Abstract
We report studies pertaining to two isomeric hexahydrocannabinols (HHCs), (9R)-HHC and (9S)-HHC, which are derivatives of the psychoactive cannabinoids Δ9- and Δ8-THC. HHCs have been known since the 1940s, but have become increasingly available to the public in the United States and are typically sold as a mixture of isomers. We show that (9R)-HHC and (9S)-HHC can be prepared using hydrogen-atom transfer reduction, with (9R)-HHC being accessed as the major diastereomer. In addition, we report the results of cannabinoid receptor studies for (9R)-HHC and (9S)-HHC. The binding affinity and activity of isomer (9R)-HHC are similar to that of Δ9-THC, whereas (9S)-HHC binds strongly in cannabinoid receptor studies but displays diminished activity in functional assays. This is notable, as our examination of the certificates of analysis for >60 commercially available HHC products show wide variability in HHC isomer ratios (from 0.2:1 to 2.4:1 of (9R)-HHC to (9S)-HHC). These studies suggest the need for greater research and systematic testing of new cannabinoids. Such efforts would help inform cannabis-based policies, ensure the safety of cannabinoids, and potentially lead to the discovery of new medicines.
Introduction
The cannabis industry has undergone remarkable evolution in recent years. Despite marijuana, cannabis having ≥0.3% (weight/weight) of Δ9-trans-tetrahydrocannabinol (Δ9-THC, see compound 1, Figure 1),1 being illegal in many parts of the world and stigmatized for decades, many states in the United States (U.S.) have now legalized or decriminalized the use of marijuana-based products.2 Similarly, there has been an increase in U.S. Federal legislation,2,3 with a particular focus on accelerating the pace of research needed to address numerous challenges in the field.4−6
Figure 1.
Cannabinoids 1–3 and emerging cannabinoids HHCs 4a and 4b.
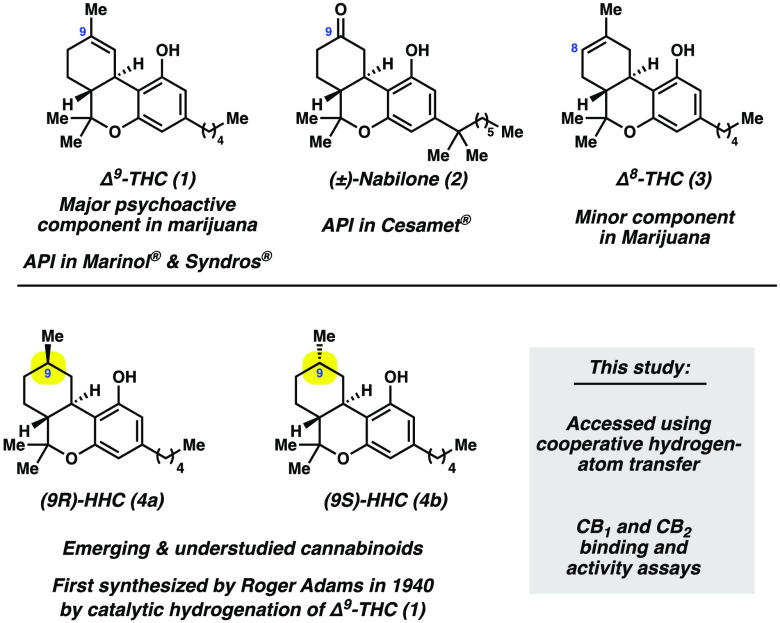
One major contemporary challenge involves the availability of derivatives of Δ9-THC (1, Figure 1),7 which is the primary active component of marijuana that is associated with intoxication. However, from a therapeutic standpoint, 1 is also the active pharmaceutical ingredient (API) in the FDA-approved drugs Marinol and Syndros. These drugs are used to treat nausea and vomiting caused by cancer chemotherapy, in addition to loss of appetite and weight loss in patients with HIV/AIDS. Whereas 1 on its own is a Schedule 1 substance, Marinol is a Schedule 3 substance, and Syndros is a Schedule 2 substance.
Not surprisingly, analogs of 1 have similarly become highly sought after for both medicinal and nonmedicinal purposes.8 THC derivatives 2 and 3 provide illustrative examples. Nabilone (2) is sold as a racemate under the trade name Cesamet and is used for the treatment of chronic pain (in Canada). It is also FDA-approved for chemotherapy-induced vomiting or nausea (in the U.S.). A contrasting example is Δ8-trans-tetrahydrocannabinol (Δ8-THC, 3), a minor constituent of cannabis with a structure similar to 1. Δ8-THC (3) has become commonly available to the public in many states, both with and without marijuana legalization, yet remains non-FDA-approved, unregulated, and generally understudied.9
The present study focuses on related cannabinoids called hexahydrocannabinols (HHCs).10,11 Despite being relatively understudied since the first synthesis by Adams in 1940,12 these compounds are now becoming increasingly widespread and can be purchased in some states in the U.S. Conflicting views regarding the federal legality of HHCs exist. One perspective is that HHCs are legal as a result of the U.S. 2018 Farm Bill. On the other hand, the U.S. Drug Enforcement Administration (DEA) has informed us that they consider HHCs of the type described herein Schedule 1 substances.
