing rats. Brain Dev. 2006;28:109–114. doi: 10.1016/j.braindev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Diamond D.M. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim T.-W., Shin M.-S., Park J.-K., Shin M.-A., Lee H.-H., Lee S.-J. Treadmill exercise alleviates prenatal noise stress-induced impairment of spatial learning ability through enhancing hippocampal neurogenesis in rat pups. J. Exerc. Rehabil. 2013;9:451–456. doi: 10.12965/jer.130064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble D.P. Hippocampus and internal inhibition. Psychol. Bull. 1968;70:285–295. doi: 10.1037/h0026470. [DOI] [PubMed] [Google Scholar]
- Kirste I., Nicola Z., Kronenberg G., Walker T.L., Liu R.C., Kempermann G. Is silence golden? Effects of auditory stimuli and their absence on adult hippocampal neurogenesis. Brain Struct. Funct. 2015;220:1221–1228. doi: 10.1007/s00429-013-0679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee J.L., Souza B.C., Battaglia F.P. Learning differentially shapes prefrontal and hippocampal activity during classical conditioning. eLife. 2021;10 doi: 10.7554/eLife.65456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliuchko M., Puoliväli T., Heinonen-Guzejev M., Tervaniemi M., Toiviainen P., Sams M., Brattico E. Neuroanatomical substrate of noise sensitivity. NeuroImage. 2018;167:309–315. doi: 10.1016/j.neuroimage.2017.11.041. [DOI] [PubMed] [Google Scholar]
- Knierim J.J. The hippocampus. Curr. Biol. 2015;25:R1116–R1121. doi: 10.1016/j.cub.2015.10.049. [DOI] [PubMed] [Google Scholar]
- Knösche T.R., Neuhaus C., Haueisen J., Alter K., Maess B., Witte O.W., Friederici A.D. Perception of phrase structure in music: perception of phrase structure in music. Hum. Brain Mapp. 2005;24:259–273. doi: 10.1002/hbm.20088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S., Fritz T., V. Cramon D.Y., Müller K., Friederici A.D. Investigating emotion with music: an fMRI study. Hum. Brain Mapp. 2006;27:239–250. doi: 10.1002/hbm.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S., Remppis A., Sammler D., Jentschke S., Mietchen D., Fritz T., Bonnemeier H., Siebel W.A. A cardiac signature of emotionality: a cardiac signature of emotionality. Eur. J. Neurosci. 2007;26:3328–3338. doi: 10.1111/j.1460-9568.2007.05889.x. [DOI] [PubMed] [Google Scholar]
- Koelsch S., Skouras S. Functional centrality of amygdala, striatum and hypothalamus in a “small-world” network underlying joy: an fMRI study with music: a Neural Network Underlying Joy. Hum. Brain Mapp. 2014;35:3485–3498. doi: 10.1002/hbm.22416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S., Skouras S., Fritz T., Herrera P., Bonhage C., Küssner M.B., Jacobs A.M. The roles of superficial amygdala and auditory cortex in music-evoked fear and joy. NeuroImage. 2013;81:49–60. doi: 10.1016/j.neuroimage.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Koelsch S., Skouras S., Lohmann G. The auditory cortex hosts network nodes influential for emotion processing: An fMRI study on music-evoked fear and joy. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike A., Ishijima B. Preserved musical abilities following right temporal lobectomy. J. Neurosurg. 1996;85:5. doi: 10.3171/jns.1996.85.6.1000. [DOI] [PubMed] [Google Scholar]
- Kok P., Rait L.I., Turk-Browne N.B. Content-based dissociation of hippocampal involvement in prediction. J. Cogn. Neurosci. 2020;32:527–545. doi: 10.1162/jocn_a_01509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok P., Turk-Browne N.B. Associative prediction of visual shape in the hippocampus. J. Neurosci. 2018;38:6888–6899. doi: 10.1523/JNEUROSCI.0163-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb E., Finkbeiner S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 2011;34:591–598. doi: 10.1016/j.tins.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblith S., Quian Quiroga R., Koch C., Fried I., Mormann F. Persistent single-neuron activity during working memory in the human medial temporal lobe. Curr. Biol. 2017;27:1026–1032. doi: 10.1016/j.cub.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelenko L.M., Nikitin N.I., Altman Ya.A. Estimation by humans of signals simulating different sound movement directions and specificity of the perception of these signals by patients with temporal epilepsy. Hum. Physiol. 2013;39:241–247. doi: 10.1134/S0362119713030122. [DOI] [PubMed] [Google Scholar]
- Kotz S.A., Kalberlah C., Bahlmann J., Friederici A.D., Haynes J.-D. Predicting vocal emotion expressions from the human brain. Hum. Brain Mapp. 2013;34:1971–1981. doi: 10.1002/hbm.22041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska D.M. Effects of the anterior temporal lobe lesions, separate or combined with hippocampal damage, on spatial delayed responses guided by auditory stimulus. Acta Neurobiol. Exp. 1999;59:303–313. doi: 10.55782/ane-1999-1315. [DOI] [PubMed] [Google Scholar]
- Kowalska D.M., Kuśmierek P., Kosmal A., Mishkin M. Neither perirhinal/entorhinal nor hippocampal lesions impair short-term auditory recognition memory in dogs. Neuroscience. 2001;104:965–978. doi: 10.1016/S0306-4522(01)00140-3. [DOI] [PubMed] [Google Scholar]
- Kragel J.E., Schuele S., VanHaerents S., Rosenow J.M., Voss J.L. Rapid coordination of effective learning by the human hippocampus. Sci. Adv. 2021:7. doi: 10.1126/sciadv.abf7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus K.S., Canlon B. Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear. Res. 2012;288:34–46. doi: 10.1016/j.heares.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Kraus K.S., Mitra S., Jimenez Z., Hinduja S., Ding D., Jiang H., Gray L., Lobarinas E., Sun W., Salvi R.J. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyberg S., Torvik A., Bjørneboe A., Wilk-Larsen W., Jacobsen D. Trimethyltin poisoning: report of a case with postmortem examination. Clin. Neuropathol. 1992;11:256–259. [PubMed] [Google Scholar]
- Kropotov J.D., Alho K., Näätänen R., Ponomarev V.A., Kropotova O.V., Anichkov A.D., Nechaev V.B. Human auditory-cortex mechanisms of preattentive sound discrimination. Neurosci. Lett. 2000;280:87–90. doi: 10.1016/S0304-3940(00)00765-5. [DOI] [PubMed] [Google Scholar]
- Kropotov J.D., Naatanen R., Sevostianov A.V., Alho K., Reinikainen K., Kropotova O.V. Mismatch negativity to auditory stimulus change recorded directly from the human temporal cortex. Psychophysiology. 1995;32:418–422. doi: 10.1111/j.1469-8986.1995.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Kubit B., Janata P. Listening for memories: attentional focus dissociates functional brain networks engaged by memory-evoking music. Psychomusicology: Music, Mind, Brain. 2018;28:82–100. doi: 10.1037/pmu0000210. [DOI] [Google Scholar]
- Kubovy M., Van Valkenburg D. Auditory and visual objects. Cognition. 2001;80:97–126. doi: 10.1016/S0010-0277(00)00155-4. [DOI] [PubMed] [Google Scholar]
- Kumar, S., Bonnici, H.M., Teki, S., Agus, T.R., Pressnitzer, D., Maguire, E.A., Griffiths, T.D., 2014. Representations of specific acoustic patterns in the auditory cortex and hippocampus. Proceedings of the Royal Society B: Biological Sciences 281, 20141000–20141000. 10.1098/rspb.2014.1000. [DOI] [PMC free article] [PubMed]
- Kumar S., Gander P.E., Berger J.I., Billig A.J., Nourski K.V., Oya H., Kawasaki H., Howard M.A., Griffiths T.D. Oscillatory correlates of auditory working memory examined with human electrocorticography. Neuropsychologia. 2021;150 doi: 10.1101/2020.06.19.161901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Joseph S., Gander P.E., Barascud N., Halpern A.R., Griffiths T.D. A brain system for auditory working memory. J. Neurosci. 2016;36:4492–4505. doi: 10.1523/JNEUROSCI.4341-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Nayak D.S., Basrur R.M.R., Vishwanathan L.G., Govindappa S.K.G., Pramod M.N.B. Auditory aura from the hippocampus – not all that ‘rings’ is neocortical temporal lobe epilepsy. Epilepsy Behav. Rep. 2022;19 doi: 10.1016/j.ebr.2022.100548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stephan K.E., Warren J.D., Friston K.J., Griffiths T.D. Hierarchical processing of auditory objects in humans. PLoS Comput. Biol. 2007;3:9. doi: 10.1371/journal.pcbi.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Tansley-Hancock O., Sedley W., Winston J.S., Callaghan M.F., Allen M., Cope T.E., Gander P.E., Bamiou D.-E., Griffiths T.D. The brain basis for misophonia. Curr. Biol. 2017;27:527–533. doi: 10.1016/j.cub.2016.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D., Maguire E.A. Which computational mechanisms operate in the hippocampus during novelty detection? Hippocampus. 2007;17:735–748. doi: 10.1002/hipo.20326. [DOI] [PubMed] [Google Scholar]
- Kurczek J., Brown-Schmidt S., Duff M. Hippocampal contributions to language: Evidence of referential processing deficits in amnesia. J. Exp. Psychol.: Gen. 2013;142:1346–1354. doi: 10.1037/a0034026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka T., Mogi S., Yamashita T. Decreasing auditory input induces neurogenesis impairment in the hippocampus. Sci. Rep. 2021;11:423. doi: 10.1038/s41598-020-80218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad M., Mullally S.L., Houston A.L., Kelly T., Griffiths T.D. Characterizing memory loss in patients with autoimmune limbic encephalitis hippocampal lesions. Hippocampus. 2019;29:1114–1120. doi: 10.1002/hipo.23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H. Effects of repeated exposure to white noise on central cholinergic activity in the rat. Brain Res. 1988;442:403–406. doi: 10.1016/0006-8993(88)91535-1. [DOI] [PubMed] [Google Scholar]
- Lai H. Acute exposure to noise affects sodium-dependent high-affinity choline uptake in the central nervous system of the rat. Pharmacol. Biochem. Behav. 1987;28:147–151. doi: 10.1016/0091-3057(87)90205-X. [DOI] [PubMed] [Google Scholar]
- Lai H., Carino M.A. Acute White Noise Exposure Affects the Concentration of Benzodiazepine Receptors in the Brain of the Rat. Pharmacol. Biochem. Behav. 1990;36:985–987. doi: 10.1016/0091-3057(90)90110-4. [DOI] [PubMed] [Google Scholar]
- Lai H., Carino M.A., Wen Y.-F. Repeated noise exposure affects muscarinic cholinergic receptors in the rat brain. Brain Res. 1989;488:361–364. doi: 10.1016/0006-8993(89)90731-2. [DOI] [PubMed] [Google Scholar]
- Lan L., Chen Y.-C., Shang S., Lu L., Xu J.-J., Yin X., Wu Y., Cai Y. Topological features of limbic dysfunction in chronicity of tinnitus with intact hearing: New hypothesis for ‘noise-cancellation’ mechanism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2022;113 doi: 10.1016/j.pnpbp.2021.110459. [DOI] [PubMed] [Google Scholar]
- Lancelot C., Ahad P., Noulhiane M., Hasboun D., Baulac M., Samson S. Loss of memory for auditory–spatial associations following unilateral medial temporal-lobe damage. Neuropsychologia. 2005;43:1975–1982. doi: 10.1016/j.neuropsychologia.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lancelot C., Samson S., Ahad P., Baulac M. Effect of unilateral temporal lobe resection on short-term memory for auditory object and sound location. Ann. N. Y. Acad. Sci. 2003;999:377–380. doi: 10.1196/annals.1284.046. [DOI] [PubMed] [Google Scholar]
- Landgrebe M., Langguth B., Rosengarth K., Braun S., Koch A., Kleinjung T., May A., de Ridder D., Hajak G. Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. NeuroImage. 2009;46:213–218. doi: 10.1016/j.neuroimage.2009.01.069. [DOI] [PubMed] [Google Scholar]
