Abstract
Background
An estimated 1% to 3% of all individuals will receive a diagnosis of epilepsy during their lives, which corresponds to approximately 50 million affected people worldwide. The real prevalence is possibly higher because epilepsy is underreported in developing countries. Although most will achieve adequate control of their disease though the use of medication, approximately 25% to 30% of all those with epilepsy are refractory to pharmacological treatment and will continue to have seizures despite the use of two or more agents in adequate dosages. Over the last decade, researchers have tested the use of polyunsaturated fatty acid (PUFA) supplements for the treatment of refractory epilepsy, with inconsistent results. There have also been some concerns about the use of omega‐3 PUFA compounds because they reduce platelet aggregation and could, in theory, cause bleeding.
Objectives
To assess the effectiveness and tolerability of omega‐3 polyunsaturated fatty acids (eicosapentaenoic acid‐EPA and docosahexanoic acid‐DHA) in the control of seizures in people with refractory epilepsy.
Search methods
We searched the Cochrane Epilepsy Group Specialised Register (from inception up to November 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, issue 11), MEDLINE (1948 to November 2015), EMBASE (1980 to November 2015), SCOPUS (1823 to November 2015); LILACS (Literatura Latino‐Americana e do Caribe de Informação em Ciências da Saúde) (1982 to November 2015); ClinicalTrials.gov; World Health Organization (WHO) International Clinical Trials Registry Platform (November 2015). No language restrictions were imposed. We contacted study authors for additional and unpublished information and screened the reference lists of retrieved citations for potentially eligible studies not identified through the electronic search.
Selection criteria
All randomised and quasi‐randomised studies using PUFAs for the treatment of drug‐resistant epilepsy.
Data collection and analysis
Two review authors were involved in study selection, data extraction and quality assessment of the included trials. The following outcomes were assessed: seizure freedom, seizure reduction, improvement in quality of life, potential adverse effects, gastrointestinal effects, drop‐out rates and changes in plasma lipid profile. Primary analyses were by intention to treat.
Main results
Eight studies were identified as potentially relevant; three fulfilled the selection criteria and were included in the review. Two placebo‐controlled, double blind trials involving adult participants were conducted in developed countries, while one placebo‐controlled, single blind trial involving children was conducted in a developing country (Egypt). Bromfield 2008 randomised 27 American adults to receive 2.2 g/day of omega‐3 PUFAs (EPA:DHA in a 3:2 ratio) or placebo. Yuen 2005 randomised 58 people in the UK to approximately 1.7 g/day omega‐3 PUFAs (1g EPA and 0.7g DHA) or placebo. Reda 2015 randomised 70 Egyptian children to receive 3 ml/day of 1200 mg fish oil (providing 0.24 g DHA and 0.36 g EPA) or placebo. The three studies recruited a total of 155 subjects (85 adults and 70 children); 78 of them (43 adults and 35 children) were randomised to PUFAs and 77 (42 adults and 35 children) to placebo. All participants were followed for up to 12 weeks. Seizure freedom was reported by only one study, with a high risk of bias, involving exclusively children. The risk estimate for this outcome was significantly higher in the children receiving PUFA compared to the control group (risk ratio (RR) 20.00, 95% confidence interval (CI) 2.84 to 140.99, 1 study, 70 children). Similarly, PUFA supplementation was associated with a significant difference in the proportion of children with at least 50% reduction in seizure frequency (RR 33.00 95% CI 4.77 to 228.15, 1 study with a high risk of bias, 70 children). However, this effect was not observed when the data from two studies including adult participants were pooled (RR 0.57, 95% CI 0.19 to 1.75, I² 0%, 2 studies, 78 participants, low‐quality evidence). One of our three primary outcomes (adverse effects related to bleeding) was not assessed in any of the studies included in this review. There were no significant differences between the PUFA and control groups in relation to gastrointestinal effects (RR 0.78, 95% CI 0.32 to 1.89, 2 studies, 85 participants, low‐quality evidence).
Supplementation with PUFA did not produce significant differences in mean frequency of seizures, quality of life or other side effects.
Authors' conclusions
In view of the limited number of studies and small sample sizes, there is not enough evidence to support the use of PUFA supplementation in people with refractory epilepsy. More trials are needed to assess the benefits of PUFA supplementation in the treatment of drug‐resistant epilepsy.
Keywords: Adult; Child; Humans; Docosahexaenoic Acids; Docosahexaenoic Acids/therapeutic use; Drug Resistant Epilepsy; Drug Resistant Epilepsy/therapy; Eicosapentaenoic Acid; Eicosapentaenoic Acid/therapeutic use; Fatty Acids, Omega‐3; Fatty Acids, Omega‐3/therapeutic use; Fatty Acids, Unsaturated; Fatty Acids, Unsaturated/therapeutic use; Randomized Controlled Trials as Topic; Treatment Outcome
Plain language summary
Polyunsaturated fatty acid supplementation for drug‐resistant epilepsy
Background
Approximately 25% to 30% of all people with epilepsy continue to have seizures despite taking two or more medications in adequate doses. This condition is called drug‐resistant or refractory epilepsy. Different treatments, including the use of vitamins and other supplements, are being tested to see if they can help to improve the control of seizures in this group.
Methods
We searched for studies that tested the effects of supplementation with polyunsaturated fatty acids (PUFAs) compared to placebo in the treatment of seizures in people with drug‐resistant epilepsy, in addition to their routine antiepileptic medications.
Key results
We identified only three randomised trials, which included 155 persons in total (85 adults and 70 children). Two of these studies included only adults and were conducted in more developed countries (US and UK) while the third study involved only children and was conducted in Egypt. In the study involving only children, after 12 weeks of treatment there were more children who were free of seizures in the group that received PUFAs than in the group that received a placebo (pills that do not contain active substance). Likewise, the proportion of children who had a 50% reduction in the number of seizures was higher in the group that received PUFAs than in the group that received placebo. The two studies involving adults did not estimate the number of participants who were free from seizures after the treatment. The analyses of the two studies involving adults showed no differences in the proportion of participants that had a 50% reduction in the number of seizures (low‐quality evidence), the mean frequency of seizures, quality of life or side effects, compared to those who received a placebo.
Conclusion
The existing evidence, which consists of only three small studies, is not enough to support the use of PUFA supplementation in addition to routine antiepileptic medications to improve seizure control or quality of life in people with drug‐resistant epilepsy.
The evidence is current to 5 November 2015.
Summary of findings
Summary of findings for the main comparison. PUFA compared to Placebo for drug‐resistant epilepsy.
| PUFA compared to Placebo for drug‐resistant epilepsy | ||||||
| Patient or population: patients with drug‐resistant epilepsy Settings: Intervention: PUFA Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | PUFA | |||||
| Seizure freedom | 29 per 10001 | 571 per 1000 (81 to 1000) | RR 20 (2.84 to 140.99) | 70 (1 study) | ⊕⊕⊝⊝ low2,3 | |
| 50% reduction in seizures frequency (12 weeks) ‐ Per protocol data ‐ Older (19 years or more) | 194 per 10001 | 111 per 1000 (37 to 340) | RR 0.57 (0.19 to 1.75) | 78 (2 studies) | ⊕⊕⊝⊝ low3,4 | |
| 50% reduction in seizures frequency (12 weeks) ‐ Per protocol data ‐ Younger (< 19 years) | 29 per 10001 | 943 per 1000 (136 to 1000) | RR 33 (4.77 to 228.15) | 70 (1 study) | ⊕⊕⊝⊝ low2,3 | |
| 50% reduction in seizures frequency (6 weeks) | 36 per 10001 | 167 per 1000 (21 to 1000) | RR 4.67 (0.58 to 37.52) | 58 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Potential adverse effects ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Gastrointestinal effects | 190 per 10001 | 149 per 1000 (61 to 360) | RR 0.78 (0.32 to 1.89) | 85 (2 studies) | ⊕⊕⊝⊝ low3,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 We used the event rate in the control group from the included studies as basis for assumed risk. 2 One of the studies was classified as having a high risk of bias for randomisation. 3 Low precision due to small number of events. 4 One of the studies was classified as having an unclear risk of bias for randomisation.
Background
Description of the condition
Epilepsy is a common neurological disorder characterised by recurrent convulsions. These are caused by spontaneous and rhythmic changes in the electrical activity of neurons, which are unrelated to toxic, metabolic or infectious factors (Berg 2010). An estimated 1% to 3% of people will receive a diagnosis of epilepsy during their lives, which corresponds to approximately 50 million affected people worldwide (Kwan 2000). In developed countries, each year 50 new cases of epilepsy are diagnosed for every 100,000 persons; while in developing countries there are 100 to 190 new cases per 100,000 persons per year (Sander 2003).
