Summary
This study was aimed to investigate the association between remnant cholesterol (RC) and low-density lipoprotein cholesterol (LDL-C) concentrations and long-term bleeding. A total of 10,724 consecutive patients who underwent percutaneous coronary intervention in 2013 were prospectively enrolled. During a median follow-up of 5.1 years, 411 bleeding events and 42 intracranial hemorrhages (ICH) were recorded. The findings revealed that lower RC concentrations were independently associated with an increased risk of long-term bleeding events (continuous RC hazard ratio [HR]: 0.47, 95% confidence interval [CI]: 0.26–0.85; Q4 vs. Q1 HR: 0.66, 95% CI: 0.45–0.98), whereas lower LDL-C concentrations did not show a similar association. Additionally, a non-linear relationship was observed between RC concentrations and the risk of ICH (P for non-linear trend = 0.014), but no such relationship was found for LDL-C concentrations. These results provided insights into the safety of LDL-C-lowering therapy and emphasized the significance of RC concentrations in lipid management.
Subject areas: Cardiovascular medicine, Public health, Pathophysiology, Molecular physiology
Graphical abstract
Highlights
-
•
Lower RC but not LDL-C was independently associated with long-term bleeding
-
•
Our finding indicated that lowering LDL-C after PCI was a safe strategy
-
•
To prevent bleeding following PCI, more attention should be paid to RC
Cardiovascular medicine; Public health; Pathophysiology; Molecular physiology
Introduction
Dyslipidemia, particularly elevated levels of low-density lipoprotein cholesterol (LDL-C), has been widely recognized as a significant predictor of atherosclerotic cardiovascular disease.1,2,3 In the secondary prevention population with coronary heart disease (CHD), the adverse effects of LDL-C has been well-established, leading guidelines to emphasize the importance of lowering its levels as much as possible. However, recent investigations have revealed that reduced LDL-C levels were associated with increased risks of intracranial hemorrhage (ICH)4,5,6,7 and short-term bleeding events (during hospitalization8 and within one year9) following percutaneous coronary intervention (PCI) in patients with acute coronary syndrome (ACS). Notably, the impact of intensive lipid-lowering treatment on long-term bleeding risk has remained a topic of debate. Therefore, there has been an urgent need to investigate the relationship between plasma lipid concentrations and the long-term bleeding risk following PCI. Additionally, it has remained unclear which component of the lipid profile is exactly related to the bleeding risk.
In recent years, there has been a growing interest in the role of remnant cholesterol (RC) in cardiovascular risk. Recent studies have demonstrated that RC, rather than LDL-C, was independently associated with an elevated risk of major adverse cardiovascular events (MACE).10,11 However, to date, no investigations have explored the potential relationship between RC and bleeding events following PCI. Intensive antithrombotic therapies, such as dual antiplatelet therapy (DAPT) in PCI patients, have presented a double-edged sword. While DAPT can effectively reduce the risk of ischemic events, it also increased the likelihood of bleeding events.12,13 Similarly, it is of interest to examine whether low LDL-C and/or low RC levels contribute to increased long-term bleeding risk in the era of intensive lipid-lowering therapy. Therefore, the objective of our study was to investigate, using a large cohort, the association between LDL-C and RC concentrations and the risk of bleeding in patients who undergo PCI and receive DAPT, with long-term follow-up data. Our findings may provide useful clinical data for the safety of lipid-lowering therapy.
Results
Baseline characteristics
Eventually, there were 9,697 eligible patients included (Figure S1). The mean age of 9,697 participants was 58.4 ± 10.2 years and 7479 (77.1%) were men. 5,784 (59.6%) clinically presented with ACS. Table 1 shows the comparison of baseline characteristics between patients who experienced bleeding events and those who did not. Individuals with bleeding events had significantly older age, a higher prevalence of high PRECISE-DAPT score, Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria, hypertension, prior bleeding, prior stroke, femoral artery approach, higher baseline concentrations of RC and HDL-C, lower baseline concentrations of TG, as well as lower levels of hemoglobin, estimated glomerular filtration rate, and serum glucose than patients without bleeding events. In addition, Tables S1 and S2 depicts the comparisons of baseline characteristics of patients with different RC concentrations, and that of patients with different LDL-C concentrations.
Table 1.
Baseline characteristics in patients with and without bleeding events
| Parameter | Total population (n = 9,697) | With bleeding events (n = 411) | Without bleeding events (n = 9,286) | p value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 58.4 ± 10.2 | 60.7 ± 10.2 | 58.3 ± 10.2 | <0.001 |
| Male sex, n (%) | 7,479 (77.1) | 308 (74.9) | 7,171 (77.2) | 0.281 |
| BMI, kg/m2 | 26.0 ± 3.7 | 25.7 ± 3.6 | 26.0 ± 3.1 | 0.134 |
| Diagnosis on admission | ||||
| ACS, n (%) | 5,784 (59.6) | 247 (60.1) | 5,537 (59.6) | 0.849 |
| aHigh PRECISE-DAPT score, n (%) | 496 (5.1) | 34 (8.3) | 462 (5.0) | 0.003 |
| ARC-HBR criteria, n (%) | 844 (8.7) | 54 (13.1) | 790 (8.5) | 0.001 |
| Medical history | ||||
| Smoking, n (%) | 5,671 (58.5) | 256 (62.3) | 5,415 (58.3) | 0.110 |
| Hypertension, n (%) | 6,249 (64.4) | 288 (70.1) | 5,961 (64.2) | 0.015 |