- Lane R.D., Reiman E.M., Bradley M.M., Lang P.J., Ahern G.L., Davidson R.J., Schwartz G.E. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/S0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Langers D.R.M., Melcher J.R. Hearing without listening: functional connectivity reveals the engagement of multiple nonauditory networks during basic sound processing. Brain Connect. 2011;1:233–244. doi: 10.1089/brain.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche S., Neuenschwander-el Massioui N., Edeline J.-M., Dutrieux G. Hippocampal associative cellular responses: dissociation with behavioral responses revealed by a transfer-of-control technique. Behav. Neural Biol. 1987;47:356–368. doi: 10.1016/S0163-1047(87)90474-2. [DOI] [PubMed] [Google Scholar]
- Laureano M.R., Onishi E.T., Bressan R.A., Castiglioni M.L.V., Batista I.R., Reis M.A., Garcia M.V., de Andrade A.N., de Almeida R.R., Garrido G.J., Jackowski A.P. Memory networks in tinnitus: a functional brain image study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavasani A.N., Mohammadkhani G., Motamedi M., Karimi L.J., Jalaie S. Evaluation of temporal resolution in patients with unilateral temporal lobe epilepsy by the gaps-in-noise test. Audiology. 2013;22:75–84. [Google Scholar]
- LeDoux J.E., Ruggiero D.A., Reis, Donald J. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J. Comp. Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- Lee A.C.H., Yeung L.-K., Barense M.D. The hippocampus and visual perception. Front. Hum. Neurosci. 2012:6. doi: 10.3389/fnhum.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., Kim J. The shift from a response strategy to object-in-place strategy during learning is accompanied by a matching shift in neural firing correlates in the hippocampus. Learn. Mem. 2010;17:381–393. doi: 10.1101/lm.1829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-M., Kim B.-K., Kim T.-W., Ji E.-S., Choi H.-H. Music application alleviates short-term memory impairments through increasing cell proliferation in the hippocampus of valproic acid-induced autistic rat pups. J. Exerc. Rehabil. 2016;12:148–155. doi: 10.12965/jer.1632638.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H., Sparks F.T., Spanswick S.C., Hadikin C., McDonald R.J., Sutherland R.J. Making context memories independent of the hippocampus. Learn. Mem. 2009;16:417–420. doi: 10.1101/lm.1385409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehne M., Rohrmeier M., Koelsch S. Tension-related activity in the orbitofrontal cortex and amygdala: an fMRI study with music. Soc. Cogn. Affect. Neurosci. 2014;9:1515–1523. doi: 10.1093/scan/nst141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman “It’s not what you say, but how you say it”: a reciprocal temporo-frontal network for affective prosody. Front. Hum. Neurosci. 2010 doi: 10.3389/fnhum.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox B.R., Park S.B.G., Medley I., Morris P.G., Jones P.B. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res.: Neuroimaging. 2000;100:13–20. doi: 10.1016/S0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Lerner Y., Papo D., Zhdanov A., Belozersky L., Hendler T. Eyes wide shut: amygdala mediates eyes-closed effect on emotional experience with music. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S., Leutgeb J.K., Treves A., Moser M.-B., Moser E.I. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Lever C., Burton S., Jeewajee A., O’Keefe J., Burgess N. Boundary Vector Cells In The Subiculum Of The Hippocampal Formation. J. Neurosci. 2009;29:9771–9777. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Jia H., She X., Cui B., Zhang N., Chen X., Xu C., An G., Ma Q. Role of NMDA receptors in noise-induced tau hyperphosphorylation in rat hippocampus and prefrontal cortex. J. Neurol. Sci. 2014;340:191–197. doi: 10.1016/j.jns.2014.03.027. [DOI] [PubMed] [Google Scholar]
- Li S., Ma L., Wang Y., Wang X., Li Y., Qin L. Auditory steady-state responses in primary and non-primary regions of the auditory cortex in neonatal ventral hippocampal lesion rats. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J.C., Wagner A.D., Preston A.R. Content representation in the human medial temporal lobe. Cereb. Cortex. 2013;23:80–96. doi: 10.1093/cercor/bhr379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman T., Velluti R.A., Pedemonte M. Temporal correlation between auditory neurons and the hippocampal theta rhythm induced by novel stimulations in awake guinea pigs. Brain Res. 2009;1298:70–77. doi: 10.1016/j.brainres.2009.08.061. [DOI] [PubMed] [Google Scholar]
- Liberson W.T., Cadilhac J.G. Electroshock and rhinencephalic seizure states. Stereotact. Funct. Neurosurg. 1953;13:278–286. doi: 10.1159/000105425. [DOI] [PubMed] [Google Scholar]
- Liddle P.F., Friston K.J., Frith C.D., Hirsch S.R., Jones T., Frackowiak R.S.J. Patterns of cerebral blood flow in schizophrenia. Br. J. Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- Lidsky T.I., Levine M.S., MacGregor S. Tonic and phasic effects evoked concurrently by sensory stimuli in hippocampal units. Exp. Neurol. 1974;44:130–134. doi: 10.1016/0014-4886(74)90053-3. [DOI] [PubMed] [Google Scholar]
- Lisman J., Buzsáki G., Eichenbaum H., Nadel L., Ranganath C., Redish A.D. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci. 2017;20:1434–1447. doi: 10.1038/nn.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J.E., Grace A.A. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- J. Liu, J., Lin, T., Yan, X., Jiang, W., Shi, M., Ye, R., Rao, Z., Zhao, G., 2010. Eff. infrasound Cell Prolif. Dent. gyrus adult rats: NeuroReport, 21, pp. 585–589 doi: 10.1097/WNR.0b013e32833a7dc4. [DOI] [PubMed]
- Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D., Ballard C., Banerjee S., Burns A., Cohen-Mansfield J., Cooper C., Fox N., Gitlin L.N., Howard R., Kales H.C., Larson E.B., Ritchie K., Rockwood K., Sampson E.L., Samus Q., Schneider L.S., Selbæk G., Teri L., Mukadam N. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Lockwood A.H., Salvi R.J., Coad M.L., Towsley M.L., Wack D.S., Murphy B.W. The functional neuroanatomy of tinnitus. Neurology. 1998;50:114–120. doi: 10.1212/WNL.50.1.114. [DOI] [PubMed] [Google Scholar]
- Loechner K.J., Weisz D.J. Hippocampectomy and feature-positive discrimination. Behav. Brain Res. 1987;26:63–73. doi: 10.1016/0166-4328(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Logan C.G., Grafton S.T. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7500–7504. doi: 10.1073/pnas.92.16.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L.L., Hinman J.R., Chen C.-M.A., Stevenson I.H., Read H.L., Escabi M.A., Chrobak J.J. Novel acoustic stimuli can alter locomotor speed to hippocampal theta relationship: sound and hippocampal theta. Hippocampus. 2014;24:1053–1058. doi: 10.1002/hipo.22308. [DOI] [PubMed] [Google Scholar]
- Lositsky O., Chen J., Toker D., Honey C.J., Shvartsman M., Poppenk J.L., Hasson U., Norman K.A. Neural pattern change during encoding of a narrative predicts retrospective duration estimates. eLife. 2016;5:40. doi: 10.7554/eLife.16070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S., Pollick F.E., Latinus M. Cerebral correlates and statistical criteria of cross-modal face and voice integration. Seeing Perceiving. 2011;24:351–367. doi: 10.1163/187847511X584452. [DOI] [PubMed] [Google Scholar]
- Mably A.J., Colgin L.L. Gamma oscillations in cognitive disorders. Curr. Opin. Neurobiol. 2018;52:182–187. doi: 10.1016/j.conb.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald C.J., Carrow S., Place R., Eichenbaum H. Distinct hippocampal time cell sequences represent odor memories in immobilized rats. J. Neurosci. 2013;33:14607–14616. doi: 10.1523/JNEUROSCI.1537-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald C.J., Lepage K.Q., Eden U.T., Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo C.E., Angst M.-J., Guiberteau T., Brasse D., O’Brien T.J., Sandner G. Acoustic hypersensitivity in adult rats after neonatal ventral hippocampus lesions. Behav. Brain Res. 2010;207:161–168. doi: 10.1016/j.bbr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Maguire E.A., Gadian D.G., Johnsrude I.S., Good C.D., Ashburner J., Frackowiak R.S.J., Frith C.D. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E.A., Mullally S.L. The hippocampus: a manifesto for change. J. Exp. Psychol.: Gen. 2013;142:1180–1189. doi: 10.1037/a0033650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J.J., Daskalakis Z.J., Thomson R.H.S., Daigle M., Barr M.S., Fitzgerald P.B. Hippocampal volumetrics in treatment-resistant depression and schizophrenia: the devil’s in De-Tail. Hippocampus. 2012;22:9–16. doi: 10.1002/hipo.20873. [DOI] [PubMed] [Google Scholar]
- Maller J.J., Welton T., Middione M., Callaghan F.M., Rosenfeld J.V., Grieve S.M. Revealing the hippocampal connectome through super-resolution 1150-direction diffusion MRI. Sci. Rep. 2019:9. doi: 10.1038/s41598-018-37905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manan H.A., Franz E.A., Yusoff A.N., Sarah Mukari S.Z.-M. Hippocampal-cerebellar involvement in enhancement of performance in word-based BRT with the presence of background noise: an initial fMRI study. Psychol. Neurosci. 2012;5:247–256. doi: 10.3922/j.psns.2012.2.16. [DOI] [Google Scholar]
- Manikandan S., Padma M.K., Srikumar R., Jeya Parthasarathy N., Muthuvel A., Devi R.S. Effects of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial prefrontal cortex. Neurosci. Lett. 2006;399:17–22. doi: 10.1016/j.neulet.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Manns J.R., Howard M.W., Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manukyan A.L. Noise as a cause of neurodegenerative disorders: molecular and cellular mechanisms. Neurol. Sci. 2022 doi: 10.1007/s10072-022-05948-6. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Martins M.J.D., Fischmeister F.Ph.S., Gingras B., Bianco R., Puig-Waldmueller E., Villringer A., Fitch W.T., Beisteiner R. Recursive music elucidates neural mechanisms supporting the generation and detection of melodic hierarchies. Brain Struct. Funct. 2020;225:1997–2015. doi: 10.1007/s00429-020-02105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell A.J., Paulson A.L., Suk H.-J., Abdurrob F., Drummond G.T., Guan W., Young J.Z., Kim D.N.-W., Kritskiy O., Barker S.J., Mangena V., Prince S.M., Brown E.N., Chung K., Boyden E.S., Singer A.C., Tsai L.-H. Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-associated pathology and improves cognition. Cell. 2019;177(256–271) doi: 10.1016/j.cell.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T., Ogata K., Hironaga N., Kikuchi Y., Uehara T., Chatani H., Mitsudo T., Shigeto H., Tobimatsu S. Altered neural synchronization to pure tone stimulation in patients with mesial temporal lobe epilepsy: an MEG study. Epilepsy Behav. 2018;88:96–105. doi: 10.1016/j.yebeh.2018.08.036. [DOI] [PubMed] [Google Scholar]
- Matt L., Eckert P., Panford-Walsh R., Geisler H.-S., Bausch A.E., Manthey M., Müller N.I.C., Harasztosi C., Rohbock K., Ruth P., Friauf E., Ott T., Zimmermann U., Rüttiger L., Schimmang T., Knipper M., Singer W. Visualizing BDNF transcript usage during sound-induced memory linked plasticity. Front. Mol. Neurosci. 2018:11. doi: 10.3389/fnmol.2018.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau W., Sullivan D.W., Kinsky N.R., Hasselmo M.E., Howard M.W., Eichenbaum H. The Same Hippocampal CA1 Population Simultaneously Codes Temporal Information Over Multiple Timescales. Curr. Biol. 2018;28(1499–1508) doi: 10.1016/j.cub.2018.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes A.R., Holdstock J.S., Isaac C.L., Montaldi D., Grigor J., Gummer A., Cariga P., Downes J.J., Tsivilis D., Gaffan D., Gong Q., Norman K.A. Associative recognition in a patient with selective hippocampal lesions and relatively normal item recognition. Hippocampus. 2004;14:763–784. doi: 10.1002/hipo.10211. [DOI] [PubMed] [Google Scholar]