Although most people with epilepsy achieve adequate control of their disease through the use of medication (Duncan 2006; Ranganathan 2009), approximately 25% to 30% of individuals with epilepsy are refractory to pharmacological treatment (Lefevre 2000). Drug‐resistant epilepsy is defined as unsatisfactory control of seizures despite the use of at least two tolerated antiepileptic drugs at an adequate strength/dosage for a sufficient length of time, whether as monotherapy or in combination (Kwan 2010).
Drug‐resistant epilepsy, also known as refractory, pharmacoresistant, intractable, incapacitating, disabling or severe epilepsy (Cardenas‐Rodriguez 2013), can be distressing and affect the quality of life of the individuals concerned and their families (Duncan 2006; Kwan 2000; Lefevre 2000). Drug‐resistant epilepsy is also associated with an increased risk of sudden death (Surges 2009). Sudden unexpected death in epilepsy (SUDEP) is up to five times more common in the population with medically intractable seizures than in people with well‐controlled epilepsy (DeGiorgio 2008; Opeskin 2003; Surges 2009; Walczak 2001).
Description of the intervention
Polyunsaturated fatty acids (PUFAs) are hydrocarbon chain molecules with multiple double bonds. PUFAs are divided into two major groups (omega‐3 and omega‐6), depending on the position of the double bonds (Taha 2010).
Eicosapentaenoic and docosahexaenoic acid are long‐chain omega‐3 (n‐3) PUFAs derived from α‐linolenic acid. These long‐chain PUFAs can be synthesised in the body to a small extent but the main source is diet (Gao 2009; Gao 2010). n‐3 PUFAs are found mainly in foods such as cold‐water fish (salmon, mackerel, tuna and sardines), fish oils, some nuts and flaxseed (Clayton 2007).
PUFAs are abundant in the brain and regulate many brain functions (Musto 2011). Over the last decade, researchers have tested the use of PUFA supplements for the treatment of refractory epilepsy, with inconsistent results. While some studies have reported a significant reduction in the number of convulsions in people receiving omega‐3 (Schlanger 2002; Yuen 2005), this has not been confirmed by other investigators (Bromfield 2008).
Although high doses of eicosapentaenoic and docosahexaenoic acid are apparently safe, omega‐3 PUFA compounds reduce platelet aggregation and could, in theory, cause bleeding (Phang 2013).
How the intervention might work
The beneficial effects of n‐3 PUFAs for epilepsy are related to their anti‐excitatory and neuro protective mechanisms (Porta 2009; Schlanger 2002; Taha 2010). Studies from the 1980s and 1990s showing that PUFAs helped to control cardiac arrhythmias led to the hypothesis that they could also help to reduce convulsive activity (Schlanger 2002; Yehuda 1994). In animal models n‐3 PUFAs have been shown to reduce the electrical activity of neurons, inhibit the repeated excitatory activity of these cells and modulate the propagation of epileptic crises (Freeman 2006; Voskuyl 1998; Xiao 1999; Yehuda 1994).
The anti‐excitatory effect of n‐3 PUFAs is thought to be related to the partial inhibition of ion channels on cell membranes, which reduces the influx of positive ions, such as sodium and calcium, into the cell (Börjesson 2011; Taha 2010; Xiao 1999). There is evidence to support the hypothesis that increased levels of PUFAs may contribute to the anticonvulsive effects of ketogenic diets (Fraser 2003; Freeman 2006). This diet was derived from historical observations that seizures ceased when people with epilepsy were fasting (Geyelin 1921; Guelpa 1911). This was attributed to ketosis, which led to the creation of a diet high in fat and low in carbohydrates, usually with a ratio of 4:1, to produce the same effect (Wilder 1921). Although the exact mechanisms of action are still unclear, the increased production of ketone bodies and changes in energy metabolism induced by this diet can have a neuroprotective effect (Levy 2012).
PUFAs are important structural components of neuronal membranes and in their free form they are also involved in the regulation of neuronal function (Taha 2010). n‐3 PUFAs reduce the production of reactive oxygen species, bioproducts of the energetic metabolism which can cause oxidative damage to membrane phospholipids, thus contributing to the inflammation and neurodegeneration present in people with epilepsy (Chuang 2010; Freeman 2006). n‐3 PUFAs also inhibit the synthesis of cyclooxygenase‐2 (COX‐2), an enzyme involved in the production of pro‐inflammatory substances (Dahlin 2007).
Why it is important to do this review
Drug‐resistant epilepsy affects millions of individuals worldwide and is associated with important adverse outcomes including an increased risk of death. The search for new and effective therapies for these people is a priority for the neurology research agenda. Existing options for the treatment of drug‐resistant epilepsy include surgery, which is not suitable for all cases and involves risks (Zhang 2013); and a ketogenic diet, which is difficult to tolerate and has a high rate of drop‐out (Levy 2012). If PUFA supplements have a similar effect to a ketogenic diet, this would be a significantly easier option for most people with drug‐resistant epilepsy and their families. There are studies on the use of PUFAs in people with drug‐resistant epilepsy, but as yet they have not been critically appraised and synthesised though a systematic review of the literature.
If supplementation with PUFAs is effective in controlling drug‐resistant epilepsy, this could be an important therapeutic alternative for people with this condition. The findings of this review will help to inform them and healthcare professionals on the effectiveness and tolerability of PUFAs for the treatment of drug‐resistant epilepsy.
Objectives
To assess the effectiveness and tolerability of omega‐3 polyunsaturated fatty acids (eicosapentaenoic acid‐EPA and docosahexanoic acid‐DHA) in the control of seizures in people with refractory epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised studies using PUFAs for the treatment of drug‐resistant epilepsy.
Types of participants
All individuals (adults and children) with a diagnosis of drug‐resistant epilepsy, irrespective of their seizure type or epilepsy syndrome. A diagnosis of 'drug‐resistant' was defined as unsatisfactory seizure control despite the use of at least two antiepileptic drugs in adequate dosages. People under dietary treatments (e.g. ketogenic diet) or who have previously used PUFA supplements were not included.
Types of interventions
Supplementation with PUFAs belonging to the omega‐3 series (eicosapentaenoic and docosahexaenoic acid) at any dosage and over any period of time, combined with antiepileptic drugs. The intervention was compared to no supplementation, placebo treatment or other treatment.
Types of outcome measures
Primary outcomes
Seizure freedom: proportion of participants with complete cessation of seizures during the treatment period.
Seizure reduction: proportion of participants with at least 50% or greater reduction in seizure frequency at the end of the study compared with baseline.
Potential adverse effects related to PUFA supplementation due to reduced platelet aggregation, such as bleeding symptoms.
Secondary outcomes
Absolute or percentage reduction in seizure frequency and duration.
Change in quality of life, assessed through validated scales.
Drop‐out rates due to non‐compliance or other reasons.
Gastrointestinal effects (nausea, flatulence and diarrhoea).
Changes in plasma lipid profile (triglycerides, low‐ and high‐density lipoprotein cholesterol).
Other side effects.
Search methods for identification of studies
Electronic searches
We searched the following databases for randomised trials:
Cochrane Epilepsy Group Specialized Register (from inception up to 5 November 2015) using the search strategy shown in Appendix 1;
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2015, issue 11) using the search strategy shown in Appendix 2;
MEDLINE (Ovid, 1946 to 5 November 2015) using the search strategy shown in Appendix 3;
SCOPUS (1823 to 5 November 2015) using the search strategy shown in Appendix 4;
LILACS (Literatura Latino‐Americana e do Caribe de Informação em Ciências da Saúde) (1982 to 5 November 2015) using the search strategy shown in Appendix 5;
ClinicalTrials.gov (5 November 2015) using the search terms: "fatty acids" AND epilepsy;
World Health Organization (WHO)‐International Clinical Trials Registry Platform ICTRP (5 November 2015) using the search terms: "fatty acids" AND epilepsy.
Searching other resources
We contacted study authors for additional and unpublished information. We screened reference lists of retrieved citations for potentially eligible studies not identified through the electronic search. We contacted experts in the field for information on additional relevant studies that could have been missed.
Data collection and analysis
Selection of studies
Two review authors (VSV and EPGM) screened the titles and abstracts of the citations identified through the searches. We read the full texts of potentially relevant studies and included them if they fulfilled the aforementioned selection criteria. We carried out the process of study selection in duplicate and resolved disagreements by discussion. In case of persistent disagreement, a third review author (MRT) was contacted.