| Dyslipidaemia, n (%) | 6,520 (67.2) | 284 (69.1) | 6,236 (67.2) | 0.411 |
| Diabetes mellitus, n (%) | 2,935 (30.3) | 122 (29.7) | 2,813 (30.3) | 0.792 |
| COPD, n (%) | 22 2(2.3) | 6 (1.5) | 216 (2.3) | 0.251 |
| Prior bleeding, n (%) | 78 (0.8) | 11 (2.7) | 67 (0.7) | <0.001 |
| Prior stroke, n (%) | 1,037 (10.7) | 70 (17.0) | 967 (10.4) | <0.001 |
| Peripheral vascular disease, n (%) | 260 (2.7) | 14 (3.4) | 246 (2.6) | 0.352 |
| Prior MI, n (%) | 1,880 (19.4) | 73 (17.8) | 1,807 (19.5) | 0.394 |
| Prior PCI, n (%) | 2,402 (24.8) | 100 (24.3) | 2,302 (24.8) | 0.833 |
| Prior CABG, n (%) | 394 (4.1) | 21 (5.1) | 373 (4.0) | 0.272 |
| Family history of coronary heart disease, n (%) | 2,381 (24.6) | 98 (24.6) | 2,381 (23.8) | 0.733 |
| Laboratory variables | ||||
| RC, mmol/L | 0.67 ± 0.38 | 0.61 ± 0.35 | 0.67 ± 0.38 | 0.001 |
| TC, mmol/L | 4.21 ± 1.07 | 4.16 ± 1.06 | 4.21 ± 1.07 | 0.333 |
| HDL-C, mmol/L | 1.03 ± 0.27 | 1.07 ± 0.31 | 1.03 ± 0.27 | 0.024 |
| LDL-C, mmol/L | 2.51 ± 0.90 | 2.49 ± 0.88 | 2.51 ± 0.91 | 0.569 |
| TG, mmol/L | 1.78 ± 1.05 | 1.67 ± 0.82 | 1.79 ± 1.06 | 0.006 |
| Hemoglobin, g/L | 142.95 ± 15.40 | 141.04 ± 16.20 | 143.03 ± 15.36 | 0.010 |
| WBC, 109/L | 6.89 ± 2.02 | 6.71 ± 1.78 | 6.90 ± 2.03 | 0.060 |
| Platelet count, 109/L | 205.06 ± 54.64 | 201.35 ± 56.15 | 205.23 ± 53.57 | 0.159 |
| eGFR, mL/min | 91.24 ± 15.09 | 88.07 ± 15.08 | 91.38 ± 15.08 | <0.001 |
| Serum glucose, mmol/L | 5.53 (4.95, 6.63) | 5.37 (4.90, 6.32) | 5.54 (4.95, 6.64) | 0.027 |
| Uric acid, μmol/L | 343.07 ± 85.00 | 343.05 ± 84.95 | 343.55 ± 85.49 | 0.908 |
| LVEF, % | 63.0 (60.0, 67.0) | 63.0 (60.0, 66.2) | 63.0 (60.0, 67.0) | 0.285 |
| Medications | ||||
| Statin | 9,323 (96.1) | 397 (96.6) | 8,926 (96.1) | 0.628 |
| Aspirin | 9,697 (100) | 411 (100) | 9,286 (100) | NA |
| P2Y12 inhibitor | 0.176 | |||
| Ticagrelor | 18 (0.2) | 2 (0.5) | 16 (0.2) | |
| Clopidogrel | 9,679 (99.8) | 409 (99.5) | 9,270 (99.8) | |
| Glycoprotein Ⅱb/Ⅲa inhibitor | 1,561 (16.1) | 69 (16.8) | 1,492 (16.1) | 0.697 |
| PPI | 1,910 (19.7) | 82 (20.0) | 1,828 (19.7) | 0.895 |
| Duration of DAPT | 0.093 | |||
| ≤ 1 year | 2,982 (30.8) | 145 (35.3) | 2,837 (30.6) | |
| 1–2 years | 3,797 (39.2) | 144 (35.0) | 3,653 (39.3) | |
| ≥ 2 years | 2,918 (30.1) | 122 (29.7) | 2,796 (30.1) | |
| Femoral artery approach | 737 (7.6) | 43 (10.5) | 694 (7.5) | 0.025 |
Values are presented as mean ± standard deviation, median (IQR) or frequency (percentage).
ACS, acute coronary syndrome; ARC-HBR, Academic Research Consortium for High Bleeding Risk; BMI: body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricle ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; PRECISE-DAPT, predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy; RC, remnant cholesterol; TC, total cholesterol; TG, triglyceride; WBC, white blood cell count; NA, not applicable.
The high PRECISE-DAPT score was defined as the score greater than or equal to 25 and the PRECISE-DAPT score for each patient was calculated by an online web calculator (http://www.precisedaptscore.com).
Incidence of long-term outcomes
Over a median follow-up of 5.1 (25th–75th: 5.0–5.1) years, 411 (4.2%) patients experienced bleeding events (200 BARC type 2 bleeding, 27 BARC type 3a bleeding, 9 BARC type 3b bleeding, 65 BARC type 3c bleeding, 1 BARC type 5a bleeding, and 9 BARC type 5b bleeding). Of 422 patients with bleeding events, there were 42 ICHs, 53 intraocular bleeds compromising vision, 150 gastrointestinal bleeding, 134 dental/nasal/skin mucosa bleeding, and 32 hemorrhages at other sites.
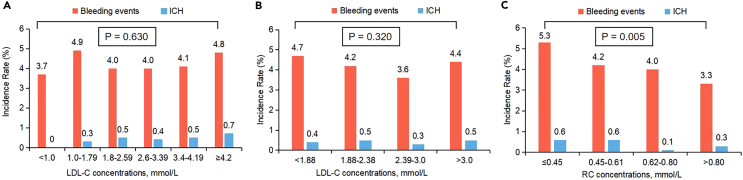
There was no significant reduction in the occurrence of bleeding events or ICH as the LDL-C concentrations increased incrementally (Figures 1A and 1B). However, a significant decrease in the incidence of bleeding events was observed with increasing concentrations of RC (Figure 1C; p = 0.005). Conversely, no such significant association was found between RC concentrations and the occurrence of ICH, but notably, the lowest incidence of ICH was observed within the RC range of 0.62–0.80 mmol/L (Figure 1C).
Figure 1.
Incidences of long-term outcomes according to LDL-C and RC
(A–C) The incidence rates of long-term bleeding events and ICH were calculated by strata of (A) clinical cut-off points of LDL-C, (B) quartiles of LDL-C or (C) quartiles of RC. Abbreviations: RC, remnant cholesterol; LDL-C, low-density lipoprotein cholesterol; ICH, intracranial hemorrhage.
Dose-response analysis
The restricted cubic splines revealed that there was no non-linear relationship between LDL-C levels and the incidence of long-term bleeding events (Figure S2A) or ICH (Figure S2B). Similarly, no non-linear association was found between RC concentrations and the risk of long-term bleeding events (Figure S2C). However, a significant non-linear association was identified between RC levels and the risk of long-term ICH. Specifically, within the RC range of 0.62–0.80 mmol/L, lower RC concentrations were associated with a reduced risk of ICH (Figure S2D; P for non-linear trend = 0.015).
Survival analysis
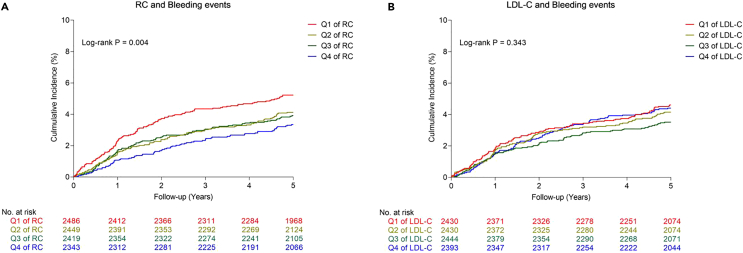
The univariate Cox analyses between clinical variables and bleeding events and ICH are shown in Table S3. The Kaplan-Meier estimates of bleeding events are shown in Figure 2.
Figure 2.
The Kaplan-Meier estimates of bleeding events with different RC and LDL-C
(A and B) The Kaplan-Meier plot of long-term outcomes were calculated by strata of (A) quartiles of RC and (B) quartiles of LDL-C. Abbreviations: RC, remnant cholesterol; LDL-C, low-density lipoprotein cholesterol.