- Mays L.E., Best P.J. Hippocampal unit activity to tonal stimuli during arousal from sleep and in awake rats. Exp. Neurol. 1975;47:268–279. doi: 10.1016/0014-4886(75)90256-3. [DOI] [PubMed] [Google Scholar]
- McCarthy G., Wood C., Williamson P., Spencer D. Task-dependent field potentials in human hippocampal formation. J. Neurosci. 1989;9:4253–4268. doi: 10.1523/JNEUROSCI.09-12-04253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland J.L., O’Reilly R.C. Why there are complementary learning systems in the hippocampus and neocortex:insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McCormick C., Dalton M.A., Zeidman P., Maguire E.A. Characterising the hippocampal response to perception, construction and complexity. Cortex. 2021;137:1–17. doi: 10.1016/j.cortex.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D.A., Clark G.A., Lavond D.G., Thompson R.F. Initial localization of the memory trace for a basic form of learning. Proc. Natl. Acad. Sci. U.S.A. 1982;79:2731–2735. doi: 10.1073/pnas.79.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron M.D., Disterhoft J.F. Sequence of Single Neuron Changes in CA1 Hippocampus Of Rabbits During Acquisition Of Trace Eyeblink Conditioned Responses. J. Neurophysiol. 1997;78:1030–1044. doi: 10.1152/jn.1997.78.2.1030. [DOI] [PubMed] [Google Scholar]
- McEchron M.D., Tseng W., Disterhoft J.F. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (Fear) conditioning in rabbit. J. Neurosci. 2003;23:1535–1547. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire P.K., Murray R.M., Shah G.M.S. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703–706. doi: 10.1016/0140-6736(93)91707-S. [DOI] [PubMed] [Google Scholar]
- McKenzie S., Frank A.J., Kinsky N.R., Porter B., Rivière P.D., Eichenbaum H. Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron. 2014;83:202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan T.M., Powell G.E., Janota I., Polkey C.E. Relationships between neuropathology and cognitive functioning in temporal lobectomy patients. J. Neurol., Neurosurg. Psychiatry. 1987;50:167–176. doi: 10.1136/jnnp.50.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton B.L., Morris R.G.M. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. doi: 10.1016/0166-2236(87)90011-7. [DOI] [Google Scholar]
- Meck W.H., Church R.M., Olton D.S. Hippocampus, time, and memory. Behav. Neurosci. 1984;98:3–22. doi: 10.1037//0735-7044.98.1.3. [DOI] [PubMed] [Google Scholar]
- Medoff D.R., Holcomb H.H., Lahti A.C., Tamminga C.A. Probing the human hippocampus using rCBF: Contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Mehta M.R., Quirk M.C., Wilson M.A. Experience-dependent asymmetric shape of hippocampal receptive fields. Neuron. 2000;25:707–715. doi: 10.1016/S0896-6273(00)81072-7. [DOI] [PubMed] [Google Scholar]
- Meister M.L.R., Buffalo E.A. Neurons in primate entorhinal cortex represent gaze position in multiple spatial reference frames. J. Neurosci. 2018;38:2430–2441. doi: 10.1523/JNEUROSCI.2432-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher J.R., Knudson I.M., Levine R.A. Subcallosal brain structure: correlation with hearing threshold at supra-clinical frequencies (>8 kHz), but not with tinnitus. Hear. Res. 2013;295:79–86. doi: 10.1016/j.heares.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Meng B., Zhu S., Li S., Zeng Q., Mei B. Global view of the mechanisms of improved learning and memory capability in mice with music-exposure by microarray. Brain Res. Bull. 2009;80:36–44. doi: 10.1016/j.brainresbull.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Mesgarani N., Cheung C., Johnson K., Chang E.F. Phonetic feature encoding in human superior temporal gyrus. Science. 2014;343:1006–1010. doi: 10.1126/science.1245994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S.S., Bonaiuto J., Lim M., Rossiter H., Waters S., Bradbury D., Bestmann S., Brookes M., Callaghan M.F., Weiskopf N., Barnes G.R. Flexible head-casts for high spatial precision MEG. J. Neurosci. Methods. 2017;276:38–45. doi: 10.1016/j.jneumeth.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer V., Yates A.J. Intellectual changes following temporal lobectomy for psychomotor epilepsy: Preliminary communication. J. Neurol., Neurosurg. Psychiatry. 1955;18:44–52. doi: 10.1136/jnnp.18.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micco D.J., Schwartz M. Effects of hippocampal lesions upon the development of Pavlovian internal inhibition in rats. J. Comp. Physiol. Psychol. 1971;76:371–377. doi: 10.1037/h0031376. [DOI] [PubMed] [Google Scholar]
- Michelmann S., Price A.R., Aubrey B., Strauss C.K., Doyle W.K., Friedman D., Dugan P.C., Devinsky O., Devore S., Flinker A., Hasson U., Norman K.A. Moment-by-moment tracking of naturalistic learning and its underlying hippocampo-cortical interactions. Nat. Commun. 2021;12:5394. doi: 10.1038/s41467-021-25376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.L., Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience. 1995;69:371–381. doi: 10.1016/0306-4522(95)00249-I. [DOI] [PubMed] [Google Scholar]
- Miller C.L., Freedman R. Medial septal neuron activity in relation to an auditory sensory gating paradigm. Neuroscience. 1993;55:373–380. doi: 10.1016/0306-4522(93)90506-B. [DOI] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Neurosurgery. 1972;19:421–446. doi: 10.1093/neurosurgery/19.CN_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Milner B. In: Passouant P., editor. Physiologie de l'Hippocampe. C.N.R.S.; Paris: 1965. Les troubles de la mémoire accompagnant des lésions hippocampiques bilatérales; pp. 257–272. [Google Scholar]
- Milner B., Teuber H.L. In: Analysis of Behavioral Change. Weiskrantz L., editor. Harper & Row; New York: 1968. Alteration of perception and memory in man: reflections on methods. [Google Scholar]
- Mirz F. Cortical networks subserving the perception of tinnitus - a PET study. Acta Oto-Laryngol. 2000;120:241–243. doi: 10.1080/000164800454503. [DOI] [PubMed] [Google Scholar]
- Mirz, F., Gjedde, A., Sdkilde-Jrgensen, H., Pedersen, C.B., 2000. Funct. brain Imaging Tinnitus- Percept. Induc. aversive Audit. stimuli: NeuroReport 11 2000 633 637 doi: 10.1097/00001756-200002280-00039. [DOI] [PubMed]
- Mitchell R.L.C., Elliott R., Barry M., Cruttenden A., Woodruff P.W.R. The neural response to emotional prosody, as revealed by functional magnetic resonance imaging. Neuropsychologia. 2003;41:1410–1421. doi: 10.1016/S0028-3932(03)00017-4. [DOI] [PubMed] [Google Scholar]
- Mitchnick K.A., Ahmad Z., Mitchnick S.D., Ryan J.D., Rosenbaum R.S., Freud E. Damage to the human dentate gyrus impairs the perceptual discrimination of complex, novel objects. Neuropsychologia. 2022 doi: 10.1016/j.neuropsychologia.2022.108238. [DOI] [PubMed] [Google Scholar]
- Modi M.N., Dhawale A.K., Bhalla U.S. CA1 cell activity sequences emerge after reorganization of network correlation structure during associative learning. eLife. 2014:3. doi: 10.7554/eLife.01982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita M.A.P., Rosis S., Zhou Y., LeDoux J.E., Blair H.T. Putting fear in its place: remapping of hippocampal place cells during fear conditioning. J. Neurosci. 2004;24:7015–7023. doi: 10.1523/JNEUROSCI.5492-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita M.A.P., Rosis S., Zhou Y., LeDoux J.E., Blair H.T. Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–497. doi: 10.1016/S0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Momennejad I. Learning structures: predictive representations, replay, and generalization. Curr. Opin. Behav. Sci. 2020;32:155–166. doi: 10.1016/j.cobeha.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount R.A., Sridhar S., Hansen K.R., Mohammed A.I., Abdulkerim M., Kessel R., Nazer B., Gritton H.J., Han X. Distinct neuronal populations contribute to trace conditioning and extinction learning in the hippocampal CA1. eLife. 2021;10 doi: 10.7554/eLife.56491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon K.A., Gerhardt G.A., Bickford P.C., Austin K., Rose G.M., Woodward D.J., Adler L.E. Multiple single units and population responses during inhibitory gating of hippocampal auditory response in freely-moving rats. Brain Res. 1999;825:75–85. doi: 10.1016/S0006-8993(99)01187-7. [DOI] [PubMed] [Google Scholar]
- Mueller K., Fritz T., Mildner T., Richter M., Schulze K., Lepsien J., Schroeter M.L., Möller H.E. Investigating the dynamics of the brain response to music: a central role of the ventral striatum/nucleus accumbens. NeuroImage. 2015;116:68–79. doi: 10.1016/j.neuroimage.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Mueller K., Mildner T., Fritz T., Lepsien J., Schwarzbauer C., Schroeter M.L., Möller H.E. Investigating brain response to music: a comparison of different fMRI acquisition schemes. NeuroImage. 2011;54:337–343. doi: 10.1016/j.neuroimage.2010.08.029. [DOI] [PubMed] [Google Scholar]
- Mueller S.G., Laxer K.D., Scanlon C., Garcia P., McMullen W.J., Loring D.W., Meador K.J., Weiner M.W. Different structural correlates for verbal memory impairment in temporal lobe epilepsy with and without mesial temporal lobe sclerosis. Hum. Brain Mapp. 2012;33:489–499. doi: 10.1002/hbm.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally S.L., Intraub H., Maguire E.A. Attenuated boundary extension produces a paradoxical memory advantage in amnesic patients. Curr. Biol. 2012;22:261–268. doi: 10.1016/j.cub.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N., Keil J., Obleser J., Schulz H., Grunwald T., Bernays R.-L., Huppertz H.-J., Weisz N. You can’t stop the music: reduced auditory alpha power and coupling between auditory and memory regions facilitate the illusory perception of music during noise. NeuroImage. 2013;79:383–393. doi: 10.1016/j.neuroimage.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Muller R., Kubie J. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M., Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) Eur. J. Neurosci. 2005;22:1368–1388. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- Munoz-Lopez M., Insausti R., Mohedano-Moriano A., Mishkin M., Saunders R.C. Anatomical pathways for auditory memory II: Information from rostral superior temporal gyrus to dorsolateral temporal pole and medial temporal cortex. Front. Neurosci. 2015:9. doi: 10.3389/fnins.2015.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Lopez M.M., Mohedano-Moriano A., Insausti R. Anatomical pathways for auditory memory in primates. Front. Neuroanat. 2010:4. doi: 10.3389/fnana.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musalek M., Podreka I., Walter H., Suess E., Passweg V., Nutzinger D., Strobl R., Lesch O.M. Regional brain function in hallucinations: a study of regional cerebral blood flow with 99m-Tc-HMPAO-SPECT in patients with auditory hallucinations, tactile hallucinations, and normal controls. Compr. Psychiatry. 1989;30:99–108. doi: 10.1016/0010-440X(89)90123-5. [DOI] [PubMed] [Google Scholar]
- Mutschler I., Wieckhorst B., Speck O., Schulze-Bonhage A., Hennig J., Seifritz E., Ball T. Time scales of auditory habituation in the amygdala and cerebral cortex. Cereb. Cortex. 2010;20:2531–2539. doi: 10.1093/cercor/bhq001. [DOI] [PubMed] [Google Scholar]