Data extraction and management
Two review authors (VSV and ASP) independently extracted data and methodological details from all included studies. We collected the following specific details: trial design, participant characteristics (age, gender, type of epilepsy, doses and types of antiepileptic drugs in use), treatment details (dose and type of PUFA), duration of treatment, follow‐up assessment and outcomes. If details were not available in the published manuscript, we contacted the authors for more information.
Assessment of risk of bias in included studies
We assessed the risk of bias of the included studies according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed six specific domains: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. Two independent review authors (VSV and ASP) judged each domain and categorised it as being at low, high or unclear risk of bias. We compared scores and resolved discrepancies through discussion or the intervention of a third review author (CRM).
Measures of treatment effect
We assessed the outcomes using risk ratio (RR) for dichotomous data, with their respective 95% confidence intervals (CIs). For continuous variables, we calculated the mean difference (MD) and their 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant (unit to be randomised for interventions to be compared). For trials comparing more than two intervention groups, we assessed the groups treated with PUFA supplementation compared to other treatment groups.For crossover studies, we would have considered only results before crossing when available in the publication or after contact with the authors.
Dealing with missing data
We used an intention‐to‐treat analysis (ITT). If there was no information on ITT, we contacted the study authors. ITT was performed in two ways: considering the losses for and against the treatment.
Assessment of heterogeneity
We assessed statistical heterogeneity among trials using the Chi² test (P < 0.1) and the I² statistic, interpreted according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We also assessed clinical heterogeneity considering differences in the participants, interventions or comparisons.
Assessment of reporting biases
If at least 10 studies had been included, we would have assessed reporting bias using a funnel plot. We assessed selective reporting by looking for the protocols of the published clinical trials.
Data synthesis
For meta‐analysis of dichotomous data we used the Mantel‐Haenszel method; for continuous data, we used the inverse variance method. We used the fixed‐effect model for meta‐analyses if studies were homogeneous. In cases of high heterogeneity (I² > 50%) between the studies, we planned to use the random‐effects model.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses if data were available: types of epilepsy (focal epilepsy, generalized epilepsy or others), age categories (< 20 vs 20 or more years), gender, different doses (< 2g/day vs > 2g/day) and types of PUFAs (eicosapentaenoic and docosahexaenoic acid versus α‐linolenic acid).
Sensitivity analysis
We planned to evaluate the robustness of the results of meta‐analyses by comparing the fixed‐effect and random‐effects estimates, removing trials with low methodological quality and excluding trials with a large effect size.
Summary of findings table
For the analysis of the quality of the evidence, we considered all the primary and secondary outcomes of major clinical importance, whether or not there were any data for those outcomes.
Results
Description of studies
Results of the search
The search strategy yielded 71 citations which were reduced to 43 after the exclusion of duplicates. The title and abstracts of these reports were screened and eight studies were selected for full‐text reading (Figure 1). Five studies were excluded (see Characteristics of excluded studies) and three studies met the selection criteria and were included in the review (Yuen 2005, Bromfield 2008, Reda 2015). The details of these studies are presented in the table Characteristics of included studies. We contacted the authors of all three studies to obtain additional details and inquire about unpublished data.
1.
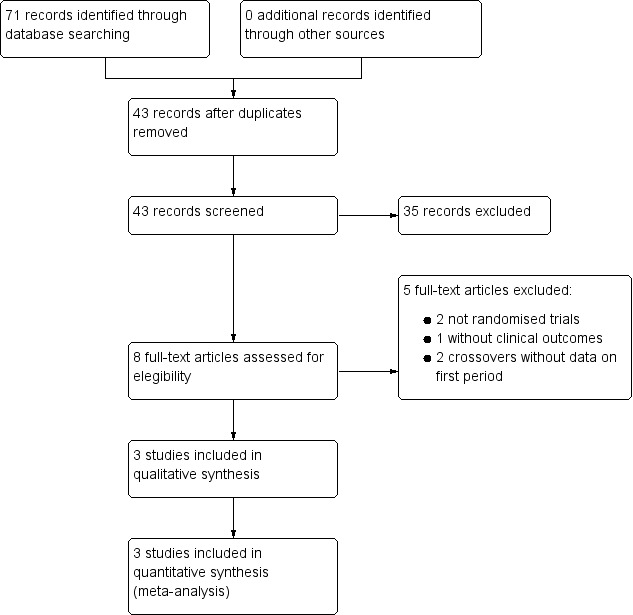
Flow Chart
Included studies
Yuen 2005 conducted a double‐blind trial in the United Kingdom involving 58 adult participants randomised to PUFA or placebo. The study had a duration of 12 weeks, followed by another 12‐week period of open maintenance phase when PUFA supplements were provided to all participants. Only data from the first, double‐blind, phase were used in this review. At baseline, all participants reported at least four seizures per month, according to a retrospective record of 12 weeks and were using two to three antiepileptic drugs (AEDs). Carbamazepine and clobazam were the most frequently used drugs . Half of the participants had localization‐related symptomatic epilepsy syndrome. The PUFA group included 30 adults (17 women and 13 men) with a mean age of 39 years (range 19 to 65 years) with onset of seizures between 1 and 21 years (mean 6 years). They received capsules containing 1000 mg of fish oils (with 171 mg EPA and 112 mg DHA, and < 100 IU vitamin A and < 40 IU vitamin D); the total daily dose corresponded to 1.7 g of omega‐3 PUFA (1 g EPA and 0.7 g DHA). The placebo group comprised 28 adults but only 27 were analysed because one individual was lost to follow up. The mean age of the 27 participants (22 men and 5 women) was 38 years (range 20 to 63 years) and they reported onset of epilepsy ranging from 1 to 14 years (mean 4 years). This group received matching placebo capsules containing mixed oils (palm olein 70%, rapeseed oil 15%, sunflower oil 15%). The outcomes were: 50% reduction of seizures, seizure frequency, serum drug concentrations, plasma fatty acids concentrations and adverse effects. One participant in the placebo group was lost to follow‐up due to an episode of status epilepticus and only 57 participants were analysed.
Bromfield 2008 randomised 27 adult Americans with drug‐resistant epilepsy defined as at least four seizures per month despite adequate doses of at least two standard AEDs and at least one electroencephalogram confirming either focal or generalized epilepsy. People who had taken PUFA supplements previously or were on special diets were ineligible. Participants were not allowed to change medication during the study. Most participants had focal epilepsy. After randomisation, the 27 subjects (13 in the PUFA group and 14 in the placebo group) were observed during a baseline period of up to 4 weeks, followed by a 1‐week titration phase when they received one capsule/day containing either 1.1 g of PUFA or placebo. After this phase, the dose was increased to two capsules of PUFA (total 2.2 g) or placebo daily. Control subjects were given identical capsules containing a mineral oil. After 12 weeks of the double‐blind phase, all participant were offered the choice of participating in a 4‐week PUFA supplementation open‐label phase. Only data from the double‐blind phase were used in this review. Only 21 of the original 27 participants were analysed because four (three in the placebo and one in the PUFA group) decided not to participate after randomisation and two other subjects (both randomised to placebo) discontinued during the treatment phase because of nausea and indigestion (one participant) or due to increase in seizure severity (one participant). The PUFA group consisted of 12 participants (seven women and five men) with a mean age of 36 years (range 25 to 55 years) who, after the titration week, received a daily dose of 2.2 mg EPA plus DHA in a 3:2 ratio. The placebo group consisted of nine participants (five women and four men) with a mean age of 38 years (range 22 to 62 years) who received identical‐looking capsules containing a mineral oil. The outcomes analysed were: 50% reduction of seizures, seizure frequency, quality of life, serum drug concentrations and side effects.
Reda 2015 conducted a single blinded, randomised trial in a paediatric unit in Egypt. The study included 70 children, between 4 and 12 years of age, weighing an average of 26.76 kg, with a diagnosis of refractory epilepsy with partial or generalized seizures. Children with hypersensitivity to fish oil, bleeding disorders, using warfarin or aspirin in the month before recruitment or who had any change in their anti‐epileptic drugs in the last 30 days prior to recruitment were excluded. At baseline (one month before supplementation), the participants had a median of four seizures per month, despite the use of anti‐epileptic drugs. Mean age of the participants was 6.9 ± 2.5 years in the intervention group (PUFA) and 6.6 ± 2.4 years in the control group. After randomisation, 35 children (19 boys and 16 girls) received daily capsules with 3 ml of fish oil providing 240 mg DHA and 360 mg EPA. These capsules also contained vitamin E to avoid oxidation of the fatty acids. The control group consisted of 35 children (16 boys and 19 girls) who received daily capsules containing 3 ml of corn oil as placebo. The study lasted 12 weeks. All participants were assessed for the number of seizures before the intervention and after 1 month, 2 months and 3 months of enrolment in the study. The outcomes were frequency and severity of seizures. The data for frequency of seizures were reported for all participants.