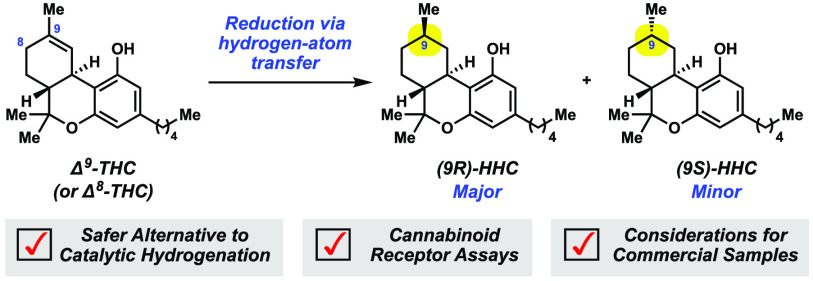
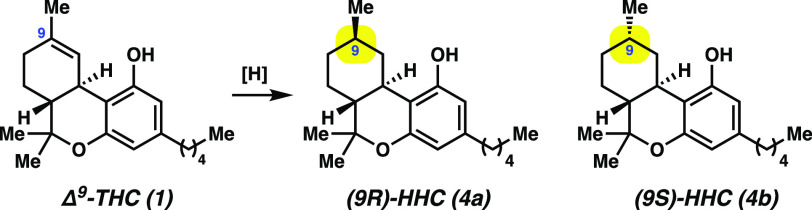
When HHCs are accessed synthetically from Δ9-THC (1) or Δ8-THC (3), two diastereomers can form: (9R)-HHC 4a and (9S)-HHC 4b (Figure 1). The 9R isomer, 4a, is sometimes referred to as the “methyl equatorial” isomer of HHC in the literature, whereas the 9S isomer, 4b, is sometimes referred to as the “methyl axial” isomer. These HHC diastereomers vary based on the stereochemistry at C9. It is expected that different properties and biological effects exist for the two diastereomers. The ratio of isomers 4a and 4b within commercially available HHC varies significantly, as will be discussed further in this Letter, presumably based on the method of production and purification. Most commonly, 4a and 4b are prepared using catalytic hydrogenation,12−15 which yields a mixture of isomers with low selectivity. In addition, fires, runaway reactions, and explosions are well-known dangers associated with catalytic hydrogenation and such dangers can vary based on the conditions employed.16−19 Lastly, trace heavy metals (e.g., Pt or Pd) may remain after catalytic hydrogenation due to leaching or dissolution. The extent to which trace metals remain can vary based on the catalyst employed, the reaction conditions used, as well as the exact purification methods.20,21 The presence of residual heavy metals bears significant toxicity concerns.
Limited biological studies of HHCs 4a and 4b are available in the literature.22−31 The psychoactive effects of 4a and 4b have been demonstrated in rabbits24 and nonhuman primates,22,23,25 using either individual isomers or mixtures. With regard to therapeutic potential, studies have shown that HHCs 4a and 4b may be valuable leads for the treatment of colon cancer26 and ocular hypotony.27 One recent study shows promising cardiac safety and cytotoxicity profiles for the mixture of HHC isomers using in vitro assays.28 There is also one report regarding the in vitro binding affinity of (±)-4a in human cannabinoid receptors.30 Systematic in vitro assay data showing potency or binding affinity of enantioenriched 4a or 4b to the cannabinoid receptors type 1 or 2 (CB1 or CB2), have yet to be published. Of note, cannabinoids that bind to either the CB1 or CB2 receptor may be associated with both adverse effects and therapeutic potential, depending on a variety of factors.32,33 Cannabinoids that bind to the CB1 receptor are also commonly associated with intoxicating effects. Individually investigating the biological characteristics of HHC isomers 4a and 4b would provide insight into their therapeutic potential.
With the aforementioned considerations, we sought to (a) develop a means to synthesize both isomers of HHC that avoids the use of potentially dangerous catalytic hydrogenation conditions and toxic heavy metals and (b) establish their affinity and activity at human CB1 and CB2 receptors relative to Δ9-THC (1). Here, we report the use of cooperative hydrogen-atom transfer (HAT) to convert Δ9-THC (1) and Δ8-THC (3) to HHCs 4a and 4b.34 In contrast to results obtained by using catalytic hydrogenation, 4a is formed as the major product using the HAT protocol. Additionally, we demonstrate via biological investigation that 4a and 4b possess significantly different binding affinities and potency, with 4a exhibiting similar activity compared with Δ9-THC (1). Finally, we examine the certificates of analyses for >60 commercially available HHC products, which show wide variability in HHC isomer ratios. These studies are expected to prompt further scientific investigation of HHCs, and also underscore the importance of performing such synthetic and biochemical studies as new cannabinoids inevitably emerge.
Reduction of Δ9-THC (1) and Δ8-THC (3)
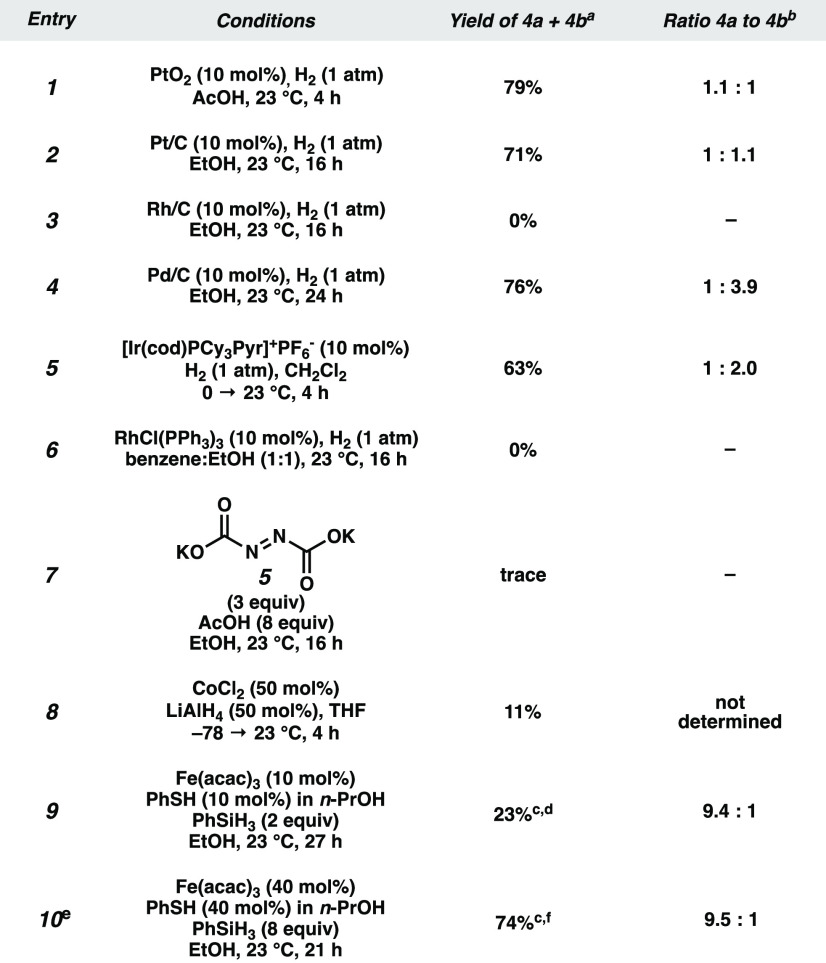
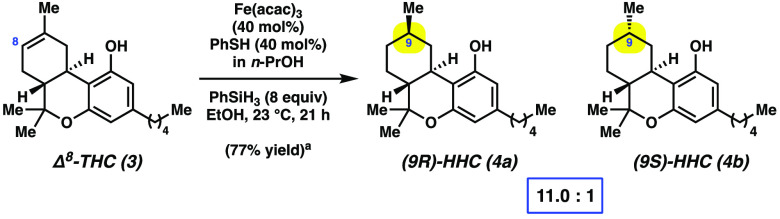