- Muzzio I.A., Levita L., Kulkarni J., Monaco J., Kentros C., Stead M., Abbott L.F., Kandel E.R. Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav. Brain Sci. 1990;13:201–233. doi: 10.1017/S0140525X00078407. [DOI] [Google Scholar]
- Nadel L., Samsonovich A., Ryan L., Moscovitch M. Multiple trace theory of human memory: computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10:352–368. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nadhimi Y., Llano D.A. Does hearing loss lead to dementia? A review of the literature. Hear. Res. 2021;402 doi: 10.1016/j.heares.2020.108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nani A., Manuello J., Liloia D., Duca S., Costa T., Cauda F. The neural correlates of time: a meta-analysis of neuroimaging studies. J. Cogn. Neurosci. 2019;31:1796–1826. doi: 10.1162/jocn_a_01459. [DOI] [PubMed] [Google Scholar]
- Nau M., Navarro Schröder T., Bellmund J.L.S., Doeller C.F. Hexadirectional coding of visual space in human entorhinal cortex. Nat. Neurosci. 2018;21:188–190. doi: 10.1038/s41593-017-0050-8. [DOI] [PubMed] [Google Scholar]
- Ngo H.-V.V., Martinetz T., Born J., Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Niki H. Progress in Brain Research. Elsevier; 1967. Effects of hippocampal ablation on learning in the rat; pp. 305–317. [DOI] [PubMed] [Google Scholar]
- Nilssen E.S., Doan T.P., Nigro M.J., Ohara S., Witter M.P. Neurons and networks in the entorhinal cortex: a reappraisal of the lateral and medial entorhinal subdivisions mediating parallel cortical pathways. Hippocampus. 2019 doi: 10.1002/hipo.23145. [DOI] [PubMed] [Google Scholar]
- Nir Y., Staba R.J., Andrillon T., Vyazovskiy V.V., Cirelli C., Fried I., Tononi G. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreña A.J. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci. Biobehav. Rev. 2011;35:1089–1109. doi: 10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Norman Y., Yeagle E.M., Khuvis S., Harel M., Mehta A.D., Malach R. Hippocampal sharp-wave ripples linked to visual episodic recollection in humans. Science. 2019;365 doi: 10.1126/science.aax1030. eaax1030. [DOI] [PubMed] [Google Scholar]
- Nourski K.V., Reale R.A., Oya H., Kawasaki H., Kovach C.K., Chen H., Howard M.A., Brugge J.F. Temporal envelope of time-compressed speech represented in the human auditory cortex. J. Neurosci. 2009;29:15564–15574. doi: 10.1523/JNEUROSCI.3065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien C.E., Bowden S.C., Bardenhagen F.J., Cook M.J. Neuropsychological correlates of hippocampal and rhinal cortex volumes in patients with mesial temporal sclerosis. Hippocampus. 2003;13:892–904. doi: 10.1002/hipo.10128. [DOI] [PubMed] [Google Scholar]
- O’Connor J.J., Rowan M.J., Anwyl R. Serotoninergic depression of auditory evoked responses recorded in the rat hippocampus: effect of repeated buspirone treatment. Brain Res. 1992;573:190–196. doi: 10.1016/0006-8993(92)90762-X. [DOI] [PubMed] [Google Scholar]
- O’Keefe J., Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe, J., Dostrovsky, J., 1971. The hippocampus as a spatial map Prelim. Evid. Unit. Act. Free. -Mov. Rat. Brain Res. 34 1971 171 175 doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed]
- O’Keefe J., Krupic J. Do hippocampal pyramidal cells respond to nonspatial stimuli? Physiol. Rev. 2021;101:1427–1456. doi: 10.1152/physrev.00014.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J., Recce M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Olds J., Hirano T. Conditioned responses of hippocampal and other neurons. Electroencephalogr. Clin. Neurophysiol. 1969;26:159–166. doi: 10.1016/0013-4694(69)90206-5. [DOI] [PubMed] [Google Scholar]
- Olsen R.K., Moses S.N., Riggs L., Ryan J.D. The hippocampus supports multiple cognitive processes through relational binding and comparison. Front. Hum. Neurosci. 2012:6. doi: 10.3389/fnhum.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar R., Henley S.M.D., Bartlett J.W., Hailstone J.C., Gordon E., Sauter D.A., Frost C., Scott S.K., Warren J.D. The structural neuroanatomy of music emotion recognition: evidence from frontotemporal lobar degeneration. NeuroImage. 2011;56:1814–1821. doi: 10.1016/j.neuroimage.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J.L., Patanaik A., Chee N.I.Y.N., Lee X.K., Poh J.-H., Chee M.W.L. Auditory stimulation of sleep slow oscillations modulates subsequent memory encoding through altered hippocampal function. Sleep. 2018:41. doi: 10.1093/sleep/zsy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K., Takahashi E., Sakata S. Event-related potentials in the frontal cortex, hippocampus, and cerebellum during a temporal discrimination task in rats. Cogn. Brain Res. 2003;17:380–387. doi: 10.1016/S0926-6410(03)00139-3. [DOI] [PubMed] [Google Scholar]
- O’Reilly R.C., McClelland J.L. Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Orsmond G.I., Miller L.K. Cognitive, musical and environmental correlates of early music instruction. Psychol. Music. 1999;27:18–37. doi: 10.1177/0305735699271003. [DOI] [Google Scholar]
- O’Sullivan A.E., Lim C.Y., Lalor E.C. Look at me when I’m talking to you: Selective attention at a multisensory cocktail party can be decoded using stimulus reconstruction and alpha power modulations. Eur. J. Neurosci. 2019 doi: 10.1111/ejn.14425. [DOI] [PubMed] [Google Scholar]
- Pallesen K.J., Brattico E., Bailey C.J., Korvenoja A., Gjedde A. Cognitive and emotional modulation of brain default operation. J. Cogn. Neurosci. 2009;21:1065–1080. doi: 10.1162/jocn.2009.21086. [DOI] [PubMed] [Google Scholar]
- Papez J.W. A proposed mechanism of emotion. Arch. Neurol. Psychiatry. 1937;38:725–743. doi: 10.1001/archneurpsyc.1937.02260220069003. [DOI] [Google Scholar]
- Papp G., Kovac S., Frese A., Evers S. The impact of temporal lobe epilepsy on musical ability. Seizure. 2014;23:533–536. doi: 10.1016/j.seizure.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Paquette S., Fournier P., Dupont S., Szabo de Edelenyi F., Galan P., Samson S. Risk of tinnitus after medial temporal lobe surgery. JAMA Neurol. 2017;74:1376. doi: 10.1001/jamaneurol.2017.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Rugg M.D. Prestimulus hippocampal activity predicts later recollection. Hippocampus. 2009;20:24–28. doi: 10.1002/hipo.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmeggiani P.L., Rapisarda C. Hippocampal output and sensory mechanisms. Brain Res. 1969;14:387–400. doi: 10.1016/0006-8993(69)90117-6. [DOI] [PubMed] [Google Scholar]
- Pastalkova E., Itskov V., Amarasingham A., Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Olah J., Clement J. Classical nictitating membrane conditioning in the awake, normal, restrained cat. Science. 1977;196:1124–1126. doi: 10.1126/science.870974. [DOI] [PubMed] [Google Scholar]
- Paz R., Gelbard-Sagiv H., Mukamel R., Harel M., Malach R., Fried I. A neural substrate in the human hippocampus for linking successive events. Proc. Natl. Acad. Sci. U.S.A. 2010;107:6046–6051. doi: 10.1073/pnas.0910834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte M., Pérez-Perera L., Peña J.L., Velluti R.A. Sleep and wakefulness auditory processing: cortical units vs. hippocampal theta rhythm. Sleep Res. Online. 2001;4:51–57. [Google Scholar]
- Pedemonte M., Pena J., Velluti RicardoA. Firing of inferior colliculus auditory neurons is phase-locked to the hippocampus theta rhythm during paradoxical sleep and waking. Exp. Brain Res. 1996:112. doi: 10.1007/BF00227176. [DOI] [PubMed] [Google Scholar]
- Penfield W., Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. AMA Arch. Neurol. 1958;79:475–497. doi: 10.1001/archneurpsyc.1958.02340050003001. [DOI] [PubMed] [Google Scholar]
- Pereira C.S., Teixeira J., Figueiredo P., Xavier J., Castro S.L., Brattico E. Music and emotions in the brain: familiarity matters. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0027241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J., Kalpouzos G., Nilsson L.-G., Ryberg M., Nyberg L. Preserved hippocampus activation in normal aging as revealed by fMRI. Hippocampus. 2011;21:753–766. doi: 10.1002/hipo.20794. [DOI] [PubMed] [Google Scholar]
- Pertzov Y., Miller T.D., Gorgoraptis N., Caine D., Schott J.M., Butler C., Husain M. Binding deficits in memory following medial temporal lobe damage in patients with voltage-gated potassium channel complex antibody-associated limbic encephalitis. Brain. 2013;136:2474–2485. doi: 10.1093/brain/awt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson K.M., Reis A., Castro-Caldas A., Ingvar M. Effective auditory–verbal encoding activates the left prefrontal and the medial temporal lobes: a generalization to illiterate subjects. NeuroImage. 1999;10:45–54. doi: 10.1006/nimg.1999.0446. [DOI] [PubMed] [Google Scholar]
- Pfeiffer B.E., Foster D.J. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G., LeDoux E. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn. Mem. 1994;1:34–44. doi: 10.1101/lm.1.1.34. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Young A.W., Scott S.K., Calder A.J., Andrew C., Giampietro V., Williams S.C.R., Bullmore E.T., Brammer M., Gray J.A. Neural responses to facial and vocal expressions of fear and disgust. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piai V., Anderson K.L., Lin J.J., Dewar C., Parvizi J., Dronkers N.F., Knight R.T. Direct brain recordings reveal hippocampal rhythm underpinnings of language processing. Proc. Natl. Acad. Sci. U.S.A. 2016;113:11366–11371. doi: 10.1073/pnas.1603312113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton T.W., Hillyard S.A., Galambos R. In: Auditory System, Handbook of Sensory Physiology. Keidel W.D., Neff W.D., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 1976. Habituation and attention in the auditory system; pp. 343–389. [DOI] [Google Scholar]
- Pietschnig J., Voracek M., Formann A.K. Mozart effect–Shmozart effect: a meta-analysis. Intelligence. 2010;38:314–323. doi: 10.1016/j.intell.2010.03.001. [DOI] [Google Scholar]
- Pizzo F., Roehri N., Medina Villalon S., Trébuchon A., Chen S., Lagarde S., Carron R., Gavaret M., Giusiano B., McGonigal A., Bartolomei F., Badier J.M., Bénar C.G. Deep brain activities can be detected with magnetoencephalography. Nat. Commun. 2019;10:971. doi: 10.1038/s41467-019-08665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plailly J., Tillmann B., Royet J.-P. The feeling of familiarity of music and odors: the same neural signature? Cereb. Cortex. 2007;17:2650–2658. doi: 10.1093/cercor/bhl173. [DOI] [PubMed] [Google Scholar]
- Plakke B., Hwang J., Romanski L.M. Inactivation of primate prefrontal cortex impairs auditory and audiovisual working memory. J. Neurosci. 2015;35:9666–9675. doi: 10.1523/JNEUROSCI.1218-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plakke B., Romanski L.M. Auditory connections and functions of prefrontal cortex. Front. Neurosci. 2014:8. doi: 10.3389/fnins.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar J., Johnson E.G., Nordahl C.W., Ghetti S. Memory-related hippocampal activation in the sleeping toddler. Proc. Natl. Acad. Sci. 2018;115:6500–6505. doi: 10.1073/pnas.1805572115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasim S.E., Fried I., Jacobs J. Phase precession in the human hippocampus and entorhinal cortex. Cell. 2021;184(3242–3255) doi: 10.1016/j.cell.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga R.Q. No pattern separation in the human hippocampus. Trends Cogn. Sci. 2020;24:994–1007. doi: 10.1016/j.tics.2020.09.012. [DOI] [PubMed] [Google Scholar]