In total, these three studies enrolled 155 participants (85 adults and 70 children) with drug‐resistant epilepsy: 78 of the participants (43 adults and 35 children) were randomised to PUFA supplementation and 77 received placebo.
Excluded studies
Two studies (De Giorgio 2010 and Schlanger 2002) were excluded because they were not randomised trials. Puri 2007 was excluded because it only provided biochemical outcomes. DeGiorgio 2008 and DeGiorgio 2015 were excluded because they were cross‐over studies and the authors did not describe separately the results for the first period (before the crossing). We contacted the authors asking for this data but they did not provide it. See Characteristics of excluded studies for more details.
Risk of bias in included studies
See Figure 2 for the review authors´ judgements about each risk of bias across all included studies. See Figure 3 for a summary of the risk of bias for each of the included studies.
2.
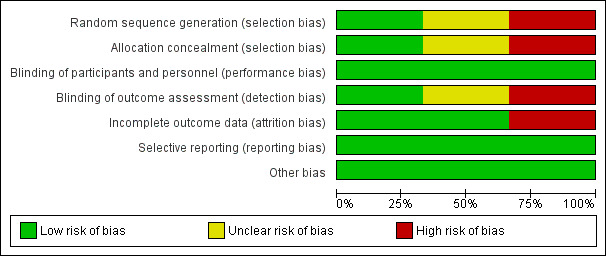
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
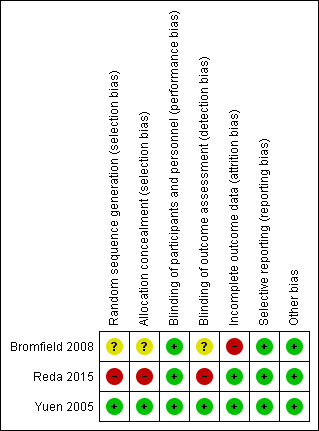
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Bromfield 2008 did not describe the method for generating the sequence of randomisation or allocation concealment and was classified as having an unclear risk of bias for these domains. After contact, Yuen 2005 reported that the randomisation was generated by computer using random numbers and the study was classified as having a low risk of bias for this domain. Yuen 2005 used an independent pharmacy and pharmacist for allocation concealment and was classified as having a low risk of bias for this domain. After contact Reda 2015 informed us that the method used for randomisation was simple random sampling and no allocation concealment took place; therefore the study was judged to be at high risk of bias for both domains.
Blinding
Bromfield 2008 was classified as having a low risk of bias on blinding of participants because the subjects were given identical capsules in both groups. The article provided no information on blinding of outcome assessors and the authors did not reply to our email; therefore this domain was judged as having an unclear risk of bias. Yuen 2005 reported the use of matching supplement and placebo capsules flavoured with peppermint oil, ensuring blinding of participants. There was no information in the publication about blinding of outcome assessors but in a personal reply to our inquiries, the authors confirmed that they were blinded and therefore we classified this study as having a low risk of bias for both domains. Reda 2015 reported that the parents of the participants were blinded to the assigned intervention and the study was classified as having a low risk of bias for this domain. However the outcome assessors were not blinded to the assigned intervention and the study was classified as having a high risk of bias for this domain.
Incomplete outcome data
Yuen 2005 was classified as having a low risk of bias for this domain because only one participant in the placebo group withdrew due to status epilepticus. In Bromfield 2008 there were six losses: four subjects decided not to participate after randomisation in the baseline period (three in the placebo and one in the PUFA group); and there were two additional discontinuations in the treatment phase, both in the placebo group, one due to nausea and indigestion and the other because of increased seizure severity. Because of these losses and imbalance between groups, this study was classified as having a high risk of bias. Reda 2015 was classified as having a low risk of bias for this domain because there were no dropouts.
Selective reporting
Bromfield 2008, Yuen 2005 and Reda 2015 did not have any reporting issues and all studies were considered as having a low risk of bias for this domain.
Other potential sources of bias
No other biases were observed in the included studies.
Effects of interventions
See: Table 1
Primary outcomes
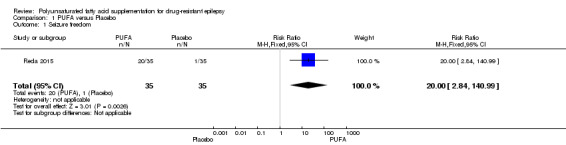
The proportion of participants with complete cessation of seizures during the treatment period (seizure freedom) was reported only by Reda 2015. In this study, after 12 weeks of treatment, 57% of the children (N = 20) in the PUFA group were free of seizures compared to 2.9% (N = 1) in the placebo group. The risk estimate for seizure freedom was RR 20.00 (95% CI 2.84 to 140.99) indicating a significant difference in this outcome in favour of the PUFA group (Figure 4).
4.
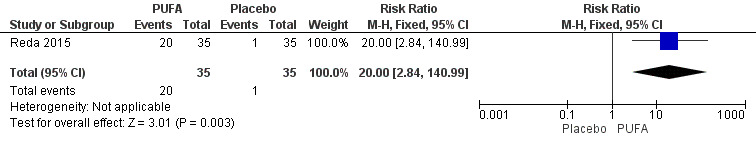
Forest plot of comparison: 1 PUFA vs Placebo, outcome: 1.1 Seizure freedom.
Bromfield 2008 and Yuen 2005 analysed the proportion of adult participants with 50% reduction in seizure frequency after 12 weeks of treatment compared with baseline; and Reda 2015, after contact, provided additional information on the 50% reduction in seizure frequency in children after 12 weeks of treatment. It would have been possible to perform a meta‐analysis for this outcome including all three studies. However, due to important differences in the characteristics of the participants (children versus adults), we decided to perform only subgroup analysis and not an overall pooled analysis of the three studies.
Due to the high number of losses in Bromfield 2008, we performed separate meta‐analyses with and without intention to treat.
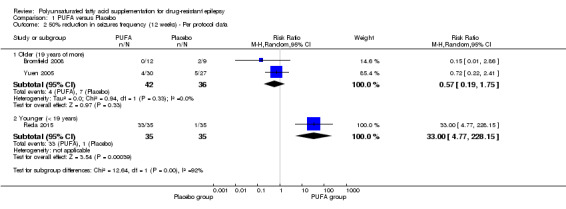
(i) Meta‐analysis per protocol: in the subgroup analysis of the two studies involving 78 adults, the risk for 50% reduction in seizure frequency was RR 0.57, 95% CI 0.19 to 1.75 (I² = 0%) indicating no significant difference for this outcome between the PUFA and placebo groups, after 12 weeks of treatment. The estimate for 50% reduction in seizure frequency after 12 weeks in the study involving only children was RR 33.00 (95% CI 4.77 to 228.15) indicating significant difference in favour of the PUFA group (Figure 5).
5.
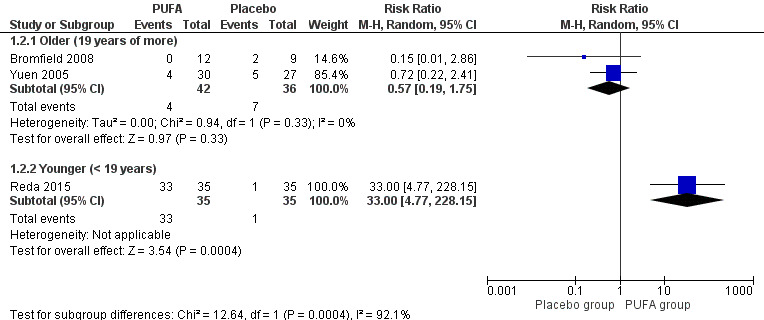
Forest plot of comparison: 1 PUFA vs Placebo, outcome: 1.2 50% reduction in seizures frequency (12 weeks) ‐ Per protocol data.