To initiate our studies, we investigated the reduction of Δ9-THC (1) to afford HHCs 4a and 4b (see Table 1). We first attempted catalytic hydrogenation conditions, since literature data did not consistently report diastereoselectivities or yields. We began with the use of PtO2, which Adams had demonstrated in the 1940s for the reduction of 1.12 This gave roughly equimolar amounts of 4a and 4b (entry 1 in Table 1). Similar results were seen when Pt/C was used (entry 2 in Table 1). Although the use of Rh/C proved to be ineffective (entry 3 in Table 1), hydrogenation using Pd/C delivered 4a and 4b in a ratio of 1 to 3.9, respectively (entry 4 in Table 1). We then examined homogeneous hydrogenation conditions that were not known for the reduction of 1 in the literature. The use of Ir catalyst (Crabtree’s catalyst) proved less effective (entry 5 in Table 1), while Rh catalyst (Wilkinson’s catalyst) was unsuccessful (entry 6 in Table 1). The reduction with diimide 5 also proved unproductive (entry 7 in Table 1). Stoichiometric metal hydride conditions gave low yields (entry 8 in Table 1).35 Lastly, we evaluated HAT conditions, which are attractive due to the avoidance of pyrophoric reagents and high levels of thermodynamically controlled diastereoselectivity.36 Such conditions had not been reported for the reduction of 1. The Fe-based protocol reported by West was especially attractive,37 as the necessary reagents are commercially available and iron is considered a metal of minimal concern.20 Employment of the West conditions gave good diastereoselectivity (entry 9 in Table 1) but a low yield. By increasing the loading of reagents and adding them portionwise over two additions, the combined yield of 4a and 4b increased to 74%, with a 4a:4b ratio of 9.5:1 (entry 10 in Table 1). It is notable that the HAT conditions provide the highest selectivity and favor the formation of isomer 4a. In consideration of the results described in the subsequent section pertaining to biological assays, the observed preference for 4a proved to be a fortuitous result. We also examined the HAT reduction of the Δ9-THC (1) isomer Δ8-THC (3). As shown in Scheme 1, treatment of 3 under our previously optimized conditions afforded HHCs 4a and 4b in 77% yield. Of note, the 4a:4b ratio was 11.0 to 1, respectively, which reflects comparable selectivity to the diastereoselectivity observed when Δ9-THC (1) is employed. These results show that either THC isomer (i.e., 1 or 3) can be used in the reduction.
Table 1. Reduction Studies of Δ9-THC (1) To Furnish HHCs 4a and 4b.
Isolated yields.
Ratios determined from isolated material using 1H NMR.
Yields reflect the average of two isolation experiments.
38% yield of recovered 1, average from two isolation experiments.
20 mol % of Fe(acac)2, 20 mol % of PhSH, and 4 equiv PhSiH3 were initially added portionwise, followed by the second portion after 17 h.
10% yield of recovered 1, average from two isolation experiments.
Scheme 1. HAT Reduction of Δ8-THC (3) To Give HHCs 4a and 4b.
Yield reflects the average of two isolation experiments.
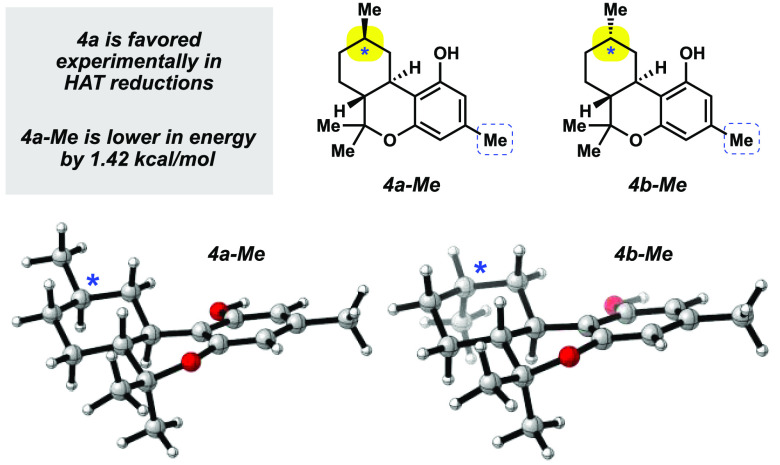
In order to rationalize the diastereoselectivity in the HAT reduction of 1 and 3, we considered the relative energies of 4a and 4b. A 1989 study by Reggio and co-workers suggested that 4a is energetically favorable,38 but computations using higher levels of theory now accessible have not been performed. As such, we conducted density functional theory (DFT) calculations using ωB97X-D (6-31G*) for 4a-Me and 4b-Me, which are the simplified structures (i.e., methyl in place of pentyl) shown in Figure 2. Following conformational searching, the lowest ground-state energies were compared. Of note, 4a-Me was found to be thermodynamically favored by 1.42 kcal/mol. This difference in energy corresponds to a diastereomeric ratio (dr) of ∼10:1, which is consistent with our experimental observations. In accordance with general mechanistic considerations for HAT reductions,36 thermodynamic control is presumably operative in the reductions of 1 or 3 to favor formation of the product bearing an equatorial methyl group (i.e., 4a, analogous to computed structure 4a-Me).
Figure 2.
Lowest energy conformers for 4a-Me and 4b-Me. Structures are displayed by using CYLview.