With bleeding events as the endpoint in multivariable Cox analysis with LDL-C and RC as continuous variables, a significant association between RC and bleeding events (HR Model 1: 0.67, 95% CI: 0.49–0.92, p = 0.013; HR Model 2: 0.47, 95% CI: 0.26–0.85, p = 0.012; HR Model 3: 0.47, 95% CI: 0.26–0.85, p = 0.012; HR Model 4: 0.47, 95% CI: 0.26–0.84, p = 0.012) (Table 2) was observed, but no such relationship was observed for LDL-C and bleeding events (all models in Table 2).
Table 2.
Predictive value of LDL-C and RC (continuous variables) for 5-year outcomes in multivariate Cox analysis
| Variables, mmol/L | Bleeding events |
ICH |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| LDL-C | ||||
| Model 1a | 0.98 (0.88–1.10) | 0.728 | 1.17 (0.85–1.61) | 0.351 |
| Model 2b | 0.99 (0.89–1.11) | 0.899 | 1.12 (0.80–1.57) | 0.515 |
| Model 3c | 0.99 (0.89–1.11) | 0.906 | 1.12 (0.80–1.57) | 0.517 |
| Model 4d | 0.99 (0.89–1.11) | 0.900 | 1.12 (0.80–1.57) | 0.514 |
| RC | ||||
| Model 1a | 0.67 (0.49–0.92) | 0.013 | 1.06 (0.45–2.52) | 0.890 |
| Model 2b | 0.47 (0.26–0.85) | 0.012 | 1.48 (0.26–8.33) | 0.660 |
| Model 3c | 0.47 (0.26–0.85) | 0.012 | 1.49 (0.26–8.42) | 0.655 |
| Model 4d | 0.47 (0.26–0.84) | 0.012 | 1.47 (0.26–8.30) | 0.662 |
RC, remnant cholesterol; LDL-C, low-density lipoprotein cholesterol; ICH, intracranial hemorrhage; HR, hazard ratio; CI, confidence interval.
Model 1 was adjusted for age, sex, and body mass index.
Model 2 was adjusted for Model 1 + hypertension, previous bleeding, previous stroke, femoral artery approach, hemoglobin, eGFR, duration of DAPT, HDL-C, and TG, because of their statistical significances (p < 0.05) in univariate analysis.
Model 3 was adjusted for Model 2 + high PRECISE-DAPT score.
Model 4 was adjusted for Model 2 + ARC-HBR criteria.
Multivariate Cox analysis showed that the highest quartile (Q4) of RC compared with the lowest quartile of RC (Q1) was significantly associated with bleeding events (HR Model 1: 0.67, 95% CI: 0.51–0.89, p = 0.006; HR Model 2: 0.66, 95% CI: 0.45–0.97, p = 0.034; HR Model 3: 0.66, 95% CI: 0.45–0.97, p = 0.035; HR Model 4: 0.66, 95% CI: 0.44–0.97, p = 0.033) (Table 3). However, no higher quartile (Q2, Q3, and Q4) of LDL-C relative to the lowest quartile of LDL-C was associated with bleeding events (all models in Table 3).
Table 3.
Predictive value of LDL-C and RC (categorical variables) for 5-year outcomes in multivariate Cox analysis
| Variables, mmol/L | Bleeding events |
ICH |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| LDL-C | ||||
| Model 1a | ||||
| Q1 | Reference | NA | Reference | NA |
| Q2 | 0.90 (0.69–1.18) | 0.457 | 1.35 (0.57–3.21) | 0.494 |
| Q3 | 0.79 (0.60–1.04) | 0.089 | 0.93 (0.36–2.40) | 0.872 |
| Q4 | 0.95 (0.73–1.24) | 0.715 | 1.56 (0.66–3.67) | 0.308 |
| Model 2b | ||||
| Q1 | Reference | NA | Reference | NA |
| Q2 | 0.92 (0.70–1.19) | 0.506 | 1.26 (0.53–3.01) | 0.599 |
| Q3 | 0.80 (0.60–1.06) | 0.113 | 0.87 (0.33–2.27) | 0.770 |
| Q4 | 0.98 (0.75–1.30) | 0.898 | 1.38 (0.57–3.30) | 0.473 |
| Model 3c | ||||
| Q1 | Reference | NA | Reference | NA |
| Q2 | 0.92 (0.70–1.20) | 0.518 | 1.27 (0.53–3.02) | 0.594 |
| Q3 | 0.80 (0.60–1.06) | 0.116 | 0.87 (0.33–2.27) | 0.771 |
| Q4 | 0.98 (0.75–1.30) | 0.905 | 1.38 (0.57–3.30) | 0.476 |
| Model 4d | ||||
| Q1 | Reference | NA | Reference | NA |
| Q2 | 0.91 (0.70–1.19) | 0.506 | 1.27 (0.53–3.02) | 0.594 |
| Q3 | 0.80 (0.60–1.06) | 0.113 | 0.87 (0.33–2.27) | 0.772 |
| Q4 | 0.98 (0.75–1.29) | 0.899 | 1.38 (0.58–3.31) | 0.472 |
| RC | ||||
| Model 1a | ||||
| Q1 | Reference | NA | Reference | NA |
| Q2 | 0.80 (0.61–1.03) | 0.081 | 1.02 (0.50–2.06) | 0.964 |
| Q3 | 0.79 (0.61–1.02) | 0.075 | 0.22 (0.06–0.76) | 0.017 |
| Q4 | 0.67 (0.51–0.89) | 0.006 | 0.66 (0.28–1.58) | 0.351 |
| Model 2b | ||||
| Q1 | Reference | NA | Reference | NA |
| Q2 | 0.80 (0.62–1.04) | 0.101 | 1.07 (0.52–2.21) | 0.850 |
| Q3 | 0.79 (0.59–1.05) | 0.102 | 0.23 (0.06–0.80) | 0.022 |
| Q4 | 0.66 (0.45–0.97) | 0.034 | 0.53 (0.17–1.65) | 0.274 |
| Model 3c | ||||
| Q1 | Reference | NA | Reference | NA |
| Q2 | 0.80 (0.61–1.04) | 0.098 | 1.08 (0.52–2.23) | 0.845 |
| Q3 | 0.79 (0.59–1.05) | 0.105 | 0.23 (0.06–0.81) | 0.022 |
| Q4 | 0.66 (0.45–0.97) | 0.035 | 0.53 (0.17–1.66) | 0.279 |
| Model 4d | ||||
| Q1 | Reference | NA | Reference | NA |
| Q2 | 0.80 (0.61–1.04) | 0.099 | 1.07 (0.52–2.22) | 0.852 |
| Q3 | 0.79 (0.59–1.05) | 0.100 | 0.23 (0.06–0.80) | 0.022 |
| Q4 | 0.66 (0.44–0.97) | 0.033 | 0.53 (0.17–1.65) | 0.273 |
RC, remnant cholesterol; LDL-C, low-density lipoprotein cholesterol; ICH, intracranial hemorrhage; HR, hazard ratio; CI, confidence interval; NA, not applicable.