- Quiroga R.Q., Kraskov A., Koch C., Fried I. Explicit encoding of multimodal percepts by single neurons in the human brain. Curr. Biol. 2009;19:1308–1313. doi: 10.1016/j.cub.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo C., Weihing J., Schochat E. Temporal resolution in individuals with neurological disorders. Clinics. 2015;70:606–611. doi: 10.6061/clinics/2015(09)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij T.T., Valkonen-Korhonen M., Holi M., Therman S., Lehtonen J., Hari R. Reality of auditory verbal hallucinations. Brain. 2009;132:2994–3001. doi: 10.1093/brain/awp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Escobar N., Mercier M., Trébuchon-Fonséca A., Rodriguez-Fornells A., François C., Schön D. Hippocampal and auditory contributions to speech segmentation. Cortex. 2022;150:1–11. doi: 10.1016/j.cortex.2022.01.017. [DOI] [PubMed] [Google Scholar]
- Ranck J.B. Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. Exp. Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Rausch R., Crandall P.H. Psychological status related to surgical control of temporal lobe seizures. Epilepsia. 1982;23:191–202. doi: 10.1111/j.1528-1157.1982.tb05067.x. [DOI] [PubMed] [Google Scholar]
- Rauschecker J.P., Leaver A.M., Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J.P., Scott S.K. Neurobiology of Language. Elsevier; 2016. Pathways and streams in the auditory cortex; pp. 287–298. [DOI] [Google Scholar]
- Rauschecker J.P., Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher F.H., Shaw G.L., Ky K.N. Music and spatial task performance. Nature. 1993;365:611. doi: 10.1038/365611a0. [DOI] [PubMed] [Google Scholar]
- Recasens M., Gross J., Uhlhaas P.J. Low-frequency oscillatory correlates of auditory predictive processing in cortical-subcortical networks: a MEG-study. Sci. Rep. 2018:8. doi: 10.1038/s41598-018-32385-3. 14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding F.K. Modification of sensory cortical evoked potentials by hippocampal stimulation. Electroencephalogr. Clin. Neurophysiol. 1967;22:74–83. doi: 10.1016/0013-4694(67)90009-0. [DOI] [PubMed] [Google Scholar]
- Reddy L., Zoefel B., Possel J.K., Peters J., Dijksterhuis D.E., Poncet M., van Straaten E.C.W., Baayen J.C., Idema S., Self M.W. Human hippocampal neurons track moments in a sequence of events. J. Neurosci. 2021;41:6714–6725. doi: 10.1523/JNEUROSCI.3157-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler R.L., Goode T.D., Kim S., Ramanathan K.R., Maren S. Covert capture and attenuation of a hippocampus-dependent fear memory. Nat. Neurosci. 2021;24:677–684. doi: 10.1038/s41593-021-00825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey C., Reinecke H., Besser R. Methyltin intoxication in six men; toxicologic and clinical aspects. Vet. Hum. Toxicol. 1984;26:121–122. [PubMed] [Google Scholar]
- Rickert E.J., Bennett T.L., Lane P., French J. Hippocampectomy and the attenuation of blocking. Behav. Biol. 1978;22:147–160. doi: 10.1016/S0091-6773(78)92170-3. [DOI] [PubMed] [Google Scholar]
- Rickert E.J., Lorden J.F., Dawson R., Smyly E., Callahan M.F. Stimulus processing and stimulus selection in rats with hippocampal lesions. Behav. Neural Biol. 1979;27:454–465. doi: 10.1016/S0163-1047(79)92040-5. [DOI] [PubMed] [Google Scholar]
- Rizzolo L., Leger M., Corvaisier S., Groussard M., Platel H., Bouet V., Schumann-Bard P., Freret T. Long-term music exposure prevents age-related cognitive deficits in rats independently of hippocampal neurogenesis. Cereb. Cortex. 2021;31:620–634. doi: 10.1093/cercor/bhaa247. [DOI] [PubMed] [Google Scholar]
- Roberts B.M., Clarke A., Addante R.J., Ranganath C. Entrainment enhances theta oscillations and improves episodic memory. Cogn. Neurosci. 2018;9:181–193. doi: 10.1080/17588928.2018.1521386. [DOI] [PubMed] [Google Scholar]
- Rocchi F., Oya H., Balezeau F., Billig A.J., Kocsis Z., Jenison R., Nourski K.V., Kovach C.K., Steinschneider M., Kikuchi Y., Rhone A.E., Dlouhy B.J., Kawasaki H., Adolphs R., Greenlee J.D.W., Griffiths T.D., Howard M.A., Petkov C.I. Common fronto-temporal effective connectivity in humans and monkeys. Neuron. 2021;109:1–17. doi: 10.1101/2020.04.03.024042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers C.C., DeWeese M.R. Neural correlates of task switching in prefrontal cortex and primary auditory cortex in a novel stimulus selection task for rodents. Neuron. 2014;82:1157–1170. doi: 10.1016/j.neuron.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. The mechanisms for pattern completion and pattern separation in the hippocampus. Frontiers in Systems. Neuroscience. 2013:7. doi: 10.3389/fnsys.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Romanski L.M., Tian B., Fritz J., Mishkin M., Goldman-Rakic P.S., Rauschecker J.P. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosburg T., Trautner P., Ludowig E., Schaller C., Kurthen M., Elger C.E., Boutros N.N. Hippocampal event-related potentials to tone duration deviance in a passive oddball paradigm in humans. NeuroImage. 2007;37:274–281. doi: 10.1016/j.neuroimage.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R.T., Orr W.B., Holland P.C., Berger T.W. Hippocampectomy disrupts acquisition and retention of learned conditional responding. Behav. Neurosci. 1984;98:211–225. doi: 10.1037//0735-7044.98.2.211. [DOI] [PubMed] [Google Scholar]
- Rothschild G., Eban E., Frank L.M. A cortical–hippocampal–cortical loop of information processing during memory consolidation. Nat. Neurosci. 2017;20:251–259. doi: 10.1038/nn.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R.D., Brown-Schmidt S., Duff M.C., Tranel D., Cohen N.J. How do i remember that i know you know that i know? Psychol. Sci. 2011;22:1574–1582. doi: 10.1177/0956797611418245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy J.W. Context representations, context functions, and the parahippocampal-hippocampal system. Learn. Mem. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy J.W., O’Reilly R.C. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn., Affect., Behav. Neurosci. 2001;1:66–82. doi: 10.3758/CABN.1.1.66. [DOI] [PubMed] [Google Scholar]
- Rummell B.P., Klee J.L., Sigurdsson T. Attenuation of responses to self-generated sounds in auditory cortical neurons. J. Neurosci. 2016;36:12010–12026. doi: 10.1523/JNEUROSCI.1564-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusuvirta T., Korhonen T., Arikoski J., Kivirikko K. ERPs to pitch changes: a result of reduced responses to standard tones in rabbits. NeuroReport. 1996;7:413–416. [PubMed] [Google Scholar]
- Ruusuvirta T., Lipponen A., Pellinen E., Penttonen M., Astikainen P. Auditory cortical and hippocampal-system mismatch responses to duration deviants in urethane-anesthetized rats. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y. Coding of auditory temporal and pitch information by hippocampal individual cells and cell assemblies in the rat. Neuroscience. 2002;115:1153–1163. doi: 10.1016/S0306-4522(02)00509-2. [DOI] [PubMed] [Google Scholar]
- Salimpoor V.N., van den Bosch I., Kovacevic N., McIntosh A.R., Dagher A., Zatorre R.J. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science. 2013;340:216–219. doi: 10.1126/science.1231059. [DOI] [PubMed] [Google Scholar]
- Säljö A., Bao F., Haglid K.G., Hansson H.-A. Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. J. Neurotrauma. 2000;17:719–726. doi: 10.1089/089771500415454. [DOI] [PubMed] [Google Scholar]
- Säljö A., Bao F., Jingshan S., Hamberger A., Hansson H.-A., Haglid K.G. Exposure to short-lasting impulse noise causes neuronal c-jun expression and induction of apoptosis in the adult rat brain. J. Neurotrauma. 2002;19:985–991. doi: 10.1089/089771502320317131. [DOI] [PubMed] [Google Scholar]
- Samson S., Peretz I. Effects of prior exposure on music liking and recognition in patients with temporal lobe lesions. Ann. N. Y. Acad. Sci. 2005;1060:419–428. doi: 10.1196/annals.1360.035. [DOI] [PubMed] [Google Scholar]
- Samson S., Zatorre R.J. Contribution of the right temporal lobe to musical timbre discrimination. Neuropsychologia. 1994;32:231–240. doi: 10.1016/0028-3932(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Samson S., Zatorre R.J. Learning and retention of melodic and verbal information after unilateral temporal lobectomy. Neuropsychologia. 1992;30:815–826. doi: 10.1016/0028-3932(92)90085-Z. [DOI] [PubMed] [Google Scholar]
- Samson S., Zatorre R.J. Recognition memory for text and melody of songs after unilateral temporal lobe lesion evidence for dual encoding. J. Exp. Psychol.: Learn. Mem. Cogn. 1991;17:793–804. doi: 10.1037//0278-7393.17.4.793. [DOI] [PubMed] [Google Scholar]
- Sander D., Grandjean D., Pourtois G., Schwartz S., Seghier M.L., Scherer K.R., Vuilleumier P. Emotion and attention interactions in social cognition: Brain regions involved in processing anger prosody. NeuroImage. 2005;28:848–858. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Sanyal T., Kumar V., Nag T.C., Jain S., Sreenivas V., Wadhwa S. Prenatal loud music and noise: differential impact on physiological arousal, hippocampal synaptogenesis and spatial behavior in one day-old chicks. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal T., Palanisamy P., Nag T.C., Roy T.S., Wadhwa S. Effect of prenatal loud music and noise on total number of neurons and glia, neuronal nuclear area and volume of chick brainstem auditory nuclei, field L and hippocampus: a stereological investigation. Int. J. Dev. Neurosci. 2013;31:234–244. doi: 10.1016/j.ijdevneu.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Savelli F., Knierim J.J. Origin and role of path integration in the cognitive representations of the hippocampus: computational insights into open questions. J. Exp. Biol. 2019;222 doi: 10.1242/jeb.188912. jeb188912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R., Kempter R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. Eur. J. Neurosci. 2006;23:3124–3138. doi: 10.1111/j.1460-9568.2006.04774.x. [DOI] [PubMed] [Google Scholar]
- Schapiro A.C., Gregory E., Landau B., McCloskey M., Turk-Browne N.B. The necessity of the medial temporal lobe for statistical learning. J. Cogn. Neurosci. 2014;26:1736–1747. doi: 10.1162/jocn_a_00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro A.C., Kustner L.V., Turk-Browne N.B. Shaping of object representations in the human medial temporal lobe based on temporal regularities. Curr. Biol. 2012;22:1622–1627. doi: 10.1016/j.cub.2012.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro A.C., Turk-Browne N.B., Botvinick M.M., Norman K.A. Complementary learning systems within the hippocampus: A neural network modeling approach to reconciling episodic memory with statistical learning. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M., Landgrebe M., Poeppl T.B., Kreuzer P., Männer P., Marienhagen J., Wack D.S., Kleinjung T., Hajak G., Langguth B. Neural correlates of tinnitus duration and distress: a positron emission tomography study. Hum. Brain Mapp. 2013;34:233–240. doi: 10.1002/hbm.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaltz L.W., Theios J. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbits (Oryctolaous Cuniculus) J. Comp. Physiol. Psychol. 1972;79:328–333. doi: 10.1037/h0032531. [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J. Separate cortical networks involved in music perception: preliminary functional MRI evidence for modularity of music processing. NeuroImage. 2005;25:444–451. doi: 10.1016/j.neuroimage.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K., Plante E. Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. NeuroImage. 2006;29:254–266. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnupp J.W.H., Honey C., Willmore B.D.B. In: Neural Correlates of Auditory Cognition, Springer Handbook of Auditory Research. Cohen Y.E., Popper A.N., Fay R.R., editors. Springer; New York, New York, NY: 2013. Neural correlates of auditory object perception; pp. 115–149. [DOI] [Google Scholar]