(ii) Meta‐analyses according to intention to treat were conducted using two possible scenarios. In the first scenario (the 'worst scenario'), supposing that all losses had not had a 50% reduction in seizure frequency, the RR using the random‐effects model would be 0.29 (95% CI 0.03 to 2.63) (Figure 6) indicating no significant differences between the groups at 12 weeks, with substantial statistical heterogeneity (I² = 58%). The high heterogeneity can be explained by an overestimation of the placebo effect. In the second scenario (the 'best scenario'), supposing that all losses had a 50% reduction in the frequency of seizures, the RR using the random effects model would be 0.69 (95% CI 0.24 to 2.01) (Figure 7) indicating no significant differences between the PUFA or placebo groups at 12 weeks, with no statistical heterogeneity (I² = 0%).
6.
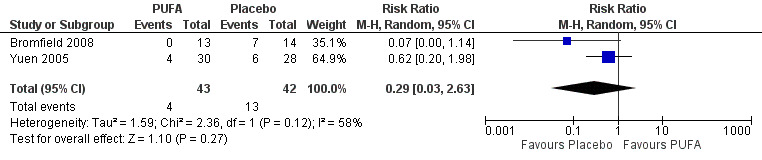
Forest plot of comparison: 1 PUFA vs Placebo, outcome: 1.2 50% reduction in seizure frequency (12 weeks) ‐ Losses in favour of the placebo group ‐ ITT.
7.
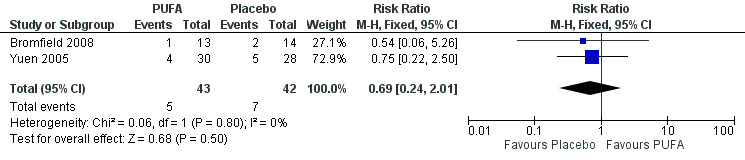
Forest plot of comparison: 1 PUFA vs Placebo, outcome: 1.3 50% reduction in seizures frequency (12 weeks) ‐ Losses in favour of the PUFA group ‐ ITT.
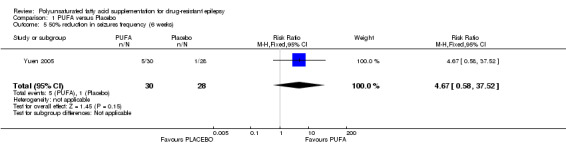
At 6 weeks, the RR for 50% reduction in the frequency of seizures was 4.67 (95% CI 0.58 to 37.52) according to one study (Yuen 2005), indicating no significant benefit from supplementation compared to placebo.
Potential adverse effects related to PUFA supplementation caused by reduced platelet aggregation were not reported in the included studies.
Secondary outcomes
According to unpublished data provided by Yuen 2005, after 12 weeks the mean seizure frequency was 41.55 (SD 75.76) for participants in the PUFA group (n = 29); and 38.20 (SD 29.99) for those in the placebo group (n = 27). Mean difference between the groups was not significant (MD 3.35, 95% CI −26.45 to 33.15). Bromfield 2008 did not provide the absolute mean frequency of seizures at 12 weeks but stated that participants in the placebo group had a 12% reduction in the median frequency of seizures compared to baseline while participants in the PUFA group had a median increase of 6%, but this difference was not statistically significant (P = 0.21).
Only Bromfield 2008 used the Quality of Life in Epilepsy (QOLIE‐31) questionnaire and reported a decrease of 6 points in the placebo group (n = 9) and a 1‐point increase in the PUFA group (n = 12); this difference did not reach statistical significance (P = 0.23).
There were no dropouts due to non‐compliance in Bromfield 2008 and Yuen 2005. There was one loss due to gastrointestinal effects (nausea and indigestion) and two losses due to increased seizure severity or status epilepticus, all in the placebo groups of the two studies. There were no dropouts in Reda 2015.
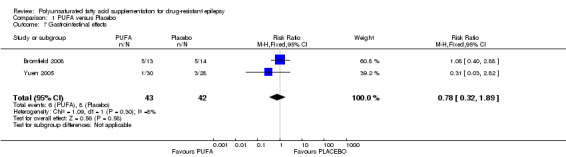
Bromfield 2008 and Yuen 2005 analysed gastrointestinal effects and there were no significant differences between PUFA versus placebo (RR 0.78, 95% CI 0.32 to 1.89) (I² = 8%). The most frequent side effects were nausea and diarrhoea but authors did not give details of the severity of these symptoms.
Changes in plasma lipid profile (triglycerides, low‐ and high‐density lipoprotein cholesterol) were not reported in the included studies.
There were a few reports of other adverse events, all in Yuen 2005. In the PUFA group there was one case of sleepiness, one participant complained of fatigue and breathlessness and one had recurrence of depression and paranoia. In the placebo group there were two cases of sleepiness and one case of aggression and fatigue.
Reda 2015 did not provide any information on adverse effects.
Discussion
Summary of main results
This review identified only three trials which compared PUFA supplementation to placebo for the control of seizures in 155 individuals (85 adults and 70 children) with drug‐resistant epilepsy. Bromfield 2008 had an overall unclear risk of bias, Yuen 2005 had a low risk of bias and Reda 2015 had a high risk of bias.
Only one study involving 70 children addressed seizure freedom and reported that the use of PUFA supplements increased the proportion of children free of seizures after 12 weeks from 0% to 57%, which was not observed in the control group (Reda 2015). The same study reported a significant difference in the proportion of children with 50% reduction in seizure frequency in favour of the PUFA group (RR 33.00, 95% CI 4.77 to 228.15). However there were no significant differences in the proportion of adults with 50% reduction in seizure frequency, based on the analysis of two studies involving adults (RR 0.52, 95% CI 0.18 to 1.53). No studies addressed the effects of PUFA supplementation on symptoms related to platelet aggregation, i.e. bleeding episodes. Gastrointestinal effects were observed mainly in the placebo group but there were no significant differences between the groups (RR 0.78, 95% CI 0.32 to 1.89) (Table 1).
Supplementation with PUFA did not produce significant differences in mean frequency of seizures, quality of life or other side effects. All cases of treatment withdrawals occurred in the placebo groups. Changes in plasma lipid profile were not evaluated in the included studies.
Overall completeness and applicability of evidence
There were only three trials included in this review. The two studies involving adults were conducted in developed countries where the prevalence of epilepsy is lower than in developing countries. Only one study was conducted in a developing country but it included only children. All three studies had small sample sizes, resulting in a total of only 155 participants. The limited number of studies involving mostly participants in countries where the prevalence of refractory epilepsy is lower and with different socioeconomic characteristics, limits the applicability of the findings to other settings. PUFA supplementation was effective in reducing seizure frequency in children, but this result is based on only one study of low methodological quality and this result was not seen in adults. The duration of treatment (three months) and the dose of PUFA in the two studies involving adults ranged from 1.7 to 2.2 g per day which is lower than the usual doses used for the treatment of other conditions such as cancer (Barber 2000) or cardiovascular diseases which range from 0.4 g to 7.0 g per day and for at least six months (Hooper 2006). This may have contributed to the lack of effect of the intervention in these two studies.
Quality of the evidence
We present the quality of the evidence in the Table 1 using the GRADE approach for the comparison PUFA versus placebo. There is low‐quality evidence suggesting that PUFA supplementation does not produce a 50% reduction in seizure frequency at 12 weeks for older (19 years of more) or for younger (< 19 years) patients. For this outcome, the quality was downgraded because of imprecision and risk of bias. There is moderate‐quality evidence indicating that PUFA supplementation does not produce a 50% reduction in seizure frequency at 6 weeks. The quality of evidence for this outcome was downgraded due to imprecision. We found no gastrointestinal effects significantly associated with PUFA supplementation but the quality of this evidence was also low due to unclear risk of bias and high imprecision.
Potential biases in the review process
Despite a sensitive search strategy, the number of included studies was small. Two potentially eligible studies were not included because they were crossover studies without data for the first phase and although the authors replied to our email, they did not provide the information that we requested (DeGiorgio 2008; DeGiorgio 2015).
Agreements and disagreements with other studies or reviews
There are no others reviews on omega‐3 for drug‐resistant epilepsy.
Authors' conclusions
Implications for practice.
In view of the limited number of studies and small sample sizes, there is not enough evidence to support the use of PUFA supplementation in people with refractory epilepsy. More trials are needed to assess the benefits of PUFA supplementation in the treatment of drug‐resistant epilepsy.
Implications for research.
More studies, involving a larger number of adults and children in different socioeconomic settings are needed to assess the effectiveness of PUFA supplementation in controlling seizures in people with drug‐resistant epilepsy. It would also be relevant to test higher dosages of PUFA (2.5 to 7 g), for longer periods of time in these individuals.
Acknowledgements
We are grateful to the Cochrane Epilepsy Group for their support.