The Q1, Q2, Q3, and Q4 of LDL-C were <1.88, 1.88–2.38, 2.39–3.0 and >3.0 mmol/L, respectively. The Q1, Q2, Q3, and Q4 of RC were <0.45, 0.45–0.61, 0.62–0.80 and >0.80 mmol/L, respectively.
Model 1 was adjusted for age, sex, and body mass index.
Model 2 was adjusted for Model 1 + hypertension, previous bleeding, previous stroke, femoral artery approach, hemoglobin, eGFR, duration of DAPT, HDL-C, and TG, because of their statistical significances (p < 0.05) in univariate analysis.
Model 3 was adjusted for Model 2 + high PRECISE-DAPT score.
Model 4 was adjusted for Model 2 + ARC-HBR criteria.
When ICH was the endpoint in multivariable Cox analysis with LDL-C and RC as continuous variables, there was no significant association between LDL-C or RC and ICH (all models in Table 2).
With independent variables (LDL-C and RC) as categorical variables, multivariate Cox analysis showed that the Q3 of RC was significantly associated with ICH compared with the lowest quartile of RC (Q1) (HR Model 1: 0.22, 95% CI: 0.06–0.76, p = 0.017; HR Model 2: 0.23, 95% CI: 0.06–0.80, p = 0.023; HR Model 3: 0.23, 95% CI: 0.06–0.81, p = 0.022; HR Model 4: 0.23, 95% CI: 0.06–0.80, p = 0.022) (Table 3). No higher quartile (Q2, Q3, and Q4) of LDL-C relative to the lowest quartile of LDL-C (Q1) was associated with ICH (all models in Table 3).
Risk of bleeding across LDL-C with clinical cut-off points
In multivariate Cox analysis, different LDL-C (<1.0, 1.0–1.79, 1.8–2.59, 3.4–4.19, and ≥ 4.2 mmol/L) relative to the reference (2.6–3.39 mmol/L) were not associated with bleeding events and ICH (all models in Table S4).
Subgroup analysis
The effect of RC concentrations on the bleeding events was consistent, regardless of age, sex, ACS, prior bleeding, prior stroke, PRECISE-DAPT score, and treated with glycoprotein Ⅱb/Ⅲa inhibitor (Figure S3).
Sensitivity analysis
The results were consistent after excluding patients with poor nutrition status, severe infection, or malignancy (n = 173) (Table S5).
Discussion
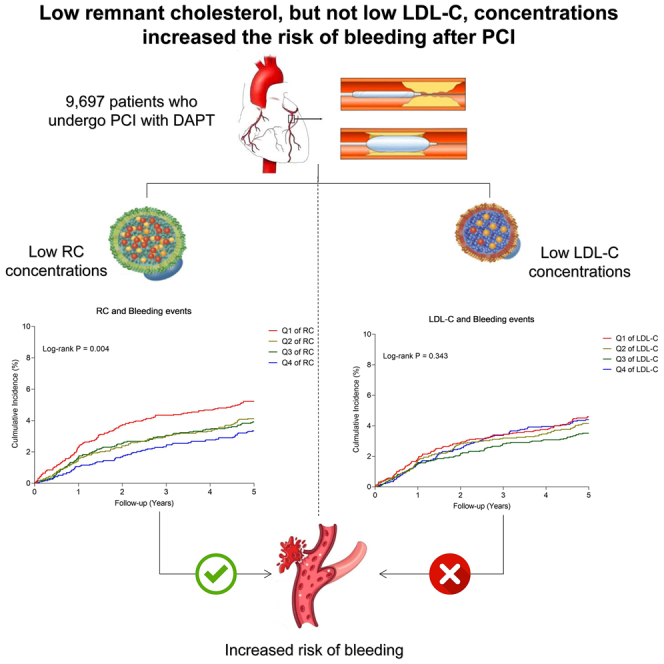
The present study, which involved a large real-world cohort (n = 9,697) with a median follow-up duration of 5.1 years, yielded three significant findings. (1) Lower concentrations of RC were an independent predictor of long-term bleeding events. (2) A non-linear association between RC concentrations and the risk of long-term ICH was observed. (3) Lower LDL-C concentrations were not independently associated with either long-term bleeding events or ICH.
RC and bleeding
RC, also known as TG-rich lipoprotein cholesterol, differs significantly from LDL-C. Fasting RC specifically refers to the cholesterol present in very low-density lipoprotein (VLDL) and intermediate-density lipoprotein residual particles following hydrolysis. Postprandial RC includes cholesterol in chylomicron residual particles as well. In recent years, there has been growing interest in the association between RC and the prognosis of individuals at high risk for cardiovascular events. Previous studies have indicated that RC, rather than LDL-C, independently predicted an elevated risk of MACE.10,11 Nevertheless, the correlation between RC and bleeding risk following PCI has remained unexplored in existing the literature. Our study represented the first investigation to demonstrate a significant relationship between lower RC levels, rather than LDL-C, and an increased long-term risk of bleeding.
This finding was a contribution with significant implications for the delicate balance between bleeding and ischemic risk in patients with PCI undergoing lipid-lowering therapy. Traditionally, LDL-C has been regarded as an independent risk factor for ischemic events, and its reduction has been emphasized with a “lower is better” approach. However, recent skepticism has arisen regarding LDL-C’s ability to accurately assess ischemic risk. Conversely, RC has emerged as an independent predictor of an elevated risk of MACE, indicating its capacity to evaluate ischemic risk is superior LDL-C.10,11 Furthermore, low LDL-C levels have been associated with an increased risk of short-term bleeding post-PCI.8,9 Nevertheless, our study suggested that LDL-C may not effectively evaluate bleeding risk, whereas RC demonstrated a more accurate assessment. Thus, RC showed promise in surpassing LDL-C for evaluating both ischemic and bleeding risks, thereby aiding clinicians in the development of personalized treatment strategies.
LDL-C and bleeding
Although current guidelines recommend increasingly lower targets for LDL-C reduction in high-risk patients,14,15,16 the effect of lower LDL-C on long-term bleeding has been unclear. The results on the association of LDL-C and bleeding were inconsistent. Notably, LDL-C has not been regarded as a predictor of bleeding in the following tools recommended by guidelines for evaluating the bleeding risk, including the CRUSADE score, ACUITY score, PRECISE-DAPT score, PARIS bleeding score, and ARC-HBR criteria. In line with these bleeding scores partially, our study findings demonstrated that LDL-C may not serve as a reliable risk marker for bleeding events, whereas RC emerged as an independent predictor of bleeding risk. This perspective highlighted the significance of incorporating RC into the assessment of bleeding risk.