CB1 and CB2 Receptor Studies
As discussed earlier, two receptors, CB1 and CB2, are important for assessing the biological activities of cannabinoids. Interactions with CB1 or CB2 can be useful for therapeutic purposes.32,33 Given that the individual activities of 4a and 4b in human CB1 and CB2 receptor assays are not described in the literature, we sought to study this further. Pure synthetic samples of 4a and 4b were prepared (>19:1 dr) for use in cannabinoid receptor studies. Given the relative product distribution of the different reduction protocols, 4a was accessed using HAT reduction of 1, whereas 4b was prepared using catalytic hydrogenation of 1 (see the SI for details). Biological assays were performed by Eurofins Discovery. A radioligand binding assay was used to determine Ki and IC50 values.39,40 A G-protein coupled receptor (GPCR) functional assay was used to study potency and determine EC50 values.41 Both assays use human cannabinoid receptors with the appropriate reference standards (see the SI for details). Δ9-THC (1), a known partial agonist for both CB1 and CB2, was simultaneously evaluated to provide a point of comparison under the same assay conditions.42
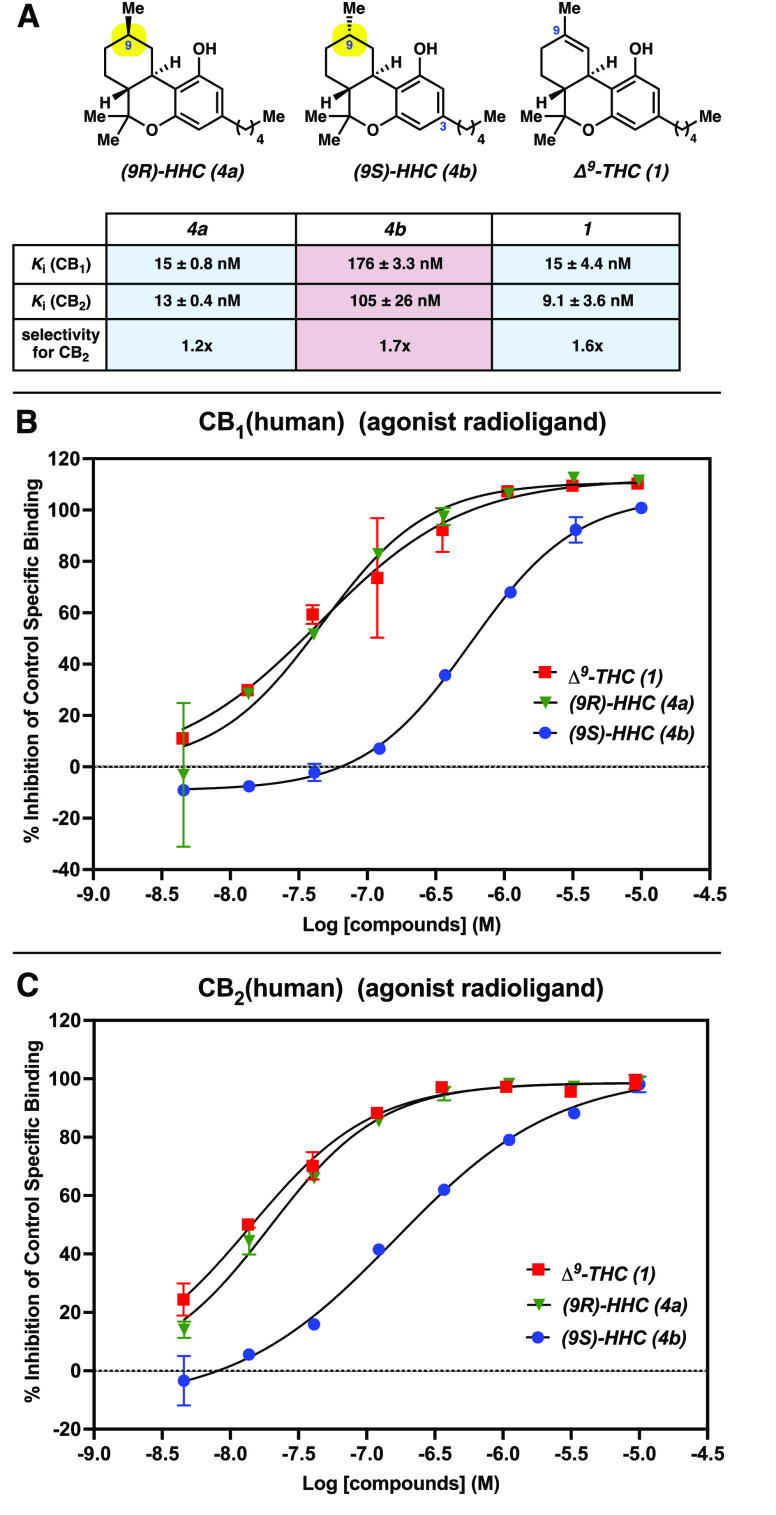
The CB1 binding assay was conducted with cellular lysates of Chem-1 cells transfected with human CB1 cannabinoid receptor. Displacement of radio-labeled [3H]CP 5594043 by the tested compounds measured specific binding and WIN 55212-244 was used to determine nonspecific binding. The CB2 binding assay was conducted with cellular lysates of CHO cells transfected with the human CB2 cannabinoid receptor. Displacement of radio-labeled [3H]WIN 55212-2 by the tested compounds measured specific binding and WIN 55212-2 was used to determine nonspecific binding. Both 4a (Ki = 15 nM at CB1 and 13 nM at CB2) and 4b (Ki = 176 nM at CB1 and 105 nM at CB2) bind to the CB1 and CB2 receptors with nanomolar affinity in the radioligand assay (Figure 3A). A comparison of the binding of each individual diastereomer to the CB1 and CB2 receptors shows minimal selectivity (1.2–1.7x for CB2) for binding to one receptor over the other. However, as shown in Figures 3B and 3C, 4a (green triangle) binds both receptors with an affinity an order of magnitude higher than that of 4b (blue circle), demonstrating stronger binding of the (9R)-HHC 4a diastereomer. Of note, the binding affinity of 4a (green triangle) is similar to that of Δ9-THC (1, red square) for both cannabinoid receptors.
Figure 3.
(A) Summary of radioligand binding affinity studies; error values represent the standard deviation. (B) Plotted inhibition of binding for human CB1 cannabinoid receptor in transfected Chem-1 cell lysate after treatment with 1, 4a, and 4b. (C) Plotted inhibition of binding for human CB2 cannabinoid receptor in transfected CHO cell lysate after treatment with 1, 4a, and 4b. For all experiments, data represent two replicate experiments, with error bars showing standard deviation (error bars omitted for clarity if the range is smaller than the data symbol), and the Y-intercept was constrained to zero, unless otherwise noted (see the SI for details).