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 Omega‐3 fatty acids
#2 MeSH DESCRIPTOR Fatty Acids, Omega‐3 Explode All
#3 "Eicosapentaenoic acid" or "docosahexaenoic acid"
#4 "Polyunsaturated fatty acids" or "n‐3 PUFAs":TI
#5 "Polyunsaturated fatty acids" or "n‐3 PUFAs":AB
#6 #1 OR #2 OR #3 OR #4 OR #5
Appendix 2. CENTRAL search strategy
#1 (epilep* or seizure* or convuls*):ti,ab,kw (Word variations have been searched)
#2 MeSH descriptor: [Epilepsy] explode all trees
#3 MeSH descriptor: [Seizures] explode all trees
#4 (#1 or #2 or #3) in Trials
#5 Omega‐3 fatty acids
#6 MeSH descriptor: [Fatty Acids, Omega‐3] explode all trees
#7 "Eicosapentaenoic acid" or "docosahexaenoic acid"
#8 ("Polyunsaturated fatty acids" or "n‐3 PUFAs"):ti,ab
#9 (#5 or #6 or #7 or #8)
#10 #4 and #9
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011).
1. exp Epilepsy/
2. exp Seizures/
3. (epilep$ or seizure$ or convuls$).tw.
4. 1 or 2 or 3
5. exp Pre‐Eclampsia/ or exp Eclampsia/
6. 4 not 5
7. (randomized controlled trial or controlled clinical trial).pt. or (randomized or placebo or randomly).ab.
8. clinical trials as topic.sh.
9. trial.ti.
10. 7 or 8 or 9
11. exp animals/ not humans.sh.
12. 10 not 11
13. Omega‐3 fatty acids.mp. or exp Fatty Acids, Omega‐3/
14. (Eicosapentaenoic acid or docosahexaenoic acid).mp.
15. (Polyunsaturated fatty acids or n‐3 PUFAs).ab,ti.
16. 13 or 14 or 15
17. 6 and 12 and 16
Appendix 4. SCOPUS search strategy
((TITLE‐ABS‐KEY(Omega‐3 fatty acids)) OR (TITLE‐ABS‐KEY("Eicosapentaenoic acid" OR "docosahexaenoic acid")) OR (TITLE‐ABS‐KEY("Polyunsaturated fatty acids" OR "n‐3 PUFAs"))) AND (((((TITLE‐ABS‐KEY(epilep* OR "infantile spasm" OR "ring chromosome 20" OR "R20" OR "myoclonic encephalopathy" OR "pyridoxine dependency")) OR (TITLE‐ABS‐KEY(syndrome W/2 (aicardi OR angelman OR doose OR dravet OR janz OR jeavons OR "landau kleffner" OR "lennox gastaut" OR ohtahara OR panayiotopoulos OR rasmussen OR rett OR "sturge weber" OR tassinari OR "unverricht lundborg" OR west)))) OR (TITLE(seizure OR convuls*))) OR ((TITLE‐ABS‐KEY(lafora* W/4 (disease OR epilep*))) AND NOT (TITLE(dog OR canine) OR INDEXTERMS(dog OR canine)))) AND NOT (TITLE(*eclampsia) OR INDEXTERMS(*eclampsia))) AND (TITLE((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)) OR ABS((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)))
Appendix 5. LILACS search strategy
Omega‐3 fatty acids OR Eicosapentaenoic acid OR docosahexaenoic acid OR polyunsaturated fatty acids [Words] and Epilepsy [Words] and "CONTROLLED CLINICAL TRIAL" or "RANDOMIZED CONTROLLED TRIAL" [Publication type]
Data and analyses
Comparison 1. PUFA versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure freedom | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 20.0 [2.84, 140.99] |
| 2 50% reduction in seizures frequency (12 weeks) ‐ Per protocol data | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Older (19 years of more) | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.19, 1.75] |
| 2.2 Younger (< 19 years) | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 33.0 [4.77, 228.15] |
| 3 50% reduction in seizure frequency (12 weeks) ‐ Worst case scenario ‐ ITT | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.63] |
| 4 50% reduction in seizures frequency (12 weeks) ‐ Best case scenario ‐ ITT | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.24, 2.01] |
| 5 50% reduction in seizures frequency (6 weeks) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.67 [0.58, 37.52] |
| 6 Seizures frequency 12 weeks double‐blind | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 3.35 [‐26.45, 33.15] |
| 7 Gastrointestinal effects | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.32, 1.89] |
1.1. Analysis.
Comparison 1 PUFA versus Placebo, Outcome 1 Seizure freedom.
1.2. Analysis.
Comparison 1 PUFA versus Placebo, Outcome 2 50% reduction in seizures frequency (12 weeks) ‐ Per protocol data.
1.3. Analysis.

Comparison 1 PUFA versus Placebo, Outcome 3 50% reduction in seizure frequency (12 weeks) ‐ Worst case scenario ‐ ITT.
1.4. Analysis.

Comparison 1 PUFA versus Placebo, Outcome 4 50% reduction in seizures frequency (12 weeks) ‐ Best case scenario ‐ ITT.
1.5. Analysis.
Comparison 1 PUFA versus Placebo, Outcome 5 50% reduction in seizures frequency (6 weeks).
1.6. Analysis.

Comparison 1 PUFA versus Placebo, Outcome 6 Seizures frequency 12 weeks double‐blind.
1.7. Analysis.
Comparison 1 PUFA versus Placebo, Outcome 7 Gastrointestinal effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bromfield 2008.
| Methods | Randomised, double‐blinded trial, USA, one centre | |
| Participants | 27 adults (13 PUFA, 14 Placebo), 9 men and 12 women (5 without information), mean age 37 (range 22 to 62 years). Most had focal epilepsy | |
| Interventions | PUFA group: one capsule (1.1 g) taken twice a day, total dose 2.2 g PUFAs daily (EPA plus DHA in a 3:2 ration). Placebo: identical capsules containing a mineral oil |
|
| Outcomes | 50% reduction of seizures, seizure frequency, quality of life, gastrointestinal effects | |
| Notes | After 12 weeks, an open phase was implemented and PUFA was offered to all participants. We included only data of the 1st, double‐blind phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not describe random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Does not describe allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Control subjects were given identical capsules containing a mineral oil placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Does not describe blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 people (3 in the placebo and 1 in the PUFA group) decided not to participate after randomisation and 2 others, both randomised to placebo, discontinued during the treatment phase because of nausea and indigestion (1 participant) or due to increase seizure severity (1 participant) |
| Selective reporting (reporting bias) | Low risk | Presents the results of the outcomes proposed |
| Other bias | Low risk | None |
Reda 2015.
| Methods | Randomized, single‐blinded, Egypt, one centre, duration 12 weeks | |
| Participants | 70 children (PUFA = 35, Placebo = 35), 35 boys and 35 girls with partial or generalised seizures, between 4 and 12 years of age, mean age 6.7 years, average weight of 26.76 kg | |
| Interventions | PUFA group: capsules with 3 ml of 1200 mg fish oil providing 240 mg DHA and 360 mg EPA per day. Supplements also contained Vitamin E to prevent oxidation of the fatty acids Control group: capsules with 3 ml per day of corn oil as placebo |
|
| Outcomes | Seizure freedom, seizure severity, 50% reduction in seizures (this last outcome was provided after contact with authors) | |
| Notes | Authors provided additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The method used for randomisation was simple random sampling |
| Allocation concealment (selection bias) | High risk | No allocation concealment took place |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The parents of the participants were blinded to the assigned intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were not blinded to the assigned intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Presents the results of the outcomes proposed |
| Other bias | Low risk | None |
Yuen 2005.
| Methods | Randomised, placebo‐controlled parallel group, double‐blind trial, UK, one centre | |
| Participants | 58 adults (30 PUFA, 28 Placebo ), 35 men and 22 women, mean age 38.5 (range 19 to 65 years) | |
| Interventions | PUFA group: 1000 mg fish oil capsules, total daily dose 1.7 g omega‐3 PUFAs (1 g EPA and 0.7 g DHA). Each capsule contained 171 mg EPA and 112 mg DHA, and < 100 IU vitamin A and < 40 IU vitamin D) Placebo: matching capsules containing mixed oils (palm olein 70%, rapeseed oil 15%, sunflower oil 15%) |
|
| Outcomes | 50% reduction in seizures, seizure frequency, gastrointestinal effects, other side effects | |
| Notes | After 12 weeks, an open maintenance phase was implemented and PUFA was given to all participants. We included only data of the 1st, double‐blind phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was by computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Allocation was through an independent pharmacy and pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Supplement and placebo capsules were identical and flavoured with peppermint oil |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was 1 drop‐out in the placebo group due an episode of status epilepticus |
| Selective reporting (reporting bias) | Low risk | Presents the results of outcomes proposed |
| Other bias | Low risk | None |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| De Giorgio 2010 | This is not a randomised trial |
| DeGiorgio 2008 | Crossover trial without separate data for the 1st phase of the study. We contacted the authors but they did not provide the data requested |
| DeGiorgio 2015 | Crossover trial without separate data for the 1st phase of the study |
| Puri 2007 | Does not report outcomes of interest for this review, only biochemical outcomes |
| Schlanger 2002 | This is not a randomised trial |
Characteristics of ongoing studies [ordered by study ID]
IRCT201012115365N1.