To the best of our knowledge, only two studies have reported LDL-C was associated with bleeding after PCI. Yang et al.8 discovered that lower LDL-C was significantly related to an increased bleeding risk after PCI during hospitalization (median hospitalization time: 8 days). A study by Chen et al.9 found that the out-of-hospital bleeding risk within one year increased as LDL-C decreased. Their results8,9 differed from our findings, which can be attributed to several factors. Firstly, the study participants were different. They focused on patients with ACS, where LDL-C levels tend to decrease by approximately 10%–20% in the acute phase.17 Moreover, ACS per se is a stress state that may increase the risk of stress-related gastrointestinal bleeding during hospitalization.18 To ensure comparability, we conducted a subgroup analysis in patients with ACS; however, no association of low LDL-C concentrations with long-term bleeding was observed. Secondly, the usage of ticagrelor varied across studies. Ticagrelor was associated with a higher bleeding risk compared to clopidogrel.8 It should be noted that in our study, only 0.2% of patients used ticagrelor, as it entered the Chinese market in 2013, in contrast to Chen et al.'s study (10.0% of patients)9 and Yang et al.'s study (31.6% of patients).8 Finally, the duration of follow-up was different. While our study had a 5-year follow-up period, Yang et al.8 focused on in-hospital bleeding events, and Chen et al.9 observed bleeding within one year after PCI. As far as we know, our study was the first to examine the long-term relationship between LDL-C and bleeding risk in patients who undergo PCI treated with DAPT (mainly clopidogrel). Our results indicated no association between lowering LDL-C concentrations after PCI and bleeding risk, suggesting that reducing LDL-C levels post-PCI is a safe strategy. Additionally, given the dose-response link between LDL-C and in-hospital bleeding, we conducted an analysis that showed no correlation between lower LDL-C (regardless of being lower than 1.4 or lower than 1.0 mmol/L) and long-term bleeding in patients taking clopidogrel.
RC and LDL-C and the risk of ICH
To date, there have been no studies specifically examining the association between RC and ICH, with the exception of a genetic-related study that suggests a potential connection. Jiang et al.19 reported that mutations in the APOC3 gene were related to increased levels of plasma TG and VLDL-C, as well as an increased risk of ICH. They speculated that the increased risk of ICH may be attributed to the elevated plasma TG and VLDL-C resulting from APOC3 mutations, although no direct association analysis was conducted. Importantly, our study revealed a non-linear relationship between RC and long-term ICH, while no such association was observed for LDL-C. These findings provide compelling evidence supporting the safety of long-term LDL-C reduction therapy.
Multiple studies have focused on the relationship between LDL-C and ICH, but their results have been inconsistent. Several prospective studies mainly in the field of CHD have demonstrated no significant association between them,20,21,22,23,24,25,26,27,28 which were consistent with our results. Of notice, many of these studies were the prespecified analyses of well-known clinical randomized trials where ICH was evaluated as one of the safety endpoints (e.g., IMPROVE-IT,27 FOURIER, 26 and SPARCL trials28). In addition, a basic research study showed that although LDL-C levels in proprotein convertase subtilisin/kexin type 9 (PCSK9)−/− mice were lower relative to PCSK9+/+ mice after feeding with a high-fat diet, there was no difference in hemoglobin concentrations in ischemic brain tissue after cerebral ischemia/reperfusion.29 However, there were also studies that reported a negative correlation between LDL-C and ICH.4,5,6,7 Of notice, these studies did not exclusively focus on patients with CHD. In summary, studies primarily focused on patients with CHD, such as those mentioned previously,20,21,22,23,24,25,26,27,28 yielded results consistent with our findings.
Potential mechanisms and clinical implications
Some basic studies have suggested potential mechanisms linking lipid metabolism to endothelial dysfunction, inflammation, thrombosis, and bleeding.30,31,32,33 Moreover, clinical randomized trials have provided evidence that reduced RC levels were metabolically associated with decreased concentrations of PCSK9.34,35,36 Plasma PCSK9 levels can directly enhance platelet activation and thrombosis by binding to platelet CD36 and subsequently activating downstream signaling pathways. Decreased RC levels may interfere with the downstream pathway of PCSK9, thereby enhancing platelet inhibition and impairing hemostasis.32,33 Besides, cholesterol, a crucial constituent of platelet membrane lipid rafts, plays a vital role in platelet signaling. For instance, in vitro cholesterol depletion can disrupt platelet aggregation by altering the critical ultrastructure involved in mediating secretion.37 Interestingly, our study found that as RC concentrations decreased, the risk of bleeding increased, providing further support for the notion that RC affects the process of bleeding and coagulation. It was important to point out that these potential mechanisms were hypothetical and the underlying mechanisms behind this finding warrant further exploration.
It is significant to acknowledge that the established benefits of LDL-C reduction in preventing ischemic events remain unquestionable, and the intensive lowering of LDL-C concentrations is an inevitable trend in the future. Furthermore, this study reinforced that there was no need to worry about bleeding brought on by low LDL-C since it is not the case, providing important clinical data for the safety of intensive LDL-C reduction therapy. Meanwhile, our study provides insights into how lipid-lowering therapy can be optimized in the future. This innovative study suggested greater attention should be given to RC in the context of lipid-lowering strategies. Rather than solely focusing on LDL-C, RC should be considered as one of the therapeutic targets. The concentrations of RC should be monitored regularly to evaluate treatment effectiveness and adjust the therapeutic approach since RC can provide more accurate predictive value of ischemic and bleeding risks. In this way can we contribute to improving patient outcomes and enhancing the effectiveness and safety of lipid-lowering therapy.
In conclusion, this large-scale and real-world study demonstrated that lower RC concentrations, but not lower LDL-C concentrations, were the independent risk predictor of long-term bleeding in the PCI population. This finding may shed light on the safety of LDL-C-lowering therapy in the current era of intensive lipid lowering. The current study indicated that we should pay more attention to the lipid marker RC in lipid management.