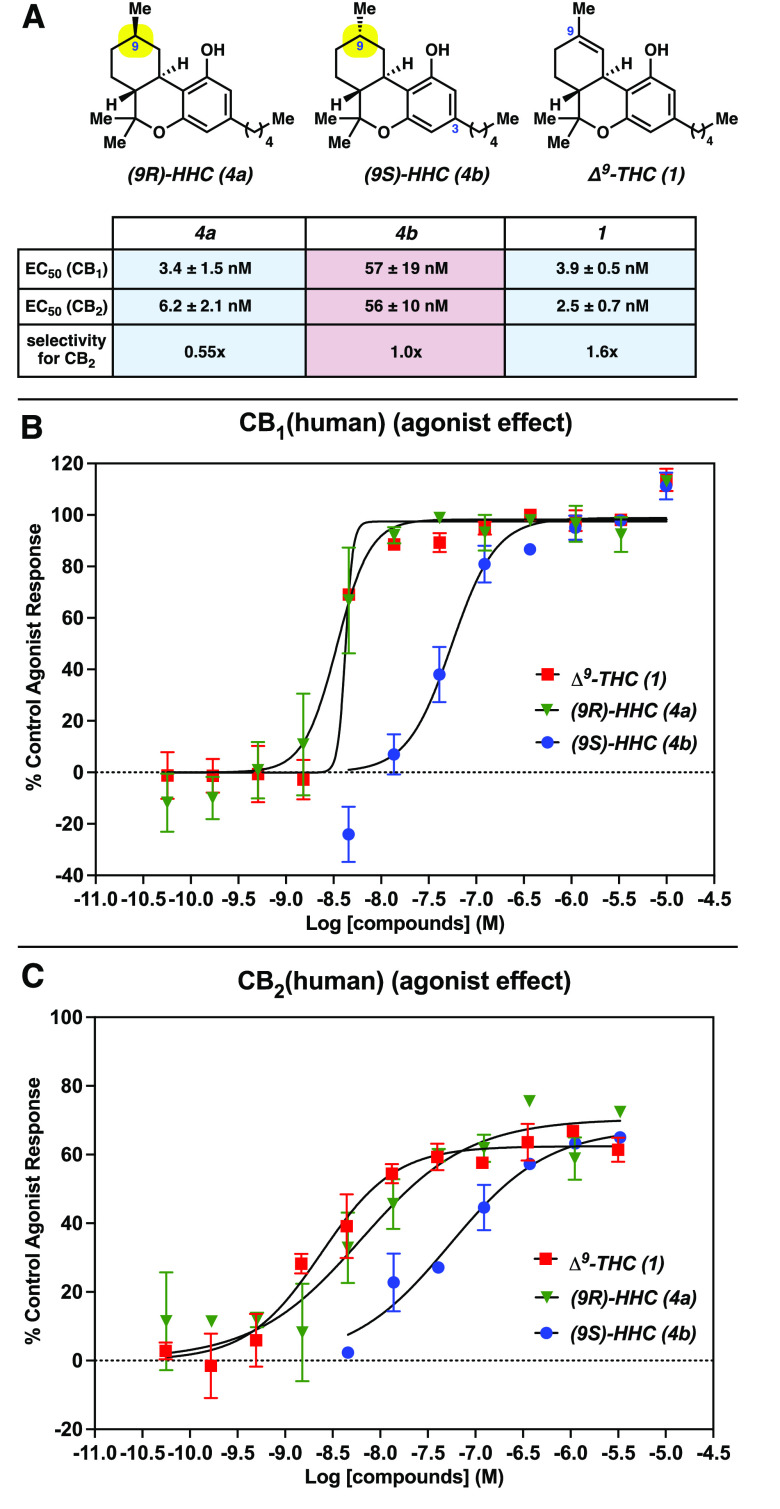
The CB1 and CB2 functional assays were conducted using CHO cells transfected with human CB1 and CB2 cannabinoid receptors, respectively. Efficacy of the tested compounds was determined by measuring changes in cAMP concentrations relative to controls using homogeneous time-resolved fluorescence45 (HTRF). In the functional assay, 4a (EC50 = 3.4 nM at CB1 and 6.2 nM at CB2) and 4b (EC50 = 57 nM at CB1 and 56 nM at CB2) demonstrated excitatory activity at the CB1 and CB2 receptors (Figure 4A). Of note, 4a shows modest selectivity (1.8x) for CB1 whereas there is no significant selectivity of 4b between the CB1 or CB2 receptors. Figures 4B and 4C show that 4a (green triangle) has 17- and 9-fold increases in potency, compared to 4b (blue circle) for CB1 and CB2, respectively. This activity of 4a (green triangle) is similar to that of Δ9-THC (1, red square). However, both HHCs 4a and 4b, such as Δ9-THC (1), are partial agonists of both receptors. Collectively, the results of the binding and functional assays demonstrate significant biological differences between HHC diastereomers 4a and 4b. These two diastereomers differ only in the directionality of the C9 methyl group, highlighting the effect of subtle structural modifications to the cannabinoid scaffold. These data also illustrate the need to understand the activity of individual compounds instead of mixtures, especially as new cannabinoids become available.
Figure 4.
(A) Summary of functional activity studies; error values represent the standard deviation. (B) Plotted response of human CB1 receptor expressed in transfected CHO cells after treatment with 1, 4a, and 4b, determined by measuring their effects on cAMP concentration. (C) Plotted response of human CB2 receptor expressed in transfected CHO cells after treatment with 1, 4a, and 4b, determined by measuring their effects on cAMP concentration. For all experiments, data represent two replicate experiments, with error bars showing the standard deviation (error bars omitted for clarity if the range is smaller than the data symbol) and the Y-intercept was constrained to zero unless otherwise noted (see the SI for details).