- Schwartzbaum J.S., Thompson J.B., Kellicutt M.H. Auditory frequency discrimination and generalization following lesions of the amygdaloid area in rats. J. Comp. Physiol. Psychol. 1964;57:257–266. doi: 10.1037/h0039921. [DOI] [PubMed] [Google Scholar]
- Schwenzer M., Mathiak K. Numeric aspects in pitch identification: an fMRI study. BMC Neurosci. 2011:12. doi: 10.1186/1471-2202-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville W.B., Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedley W., Friston K.J., Gander P.E., Kumar S., Griffiths T.D. An integrative tinnitus model based on sensory precision. Trends Neurosci. 2016;39:799–812. doi: 10.1016/j.tins.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedley W., Gander P.E., Kumar S., Oya H., Kovach C.K., Nourski K.V., Kawasaki H., Howard M.A., Griffiths T.D. Intracranial mapping of a cortical tinnitus system using residual inhibition. Curr. Biol. 2015;25:1208–1214. doi: 10.1016/j.cub.2015.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres M.J., Winocur G., Moscovitch M., Anderson J.A.E., Pishdadian S., Martin Wojtowicz J., St-Laurent M., McAndrews M.P., Grady C.L. Changes in patterns of neural activity underlie a time-dependent transformation of memory in rats and humans. Hippocampus. 2018;28:745–764. doi: 10.1002/hipo.23009. [DOI] [PubMed] [Google Scholar]
- Shan K.Q., Lubenov E.V., Papadopoulou M., Siapas A.G. Spatial tuning and brain state account for dorsal hippocampal CA1 activity in a non-spatial learning task. eLife. 2016;5 doi: 10.7554/eLife.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Yao P.-T., Ge S., Xiong Q. Dentate granule cells encode auditory decisions after reinforcement learning in rats. Sci. Rep. 2021;11:14360. doi: 10.1038/s41598-021-93721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Hu H., Fan C., Wang Q., Zou T., Ye B., Xiang M. Sensorineural hearing loss may lead to dementia-related pathological changes in hippocampal neurons. Neurobiol. Dis. 2021 doi: 10.1016/j.nbd.2021.105408. [DOI] [PubMed] [Google Scholar]
- Shergill S.S. Modality specific neural correlates of auditory and somatic hallucinations. J. Neurol. Neurosurg. Psychiatry. 2001;71:688–690. doi: 10.1136/jnnp.71.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill S.S., Brammer M.J., Williams S.C.R., Murray R.M., McGuire P.K. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch. Gen. Psychiatry. 2000;57:1033. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- Shigeto H. Hippocampal modulation of auditory processing in epilepsy. Neurol. Clin. Neurosci. 2021;9:17–23. doi: 10.1111/ncn3.12470. [DOI] [Google Scholar]
- Shimbo A., Izawa E.-I., Fujisawa S. Scalable representation of time in the hippocampus. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abd7013. eabd7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. The interrelationship between movement and cognition: Theta rhythm and the P300 event-related potential. Hippocampus. 2011;21:744–752. doi: 10.1002/hipo.20792. [DOI] [PubMed] [Google Scholar]
- Shinba T. Neuronal firing activity in the dorsal hippocampus during the auditory discrimination oddball task in awake rats: relation to event-related potential generation. Cogn. Brain Res. 1999;8:241–250. doi: 10.1016/S0926-6410(99)00026-9. [DOI] [PubMed] [Google Scholar]
- Shinba T., Andow Y., Shinozaki T., Ozawa N., Yamamoto K. Event-related potentials in the dorsal hippocampus of rats during an auditory discrimination paradigm. Electroencephalogr. Clin. Neurophysiol. 1996:6. doi: 10.1016/s0168-5597(96)95205-3. [DOI] [PubMed] [Google Scholar]
- Shulman A. A final common pathway for tinnitus - the medial temporal lobe system. Journal: Int. Tinnitus J. 1995;1:115–126. [PubMed] [Google Scholar]
- Shulman A., Strashun A.M., Afriyie M., Aronson F., Abel W., Goldstein B. SPECT imaging of brain and tinnitus - neurotologic/neurologic implications. Int. Tinnitus J. 1995;1:13–29. [PubMed] [Google Scholar]
- Silbersweig D.A., Stern E., Frith C., Cahill C., Holmes A., Grootoonk S., Seaward J., McKenna P., Chua S.E., Schnorr L., Jones T., Frackowiak R.S.J. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995:378. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Simon J.Z. The encoding of auditory objects in auditory cortex: Insights from magnetoencephalography. Int. J. Psychophysiol. 2015;95:184–190. doi: 10.1016/j.ijpsycho.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti P., Ono C.R., Godoi Carneiro C., de, Ali Khan R., Shahsavarani S., Husain F.T., Oiticica J. Evaluating the efficacy of hearing aids for tinnitus therapy – a positron emission tomography study. Brain Res. 2022;1775 doi: 10.1016/j.brainres.2021.147728. [DOI] [PubMed] [Google Scholar]
- Singer W., Zuccotti A., Jaumann M., Lee S.C., Panford-Walsh R., Xiong H., Zimmermann U., Franz C., Geisler H.-S., Köpschall I., Rohbock K., Varakina K., Verpoorten S., Reinbothe T., Schimmang T., Rüttiger L., Knipper M. Noise-induced inner hair cell ribbon loss disturbs central Arc mobilization: a novel molecular paradigm for understanding tinnitus. Mol. Neurobiol. 2013;47:261–279. doi: 10.1007/s12035-012-8372-8. [DOI] [PubMed] [Google Scholar]
- Sliwa J., Planté A., Duhamel J.-R., Wirth S. Independent neuronal representation of facial and vocal identity in the monkey hippocampus and inferotemporal cortex. Cereb. Cortex. 2014;26:950–966. doi: 10.1093/cercor/bhu257. [DOI] [PubMed] [Google Scholar]
- Smith C.N., Jeneson A., Frascino J.C., Kirwan C.B., Hopkins R.O., Squire L.R. When recognition memory is independent of hippocampal function. Proc. Natl. Acad. Sci. U.S.A. 2014;111:9935–9940. doi: 10.1073/pnas.1409878111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.E., Halgren E., Sokolik M., Baudena P., Musolino A., Liegeois-Chauvel C., Chauvel P. The intracranial topography of the P3 event-related potential elicited during auditory oddball. Electroencephalogr. Clin. Neurophysiol. 1990;76:235–248. doi: 10.1016/0013-4694(90)90018-F. [DOI] [PubMed] [Google Scholar]
- Smith M.E., Stapleton J.M., Halgren E. Human medial temporal lobe potentials evoked in memory and language tasks. Electroencephalogr. Clin. Neurophysiol. 1986;63:145–159. doi: 10.1016/0013-4694(86)90008-8. [DOI] [PubMed] [Google Scholar]
- Snyder J.S., Elhilali M. Recent advances in exploring the neural underpinnings of auditory scene perception. Ann. N. Y. Acad. Sci. 2017;1396:39–55. doi: 10.1111/nyas.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon E.A., Lega B.C., Sperling M.R., Kahana M.J. Hippocampal theta codes for distances in semantic and temporal spaces. Proc. Natl. Acad. Sci. U.S.A. 2019;116:24343–24352. doi: 10.1073/pnas.1906729116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon P.R. Role of the hippocampus in blocking and conditioned inhibition of the rabbit’s nictitating membrane response. J. Comp. Physiol. Psychol. 1977;91:407–417. doi: 10.1037/h0077330. [DOI] [PubMed] [Google Scholar]
- Solomon P.R., Moore J.W. Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctolagus cuniculus) following dorsal hippocampal ablation. J. Comp. Physiol. Psychol. 1975;89:1192–1203. doi: 10.1037/h0077183. [DOI] [PubMed] [Google Scholar]
- Solomon P.R., Schaaf E.R.V., College W., Thompson R.F., Weisz D.J. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Sommer I.E.C., Diederen K.M.J., Blom J.-D., Willems A., Kushan L., Slotema K., Boks M.P.M., Daalman K., Hoek H.W., Neggers S.F.W., Kahn R.S. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131:3169–3177. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- Song J.-J., De Ridder D., Van de Heyning P., Vanneste S. Mapping tinnitus-related brain activation: an activation-likelihood estimation metaanalysis of PET studies. J. Nucl. Med. 2012;53:1550–1557. doi: 10.2967/jnumed.112.102939. [DOI] [PubMed] [Google Scholar]
- Squire L., Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Squire L.R., Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr. Opin. Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire L.R., Schmolck H., Stark S.M. Impaired auditory recognition memory in amnesic patients with medial temporal lobe lesions. Learn. Mem. 2001;8:252–256. doi: 10.1101/lm.42001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan K.K., D’Esposito M. The what, where and how of delay activity. Nat. Rev. Neurosci. 2019;20:466–481. doi: 10.1038/s41583-019-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachenfeld K.L., Botvinick M.M., Gershman S.J. The hippocampus as a predictive map. Nat. Neurosci. 2017;20:1643–1653. doi: 10.1038/nn.4650. [DOI] [PubMed] [Google Scholar]
- Stapleton J.M., Halgren E., Moreno K.A. Endogenous potentials after anterior temporal lobectomy. Neuropsychologia. 1987;25:549–557. doi: 10.1016/0028-3932(87)90079-0. [DOI] [PubMed] [Google Scholar]
- Stefanacci L., Buffalo E.A., Schmolck H., Squire L.R. Profound amnesia after damage to the medial temporal lobe: a neuroanatomical and neuropsychological profile of patient E. P. J. Neurosci. 2000;20:7024–7036. doi: 10.1523/JNEUROSCI.20-18-07024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Laurent M., Moscovitch M., Jadd R., McAndrews M.P. The perceptual richness of complex memory episodes is compromised by medial temporal lobe damage. Hippocampus. 2014;24:560–576. doi: 10.1002/hipo.22249. [DOI] [PubMed] [Google Scholar]
- Sundaramahalingam M., Ramasundaram S., Rathinasamy S.D., Natarajan R.P., Somasundaram T. Role of acorus calamus and α-asarone on hippocampal dependent memory in noise stress exposed rats. Pak. J. Biol. Sci. 2013;16:770–778. doi: 10.3923/pjbs.2013.770.778. [DOI] [PubMed] [Google Scholar]
- Sutton S., Braren M., Zubin J., John E.R. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Takei N., Toyoda T., Iwata Y., Hoshino R., Minabe Y., Mori N. Auditory hallucinations and cognitive impairment in a patient with a lesion restricted to the hippocampus. Schizophr. Res. 2003;64:87–89. doi: 10.1016/S0920-9964(02)00386-9. [DOI] [PubMed] [Google Scholar]
- Suzuki W.L., Amaral D.G. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J. Comp. Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Svoboda E., Levine B. The effects of rehearsal on the functional neuroanatomy of episodic autobiographical and semantic remembering: a functional magnetic resonance imaging study. J. Neurosci. 2009;29:3073–3082. doi: 10.1523/JNEUROSCI.3452-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson A.M., Isaacson R.L. Hippocampal ablation and performance during withdrawal of reinforcement. J. Comp. Physiol. Psychol. 1967;64:30–35. doi: 10.1037/h0024797. [DOI] [PubMed] [Google Scholar]
- Szameitat D.P., Kreifelts B., Alter K., Szameitat A.J., Sterr A., Grodd W., Wildgruber D. It is not always tickling: Distinct cerebral responses during perception of different laughter types. NeuroImage. 2010;53:1264–1271. doi: 10.1016/j.neuroimage.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Tachibana H., Miyata Y., Takeda M., Minamoto H., Sugita M., Okita T. Auditory event-related potentials in an amnesic patient with a left temporal lobe lesion. J. Neurol. Sci. 1999;168:52–56. doi: 10.1016/S0022-510X(99)00182-3. [DOI] [PubMed] [Google Scholar]