Limitations of the study
Several limitations existed in this study. First, as this was a single-centre, observational study, more research is needed to confirm the conclusion’s generalization. Second, we did not routinely evaluate plasma lipid concentrations during the follow-up. Third, the subjects were patients who had taken statins before admission and those given statins after admission, and we only recorded the use of statins at discharge, but not before hospital. However, the use of statins before hospital may not have made a difference to our results since a previous study has shown that the use of statins has little effect on the bleeding risk following PCI in patients with hypercholesterolemia or non-hypercholesterolemia.38 Fourth, lipid-lowering therapy was managed with moderate-intensity statins recommended by the Chinese guideline. Few patients were treated with non-statin lipid-lowering drugs in this study, since the subjects were enrolled before the publication of the IMPROVE-IT trial in 2015 and the introduction of PCSK9 inhibitor to the Chinese market in 2018. Further validation should be done in the setting of high-intensity statin therapy and future attention should be paid to whether the use of non-statin lipid-lowering drugs will affect the results. Fifth, the DAPT regimen in our study was in line with contemporary guidelines in China.39 At present, antithrombotic therapy needs individualization. If the patient is at high bleeding risk, the DAPT duration could be shortened. It can be seen that few patients were at high risk of bleeding in our study (5.1% had high PRECISE-DAPT score and 8.1% met ARC-HBR criteria). Therefore, the prolonged DAPT was still plausible to our study population, although short-term DAPT has recently been suggested to apply more often. Moreover, although we recommended patient strategies following the guidelines, the follow-up found there were also 30.8% of patients with DAPT ≤ 1 year in the real world. Therefore, our findings still had a certain guiding significance for the current clinical practice. Last, even though we made every effort to account for significant confounding factors, unmeasured confounding factors is still conceivable to link to the risk of bleeding.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical commercial assays | ||
| TC | CHOD-PAP method (CHO Assay Kit, Biosino Bio-Technology and Science Incorporation, Beijing, China) | N/A |
| LDL-C | Selective melt method (LDL-C Assay Kit, Kyowa Medex Co., Ltd., Tokyo, Japan) | N/A |
| HDL-C | Chemistry modify enzyme method (Determiner L HDL, Kyowa Medex Co., Ltd., Tokyo, Japan) | N/A |
| TG | GPO-PAP method (TG Assay Kit, Biosino Bio-Technology and Science Incorporation, Beijing, China) | N/A |
| Hemoglobin | A latex turbidimetric method [LASAY Lp(a) auto; SHIMA laboratories; Tokyo, Japan] | N/A |
| White blood cell count | A latex turbidimetric method [LASAY Lp(a) auto; SHIMA laboratories; Tokyo, Japan] | N/A |
| Platelet count | A latex turbidimetric method [LASAY Lp(a) auto; SHIMA laboratories; Tokyo, Japan] | N/A |
| eGFR | Sarcosine oxidase method (creatinine assay kit, Weihai Weigao Biotech Co., Ltd., Shandong, China) | N/A |
| Serum glucose | Glucose oxidase method (glucose assay kit, Biosino Bio-Technology And Science Incorporation, Beijing, China) | N/A |
| Uric acid | A latex turbidimetric method [LASAY Lp(a) auto; SHIMA laboratories; Tokyo, Japan] | N/A |
| Software and algorithms | ||
| SPSS Statistics Version 23.0 | IBM, Chicago, IL, USA | https://www.ibm.com/products/spss-statistics |
| R Programming Language version 4.0.3 | Foundation for Statistical Computing, Vienna, Austria | https://www.r-project.org |
| GraphPad Software | San Diego, California, USA | https://www.graphpad.com |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Xueyan Zhao (zhao_xueyan@sina.com).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Study design and participants
This was a single-centre, prospective, observational, real-world study. Totally 10,724 PCI patients were consecutively and prospectively enrolled from January 2013 to December 2013 at Fu Wai Hospital in Beijing, China. After excluding those who had oral anticoagulants, those who did not have DAPT, and those who lost to follow-up, 9,697 (91.78%) participants were selected for the analysis. All participants in this study were Chinese patients [n = 9,697; males = 7479 (77.1%); mean age = 58.4 years]. Following PCI, all individuals received DAPT for at least 12 months: aspirin 100 mg a day with clopidogrel 75 mg a day, or ticagrelor 90 mg twice daily. Complied with the Helsinki Declaration, this study was approved by the ethics committee (Approval number: 2013-449) at Fu Wai Hospital (Beijing, China). Written informed consent was obtained from all participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Method details
Blood sampling and biomarker measurement
All patients were given venous blood draws at admission following a longer than 12-h fast. Using an automatic biochemistry analyser (Hitachi 7150, Tokyo, Japan) to determine triglycerides (TG), total cholesterol (TC), LDL-C, and high-density lipoprotein cholesterol (HDL-C) with strict quality control in the Clinical Chemistry Department of Fu Wai Hospital. In detail, plasma TC was determined by cholesterol oxidase-phenol aminophenazone method. Plasma TG was determined by glycerol-3-phosphate oxidase-phenol aminophenazone method. Plasma HDL-C was determined by chemistry modify enzyme method. Plasma LDL-C was assayed by selective melt method. .10,11
Clinical outcomes and follow-up
Using the definition of Bleeding Academic Research Consortium (BARC), the primary endpoint was bleeding events which included BARC type 2, BARC type 3, and BARC type 5 bleeding. The secondary endpoint was ICH, a sub-type of stroke, which was defined as any hemorrhage in intracranial vault. The time to events was calculated as the number of days from the date of performing PCI to the date of the incident bleeding, the last visit or the last recorded clinical event of participants still alive, whichever came first. At 30 days, 6 months, 1 year, 2 years, and 5 years, follow-ups were regularly carried out by clinic visits or telephone interviews. Two independent cardiologists arbitrated all endpoint events centrally, and any discrepancies were settled by reaching a consensus.
Quantification and statistical analysis
Continuous variables with a normal distribution were expressed as mean ± standard deviation, while continuous variables with a nonnormal distribution were expressed as median (25th–75th). Categorical variables were expressed as frequency (percentage). The Pearson chi-square test and Fisher’s exact test were used to compare categorical variables, and the Student’s t test, one-way ANOVA and non-parametric test were used to compare continuous variables.
LDL-C and RC concentrations were modeled as both continuous variables and quartiles. Using multivariate restricted cubic spline to evaluate non-linear association of LDL-C and RC with long-term bleeding. The incidences of bleeding events in different groups were compared via the log rank test with the Kaplan–Meier method. Using univariable and multivariable Cox regressions to evaluate the associations between LDL-C and RC (as categorical or continuous variables) and incident bleeding. The hazard ratio (HR) and 95% confidence interval (CI) were reported. The variables in univariate Cox analysis significantly related to bleeding events entered the subsequent multivariate analysis. The covariates in 4 multivariable models were as follows: (1) model 1: age, sex, and body mass index; (2) model 2: model 1 + hypertension, previous bleeding, previous stroke, femoral artery approach, hemoglobin, estimated glomerular filtration rate, duration of DAPT, HDL-C, and TG; (3) model 3: model 2 + high PRECISE-DAPT (predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy) score; (4) model 4: model 2 + ARC-HBR criteria (Academic Research Consortium for High Bleeding Risk).
To combine clinical significance, several clinical cut-off points14,15,16 were further used to divide LDL-C. Furthermore, the effect of RC on the primary endpoint was assessed in different sub-groups (age, sex, ACS, prior bleeding, prior stroke, PRECISE-DAPT score, and treated with glycoprotein Ⅱb/Ⅲa inhibitor). To avoid the low RC in this study was very severe comorbidity to the poor conditions (poor nutrition status, severe infection, or malignancy), we conducted sensitivity analysis. All of the significance levels in the statistical analyses were two-tailed p value <0.05. Data analyses were performed using SPSS Statistics Version 23.0 (IBM, Chicago, IL, USA), R Programming Language version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), and GraphPad Prism version 8.0.0 (GraphPad Software, San Diego, California, USA). Additional statistical methods were detailed in the supplementary material.