Chemical Composition of HHC Products
Given the different biological profiles of HHCs 4a and 4b described above, it is important to understand the chemical composition of recreational HHC products that are becoming increasingly widespread. We examined the online certificates of analysis for >60 HHC-containing products (see the SI for details). For 15 samples, the 4a:4b ratios were not provided. For the others, the 4a:4b ratios ranged from 0.2:1 to 2.4:1 (average of 1.4:1). As such, roughly 15%–70% of a given sample’s HHC content is composed of the more potent isomer 4a. This wide variability in chemical composition and the potential presence of heavy metals may have public health consequences.
Because catalytic hydrogenation of Δ9-THC (1) or Δ8-THC (3) is typically the final step in the synthesis of HHCs 4a and 4b, we questioned if consumer products had been analyzed for the heavy metals typically used for catalysis (i.e., Pd or Pt).20,21 Numerous cannabis analytical laboratories were contacted in different states. Although testing for arsenic, mercury, lead, and cadmium is typically available as required by cannabis laws in most or all states, we learned that testing for Pd or Pt is rarely requested or offered at most cannabis analytical testing facilities. Several laboratories reiterated that such testing is not required. It was not possible for us to acquire and test commercial samples of HHC for heavy metals due to DEA regulations. We encourage that such testing be performed by HHC producers to ensure consumer safety, perhaps drawing from well-established practices for pharmaceutical production.
Conclusions
We have performed studies pertaining to HHCs 4a and 4b, which are emerging cannabinoids first synthesized in the 1940s. Our current study shows that HHCs 4a and 4b can be prepared using HAT reduction of Δ9-THC (1) or Δ8-THC (3), thus providing an alternative to classic hydrogenation conditions. This is notable, because the HAT reduction protocols provide 4a as the major diastereomer, whereas classical hydrogenation conditions are less selective, leading to mixtures of isomers being available to consumers. Moreover, the traditional catalytic hydrogenation protocols, which bear significant safety risks, can be avoided. In addition, we have performed cannabinoid receptor studies of each diastereomer with Δ9-THC (1) as a control in the same assays. Both isomers 4a and 4b were shown to have partial CB1 and CB2 receptor agonist activity, such as Δ9-THC (1). However, isomer 4a, accessed as the major diastereomer in the HAT reduction protocol, binds with higher affinity (Ki = 15 and 13 nM at CB1 and CB2, respectively) and displays good activity in the functional assay (EC50 = 3.4 and 6.2 nM at CB1 and CB2, respectively); the activity of isomer 4a nearly matches that of Δ9-THC (1). This study demonstrates how minor modifications to the Δ9-THC (1) scaffold can lead to significant and varying differences in biological activity. Furthermore, the results of this study illustrate the importance of conducting assays on singular compounds in order to draw conclusions about how changes to the three-dimensional space in derivatives affect both their binding and potency. Lastly, this study shows that the method of Δ9-THC (1) reduction greatly affects the diastereomeric ratio of the HHC products formed. HAT provides an alternative method to access the more active (9R)-HHC isomer (4a) and could enable further biological evaluation. As highlighted in recent articles,46−48 further studies of HHCs are desirable.
Although our scientific findings should not be used on their own to create federal or state policies, this study prompts many future directions that should be considered. For example, it would benefit society to increase the pace of chemical and biochemical-based research in the cannabinoid field while maintaining scientific rigor. New cannabinoids are emerging, and increased fundamental research is needed to characterize their in vitro and in vivo pharmacology and assess psychoactivity, therapeutic potential, and safety. In the U.S., this need has already been recognized by the federal government with recent efforts aimed at helping researchers secure faster approval to perform their research. However, more attention could be paid to the rising availability of new cannabis-derived compounds and analogs.2,3
With regard to specific research areas that require attention, we offer our view that organic and medicinal chemistry can play an important role in the cannabinoid research space. Historically, efforts by organic chemists to impact cannabinoid research have been instrumental. In this rapidly evolving climate, further engagement should be encouraged. Chemists can contribute by preparing new compounds using synthesis, semisynthesis, or biocatalysis, performing careful analyses of chemical composition and contaminants, conducting assays, investigating the mechanisms of action, performing structure-activity relationship studies, exploring receptor binding using computational studies, and ultimately engaging in advanced studies, such as clinical trials. Such efforts could help inform cannabis-based policies and regulations, ensure the safe and fair use of cannabinoids, and ultimately lead to the discovery of new medicines.
Acknowledgments
The authors are grateful to the University of California, Los Angeles, ElectraTect, Inc., and the CNSI Noble Family Innovation Fund for financial support. We thank E. Darzi (ElectraTect, Inc.), Z. Cooper (UCLA), and J. Sorrentino (UCLA) for helpful discussions. We also thank D. Witkowski (UCLA) for computational assistance. Δ9-THC is a Schedule I substance and was procured using an active federal DEA license (No. RG0538338).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.3c00254.
Detailed experimental procedures, compound characterization data, description of biological assays (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): N.K.G. has an equity interest and serves as an advisor for ElectraTect, Inc., a cannabinoid detection company.
Supplementary Material
References
- Sacco L. N.Evolution of Marijuana as a Controlled Substance and the Federal-State Policy Gap. Congressional Research Service, April 7, 2022. Available via the Internet at: https://crsreports.congress.gov/product/pdf/R/R44782 (accessed April 13, 2023).
- Lampe J. R.Recent Developments in Marijuana Law. Congressional Research Service, Dec. 6, 2022. Available via the Internet at: https://crsreports.congress.gov/product/pdf/LSB/LSB10859 (accessed April 13, 2023).
- Erickson B. E.Cannabis research bill clears U.S. Congress. Chem. Eng. News, 2022. Available via the Internet at: https://cen.acs.org/biological-chemistry/natural-products/Cannabis-research-bill-clears-US/100/i43 (accessed April 13, 2023).
- Wadman M. New U.S. law aims to light up medical research on cannabis. Science 2022, 378, 1035. 10.1126/science.adg1903. [DOI] [PubMed] [Google Scholar]
- Devitt T.; Epstein P.; Phillips N.; Devitt-Lee A.. Pandora’s box the dangers of a national, unregulated, hemp-derived intoxicating cannabinoid market. California Cannabis Industry Association, 2022. Available via the Internet at: https://www.projectcbd.org/sites/projectcbd/files/downloads/white-paper_hemp_2022-10-18.pdf (accessed April 13, 2023). [White paper.]