| Trial name or title | Assess the effectiveness of omega 3 on children with refractory epilepsy |
| Methods | Randomized crossover (one‐month washout period), not blinded, not placebo controlled |
| Participants | Male or female, 2 to 13 years, with insufficient control of epilepsy (> 4 seizure episodes per month), despite at least 3 AED |
| Interventions | 1.5 grams/m² of Omega 3 in a single daily dose in addition to their routine AED. Each 5 ml of the omega 3 contained 1484 mg of omega 3, 786 mg of EPA and 524 mg of DHA |
| Outcomes | Frequency of seizures, severity of seizure and other benefits of omega 3 |
| Starting date | August 2011 |
| Contact information | Hamed Azarnoush (hamedmds@smd.iaun.ac.ir) |
| Notes | http://www.irct.ir/searchresult.php?id=5365&number=1 |
ISRCTN57643242.
| Trial name or title | Omega 3 fatty acid treatment in patients with epilepsy |
| Methods | Double‐blind, placebo‐controlled randomised intervention trial |
| Participants | People with refractory idiopathic epilepsy, aged 17 to 50 years, male or female |
| Interventions | Active supplement (contains 1.5 g DHA and 390 mg EPA) |
| Outcomes | Complete elimination or reduction in the frequency of seizures; improvements of cognition, memory, and manifestations of behavioural and psychiatric disorders; modulation of clinical markers of cardiac arrhythmias; down‐regulation of inflammatory markers |
| Starting date | May 2014 |
| Contact information | Prof Kebreab Ghebremeskel (k.ghebremeskel@londonmet.ac.uk) |
| Notes | http://isrctn.com/ISRCTN57643242 |
NCT01769092.
| Trial name or title | Clinical Trial of the Effects of DHA in the Treatment of Seizure Disorders |
| Methods | Randomised, double‐blind, placebo controlled |
| Participants | Male or female, aged 15 years or older, three or more seizures per month |
| Interventions | Daily dose of fish oil standardized to contain 3 g/day of DHA. The daily dose is divided; capsules are taken with meals for a period of 6 months |
| Outcomes | Seizure diary, serum polyunsaturated fatty acid levels, serum anticonvulsant levels, adverse reactions |
| Starting date | January 2013 |
| Contact information | Miles Thomson, PhD (epilepsypufastudy@gmail.com) |
| Notes | http://ClinicalTrials.gov/show/NCT01769092 |
Contributions of authors
Maria Regina Torloni ‐ drafting of protocol and review, screening and selection of studies, data extraction and quality assessment.
Cristiane R Macedo ‐ drafting of protocol, search strategy, screening and selection of studies.
Vivian Sarmento Vasconcelos ‐ drafting of protocol and review, search strategy, screening and selection of studies, data extraction and quality assessment.
Edna Morais ‐ screening and selection of studies, data extraction and quality assessment.
Gustavo Porfirio ‐ data extraction and quality assessment.
Alexsandra de Souza Pedrosa ‐ screening and selection of studies, data extraction and quality assessment.
Sources of support
Internal sources
Brazilian Cochrane Centre, Brazil.
External sources
-
National Institute for Health Research (NIHR), UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
None known.
New
References
References to studies included in this review
Bromfield 2008 {published data only}
- Bromfield E, Dworetzky B, Hurwitz S, Eluri Z, Lane L, Replansky S, et al. A randomized trial of polyunsaturated fatty acids for refractory epilepsy. Epilepsy & Behavior 2008;12(1):187‐90. [DOI] [PubMed] [Google Scholar]
- Bromfield E, Dworetzky B, Hurwitz S, Eluri Z, O'Brien L, Replansky S, et al. Randomized Trial of Polyunsaturated Fatty Acids for Refractory Epilepsy. Epilepsia 2006;47, S4:331‐332. [DOI] [PubMed] [Google Scholar]
Reda 2015 {published data only}
- Reda DMA, Abd‐El‐Fatah NK, Omar TESI, Darwish OAH. Fish Oil Intake and Seizure Control in Children with Medically Resistant Epilepsy. North American Journal of Medical Sciences 2015;7(7):317‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuen 2005 {published data only}
- Yuen AW, Sander JW, Fluegel D, Patsalos PN, Bell GS, Johnson T, et al. Omega 3 fatty acids supplementation in patients with chronic epilepsy: a randomized trial. Epilepsy & Behavior 2005;7(2):253‐8. [DOI] [PubMed] [Google Scholar]
- Yuen AWC, Sander JW, Fluegel D, Patsalos PN, Browning L, Bell GS, el al. Double‐blind Placebo‐controlled Parallel‐Group trial of omega‐3 fatty acids supplementation in patients with chronic epilepsy. Epilepsia 2005;46, S 6:538. [Google Scholar]
- Yuen AWC, Sander JW, Fluegel D, Patsalos PN, Browning L, Bell GS, et al. Double‐blind Placebo‐controlled Parallel‐Group trial of omega‐3 fatty acids supplementation in patients with chronic epilepsy. Epilepsia 2004;45, Suppl7:151. [Google Scholar]
- Yuen AWC, Sander JWA, Fluegel D, Patsalos PN, Browning L, Bell GS, et al. Erythrocyte fatty acid profiles in people with epilepsy and the effects of ômega‐3 fatty acid supplementation. Epilepsia 2005;46, S8:226. [Google Scholar]
- Yuen AWC, Sander JWA, Fluegel D, Patsalos PN, Browning L, Bell GS, et al. Erythrocyte fatty acid profiles in people with epilepsy and the effects of ômega‐3 fatty acid supplementation. Epilepsia 2006;47, S4:334. [Google Scholar]
References to studies excluded from this review
DeGiorgio 2008 {published data only}
- DeGiorgio CM, Miller P, Meymandi S, Gornbein JA. n‐3 fatty acids (fish oil) for epilepsy, cardiac risk factors, and risk of SUDEP: clues from a pilot, double‐blind, exploratory study. Epilepsy & Behavior 2008;13(4):681‐4. [DOI] [PubMed] [Google Scholar]
De Giorgio 2010 {published data only}
- DeGiorgio CM, Miller P, Meymandi S, Chin A, Epps J, Gordon S, et al. RMSSD, a measure of vagus‐mediated heart rate variability, is associated with risk factors for SUDEP: The SUDEP‐7 Inventory. Epilepsy & Behavior 2010;19(1):78‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeGiorgio 2015 {published data only}
- DeGiorgio CM, Miller PR, Harper R, Gornbein J, Schrader L, Soss J, et al. Fish oil (n‐3 fatty acids) in drug resistant epilepsy: a randomised placebo‐controlled crossover study. Journal of Neurology, Neurosurgery & Psychiatry 2015;86(1):65‐70. [DOI] [PubMed] [Google Scholar]
Puri 2007 {published data only}
- Puri BK, Koepp MJ, Holmes J, Hamilton G, Yuen AWC. A 31‐phosphorus neurospectroscopy study of ω‐3 long‐chain polyunsaturated fatty acid intervention with eicosapentaenoic acid and docosahexaenoic acid in patients with chronic refractory epilepsy. Prostaglandins, Leukotrienes and Essential Fatty Acids 2007;77(2):105‐7. [DOI] [PubMed] [Google Scholar]
Schlanger 2002 {published data only}
- Schlanger S, Shinitzky M, Yam D. Diet enriched with omega‐3 fatty acids alleviates convulsion symptoms in epilepsy patients. Epilepsia 2002;43(1):103‐4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
IRCT201012115365N1 {unpublished data only}
- IRCT201012115365N1. Assess the effectiveness of omega 3 on children with refractory epilepsy [Efficacy of omega 3 fatty acid supplementation in children with refractory epilepsy refer to selected Isfahan's hospitals 1389]. http://www.irct.ir/searchresult.php?id=5365&number=1 (accessed 5 November 2015). [Hamed Azarnoush, Mohammadreza Ghazavi]
ISRCTN57643242 {unpublished data only}
- ISRCTN57643242. Omega 3 fatty acid treatment in patients with epilepsy [Omega 3 fatty acid supplementation to prevent seizure in patients with refractory epilepsy]. http://isrctn.com/ISRCTN57643242 (accessed 13 February 2016). [Kebreab Ghebremeskel]
NCT01769092 {unpublished data only}