Acknowledgments
We thank all staff members for data collection, data entry, and monitoring as part of this study.
Sources of funding: This work was supported by the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences (grant No. NCRC2020013); the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant No. 2020-I2M-C&T-B-052); CS Optimizing Antithrombotic Research Fund (grant No. BJUHFCSOARF201801-06); Young and middle-aged talents in the XPCC Science and Technology Project (grant No. 2020CB012); and the National High Level Hospital Clinical Research Funding (grant 2023-GSP-GG-40).
Author contributions
L.J.W. and Z.X.Y. contributed to the concept and design of the study; L.J.W. wrote the manuscript; L.J.W. conducted the statistical analysis; Z.X.Y. and Y.J.Q. revised the intellectual content; L.J.W., L.Y.L., Z.P., X.J.J., and T.X.F. contributed to data collection; Q.S.B., Y.W.X., Y.Y.J., and G.R.L. contributed to interpretation of data; all authors approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: August 19, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107666.
Contributor Information
Jinqing Yuan, Email: jqyuanfw@163.com.
Xueyan Zhao, Email: zhao_xueyan@sina.com.
Supplemental information
Data and code availability
-
•
The complete original data reported in this study cannot be deposited in a public repository because these data are confidential medical records. To request access, contact Dr. Xueyan Zhao (zhao_xueyan@sina.com).
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Cholesterol Treatment Trialists' CTT Collaborators. Mihaylova B., Emberson J., Blackwell L., Keech A., Simes J., Barnes E.H., Voysey M., Gray A., Collins R., Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/s0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent C., Keech A., Kearney P.M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/s0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 3.Koskinas K.C., Siontis G.C.M., Piccolo R., Mavridis D., Räber L., Mach F., Windecker S. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur. Heart J. 2018;39:1172–1180. doi: 10.1093/eurheartj/ehx566. [DOI] [PubMed] [Google Scholar]
- 4.Sun L., Clarke R., Bennett D., Guo Y., Walters R.G., Hill M., Parish S., Millwood I.Y., Bian Z., Chen Y., et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat. Med. 2019;25:569–574. doi: 10.1038/s41591-019-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Dong Y., Qi X., Huang C., Hou L. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke. 2013;44:1833–1839. doi: 10.1161/strokeaha.113.001326. [DOI] [PubMed] [Google Scholar]
- 6.Sturgeon J.D., Folsom A.R., Longstreth W.T., Jr., Shahar E., Rosamond W.D., Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38:2718–2725. doi: 10.1161/strokeaha.107.487090. [DOI] [PubMed] [Google Scholar]
- 7.Rist P.M., Buring J.E., Ridker P.M., Kase C.S., Kurth T., Rexrode K.M. Lipid levels and the risk of hemorrhagic stroke among women. Neurology. 2019;92:e2286–e2294. doi: 10.1212/wnl.0000000000007454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Q., Sun D., Pei C., Zeng Y., Wang Z., Li Z., Hao Y., Song X., Li Y., Liu G., et al. LDL cholesterol levels and in-hospital bleeding in patients on high-intensity antithrombotic therapy: findings from the CCC-ACS project. Eur. Heart J. 2021;42:3175–3186. doi: 10.1093/eurheartj/ehab418. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y., Yin T., Xi S., Zhang S., Yan H., Tang Y., Qian J., Chen J., Su X., Du Z., et al. A risk score to predict postdischarge bleeding among acute coronary syndrome patients undergoing percutaneous coronary intervention: BRIC-ACS study. Catheter. Cardiovasc. Interv. 2019;93:1194–1204. doi: 10.1002/ccd.28325. [DOI] [PubMed] [Google Scholar]
- 10.Castañer O., Pintó X., Subirana I., Amor A.J., Ros E., Hernáez Á., Martínez-González M.Á., Corella D., Salas-Salvadó J., Estruch R., et al. Remnant Cholesterol, Not LDL Cholesterol, Is Associated With Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020;76:2712–2724. doi: 10.1016/j.jacc.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Quispe R., Martin S.S., Michos E.D., Lamba I., Blumenthal R.S., Saeed A., Lima J., Puri R., Nomura S., Tsai M., et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur. Heart J. 2021;42:4324–4332. doi: 10.1093/eurheartj/ehab432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao D., Mehran R., Dangas G., Baber U., Sartori S., Chandiramani R., Stefanini G.G., Angiolillo D.J., Capodanno D., Urban P., et al. Validation of the Academic Research Consortium High Bleeding Risk Definition in Contemporary PCI Patients. J. Am. Coll. Cardiol. 2020;75:2711–2722. doi: 10.1016/j.jacc.2020.03.070. [DOI] [PubMed] [Google Scholar]
- 13.Mehta S.R., Yusuf S., Peters R.J., Bertrand M.E., Lewis B.S., Natarajan M.K., Malmberg K., Rupprecht H., Zhao F., Chrolavicius S., et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 14.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 15.China Cholesterol Education Program CCEP Working Committee, Atherosclerosis Thrombosis Prevention and Control Subcommittee of Chinese International Exchange and Promotion Association for Medical and Healthcare, Cardiovascular Disease Subcommittee of China Association of Gerontology and Geriatrics, Atherosclerosis Professional Committee of Chinese College of Cardiovascular Physicians. Atherosclerosis Thrombosis Prevention and Control Subcommittee of Chinese International Exchange and Promotion Association for Medical and Healthcare, Cardiovascular Disease Subcommittee of China Association of Gerontology and Geriatrics, Atherosclerosis Professional Committee of Chinese College of Cardiovascular Physicians China cholesterol education program (CCEP) expert advice for the management of dyslipidaemias to reduce cardiovascular risk (2019) Zhonghua Nei Ke Za Zhi. 2020;59:18–22. doi: 10.3760/cma.j.issn.0578-1426.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/cir.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiele F., Farnier M., Krempf M., Bruckert E., Ferrières J., French Group A consensus statement on lipid management after acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care. 2018;7:532–543. doi: 10.1177/2048872616679791. [DOI] [PubMed] [Google Scholar]