- Legal weed, broken promises: A Times series on the fallout of legal pot in California. Los Angeles Times. Sept. 8, 2022, updated Dec. 29, 2022. Available via the Internet at: https://www.latimes.com/california/story/2022-09-08/a-series-on-the-fallout-of-legal-weed-in-california (accessed April 13, 2023).
- Iversen L.The pharmacology of delta-9-Tetrahydrocannabinol (THC). In The Science of Marijuana, 3rd Edition; Oxford University Press, 2018; p 22-C2.F7. [Google Scholar]
- Willner N.The Controlled Substances Act leaves pathway for intoxicating hemp-derived cannabinoids. MJBizDaily, Feb. 1, 2022. Available via the Internet at: https://mjbizdaily.com/the-controlled-substances-act-leaves-pathway-for-intoxicating-hemp-derived-cannabinoids/ (accessed April 13, 2023).
- Erickson B. E.Delta-8-THC craze concerns chemists. In Chem. Eng. News, 2021. Available via the Internet at: https://cen.acs.org/biological-chemistry/natural-products/Delta-8-THC-craze-concerns/99/i31 (accessed Jan. 14, 2023).
- Qureshi M. N.; Kanwal F.; Siddique M.; Inayat-ur-Rahman A. M. Estimation of biologically active cannabinoids in cannabis indica by gas chromatography-mass spectrometry (GC-MS). World Appl. Sci. J. 2012, 19, 918–923. 10.5829/idosi.wasj.2012.19.07.1922. [DOI] [Google Scholar]
- Collins A. C.; Ramirez G. A.; Tesfatsion T. T.; Ray K. P.; Cruces W.. Characterization of hexahydrocannabinol (HHC) diastereomers, and hexahydrocannabidiol (H4CBD) diastereomers using NMR, HPLC, and GC-MS. Res. Square 2022, 1, 10.21203/rs.3.rs-2322468/v1. [DOI] [Google Scholar]
- Adams R.; Pease D. C.; Cain C. K.; Clark J. H. Structure of cannabidiol. VI. Isomerization of cannabidiol to tetrahydrocannabinol, a physiologically active product. Conversion of cannabidiol to cannabinol. J. Am. Chem. Soc. 1940, 62, 2402–2405. 10.1021/ja01866a040. [DOI] [Google Scholar]
- Adams R.; Cain C. K.; McPhee W. D.; Wearn R. B. Structure of cannabidiol. XII. Isomerization to tetrahydrocannabinols. J. Am. Chem. Soc. 1941, 63, 2209–2213. 10.1021/ja01853a052. [DOI] [Google Scholar]
- Adams R.Marihuana active compounds. U.S. Patent No. US2419937A, March 27, 1944.
- Gaoni Y.; Mechoulam R. Hashish – VII The isomerization of cannabidiol to tetrahydrocannabinols. Tetrahedron 1966, 22, 1481–1488. 10.1016/S0040-4020(01)99446-3. [DOI] [Google Scholar]
- Solis N.2 dead after explosive fire at suspected hemp lab in Canoga Park. Los Angeles Times, Oct. 19, 2021. Available via the Internet at: https://www.google.com/amp/s/www.latimes.com/california/story/2021-10-19/2-dead-after-fire-at-suspected-pot-warehouse-in-canoga-park%3f_amp=true (accessed Jan. 11, 2023).
- Ruscitto A.What is HHC? Cannabis Business Times. Feb. 9, 2022. Available via the Internet at: https://www.cannabisbusinesstimes.com/article/what-is-hexahydrocannabinol-hhc-hemp-derived-cannabinoid-thc/ (accessed Jan. 11, 2023).
- Chandra T.; Zebrowski J. P. Hazards associated with laboratory scale hydrogenations. J. Chem. Health Saf. 2016, 23, 16–25. 10.1016/j.jchas.2015.10.019. [DOI] [Google Scholar]
- Fannes C.; Verbruggen S.; Janssen B.; Egle B. Influence of solvents and additives on the pyrophoricity of palladium on carbon catalyst after hydrogenation. Org. Process Res. Dev. 2021, 25, 2438–2441. 10.1021/acs.oprd.1c00190. [DOI] [Google Scholar]
- Raghuram P.; Soma Raju I. V.; Sriramulu J. Heavy metals testing in active pharmaceutical ingredients: an alternate approach. Pharmazie 2010, 65, 15–18. [PubMed] [Google Scholar]
- Miyamoto H.; Sakumoto C.; Takekoshi E.; Maeda Y.; Hiramoto N.; Itoh T.; Kato Y. Effective method to remove metal elements from pharmaceutical intermediates with polychelated resin scavenger. Org. Process Res. Dev. 2015, 19, 1054–1061. 10.1021/acs.oprd.5b00161. [DOI] [Google Scholar]
- Edery H.; Grunfeld Y.; Ben-Zvi Z.; Mechoulam R. Structural requirements for cannabinoid activity. Ann. N.Y. Acad. Sci. 1971, 191, 40–53. 10.1111/j.1749-6632.1971.tb13985.x. [DOI] [Google Scholar]
- Mechoulam R.; Lander N.; Varkony T. H.; Kimmel I.; Becker O.; Ben-Zvi Z.; Edery H.; Porath G. Stereochemical requirements for cannabinoid activity. J. Med. Chem. 1980, 23, 1068–1072. 10.1021/jm00184a002. [DOI] [PubMed] [Google Scholar]
- Consroe P.; Martin A. R.; Fish B. S. Use of a potential rabbit model for structure-behavioral activity studies of cannabinoids. J. Med. Chem. 1982, 25, 596–599. 10.1021/jm00347a021. [DOI] [PubMed] [Google Scholar]
- Edery H.; Porath G.; Mechoulam R.; Lander N.; Srebnik M.; Lewis N. Activity of novel aminocannabinoids in baboons. J. Med. Chem. 1984, 27, 1370–1373. 10.1021/jm00376a029. [DOI] [PubMed] [Google Scholar]
- Thapa D.; Babu D.; Park M.-A.; Kwak M.-K.; Lee Y.-R.; Kim J. M.; Kwon T. K.; Kim J.-A. Induction of p53-independent apoptosis by a novel synthetic hexahydrocannabinol analog is mediated via Sp1-dependent NSAID-activated gene-1 in colon cancer cells. Biochem. Pharmacol. 2010, 80, 62–71. 10.1016/j.bcp.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Elsohly M. A.; Harland E. C.; Benigni D. A.; Waller C. W. Cannabinoids in glaucoma II: The effect of different cannabinoids on intraocular pressure of the rabbit. Curr. Eye Res. 1984, 3, 841–850. 10.3109/02713688409000797. [DOI] [PubMed] [Google Scholar]