- Tae W.-S., Yakunina N., Lee W.H., Ryu Y.-J., Ham H., Pyun S.-B., Nam E.-C. Changes in the regional shape and volume of subcortical nuclei in patients with tinnitus comorbid with mild hearing loss. Neuroradiology. 2018;60:1203–1211. doi: 10.1007/s00234-018-2093-2. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Sakurai Y. Sub-millisecond firing synchrony of closely neighboring pyramidal neurons in hippocampal CA1 of rats during delayed non-matching to sample task. Front. Neural Circuits. 2009:3. doi: 10.3389/neuro.04.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura H., Umeno K., Tabuchi E., Hori E., Miyamoto K., Aso S., Watanabe Y., Ono T., Nishijo H. Differential activation in the medial temporal lobe during a sound-sequence discrimination task across age in human subjects. Neuroscience. 2003;119:517–532. doi: 10.1016/S0306-4522(03)00193-3. [DOI] [PubMed] [Google Scholar]
- Takebayashi H., Takei N., Suzuki S., Mori N. Unilateral auditory hallucinations in schizophrenia after damage to the right hippocampus. Schizophr. Res. 2002;58:329–331. doi: 10.1016/S0920-9964(01)00399-1. [DOI] [PubMed] [Google Scholar]
- Talk A., Grasby K., Rawson T., Ebejer J. Preconditioning of spatial and auditory cues: roles of the hippocampus, frontal cortex, and cue-directed attention. Brain Sci. 2016;6:63. doi: 10.3390/brainsci6040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talk A.C., Gandhi C.C., Matzel L.D. Hippocampal function during behaviorally silent associative learning: Dissociation of memory storage and expression. Hippocampus. 2002;12:648–656. doi: 10.1002/hipo.10098. [DOI] [PubMed] [Google Scholar]
- Tamura R., Ono T., Fukuda M., Nakamura K. Spatial responsiveness of monkey hippocampal neurons to various visual and auditory stimuli. Hippocampus. 1992;2:307–322. doi: 10.1002/hipo.450020309. [DOI] [PubMed] [Google Scholar]
- Tamura R., Ono T., Fukuda M., Nakamura K. Recognition of egocentric and allocentric visual and auditory space by neurons in the hippocampus of monkeys. Neurosci. Lett. 1990;109:293–298. doi: 10.1016/0304-3940(90)90010-7. [DOI] [PubMed] [Google Scholar]
- Tang W., Shin J.D., Jadhav S.P. Multiple time-scales of decision-making in the hippocampus and prefrontal cortex. eLife. 2021;10 doi: 10.7554/eLife.66227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J., Muller R., Ranck J. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 1990;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares R.M., Mendelsohn A., Grossman Y., Williams C.H., Shapiro M., Trope Y., Schiller D. A map for social navigation in the human brain. Neuron. 2015;87:231–243. doi: 10.1016/j.neuron.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teki S., Griffiths T.D. Brain bases of working memory for time intervals in rhythmic sequences. Front. Neurosci. 2016:10. doi: 10.3389/fnins.2016.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teki S., Kumar S., von Kriegstein K., Stewart L., Lyness C.R., Moore B.C.J., Capleton B., Griffiths T.D. Navigating the auditory scene: an expert role for the hippocampus. J. Neurosci. 2012;32:12251–12257. doi: 10.1523/JNEUROSCI.0082-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S., Sakurai Y., Nakahara H., Fujisawa S. Temporal and rate coding for discrete event sequences in the hippocampus. Neuron. 2017;94(1248–1262) doi: 10.1016/j.neuron.2017.05.024. [DOI] [PubMed] [Google Scholar]
- Teyler T.J., DiScenna P. The hippocampal memory indexing theory. Behav. Neurosci. 1986;100:147–154. doi: 10.1037//0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- Thompson R.F. In search of memory traces. Annu. Rev. Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- Thompson W.F., Schellenberg E.G., Husain G. Arousal, mood, and the Mozart effect. Psychol. Sci. 2001;12:248–251. doi: 10.1111/1467-9280.00345. [DOI] [PubMed] [Google Scholar]
- Tierney T.M., Levy A., Barry D.N., Meyer S.S., Shigihara Y., Everatt M., Mellor S., Lopez J.D., Bestmann S., Holmes N., Roberts G., Hill R.M., Boto E., Leggett J., Shah V., Brookes M.J., Bowtell R., Maguire E.A., Barnes G.R. Mouth magnetoencephalography: a unique perspective on the human hippocampus. NeuroImage. 2021;225 doi: 10.1016/j.neuroimage.2020.117443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobia M.J., Iacovella V., Hasson U. Multiple sensitivity profiles to diversity and transition structure in non-stationary input. NeuroImage. 2012;60:991–1005. doi: 10.1016/j.neuroimage.2012.01.041. [DOI] [PubMed] [Google Scholar]
- Tolman E.C. Cognitive maps in rats and men. Psychol. Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- Tranel D., Brady D.R., Van Hoesen G.W., Damasio A.R. Parahippocampal projections to posterior auditory association cortex (area Tpt) in Old-World monkeys. Exp. Brain Res. 1988;70:406–416. doi: 10.1007/BF00248365. [DOI] [PubMed] [Google Scholar]
- Trost W., Ethofer T., Zentner M., Vuilleumier P. Mapping aesthetic musical emotions in the brain. Cereb. Cortex. 2012;22:2769–2783. doi: 10.1093/cercor/bhr353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A., Sugar J., Lu L., Wang C., Knierim J.J., Moser M.-B., Moser E.I. Integrating time from experience in the lateral entorhinal cortex. Nature. 2018;561:57–62. doi: 10.1038/s41586-018-0459-6. [DOI] [PubMed] [Google Scholar]
- Tsivilis D., Vann S.D., Denby C., Roberts N., Mayes A.R., Montaldi D., Aggleton J.P. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat. Neurosci. 2008;11:834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Turk-Browne N.B. The hippocampus as a visual area organized by space and time: a spatiotemporal similarity hypothesis. Vis. Res. 2019;165:123–130. doi: 10.1016/j.visres.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuwaig M., Savard M., Jutras B., Poirier J., Collins D.L., Rosa-Neto P., Fontaine D., Breitner J.C.S. Deficit in central auditory processing as a biomarker of pre-clinical Alzheimer’s disease. J. Alzheimer’s Dis. 2017;60:1589–1600. doi: 10.3233/JAD-170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T., Donishi T., Ukai S., Ikeda Y., Hotomi M., Yamanaka N., Shinosaki K., Terada M., Kaneoke Y. Brain regions responsible for tinnitus distress and loudness: a resting-state fMRI study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N., Moss C.F. Dynamics of hippocampal spatial representation in echolocating bats. Hippocampus. 2011;21:150–161. doi: 10.1002/hipo.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N., Moss C.F. What the bat’s voice tells the bat’s brain. Proc. Natl. Acad. Sci. U.S.A. 2008;105:8491–8498. doi: 10.1073/pnas.0703550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N., Moss C.F. Hippocampal cellular and network activity in freely moving echolocating bats. Nat. Neurosci. 2007;10:224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- Umbach G., Kantak P., Jacobs J., Kahana M., Pfeiffer B.E., Sperling M., Lega B. Time cells in the human hippocampus and entorhinal cortex support episodic memory. Proc. Natl. Acad. Sci. U.S.A. 2020;117:28463–28474. doi: 10.1073/pnas.2013250117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungiadze A.A. Electrical activity of the hippocampus during peripheral stimulation. Neurosci. Transl. 1967;1:136–142. doi: 10.1007/BF01124392. [DOI] [Google Scholar]
- Uran S.L., Aon-Bertolino M.L., Caceres L.G., Capani F., Guelman L.R. Rat hippocampal alterations could underlie behavioral abnormalities induced by exposure to moderate noise levels. Brain Res. 2012;1471:1–12. doi: 10.1016/j.brainres.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Uran S.L., Caceres L.G., Guelman L.R. Effects of loud noise on hippocampal and cerebellar-related behaviors. Brain Res. 2010;1361:102–114. doi: 10.1016/j.brainres.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Uran S.L., Gómez-Casati M.E., Guelman L.R. Long-term recovery from hippocampal-related behavioral and biochemical abnormalities induced by noise exposure during brain development. Eval. Audit. Pathw. Integr. Int. J. Dev. Neurosci. 2014;37:41–51. doi: 10.1016/j.ijdevneu.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Urgolites Z.J., Wixted J.T., Goldinger S.D., Papesh M.H., Treiman D.M., Squire L.R., Steinmetz P.N. Two kinds of memory signals in neurons of the human hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2022;119 doi: 10.1073/pnas.2115128119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgolites Z.J., Wixted J.T., Goldinger S.D., Papesh M.H., Treiman D.M., Squire L.R., Steinmetz P.N. Spiking activity in the human hippocampus prior to encoding predicts subsequent memory. Proc. Natl. Acad. Sci. U.S.A. 2020;117:13767–13770. doi: 10.1073/pnas.2001338117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen J., Gregory E., Landau B., McCloskey M. New learning of music after bilateral medial temporal lobe damage: evidence from an amnesic patient. Front. Hum. Neurosci. 2014:8. doi: 10.3389/fnhum.2014.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven V., Waldorp L., Christoffels I. Hippocampus plays a role in speech feedback processing. NeuroImage. 2020;223 doi: 10.1016/j.neuroimage.2020.117319. [DOI] [PubMed] [Google Scholar]
- van Dongen E.V., Takashima A., Barth M., Zapp J., Schad L.R., Paller K.A., Fernandez G. Memory stabilization with targeted reactivation during human slow-wave sleep. Proc. Natl. Acad. Sci. U.S.A. 2012;109:10575–10580. doi: 10.1073/pnas.1201072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noorden, L.P.A.S., 1975. Temporal Coherence in the Perception of Tone Sequences. Univeristy of Technology, Eindhoven, The Netherlands.
- van Strien N.M., Cappaert N.L.M., Witter M.P. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat. Rev. Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F., Gadian D.G., Watkins K.E., Connelly A., Van Paesschen W., Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Vaudano E., Legg C.R., Glickstein M. Afferent and efferent connections of temporal association cortex in the rat: a horseradish peroxidase study. Eur. J. Neurosci. 1991;3:317–330. doi: 10.1111/j.1460-9568.1991.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Velasco M., Velasco F., Velasco A.L., Almanza X., Olvera A. Subcortical correlates of the P300 potential complex in man to auditory stimuli. Electroencephalogr. Clin. Neurophysiol. 1986;64:199–210. doi: 10.1016/0013-4694(86)90166-5. [DOI] [PubMed] [Google Scholar]
- Viganò S., Piazza M. Distance and direction codes underlie navigation of a novel semantic space in the human brain. J. Neurosci. 2020;40:2727–2736. doi: 10.1523/JNEUROSCI.1849-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinnik E., Antopolskiy S., Itskov P.M., Diamond M.E. Auditory stimuli elicit hippocampal neuronal responses during sleep. Front. Syst. Neurosci. 2012:6. doi: 10.3389/fnsys.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova O.S. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Vinogradova O.S. In: The Hippocampus. Isaacson R.L., Pribram K.H., editors. Springer; US, Boston, MA: 1975. Functional organization of the limbic system in the process of registration of information: facts and hypotheses; pp. 3–69. [DOI] [Google Scholar]
- Vinogradova, O.S., 1975b. Functional organization of the limbic system in the process of registration of information: Facts and hypotheses, in: The Hippocampus. Volume 2: Neurophysiology and Behavior. Plenum Press, New York, NY, pp. 3–70.