- NCT01769092. Clinical Trial of the Effects of DHA in the Treatment of Seizure Disorders [Double Blind Placebo Controlled Trial of Effects of DHA in Fish Oil for the Treatment of Seizure Disorders]. https://clinicaltrials.gov/show/NCT01769092 (accessed 5 November 2015). [Paul A Hwang, Miles Thomson, Mac Burnham]
Additional references
Barber 2000
- Barber MD, McMillan DC, Preston T, Ross JA, Fearon CH. Metabolic response to feeding in weight‐losing pancreatic cancer patients and its modulation by a fish‐oil‐enriched nutritional supplement. Clinical Science 2000;98(4):389‐99. [PubMed] [Google Scholar]
Berg 2010
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Boas WE, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005‐2009. Epilepsia 2010;51(4):676‐85. [DOI] [PubMed] [Google Scholar]
Börjesson 2011
- Börjesson SI, Elinder F. An electrostatic potassium channel opener targeting the final voltage sensor transition. Journal of General Physiology 2011;137(6):563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardenas‐Rodriguez 2013
- Cardenas‐Rodriguez N, Huerta‐Gertrudis B, Rivera‐Espinosa L, Montesinos‐Correa H, Bandala C, Carmona‐Aparicio L, et al. Role of oxidative stress in refractory epilepsy: evidence in patients and experimental models. International Journal of Molecular Sciences 2013;14(1):1455‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chuang 2010
- Chuang YC. Mitochondrial dysfunction and oxidative stress in seizure‐induced neuronal cell death. Acta Neurologica Taiwanica 2010;19(1):3‐15. [PubMed] [Google Scholar]
Clayton 2007
- Clayton EH, Hanstock TL, Garg ML, Hazell PL. Long chain omega‐3 polyunsaturated fatty acids in the treatment of psychiatrics illnesses in children and adolescents. Acta Neuropsychiatrica 2007;19(2):92‐103. [DOI] [PubMed] [Google Scholar]
Dahlin 2007
- Dahlin M, Hjelte L, Nilsson S, Amark P. Plasma phospholipid fatty acids are influenced by a ketogenic diet enriched with n‐3 fatty acids in children with epilepsy. Epilepsy Research 2007;73(2):199‐207. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Duncan 2006
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet 2006;367(9516):1087‐100. [DOI] [PubMed] [Google Scholar]
Fraser 2003
- Fraser DD, Whiting S, Andrew RD, Macdonald EA, Musa‐Veloso K, Cunnane SC. Elevated polyunsaturated fatty acids in blood serum obtained from children on the ketogenic diet. Neurology 2003;60(6):1026‐9. [DOI] [PubMed] [Google Scholar]
Freeman 2006
- Freeman J, Veggiotti P, Lanzi G, Tagliabue A, Perucca E. The ketogenic diet: from molecular mechanisms to clinical effects. Epilepsy Research 2006;68(2):145‐80. [DOI] [PubMed] [Google Scholar]
Gao 2009
- Gao F, Kiesewetter D, Chang L, Ma K, Bell JM, Rapoport SI, et al. Whole‐body synthesis‐secretion rates of long‐chain n‐3 PUFAs from circulating unesterified alpha‐linolenic acid in unanesthetized rats. Journal of Lipid Research 2009;50(4):749‐58. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Gao 2010
- Gao F, Kiesewetter D, Chang L, Rapoport SI, Igarashi M. Quantifying conversion of linoleic to arachidonic and other n‐6 polyunsaturated fatty acids in unanesthetized rats. Journal of Lipid Research 2010;51(10):29406. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Geyelin 1921
- Geyelin HR. Fasting as a method for treating epilepsy. Medical Record 1921;99:1031‐9. [Google Scholar]
Guelpa 1911
- Guelpa G, Marie A. La lutte contre l’epilepsie par ladesintoxication et par la re–education alimentaire. Revuede Therapie Medico‐Chirurgide 1911;78:8‐13. [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne ACJ (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hooper 2006
- Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ 2006;332(7544):752‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwan 2000
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New England Journal of Medicine 2000;342(5):314‐9. [DOI] [PubMed] [Google Scholar]
Kwan 2010
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Hauser WA, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51(6):1069‐77. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lefevre 2000
- Lefevre F, Aronso N. Ketogenic diet for the treatment of refractory epilepsy in children: a systematic review of efficacy. Pediatrics 2000;105(4):1‐7. [DOI] [PubMed] [Google Scholar]
Levy 2012
- Levy RG, Cooper PN, Giri P. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD001903.pub2] [DOI] [PubMed] [Google Scholar]
Musto 2011
- Musto AE, Gjorstrup P, Bazan NG. The omega‐3 fatty acid–derived neuroprotectin D1 limits hippocampal hyperexcitability and seizure susceptibility in kindling epileptogenesis. Epilepsia 2011;52(9):1601‐8. [DOI] [PubMed] [Google Scholar]
Opeskin 2003
- Opeskin K, Berkovic SF. Risk factors for sudden unexpected death in epilepsy: a controlled prospective study based on coroners cases. Seizure 2003;12(7):456–64. [DOI] [PubMed] [Google Scholar]
Phang 2013
- Phang M, Lincz LF, Garg ML. Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women. Journal of Nutrition 2013;143(4):457–63. [DOI] [PubMed] [Google Scholar]
Porta 2009
- Porta N, Bourgois B, Galabert C, Lecointe C, Cappy P, Bordet R, et al. Anticonvulsant effects of linolenic acid are unrelated to brain phospholipid cell membrane compositions. Epilepsia 2009;50(1):65‐71. [DOI] [PubMed] [Google Scholar]
Ranganathan 2009
- Ranganathan LN, Ramaratnam S. Vitamins for epilepsy. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004304.pub2] [DOI] [PubMed] [Google Scholar]
Sander 2003
- Sander JW. The epidemiology of epilepsy revisited. Current Opinion in Neurology 2003;16(2):165‐70. [DOI] [PubMed] [Google Scholar]
Surges 2009
- Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nature Reviews. Neurology 2009;5(9):492‐504. [DOI] [PubMed] [Google Scholar]
Taha 2010
- Taha AY, Burnham WM, Auvin S. Polyunsaturated fatty acids and epilepsy. Epilepsia 2010;51(8):1348‐58. [DOI] [PubMed] [Google Scholar]
Voskuyl 1998
- Voskuyl RA, Vreugdenhil M, Kang JX, Leaf A. Anticonvulsant effect of polyunsaturated fatty acids in rats, using the cortical stimulation model. European Journal of Pharmacology 1998;341(2‐3):145‐52. [DOI] [PubMed] [Google Scholar]
Walczak 2001
- Walczak TS, Leppik IE, D’Amelio M, Rarick J, So E, Ahman P, et al. Incidence and risk factors in sudden unexpected death in epilepsy. A prospective cohort study. Neurology 2001;56(4):519‐25. [DOI] [PubMed] [Google Scholar]
Wilder 1921
- Wilder RM. The effects of ketonemia on course of epilepsy. Mayo Clinic Proceedings 1921;2:307‐8. [Google Scholar]
Xiao 1999
- Xiao Y, Li X. Polyunsaturated fatty acids modify mouse hippocampal neuronal excitability during excitotoxic or convulsant stimulation. Brain Research 1999;846(1):112‐21. [DOI] [PubMed] [Google Scholar]
Yehuda 1994
- Yehuda S, Carasso RL, Mostofsky DI. Essential fatty acid preparation (SR‐3) raises the seizure threshold in rats. European Journal of Pharmacology 1994;254(1‐2):193‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2013
- Zhang J, Liu W, Chen H, Xia H, Zhou Z, Mei S, et al. Identification of common predictors of surgical outcomes for epilepsy surgery. Neuropsychiatric Disease and Treatment 2013;9:1673‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sarmento Vasconcelos 2014
- Sarmento Vasconcelos V, Macedo CR, Souza Pedrosa A, Pereira Gomes Morais E, Torloni MR. Polyunsaturated fatty acid supplementation for drug‐resistant epilepsy. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD011014] [DOI] [PMC free article] [PubMed] [Google Scholar]