- Collins A.; Tesfatsion T.; Ramirez G.; Ray K.; Cruces W. Nonclinical in vitro safety assessment summary of hemp derived (R/S)-hexahydrocannabinol ((R/S)-HHC). Cannabis Sci. Technol. 2022, 5, 23–27. [Google Scholar]
- Harvey D. J.; Brown N. K. Comparative in vitro metabolism of the cannabinoids. Pharmacol., Biochem. Behav. 1991, 40, 533–540. 10.1016/0091-3057(91)90359-A. [DOI] [PubMed] [Google Scholar]
- Sanchez Montero J. M.; Agis-Torres A.; Solano D.; Söllhuber M.; Fernandez M.; Villaro W.; Gómez-Cañas M.; García-Arencibia M.; Fernández-Ruiz J.; Egea J.; Martín M. I.; Girón R. Analogues of cannabinoids as multitarget drugs in the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 895, 173875. 10.1016/j.ejphar.2021.173875. [DOI] [PubMed] [Google Scholar]
- Nikas S. P.; Alapafuja S. P.; Papanastasiou I.; Paronis C. A.; Shukla V. G.; Papahatjis D. P.; Bowman A. L.; Halikhedkar A.; Han X.; Makriyannis A. Novel 1′,1′-chain substituted hexahydrocannabinols: 9βHydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahydrocannabinol (AM2389) a highly potent cannabinoid receptor 1 (CB1) agonist. J. Med. Chem. 2010, 53, 6996–7010. 10.1021/jm100641g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D.; Peigneur S.; Hendrickx L. A.; Tytgat J. Targeting cannabinoid receptors: Current status and prospects of natural products. Int. J. Mol. Sci. 2020, 21, 5064. 10.3390/ijms21145064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. Neurobiology of cannabinoid receptor signaling. Dialogues Clin. Neurosci. 2020, 22, 207–222. 10.31887/DCNS.2020.22.3/blutz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattamuri P. V.; West J. G. Cooperative hydrogen atom transfer: From theory to applications. Synlett 2021, 32, 1179–1186. 10.1055/a-1463-9527. [DOI] [Google Scholar]
- Ashby E. C.; Lin J. J. Selective reduction of alkenes and alkynes by the reagent lithium aluminum hydride-transition-metal halide. J. Org. Chem. 1978, 43, 2567–2572. 10.1021/jo00407a004. [DOI] [Google Scholar]
- Green S. A.; Crossley S. W. M.; Matos J. L. M.; Vaśquez–Ceśpedes S.; Shevick S. L.; Shenvi R. A. The high chemofidelity of metal-catalyzed hydrogen atom transfer. Acc. Chem. Res. 2018, 51, 2628–2640. 10.1021/acs.accounts.8b00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattamuri P. V.; West J. G. Hydrogenation of alkenes via cooperative hydrogen atom transfer. J. Am. Chem. Soc. 2020, 142, 19316–19326. 10.1021/jacs.0c09544. [DOI] [PubMed] [Google Scholar]
- Reggio P. H.; Greer K. V.; Cox S. M. The importance of the Orientation of the C9 Substituent to cannabinoid activity. J. Med. Chem. 1989, 32, 1630–1635. 10.1021/jm00127a038. [DOI] [PubMed] [Google Scholar]
- Munro S.; Thomas K. L.; Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M.; Calandra B.; Shire D.; Bouaboula M.; Oustric D.; Barth F.; Casellas P.; Ferrara P.; Le Fur G. Characterization of two cloned human CB1 cannabinoid receptor isoforms. J. Pharmacol. Exp. Ther. 1996, 278, 871–878. [PubMed] [Google Scholar]
- Felder C. C.; Joyce K. E.; Briley E. M.; Mansouri J.; Mackie K.; Blond O.; Lai Y.; Ma A. L.; Mitchell R. L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995, 48, 443–450. [PubMed] [Google Scholar]
- Howlett A. C.; Barth F.; Bonner T. I.; Cabral G.; Casellas P.; Devane W. A.; Felder C. C.; Herkenham M.; Mackie K.; Martin B. R.; Mechoulam R.; Pertwee R. G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Devane W. A.; Dysarz F. A.; Johnson M. R.; Melvin L. S.; Howlett A. C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [PubMed] [Google Scholar]
- D’Ambra T. E.; Estep K. G.; Bell M. R.; Eissenstat M. A.; Josef K. A.; Ward S. J.; Haycock D. A.; Baizman E. R.; Casiano F. M.; Beglin N.; Chippari S. M.; Grego J. D.; Kullnig R. K.; Daley G. T. Conformationally restrained analogs of pravadoline: nanomolar potent, enantioselective, (aminoalkyl) indole agonists of the cannabinoid receptor. J. Med. Chem. 1992, 35, 124–135. 10.1021/jm00079a016. [DOI] [PubMed] [Google Scholar]
- Degorce F.; Card A.; Soh S.; Trinquet E.; Knapik G. P.; Xie B. HTRF: A technology tailored for drug discovery – A review of theoretical aspects and recent applications. Curr. Chem. Genomics 2009, 3, 22–32. 10.2174/1875397300903010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujváry I. Hexahydrocannabinol and closely related semi-synthetic cannabinoids: A comprehensive review. Drug Test. Anal. 2023, 10.1002/dta.3519. [DOI] [PubMed] [Google Scholar]
- Graziano S.; Varì M. R.; Pichini S.; Busardo F. P.; Cassano T.; Di Trana A. Hexahydrocannabinol pharmacology, toxicology, and analysis: the first evidence for a recent new psychoactive substance. Curr. Neuropharmacol. 2023, 10.2174/1570159X21666230623104624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F.; Vandelli M. A.; Biagini G.; et al. Synthesis and pharmacological activity of the epimers of hexahydrocannabinol (HHC). Sci. Rep. 2023, 13, 11061. 10.1038/s41598-023-38188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.