- 18.Subherwal S., Bach R.G., Chen A.Y., Gage B.F., Rao S.V., Newby L.K., Wang T.Y., Gibler W.B., Ohman E.M., Roe M.T., et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873–1882. doi: 10.1161/circulationaha.108.828541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y., Ma J., Li H., Liu Y., You C. Effect of apolipoprotein C3 genetic polymorphisms on serum lipid levels and the risk of intracerebral hemorrhage. Lipids Health Dis. 2015;14:48. doi: 10.1186/s12944-015-0047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieberdink R.G., Poels M.M.F., Vernooij M.W., Koudstaal P.J., Hofman A., van der Lugt A., Breteler M.M.B., Ikram M.A. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam Study. Arterioscler. Thromb. Vasc. Biol. 2011;31:2982–2989. doi: 10.1161/atvbaha.111.234948. [DOI] [PubMed] [Google Scholar]
- 21.Psaty B.M., Anderson M., Kronmal R.A., Tracy R.P., Orchard T., Fried L.P., Lumley T., Robbins J., Burke G., Newman A.B., Furberg C.D. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: The Cardiovascular Health Study. J. Am. Geriatr. Soc. 2004;52:1639–1647. doi: 10.1111/j.1532-5415.2004.52455.x. [DOI] [PubMed] [Google Scholar]
- 22.Holme I., Aastveit A.H., Hammar N., Jungner I., Walldius G. Relationships between lipoprotein components and risk of ischaemic and haemorrhagic stroke in the Apolipoprotein MOrtality RISk study (AMORIS) J. Intern. Med. 2009;265:275–287. doi: 10.1111/j.1365-2796.2008.02016.x. [DOI] [PubMed] [Google Scholar]
- 23.Stoekenbroek R.M., Boekholdt S.M., Luben R., Hovingh G.K., Zwinderman A.H., Wareham N.J., Khaw K.T., Peters R.J.G. Heterogeneous impact of classic atherosclerotic risk factors on different arterial territories: the EPIC-Norfolk prospective population study. Eur. Heart J. 2016;37:880–889. doi: 10.1093/eurheartj/ehv630. [DOI] [PubMed] [Google Scholar]
- 24.Amarenco P., Kim J.S., Labreuche J., Charles H., Giroud M., Lavallée P.C., Lee B.C., Mahagne M.H., Meseguer E., Nighoghossian N., et al. Intracranial Hemorrhage in the TST Trial. Stroke. 2022;53:457–462. doi: 10.1161/strokeaha.121.035846. [DOI] [PubMed] [Google Scholar]
- 25.Gu X., Li Y., Chen S., Yang X., Liu F., Li Y., Li J., Cao J., Liu X., Chen J., et al. Association of Lipids With Ischemic and Hemorrhagic Stroke: A Prospective Cohort Study Among 267 500 Chinese. Stroke. 2019;50:3376–3384. doi: 10.1161/strokeaha.119.026402. [DOI] [PubMed] [Google Scholar]
- 26.Giugliano R.P., Pedersen T.R., Park J.G., De Ferrari G.M., Gaciong Z.A., Ceska R., Toth K., Gouni-Berthold I., Lopez-Miranda J., Schiele F., et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390:1962–1971. doi: 10.1016/s0140-6736(17)32290-0. [DOI] [PubMed] [Google Scholar]
- 27.Giugliano R.P., Wiviott S.D., Blazing M.A., De Ferrari G.M., Park J.G., Murphy S.A., White J.A., Tershakovec A.M., Cannon C.P., Braunwald E. Long-term Safety and Efficacy of Achieving Very Low Levels of Low-Density Lipoprotein Cholesterol : A Prespecified Analysis of the IMPROVE-IT Trial. JAMA Cardiol. 2017;2:547–555. doi: 10.1001/jamacardio.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amarenco P., Goldstein L.B., Szarek M., Sillesen H., Rudolph A.E., Callahan A., 3rd, Hennerici M., Simunovic L., Zivin J.A., Welch K.M.A., SPARCL Investigators Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2007;38:3198–3204. doi: 10.1161/strokeaha.107.493106. [DOI] [PubMed] [Google Scholar]
- 29.Tran-Dinh A., Levoye A., Lambert G., Louedec L., Journé C., Meilhac O., Amarenco P. Low levels of low-density lipoprotein-C associated with proprotein convertase subtilisin kexin 9 inhibition do not increase the risk of hemorrhagic transformation. Stroke. 2014;45:3086–3088. doi: 10.1161/strokeaha.114.005958. [DOI] [PubMed] [Google Scholar]
- 30.Nordestgaard B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ. Res. 2016;118:547–563. doi: 10.1161/circresaha.115.306249. [DOI] [PubMed] [Google Scholar]
- 31.Nordestgaard B.G., Tybjaerg-Hansen A., Lewis B. Influx in vivo of low density, intermediate density, and very low density lipoproteins into aortic intimas of genetically hyperlipidemic rabbits. Roles of plasma concentrations, extent of aortic lesion, and lipoprotein particle size as determinants. Arterioscler. Thromb. 1992;12:6–18. doi: 10.1161/01.atv.12.1.6. [DOI] [PubMed] [Google Scholar]
- 32.Qi Z., Hu L., Zhang J., Yang W., Liu X., Jia D., Yao Z., Chang L., Pan G., Zhong H., et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation. 2021;143:45–61. doi: 10.1161/circulationaha.120.046290. [DOI] [PubMed] [Google Scholar]
- 33.Poirier S., Mayer G., Benjannet S., Bergeron E., Marcinkiewicz J., Nassoury N., Mayer H., Nimpf J., Prat A., Seidah N.G. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J. Biol. Chem. 2008;283:2363–2372. doi: 10.1074/jbc.M708098200. [DOI] [PubMed] [Google Scholar]
- 34.Toth P.P., Sattar N., Blom D.J., Martin S.S., Jones S.R., Monsalvo M.L., Elliott M., Davis M., Somaratne R., Preiss D. Effect of Evolocumab on Lipoprotein Particles. Am. J. Cardiol. 2018;121:308–314. doi: 10.1016/j.amjcard.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Hollstein T., Vogt A., Grenkowitz T., Stojakovic T., März W., Laufs U., Bölükbasi B., Steinhagen-Thiessen E., Scharnagl H., Kassner U. Treatment with PCSK9 inhibitors reduces atherogenic VLDL remnants in a real-world study. Vascul. Pharmacol. 2019;116:8–15. doi: 10.1016/j.vph.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Masuda D., Kiyosue A., Hirayama A., Shimauchi J., López J.A.G., Miyawaki K., Yamashita S. Evolocumab Effects on Lipoproteins, Measured by High-Performance Liquid Chromatography. J. Atheroscler. Thromb. 2020;27:1183–1207. doi: 10.5551/jat.54353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grgurevich S., Krishnan R., White M.M., Jennings L.K. Role of in vitro cholesterol depletion in mediating human platelet aggregation. J. Thromb. Haemost. 2003;1:576–586. doi: 10.1046/j.1538-7836.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 38.Ueshima D., Yoshikawa S., Sasaoka T., Hatano Y., Kurihara K., Maejima Y., Isobe M., Ashikaga T. The Hypercholesterolemia Paradox in Percutaneous Coronary Intervention: An Analysis of a Multicenter PCI Registry. Intern. Med. 2019;58:345–353. doi: 10.2169/internalmedicine.1553-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Section of Interventional Cardiology, Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology Section of Interventional Cardiology, Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology Chinese guideline for percutaneous coronary intervention (pocket guideline) Zhonghua Xinxueguanbing Zazhi. 2012;40:271–277. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The complete original data reported in this study cannot be deposited in a public repository because these data are confidential medical records. To request access, contact Dr. Xueyan Zhao (zhao_xueyan@sina.com).
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.