- Vinogradova O.S., Semyonova T.P., Konovalov V.Ph. Biology of Memory. Elsevier; 1970. Trace phenomena in single neurons of hippocampus and mammiliary bodies; pp. 191–221. [DOI] [Google Scholar]
- Vohs J.L., Andrew Chambers R., Krishnan G.P., O’Donnell B.F., Berg S., Morzorati S.L. GABAergic modulation of the 40 Hz auditory steady-state response in a rat model of schizophrenia. Int. J. Neuropsychopharmacol. 2010;13:487. doi: 10.1017/S1461145709990307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs J.L., Chambers R.A., Krishnan G.P., O’Donnell B.F., Hetrick W.P., Kaiser S.T., Berg S., Morzorati S.L. Auditory sensory gating in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropsychobiology. 2009;60:12–22. doi: 10.1159/000234813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs J.L., Chambers R.A., O’Donnell B.F., Krishnan G.P., Morzorati S.L. Auditory steady state responses in a schizophrenia rat model probed by excitatory/inhibitory receptor manipulation. Int. J. Psychophysiol. 2012;86:136–142. doi: 10.1016/j.ijpsycho.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wolf A.P., van Gelder P., Brodie J.D., Overall J.E., Cancro R., Gomez-Mont F. Phenomological correlates of metabolic activity in 18 patients with chronic schizophrenia. Am. J. Psychiatry. 1987;144:151–158. doi: 10.1176/ajp.144.2.151. [DOI] [PubMed] [Google Scholar]
- Wahlstrom K.L., Huff M.L., Emmons E.B., Freeman J.H., Narayanan N.S., McIntyre C.K., LaLumiere R.T. Basolateral amygdala inputs to the medial entorhinal cortex selectively modulate the consolidation of spatial and contextual learning. J. Neurosci. 2018;38:2698–2712. doi: 10.1523/JNEUROSCI.2848-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.X., Rogers L.M., Gross E.Z., Ryals A.J., Dokucu M.E., Brandstatt K.L., Hermiller M.S., Voss J.L. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yu Y., Feng Y., Zou F., Zhang X., Huang J., Zhang Y., Zheng X., Huang X.-F., Zhu Y., Liu Y. Protective effect of the orientin on noise-induced cognitive impairments in mice. Behav. Brain Res. 2016;296:290–300. doi: 10.1016/j.bbr.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Wang S.-F., Ritchey M., Libby L.A., Ranganath C. Functional connectivity based parcellation of the human medial temporal lobe. Neurobiol. Learn. Mem. 2016;134:123–134. doi: 10.1016/j.nlm.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J.D., Jennings A.R., Griffiths T.D. Analysis of the spectral envelope of sounds by the human brain. NeuroImage. 2005;24:1052–1057. doi: 10.1016/j.neuroimage.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Yagishita S., Kikyo H. Memory of music: roles of right hippocampus and left inferior frontal gyrus. NeuroImage. 2008;39:483–491. doi: 10.1016/j.neuroimage.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Weisberg S.M., Ekstrom A.D. Hippocampal volume and navigational ability: the map(ping) is not to scale. Neurosci. Biobehav. Rev. 2021;126:102–112. doi: 10.1016/j.neubiorev.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Kronforst-Collins M.A., Disterhoft J.F. Activity of hippocampal pyramidal neurons during trace eyeblink conditioning. Hippocampus. 1996;6:192–209. doi: 10.1002/(SICI)1098-1063(1996)6:2<192::AID-HIPO9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Weitz A.J., Fang Z., Lee H.J., Fisher R.S., Smith W.C., Choy M., Liu J., Lin P., Rosenberg M., Lee J.H. Optogenetic fMRI reveals distinct, frequency-dependent networks recruited by dorsal and intermediate hippocampus stimulations. NeuroImage. 2015;107:229–241. doi: 10.1016/j.neuroimage.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston P.S.J., Nicholas J.M., Henley S.M.D., Liang Y., Macpherson K., Donnachie E., Schott J.M., Rossor M.N., Crutch S.J., Butler C.R., Zeman A.Z., Fox N.C. Accelerated long-term forgetting in presymptomatic autosomal dominant Alzheimer’s disease: a cross-sectional study. Lancet Neurol. 2018;17:123–132. doi: 10.1016/S1474-4422(17)30434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington J.C.R., Muller T.H., Mark S., Chen G., Barry C., Burgess N., Behrens T.E.J. The Tolman-Eichenbaum machine: unifying space and relational memory through generalization in the hippocampal formation. Cell. 2020;183(1249–1263) doi: 10.1016/j.cell.2020.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren W.A. Sparing of short-term memory in an amnesic patient: Implications for strength theory of memory. Neuropsychologia. 1968;6:235–244. doi: 10.1016/0028-3932(68)90022-5. [DOI] [Google Scholar]
- Wiethoff S., Wildgruber D., Kreifelts B., Becker H., Herbert C., Grodd W., Ethofer T. Cerebral processing of emotional prosody—influence of acoustic parameters and arousal. NeuroImage. 2008;39:885–893. doi: 10.1016/j.neuroimage.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Wilkins R.W., Hodges D.A., Laurienti P.J., Steen M., Burdette J.H. Network science and the effects of music preference on functional brain connectivity: from Beethoven to Eminem. Sci. Rep. 2014:4. doi: 10.1038/srep06130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills T.J., Lever C., Cacucci F., Burgess N., O’Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science. 2005;308:873–876. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G., Moscovitch M. Memory transformation and systems consolidation. J. Int. Neuropsychol. Soc. 2011;17:766–780. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- Witt J.-A., Coras R., Schramm J., Becker A.J., Elger C.E., Blümcke I., Helmstaedter C. The overall pathological status of the left hippocampus determines preoperative verbal memory performance in left mesial temporal lobe epilepsy: hippocampal neuronal cell densities and memory functions. Hippocampus. 2014;24:446–454. doi: 10.1002/hipo.22238. [DOI] [PubMed] [Google Scholar]
- Witter M.P., Amaral D.G. The entorhinal cortex of the monkey: VI. Organization of projections from the hippocampus, subiculum, presubiculum, and parasubiculum. J. Comp. Neurol. 2021;529:828–852. doi: 10.1002/cne.24983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N.I., Stefanini F., Apodaca-Montano D.L., Tan I.M.C., Biane J.S., Kheirbek M.A. The dentate gyrus classifies cortical representations of learned stimuli. Neuron. 2020;107(173–184) doi: 10.1016/j.neuron.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett K., Maguire E.A. Acquiring “the knowledge” of London’s layout drives structural brain changes. Curr. Biol. 2011;21:2109–2114. doi: 10.1016/j.cub.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Yao J., Hu B., Zhang H., Li Y., Li X., Li Q., Sui J. Reevaluating the role of the hippocampus in delay eyeblink conditioning. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C., Liu Y., Xu J., Gan X., Xiao Z. Septal and hippocampal neurons contribute to auditory relay and fear conditioning. Front. Cell. Neurosci. 2018:12. doi: 10.3389/fncel.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Chen W., Wang Y., Jing W., Gao S., Guo D., Xia Y., Yao D. Music exposure improves spatial cognition by enhancing the BDNF level of dorsal hippocampal subregions in the developing rats. Brain Res. Bull. 2016;121:131–137. doi: 10.1016/j.brainresbull.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Xing Y., Xia Y., Kendrick K., Liu X., Wang M., Wu D., Yang H., Jing W., Guo D., Yao D. Mozart, Mozart rhythm and retrograde Mozart effects: evidences from behaviours and neurobiology bases. Sci. Rep. 2016;6 doi: 10.1038/srep18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Hu L., Song T., Liu Y., Wu Q., Zhao L., Liu L., Zhao X., Zhang D., Huang C. Proteomic changes in female rat hippocampus following exposure to a terrified sound stress. J. Mol. Neurosci. 2014;53:158–165. doi: 10.1007/s12031-014-0242-6. [DOI] [PubMed] [Google Scholar]
- Yang J., Hu L., Wu Q., Liu L., Zhao L., Zhao X., Song T., Huang C. A terrified-sound stress induced proteomic changes in adult male rat hippocampus. Physiol. Behav. 2014;128:32–38. doi: 10.1016/j.physbeh.2014.01.038. [DOI] [PubMed] [Google Scholar]
- Yi G.-L., Zhu M.-Z., Cui H.-C., Yuan X.-R., Liu P., Tang J., Li Y.-Q., Zhu X.-H. A hippocampus dependent neural circuit loop underlying the generation of auditory mismatch negativity. Neuropharmacology. 2022;206 doi: 10.1016/j.neuropharm.2022.108947. [DOI] [PubMed] [Google Scholar]
- Yonelinas A.P. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav. Brain Res. 2013;254:34–44. doi: 10.1016/j.bbr.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas A.P., Ranganath C., Ekstrom A.D., Wiltgen B.J. A contextual binding theory of episodic memory: systems consolidation reconsidered. Nat. Rev. Neurosci. 2019 doi: 10.1038/s41583-019-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura T., Zhong J., Shibata D.K., Kwok W.E., Shrier D.A., Numaguchi Y. Functional MRI study of auditory and visual oddball tasks. NeuroReport. 1999;10:1683–1688. doi: 10.1097/00001756-199906030-00011. [DOI] [PubMed] [Google Scholar]
- Yu C., Moss C.F. Natural acoustic stimuli evoke selective responses in the hippocampus of passive listening bats. Hippocampus. 2022;32:298–309. doi: 10.1002/hipo.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.-F., Zhai F., Dai C.-F., Hu J.-J. The relationship between age-related hearing loss and synaptic changes in the hippocampus of C57BL/6J mice. Exp. Gerontol. 2011;46:716–722. doi: 10.1016/j.exger.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J. Musical perception and cerebral function: a critical review. Music Percept. 1984;2:196–221. doi: 10.2307/40285291. [DOI] [Google Scholar]
- Zeidman P., Mullally S.L., Maguire E.A. Constructing, perceiving, and maintaining scenes: hippocampal activity and connectivity. Cereb. Cortex. 2015;25:3836–3855. doi: 10.1093/cercor/bhu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevin J.D., McCandliss B.D. Dishabituation of the BOLD response to speech sounds. Behav. Brain Funct. 2005;1 doi: 10.1186/1744-9081-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.-W., Sun W.-J., Zingg B., Shen L., He J., Xiong Y., Tao H.W., Zhang L.I. A non-canonical reticular-limbic central auditory pathway via medial septum contributes to fear conditioning. Neuron. 2018;97:406–417. doi: 10.1016/j.neuron.2017.12.010. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Lin S.-C., Nicolelis M.A.L. A distinctive subpopulation of medial septal slow-firing neurons promote hippocampal activation and theta oscillations. J. Neurophysiol. 2011;106:2749–2763. doi: 10.1152/jn.00267.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang J., Sun H., Feng G., Gao Z. Interactions between the hippocampus and the auditory pathway. Neurobiol. Learn. Mem. 2022;189 doi: 10.1016/j.nlm.2022.107589. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu C., Martel D.T., West M., Sutton M.A., Shore S.E. Remodeling of cholinergic input to the hippocampus after noise exposure and tinnitus induction in Guinea pigs. Hippocampus. 2019;29:669–682. doi: 10.1002/hipo.23058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wu C., Martel D.T., West M., Sutton M.A., Shore S.E. Noise exposure alters glutamatergic and GABAergic synaptic connectivity in the hippocampus and its relevance to tinnitus. Neural Plast. 2021;2021:1–16. doi: 10.1155/2021/8833087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Wang L., Chen Liang, Zhang J., Sun W., Salvi R.J., Huang Y.-N., Wang M., Chen Lin. Temporary conductive hearing loss in early life impairs spatial memory of rats in adulthood. Brain Behav. 2018;8 doi: 10.1002/brb3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Olofsson J.K., Koubeissi M.Z., Menelaou G., Rosenow J., Schuele S.U., Xu P., Voss J.L., Lane G., Zelano C. Human hippocampal connectivity is stronger in olfaction than other sensory systems. Prog. Neurobiol. 2021;201 doi: 10.1016/j.pneurobio.2021.102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material